Abstract
Coated vesicles concentrate and package cargo molecules to mediate their efficient transport between intracellular compartments. Cytosolic coat proteins such as clathrin and adaptor complexes, and COPI and COPII coatomer complexes self-assemble to deform the membrane and interact directly with cargo molecules to capture them in nascent buds. The GTPases Arf, Sar1 and dynamin are core components of the coated vesicle machinery. These GTPases, which associate with and dissociate from donor membranes in a GTP-dependent manner, can also actively remodel membranes. Recent evidence suggests that although structurally diverse, Arf-family GTPases and dynamin may play mechanistically similar roles as fidelity monitors that govern cargo packaging and coated vesicle maturation, and as components of the fission machinery to mediate vesicle release.
The modular organization of the eukaryotic cytoplasm into membrane-bound organelles contributes to increased efficiency and specificity of chemical reactions. Organelles communicate with each other through vesicular transport, where information is packaged and transported in small vesicles. Cargo routing and the speed of delivery are thus key determinants for successful information transfer between compartments.
Coated vesicular transport is a specialized form of cargo transfer involving vesicles enveloped by a discernable coat. Coated vesicles achieve high selectivity as protein cargo transporters due to their high protein to lipid ratio (1). Clathrin-coated vesicles (CCVs) mediate transport between the plasma membrane, endosome and trans Golgi compartments while COPI-and COPII- (coatomer complexes I and II) coated vesicles mediate transport from the Golgi and endoplasmic reticulum (ER), respectively (2–4). Coat complexes perform the dual role of concentrating cargo and sculpting membranes in the process of generating nascent transport vesicles. Growing evidence points to a role for membrane-active GTPases in monitoring the fidelity and efficiency of these processes. Arf1 (ADP-Ribosylation Factor 1) functions in COPI vesicles and trans Golgi-derived CCVs, Sar1 (Secretion-associated RAS-related protein 1) in COPII vesicle formation at the ER and dynamin in CCV formation at the plasma membrane. Recent studies indicate that these GTPases show important mechanistic similarities in terms of their role in cargo packaging and their ability to actively remodel membranes. Here we review these findings and propose general models that might explain how these GTPases could regulate the formation of coated vesicles.
First Principles of Coated Vesicle Formation
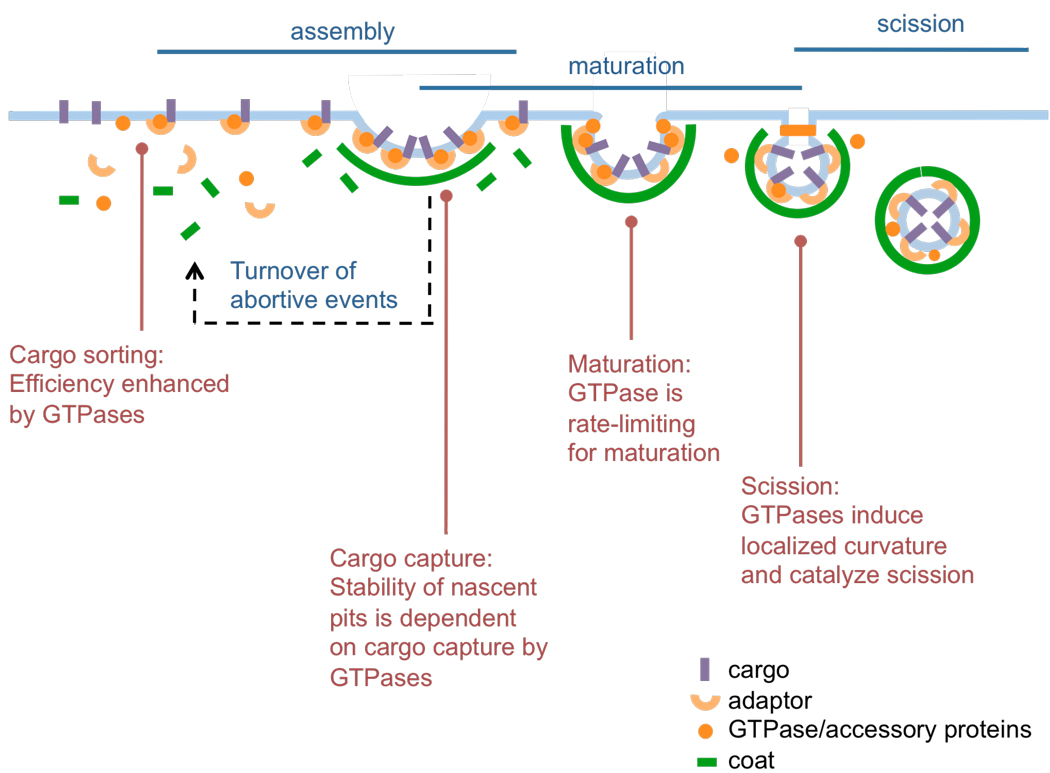
Coated vesicle formation can be viewed as a progression of three stages: 1) assembly of coat subunits on the membrane, during which cargo recognition and sorting take place; 2) maturation, during which the coat and underlying membrane acquire curvature; and 3) scission to release a coated vesicle (5, 6) (Fig. 1). Coat assembly and maturation, while conceptually distinct, are in fact intricately linked and temporally overlapping stages that are required for efficient cargo capture and packaging. The efficiency of coated vesicle-mediated transport is determined both by their ability to select and concentrate cargo molecules during assembly and maturation, and by the overall rate of the maturation process and membrane scission leading to vesicle formation.
Fig. 1.
General scheme of coated vesicle formation. This process involves the initial assembly of coat subunits on the membrane, during which cargo recognition and sorting takes place, followed by maturation, during which the coat and underlying membrane acquires curvature. Scission of the coated bud finally generates a coated vesicle. The reported involvement of the membrane active GTPases, Arf1, Sar1 and dynamin, during each of these stages is described.
The three major classes of coated vesicles (CCV, COPI and COPII) are distinct in their protein and lipid composition and recognize distinct sets of cargo. Two cytosolic protein complexes, clathrin triskelia and heterotetrameric adaptor proteins for CCVs, and the Sec13–31 and Sec23–24 complexes for COPII vesicles assemble on the membrane to form the coat. A single, multisubunit coatomer complex assembles to form the COPI coat. In spite of these compositional differences, coated vesicles show architectural similarities. For example, each coat consists of an outer cage composed of polymerized protein complexes containing N-terminal β-propeller-WD40 and C-terminal α-solenoid motifs (7, 8). Biochemical and structural studies have shown that these protein complexes exhibit a tendency to self-assemble into empty spherical cages (9–11). Cage subunits per se do not bind membranes and need to be recruited by an inner adaptor layer. The adaptor proteins display remarkable diversity in their structural characteristics but are similar in their capacity for simultaneous recognition of multiple membrane-bound signals such as sorting motifs in cargo, additional coat proteins, and negatively charged membrane lipids. Even in solution, adaptors promote self-assembly of the outer cage layer subunits and modulate their sizes by recruiting more cage subunits and/or by structurally affecting the inherent flexibility of cages (12, 13).
The membrane acts as a platform to concentrate coat proteins while at the same time imparts considerable resistance to bending during formation of coated vesicles (14). Coats recruit additional proteins to counter this resistance. COPI and COPII coated vesicles display a functional conservation in the coat components such that members of the Arf-family of GTPases appear to manage both coat recruitment and curvature generation in membranes (8). In addition to the GTPase dynamin, plasma membrane-derived CCVs recruit accessory proteins such as amphiphysin, epsin, endophilin, and/or members of the sorting nexin family, which have all been shown to bind the core coat components and to generate and/or recognize membrane curvature (8, 15). The accessory proteins interact with the membrane through structural elements such as BAR (Bin, amphiphysin, Rvs) or ENTH (epsin N-terminal homology) domains (16). Unlike the GTPases (see below) membrane binding and/or dissociation of these proteins is not known to be induced or triggered, and thus they show a "passive" mode of membrane association. While they may not be needed to generate curvature per se, they might play a role in stabilizing or locking in the coat-generated curvature, and/or in fine-tuning the rate and degree of curvature generation to enhance the efficiency of cargo loading and the ability to accommodate structurally diverse cargos. The GTPases on the other hand associate with membranes through a more "active" switch-type mechanism dependent on GTP binding and/or hydrolysis. Such switching might control the generation of membrane curvature during coat maturation and/or trigger scission. The importance of this feature is conveyed by the fact that their involvement is critical to all forms of coated vesicular transport.
Arf1 in COPI Vesicle Formation
Arf-family GTPases constitute members of the Ras superfamily (17) that display low rates of GDP-dissociation and GTP-binding and thus require extrinsic GTP exchange factors (GEFs). In addition, they have an intrinsically low rate of GTP hydrolysis that is dramatically stimulated by extrinsic GTPase activating proteins (GAPs). The GTP-bound, active state is often associated with biological activity, such as binding to effectors, while conversion to GDP-bound, inactive state leads to loss in binding to effectors. Thus, externally provided GEF and GAP activities transition the GTPases between GTP-bound, active and GDP-bound, inactive states.
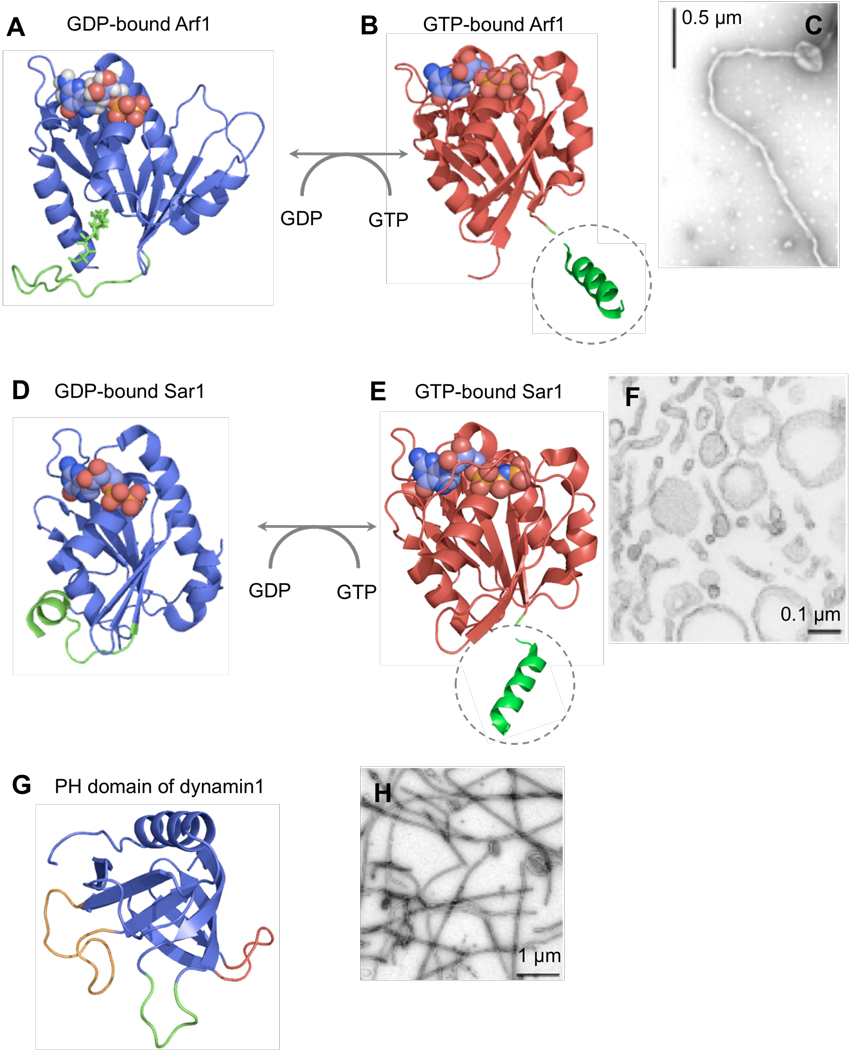
Arf1 initiates COPI coated vesicle formation by recruiting the coatomer complex to the membrane (18). Arf1 associates with the membrane in a GTP-dependent manner, via a short amphipathic N-terminal helix that is covalently modified with a myristoyl chain (Fig. 2). In its GDP-bound state the helix is buried in the molecule, but GTP binding triggers a conformational change in the molecule that exposes the amphipathic helix. As a result, Arf1 has a higher affinity for the membrane in the GTP bound state. Recent results show that upon binding, Arf1 can remodel membranes into highly curved tubules with an outer diameter of ~45 nm (Fig. 2) (19, 20). GDP-bound Arf1 or Arf mutants with polar substitutions in the amphipathic helix do not bind membranes and, as a result, are not membrane active. Curvature generation by Arf1 can be explained by the bilayer-couple model (21) where the insertion of the amphipathic N-terminal helix would cause an asymmetric expansion of the outer versus the inner leaflet of the lipid bilayer thereby causing it to bend. Recent evidence also indicates that GTP-bound, membrane-active Arf1 can form dimers, which could stabilize regions of high membrane curvature (19).
Fig. 2.
Membrane active GTPases in coated vesicular transport. (A) Structure of myristoylated Arf1 in the GDP-bound state (PDB 2K5U) (51). The myristoyl chain and the first 16 residues are shown in green. (B) Structure of Arf1 in the GTP-bound state (PDB 1O3Y) (52). Gly16 and a schematic representation of the exposed N-terminal helix without the myristoyl chain (dotted circle) are shown in green. (C) Membrane tubules formed by GTP-bound Arf1 (20). (D) Structure of GDP-bound Sar1 (PDB 2GAO). Residues 13–26 are shown in green. (E) Structure of GTP-bound Sar1 (PDB 1M2O) (53). Gly24 and a representation of the extended N-terminal helix (dotted circle) are shown in green. (F) Membrane tubules formed by GTP-bound Sar1 (24). (G) Structure of dynamin1 PH domain (PDB 1DYN) (54). The variable loops VL1 (green), VL2 (red) and VL3 (gold) are highlighted. (H) Membrane tubules formed by dynamin1 (34).
Growing evidence points to an intricate relationship between GEF- and GAP-mediated GTP binding and hydrolysis by Arf1 and membrane curvature. GBF-1 (Golgi-specific brefeldin A-resistance factor 1) and ArfGAP1, which are the Golgi-localized GEF and GAP for Arf1 are peripherally associated membrane proteins involved in COPI vesicle formation (17). GBF-1 is thought to associate with Golgi membranes through interactions with resident membrane proteins (22). GTP binding promoted by GBF-1 causes Arf1 to associate with membranes, which could result in the generation of membrane curvature. ArfGAP1, on the other hand, binds membranes through a motif termed ArfGAP1-like lipid packing sensor (ALPS). ALPS motifs are amphipathic helices lined by residues that pack favorably into membranes of high curvature (23). Thus, ArfGAP1 displays higher affinity for curved membranes thereby selectively promoting GTP hydrolysis by Arf1 on curved membranes.
Sar1 in COPII Vesicle Formation
Like Arf, Sar1 is a Ras-related GTPase that initiates COPII coated vesicle formation by recruiting the adaptor complex Sec23–24 to the membrane (18). Sar1 also shows similarities to Arf1 in terms of GTP-binding and hydrolysis dependent membrane association properties. GTP loading of Sar1 exposes a long N-terminal amphipathic helix, which is not myristoylated, but nonetheless mediates membrane binding (Fig. 2). Binding of Sar1 to liposomes induces formation of membrane tubules with an outer diameter of ~26 nm (Fig. 2) (24, 25). Neither Sar1 mutants deleted in residues that comprise the amphipathic helix, nor wild type GDP-bound Sar1 can associate with membranes. The GEF for Sar1 is Sec12, an integral ER-localized membrane protein. The COPII adaptor protein Sec23–24 and outer coat protein Sec13–31 both function as GAPs causing GTP hydrolysis on Sar1 (26).
Dynamin family of GTPases in CCV formation
The dynamin family of GTPases show biochemical properties that are distinct from those of the small Ras-family GTPases (27). They display a low affinity for GTP and high rates of GDP dissociation. Nucleotide exchange can thus occur spontaneously without external GEFs. Dynamin family members also show high basal rates of GTP hydrolysis, which is further stimulated upon self-assembly. Dynamin 1, the prototypical member of this family is involved in CCV formation from the presynaptic membrane while dynamin 2 functions in clathrin-mediated endocytosis in nonneuronal cells (28).
Dynamin is a multidomain GTPase that binds membranes via a PH domain, which provides docking sites for negatively charged lipid headgroups and contains three loops, the first of which is lined by hydrophobic residues (Fig. 2). Dynamin interacts with multiple SH3 domain-containing endocytic accessory proteins through its C-terminal proline-arginine rich domain (PRD). Despite its ability to bind directly to membranes in vitro, in vivo studies have shown that PRD-SH3 domain protein interactions are required to recruit dynamin to clathrin-coated pits (29). Dynamin has a high propensity to self-assemble under low ionic strength conditions or under physiological conditions on a membrane template. Self-assembly considerably stimulates basal GTP hydrolysis, in part mediated by an intramolecular GTPase effector domain (GED) (30, 31). Although the mechanism remains unknown, self-assembly activates an intrinsic GAP activity in dynamin. Despite its ability to bind liposomes in a nucleotide-independent manner, recent results using site-specific labeling of residues in the PH domain with environment-sensitive fluorescent probes suggest that GTP binding and hydrolysis on dynamin1 provide a reversible switch for membrane association (32). Thus, dynamin1 shows higher membrane binding in the apo or GTP-bound state and lower binding in the GDP-bound state. When analyzed in the presence of GTP, dynamin1 binds first and then dissociates from the membrane reflecting a cycle of association, spontaneous self-assembly, GTP hydrolysis, and dissociation. Residues in the PH domain show significant insertion into the lipid bilayer and this, together with dynamin’s scaffolding activity, could induce and stabilize membrane curvature (32, 33). Indeed, membrane binding of dynamin causes the formation of membrane tubules with an outer diameter of ~45 nm (Fig. 2) (34).
GTPases Regulate Cargo Sorting during Coated Vesicle Formation
Proofreading mechanisms ensure sorting of bona fide cargo and maximize cargo uptake thereby contributing to the fidelity and efficiency of vesicular transport. Coat adaptors display multiple independent binding sites for cargo, coat components, and negatively-charged lipids. Because binding affinities for polyvalent interactions are much higher compared to monovalent interactions, coincident detection of such binding partners contributes to the spatiotemporal coordination of coat assembly. Thus, coat assembly occurs on the right cellular compartment and when adaptors engage with as many partners as possible.
Several reports suggest the involvement of GTPases in regulating cargo sorting during coated vesicular transport (Fig. 1). GTP-bound, membrane-anchored Arf1 interacts with cargo-ArfGAP1 and cargo-coatomer β subunit complexes, while GTP-bound Sar1 interacts with cargo- Sec23–24 complexes to couple cargo recruitment to coat assembly (35, 36). This form of cargo delivery in complex with coat components ensures efficient incorporation of essential cargo such as soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) molecules into coated vesicles. The involvement of Arf-family GTPases in these early stages provides an avenue for regulation of cargo sorting into coated pits via a kinetic proofreading mechanism based on the stability of the cargo, GTPase, and coat complex (Fig. 1). Thus, given the intrinsic Sar1 GAP activity of the COPII coat, Sar1 and Sec23–24 complexes interacting with bona fide ER-associated cargo molecules will be longer-lived than those not interacting with cargo molecules on the membrane (37). Similarly, ArfGAP1 activity can be regulated by cargo and coat interactions and in this way coat assembly can be tightly coupled to efficient cargo recruitment (38). In the case of plasma membrane-derived CCVs, dynamin also appears to control cargo sorting but by a different mechanism (Fig. 1). Recent results using total internal reflection fluorescence microscopy (TIR-FM) to monitor the dynamics of clathrin-coats on the plasma membrane combined with statistical analyses have indicated that dynamin controls early decision-making and rate-limiting events in CCV formation (6). Thus, reducing dynamin concentrations in cells decreases the rate of turnover of early abortive species and increases the maturation time of coated pits, while expression of dynamin point mutants impaired in self-assembly leads to faster turnover of abortive species and increased endocytic uptake of cargo (30). These results have been rationalized based on a model in which dynamin acts as a fidelity sensor that monitors maturation efficiencies of coated vesicles. Live cell microscopy has also demonstrated that specific cargo molecules (e.g. G-protein coupled receptors) can regulate dynamin recruitment to CCPs and thus regulate the kinetics and fidelity of endocytic sorting events (39).
Membrane Fission During Coated Vesicular Transport
Release of coated vesicles requires the membrane necks of coated buds to undergo fission, a process requiring GTP hydrolysis. This is apparent from early studies where attached membrane buds arrested prior to the late step of scission accumulate in cells or synaptosomes perfused with non-hydrolyzable GTP analogues (40, 41). Reconstitution studies using liposomes have identified the minimal components necessary for the formation of COPI and COPII vesicles (42, 43). Early studies in these minimal systems indicated that GTP-bound Arf1 and Sar1 could both recruit coat proteins to the membrane and cause scission. However, more recent studies that monitor release of coated vesicles under milder conditions, which avoid shearing of budded membranes, suggest that GTP hydrolysis is indeed necessary for coat scission (25, 44). Similar reconstitution studies have not been performed to identify the minimum components necessary for CCV formation, although clathrin-mediated endocytosis in perforated cells is blocked at a late stage by non-hydrolyzable GTP analogues (45).
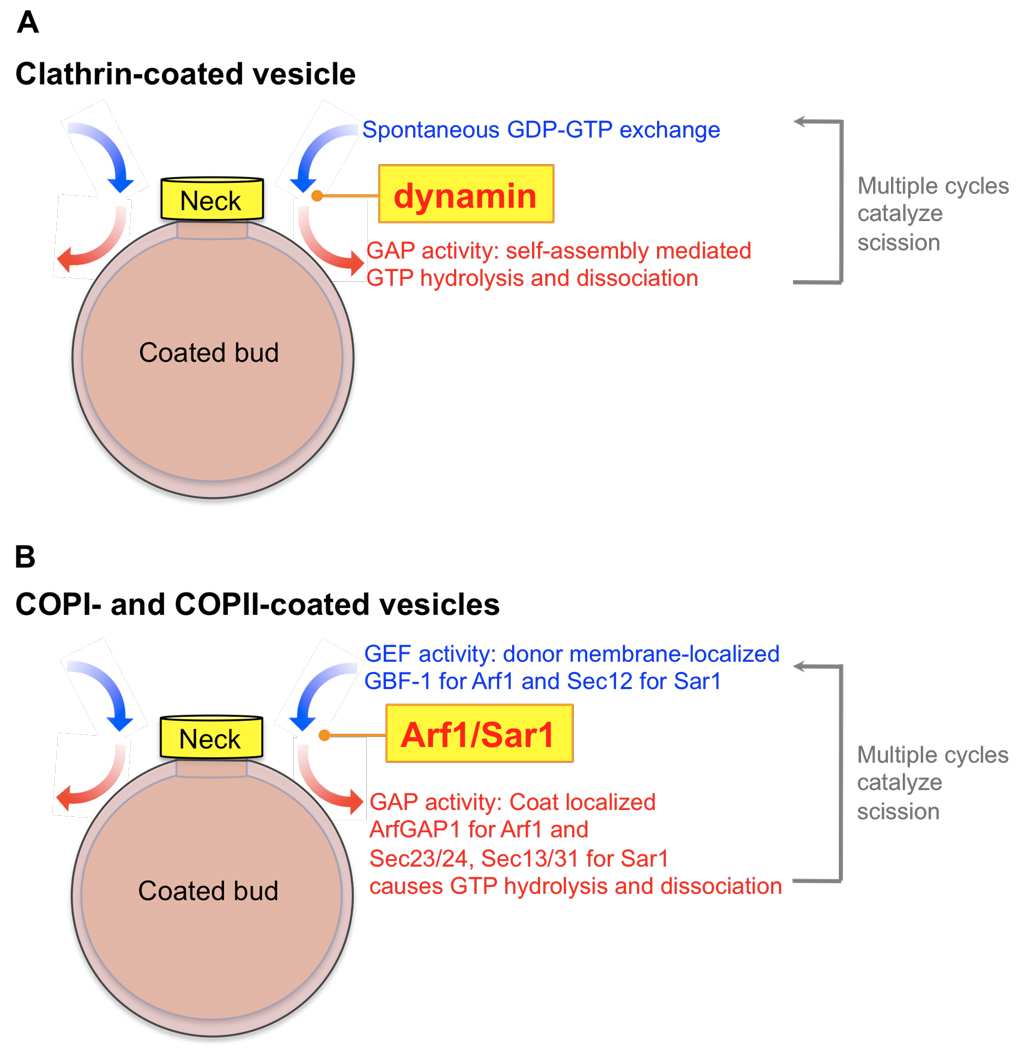
Dynamin1-mediated membrane fission has been reconstituted on liposomes and has been shown to require GTP hydrolysis (34, 46). More recently, dynamin-catalyzed membrane fission has been studied in greater detail using a system of supported bilayers with excess reservoir or SUPER templates (47) and by direct conductivity measurements of membrane tubules (48). Results from these studies could provide a general model for coated vesicle scission catalyzed by membrane active GTPases (Fig. 3). Previous models suggested that dynamin-mediated fission required a power-stroke generated by a concerted conformational change in assembled dynamin and triggered by rapid GTP hydrolysis (27, 34, 46, 49). However, more recent studies have shown that dynamin assemblies stabilize highly curved templates (48) and that membrane fission requires GTP hydrolysis-dependent reversible membrane association of dynamin (47). During these cycles, and due to dynamin's ability to induce curvature, the underlying membrane undergoes squeezing and relaxation resulting in the stochastic generation of a hemifission intermediate thereby causing fission (47, 48). Thus, a seemingly futile cycle of membrane binding and GTP hydrolysis-dependent dissociation is in fact necessary for dynamin-catalyzed membrane fission.
Fig. 3.
General model for scission of coated-buds. We propose that the restricted localization of membrane active GTPases to the necks of budding coated vesicles could catalyze membrane fission. In the case of clathrin-coated buds (A), dynamin is localized at the neck where multiple cycles of membrane association, spontaneous self-assembly, GTP hydrolysis to produce the GDP-bound state, and membrane dissociation leads to scission. The donor membrane-localized GEF activity and coat-localized GAP activity could restrict Arf1 and Sar1 localization to the necks of COPI- and COPII-coated buds (B). As a consequence, reversible membrane association of these GTPases at the neck could eventually lead to scission of the coated-bud.
It is tempting to speculate that Arf1 and Sar1 could function similarly during scission of COPI and COPII vesicles (Fig. 3). Dynamin is self-sufficient in catalyzing membrane fission because it does not require an external GEF or GAP for GTP hydrolysis and reversible membrane association. At the necks of budded COPI and COPII vesicles, both Arf1 and Sar1 could acquire properties similar to dynamin1. At late stages of vesicle formation, the GAPs for Arf1 and Sar1, i.e. the curvature-sensitive ArfGAP and coat components Sec23–24, Sec13–31, are present exclusively on the membrane bud, while the GEFs, GBF-1 and Sec12 are restricted to the more planar donor membrane. Moreover, recent studies show that lipid packing defects associated with regions of high membrane curvature could promote spontaneous insertion of Arf1's amphipathic helix thereby allowing GDP-GTP exchange (50). In principle, this effect could obviate the need for GEF activity at the necks of budded vesicles. Thus, due in part to the spatial segregation of GEFs and GAPs and to their local high curvature, the necks of budded vesicles could represent a region where the combined influence of GEFs and GAPs promote GTP-binding and hydrolysis thereby causing Arf1 and Sar1 to undergo multiple cycles of membrane binding and release (Fig. 3). This dynamic behavior would mirror that of dynamin1 and could thus catalyze scission and coated vesicle release by a common mechanism.
Future Perspectives
While we have a good knowledge of what makes a coated vesicle (i.e. its constituents), we still have little understanding of how a coated vesicle is made (i.e. the mechanisms governing vesicle formation). Better assays are needed to reconstitute the transport of cargo in a biochemically-defined environment. Fluorescence-based approaches to monitor protein-protein and protein-lipid interactions along with assays that distinguish stages of budding and fission would provide the much-needed dynamic information. Most of our understanding of vesicular transport is derived from the read-outs of bulk assays. Bulk read-outs may be insufficient to understand cargo sorting during coated vesicular transport because cargo transport is a function of both cargo density per vesicle and the number of vesicles. Results from such assays average out possible differences between cargo content among individual vesicles. It is thus important to interrogate the process of coated vesicle formation at the single vesicle level. TIR-FM based analysis is easily applied to cell surface phenomena such as clathrin-mediated endocytosis but may not be a viable option for intracellular vesicular transport. However such analysis in reconstituted systems should provide a clearer understanding of the process of cargo sorting and vesicle formation. We may find interesting mechanistic differences between the different classes of coat proteins, but a deeper understanding may also reveal more mechanistic similarities than currently appreciated.
Notes
- 1.Takamori S, et al. Cell. 2006 Nov 17;127:831. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 2.Schmid SL. Annu Rev Biochem. 1997;66:511. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 3.Rothman JE. Nature. 1994 Nov 3;372:55. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 4.Schekman R, Orci L. Science. 1996 Mar 15;271:1526. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- 5.Kirchhausen T. Nat Rev Mol Cell Biol. 2000 Dec;1:187. doi: 10.1038/35043117. [DOI] [PubMed] [Google Scholar]
- 6.Loerke D, et al. PLoS Biol. 2009 Mar 17;7:e57. doi: 10.1371/journal.pbio.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stagg SM, LaPointe P, Balch WE. Curr Opin Struct Biol. 2007 Apr;17:221. doi: 10.1016/j.sbi.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 8.McMahon HT, Mills IG. Curr Opin Cell Biol. 2004 Aug;16:379. doi: 10.1016/j.ceb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Stagg SM, et al. Nature. 2006 Jan 12;439:234. doi: 10.1038/nature04339. [DOI] [PubMed] [Google Scholar]
- 10.Kirchhausen T. Annu Rev Biochem. 2000;69:699. doi: 10.1146/annurev.biochem.69.1.699. [DOI] [PubMed] [Google Scholar]
- 11.Fath S, Mancias JD, Bi X, Goldberg J. Cell. 2007 Jun 29;129:1325. doi: 10.1016/j.cell.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 12.Stagg SM, et al. Cell. 2008 Aug 8;134:474. doi: 10.1016/j.cell.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Annu Rev Cell Dev Biol. 2001;17:517. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- 14.Ungewickell EJ, Hinrichsen L. Curr Opin Cell Biol. 2007 Aug;19:417. doi: 10.1016/j.ceb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Borner GH, Harbour M, Hester S, Lilley KS, Robinson MS. J Cell Biol. 2006 Nov 20;175:571. doi: 10.1083/jcb.200607164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoh T, De Camilli P. Biochim Biophys Acta. 2006 Aug;1761:897. doi: 10.1016/j.bbalip.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Gillingham AK, Munro S. Annu Rev Cell Dev Biol. 2007;23:579. doi: 10.1146/annurev.cellbio.23.090506.123209. [DOI] [PubMed] [Google Scholar]
- 18.Spang A. Cell Mol Life Sci. 2008 Sep;65:2781. doi: 10.1007/s00018-008-8349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck R, et al. Proc Natl Acad Sci U S A. 2008 Aug 19;105:11731. doi: 10.1073/pnas.0805182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krauss M, et al. J Biol Chem. 2008 Oct 10;283:27717. doi: 10.1074/jbc.M804528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheetz MP, Singer SJ. Proc Natl Acad Sci U S A. 1974 Nov;71:4457. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu TK, Pfeifer AC, Lippincott-Schwartz J, Jackson CL. Mol Biol Cell. 2005 Mar;16:1213. doi: 10.1091/mbc.E04-07-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bigay J, Gounon P, Robineau S, Antonny B. Nature. 2003 Dec 4;426:563. doi: 10.1038/nature02108. [DOI] [PubMed] [Google Scholar]
- 24.Lee MC, et al. Cell. 2005 Aug 26;122:605. doi: 10.1016/j.cell.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Bielli A, et al. J Cell Biol. 2005 Dec 19;171:919. doi: 10.1083/jcb.200509095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barlowe C, Schekman R. Nature. 1993 Sep 23;365:347. doi: 10.1038/365347a0. [DOI] [PubMed] [Google Scholar]
- 27.Song BD, Schmid SL. Biochemistry. 2003 Feb 18;42:1369. doi: 10.1021/bi027062h. [DOI] [PubMed] [Google Scholar]
- 28.Liu YW, Surka MC, Schroeter T, Lukiyanchuk V, Schmid SL. Mol Biol Cell. 2008 Dec;19:5347. doi: 10.1091/mbc.E08-08-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shpetner HS, Herskovits JS, Vallee RB. J Biol Chem. 1996 Jan 5;271:13. doi: 10.1074/jbc.271.1.13. [DOI] [PubMed] [Google Scholar]
- 30.Sever S, Muhlberg AB, Schmid SL. Nature. 1999 Apr 8;398:481. doi: 10.1038/19024. [DOI] [PubMed] [Google Scholar]
- 31.Chappie JS, Acharya S, Liu Y-W, Leonard M, Pucadyil TJ, Schmid SL. Mol Biol Cell. 2009 doi: 10.1091/mbc.E09-04-0318. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramachandran R, Schmid SL. EMBO J. 2008 Jan 9;27:27. doi: 10.1038/sj.emboj.7601961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burger KN, Demel RA, Schmid SL, de Kruijff B. Biochemistry. 2000 Oct 10;39:12485. doi: 10.1021/bi000971r. [DOI] [PubMed] [Google Scholar]
- 34.Sweitzer SM, Hinshaw JE. Cell. 1998 Jun 12;93:1021. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- 35.Springer S, Spang A, Schekman R. Cell. 1999 Apr 16;97:145. doi: 10.1016/s0092-8674(00)80722-9. [DOI] [PubMed] [Google Scholar]
- 36.Sato K, Nakano A. FEBS Lett. 2007 May 22;581:2076. doi: 10.1016/j.febslet.2007.01.091. [DOI] [PubMed] [Google Scholar]
- 37.Forster R, et al. Curr Biol. 2006 Jan 24;16:173. doi: 10.1016/j.cub.2005.11.076. [DOI] [PubMed] [Google Scholar]
- 38.Lee SY, Yang JS, Hong W, Premont RT, Hsu VW. J Cell Biol. 2005 Jan 17;168:281. doi: 10.1083/jcb.200404008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puthenveedu MA, von Zastrow M. Cell. 2006 Oct 6;127:113. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 40.Takei K, McPherson PS, Schmid SL, De Camilli P. Nature. 1995 Mar 9;374:186. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- 41.Weidman P, Roth R, Heuser J. Cell. 1993 Oct 8;75:123. [PubMed] [Google Scholar]
- 42.Bremser M, et al. Cell. 1999 Feb 19;96:495. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- 43.Matsuoka K, et al. Cell. 1998 Apr 17;93:263. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- 44.Yang JS, et al. J Cell Biol. 2002 Oct 14;159:69. doi: 10.1083/jcb.200206015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carter LL, Redelmeier TE, Woollenweber LA, Schmid SL. J Cell Biol. 1993 Jan;120:37. doi: 10.1083/jcb.120.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roux A, Uyhazi K, Frost A, De Camilli P. Nature. 2006 May 25;441:528. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- 47.Pucadyil TJ, Schmid SL. Cell. 2008 Dec 26;135:1263. doi: 10.1016/j.cell.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bashkirov PV, et al. Cell. 2008 Dec 26;135:1276. doi: 10.1016/j.cell.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hinshaw JE, Schmid SL. Nature. 1995 Mar 9;374:190. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- 50.Lundmark R, Doherty GJ, Vallis Y, Peter BJ, McMahon HT. Biochem J. 2008 Sep 1;414:189. doi: 10.1042/BJ20081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, Kahn RA, Prestegard JH. Structure. 2009 Jan 14;17:79. doi: 10.1016/j.str.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiba T, et al. Nat Struct Biol. 2003 May;10:386. doi: 10.1038/nsb920. [DOI] [PubMed] [Google Scholar]
- 53.Bi X, Corpina RA, Goldberg J. Nature. 2002 Sep 19;419:271. doi: 10.1038/nature01040. [DOI] [PubMed] [Google Scholar]
- 54.Ferguson KM, Lemmon MA, Schlessinger J, Sigler PB. Cell. 1994 Oct 21;79:199. doi: 10.1016/0092-8674(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 55.We thank members of the Schmid lab, A. Razvi and S. Kalipatnapu for critical comments on the manuscript. We apologize to those authors whose work we could not cite due to space constraints. This work was supported by a career development grant from the Leukemia & Lymphoma Society to T.J.P. and NIH grants (GM42455, MH61345, GM73165) to S.L.S. This is TSRI manuscript number 20182.