Abstract
Naphthalene is a volatile aromatic hydrocarbon to which humans are exposed from a variety of sources including mobile air sources and cigarette smoke. Naphthalene produces dose- (concentration) dependent injury to airway epithelial cells of murine lung which is observed at concentrations well below the current occupational exposure standard. Toxicity is dependent upon the cytochrome P450 mediated metabolic activation of the parent substrate to unstable metabolites which become bound covalently to tissue proteins. Nearly 70 proteins have been identified as forming adducts with reactive naphthalene metabolites using in vitro systems but very little work has been conducted in vivo because reasonably large amounts (100 μCi) of 14C labeled parent compound must be administered to generate detectable adduct levels on storage phosphor screens following separation of labeled proteins by 2 D gel electrophoresis. The work described here was done to provide proof of concept that protein separation by free flow electrophoresis followed by AMS detection of protein fractions containing protein bound reactive metabolites would provide adducted protein profiles in animals dosed with trace quantities of labeled naphthalene. Mice were administered 200 mg/kg naphthalene intraperitoneally at a calculated specific activity of 2 DPM/nmol (1 pCi/nmol) and respiratory epithelial tissue was obtained by lysis lavage 4 hr post injection. Free flow electrophoresis (FFE) separates proteins in the liquid phase over a large pH range (2.5–11.5) using low molecular weight acids and bases to modify the pH. The apparatus separates fractions into standard 96-well plates that can be used in other protein analysis techniques. The buffers of the fractions have very high carbon content, however, and need to be dialyzed to yield buffers compatible with 14C-AMS. We describe the processing techniques required to couple FFE to AMS for quantitation of protein adducts.
Keywords: Free flow electrophoresis, FFE, AMS, protein separation
Introduction
Lung disease is the fourth leading cause of morbidity and mortality in the US population. Cancer of the lung has the highest mortality rate of any cancer in both males and females. While death rates from heart disease, cancer and cerebrovascular disease have decreased in the past 10 years, death rates from chronic lower respiratory disease have remained steady. Although tobacco use and traffic-related pollutants have been demonstrated to be causative factors and likely account for a portion of the disease statistics, the precise etiology of lung disease remains largely unknown [1]. Causation is likely mutifactorial involving genetic predisposition and exposure to chemicals, particles, and/or allergens.
Naphthalene is a volatile, bicyclic aromatic hydrocarbon to which humans are exposed from a variety of sources including industrial (aluminum smelting and use as a starting material for various synthetic derivatives) and via combustion of fossil fuels. Short term inhalation exposures (4 hrs) to levels of naphthalene well below the current occupational limit (currently 10 ppm) results in substantial necrosis of murine airway epithelial cells and rat and murine nasal olfactory epithelial cells [2]. Rat airway epithelium is not susceptible to naphthalene even after parenteral exposures to LD50 doses [3]. Similarly, long-term cancer bioassays have revealed susceptibility of female mouse lungs (a slight increase in adenoma formation) at the highest doses tested (30 ppm) whereas in rats, no lung tumors were observed. However, there was a dramatic increase in nasal epithelial cell hyperplasia and an increase in epithelial adenoma and neuroblastoma in rats which was concentration dependent [4]. To date there is insufficient evidence to provide a clear epidemiologic link between naphthalene exposure and any long term heath effects in human lungs.
Human exposure to naphthalene occurs from a variety of sources both industrial [5] and environmental. Naphthalene is a significant component of jet fuel (1–3%) and is a byproduct of combustion of wood, gasoline and tobacco products [6]. Measurements of 1-and 2-naphthol eliminated in human urine have consistently demonstrated detectable levels in almost all samples analyzed [7,8]. Although some of this may arise as a byproduct of carbaryl exposure, the most likely source is environmental naphthalene. Cigarette smoking markedly increases the levels of naphthol in the urine [7].
The finding of nearly universal human exposure to naphthalene along with animal data showing toxicity after both acute and chronic exposure underscores the need to understand the precise mechanisms of toxicity in animal models, to develop and validate markers that are intimately associated with those mechanisms, and to apply these markers to exposed human populations. The work described here is part of a long-term effort to understand the importance of protein targets to which reactive metabolites of naphthalene become bound [9–11] as a mechanism of toxicity. The vast majority of the work conducted uses in vitro models and data is needed from in vivo studies on adducts generated in target cells. Conventional in vivo studies are impractical, particularly in large rodents, because of the large amounts of 14C labeled starting material which must be used to obtain detectable signals using current standard methodology (separation of proteins by 2D gel electrophoresis followed by protein transfer to PVDF membranes and storage phosphorimaging analysis. Accordingly the work described here couples the power of protein separation by free flow electrophoresis with the exquisite sensitivity of 14C-AMS.
Experimental
Animal dosing and treatment
Male Swiss Webster mice (20–30 g) were purchased from Harlan Laboratories, Indianapolis, IN and were allowed food and water ad libitum. Animals were housed in HEPA filtered barrier racks for at least 1 week following receipt from the supplier. All animal use was approved in advance by the IAUCC. 14C-Naphthalene (labeled in the 1,4,5,8 positions) was purchased from Moravek Radiochemicals (>99% radiochemical purity by HPLC) and was diluted with unlabeled naphthalene to achieve a specific activity of 1 pCi/nmole. This specific activity corresponds to 1 14C atom in every 62,400 naphthalene atoms. Naphthalene was administered intraperitoneally in oleic oil at a dose of 200 mg/kg, 5 ml/kg.
Four hrs after administration, animals were given an overdose of pentobarbital, the trachea was cannulated and lungs were removed from the chest cavity. Parenchymal areas of the lung were filled with low melting temperature agarose, the lungs were cooled in 5% dextrose for 10 min to allow agarose to congeal and epithelial airway proteins were selectively removed by lysis lavage. The procedure is described in detail in [12].
FFE separation
Large biological amphoteric molecules such as proteins contain both acidic and basic functional groups and thus can carry net positive or negative charge as well as no net charge. FFE separates proteins by isoelectric point (pI), the pH at which a particular protein carries no net electrical charge. At a pH below the pI, a protein carries a net positive charge while at a pH above the pI, it carries a net negative charge. The FFE uses a technique known as isoelectric focusing (IEF) which allows the proteins to migrate to their pI on a pH gradient. IEF is routinely used in 2-D gel protein separation. An advantage of FFE over gels is maintaining the protein in solution to make additional analyses easier.
The lysate of mouse lung epithelial cells (1 mL of 5 mg/mL protein) was diluted to 1 mg/mL protein in separation medium (7M urea, 2M thiourea, 0.25M mannitol, 1% CHAPS, and proprietary prolytes to create a pH gradient for separation). The diluted sample was then centrifuged at 25,000 RPM (80,000 × g) for 10 min and the supernatant was removed for separation by FFE.
A BD™ Free Flow Electrophoresis System (Becton, Dickinson and Company, Franklin Lakes, NJ USA) was used for the separations. The FFE system is capable of producing an IEF gradient from pH 2.5 to pH 11.5. We used an IEF gradient from pH 3 to pH 9 to fractionate the sample, spanning fractions 23–56 in the 96 well plate. The FFE used a voltage of 400V, current of 18 mA, buffer flow rate (excluding counter flow) of 60 mL/hr, and sample flow rate of 1 mL/hr while operating at 10°C. A pI marker test was performed after FFE equilibration to verify the pH gradient in the apparatus. The sample was applied immediately after checking the gradient. The FFE produced 96 fractions containing approximately 2 mL each. About 2 mg of protein was distributed among the fractions and no single fraction had more than a couple hundred micrograms of protein.
Analysis of FFE Fractions
We initially tried to analyze a subset of the FFE fractions using the carbon content of the buffer solution to serve as a carrier. The FFE buffer was very high in carbon, approximately 132 mg C/mL. We could use only a 10 μL aliquot of each 2 mL fraction. Although we observed variation in 14C content among fractions, the dynamic range was relatively poor due to the small amount of protein in each fraction and the low specific activity of the naphthalene (see Table 1). We decided it would be better to try replacing the buffer with one that contained less carbon so that we could improve signal-to-noise and analyze a larger portion of the fraction.
Table 1.
Analysis of FFE Fractions without changing buffers.
| Fraction Number | pH | F14C | ± |
|---|---|---|---|
| 17 | 2.60 | .2205 | .0067 |
| 18 | 2.61 | .1944 | .0069 |
| 25 | 3.60 | .3186 | .0077 |
| 26 | 3.68 | .4837 | .0081 |
| 27 | 3.93 | .3361 | .0087 |
| 28 | 4.31 | .2296 | .0069 |
| 29 | 4.53 | .2219 | .0071 |
| 30 | 4.78 | .2061 | .0076 |
| 41 | 6.39 | .2015 | .0068 |
| 49 | 7.17 | .2165 | .0068 |
| 57 | 9.62 | .1778 | .0066 |
| 64 | 9.70 | .1737 | .0065 |
F14C units defined by Reimer et al, 2004 [20].
Dialysis
Each fraction was dialyzed in 3500 MW cutoff Spectrapor membrane with a total of 4 buffer changes for 2 days each against 0.1% SDS (sodium dodecyl sulfate). This procedure removes any unbound naphthalene or naphthalene metabolites and exchanges the salts and buffer associated with the sample for 0.1% SDS. Proteins are solubilized in SDS. After dialysis, each fraction contained 0.5 mg C/mL buffer and approximately 3 mL total volume. Dialysis reduced the carbon inventory of each fraction by a factor of more than 100 and produced uniform baseline concentrations of carbon and 14C by replacing the proprietary prolytes from the FFE fraction with 0.1% SDS.
AMS sample prep and analysis
Sample prep of FFE fractions was very similar to our previous work with HPLC fractions [13–15]. Here we used 300 μL aliquots of SDS buffered samples for 14C analysis because this volume fits within our nested combustion tube system for drying samples. Each aliquot was placed in a quartz tube (~6×30 mm, 4 mm i.d.) nested inside two borosilicate glass culture tubes (10×75 mm in 12×100 mm). The insides of the borosilicate tubes were never touched by the researcher. These aliquots contained 150 μg C (25 amol 14C) from SDS, which is insufficient for reliable graphite production using our standard procedure [16]. It was necessary to add 620 μg C from trybutyrin (1μL neat trybutryin delivered with a capillary tube) carrier to ensure robust sample graphitization. The carrier tributyrin contained 10 amol 14C per fraction.
The inner quartz vials were transferred to quartz combustion tubes which were evacuated and sealed. The samples were combusted to CO2 and reduced to filamentous carbon using our standard procedure [16,17]. Graphite samples were packed into aluminum sample holders and carbon isotope ratios were measured on the compact LLNL spectrometer [18]. Typical AMS measurement times were 3–5 min/sample, with a counting precision of 0.8 – 1.4 % and a standard deviation among 3–7 measurements of 1–3%. The 14C/13C ratios of the unknowns were normalized to measurements of four identically prepared standards of known isotope concentration (IAEA-C6 formerly known as Australian National University Sucrose) [19].
Results
Limit of Quantitation
Detection limit and limit of quantitation (LOQ) can be defined in a variety of different ways. We believe a LOQ is more appropriate than a detection limit for this application because the processing of the sample introduces a baseline of 14C in all the fractions. The true LOQ is the 14C signal above this baseline. The carbon carrier contributes 35.0±1.3 (1SD) amol of 14C and 770 μg C per fraction. Six replicates of SDS + tributyrin were used to determine the carrier contribution. The fractions also contained some carbon from the proteins. Approximately 2 mg of protein was distributed across 30 fractions. If we assume uniform distribution, about 65 μg of protein was distributed in each fraction. We know that the protein did not distribute uniformly, but the limit in any one fraction was probably no more than 300 μg of protein. Only 1/10 of each fraction was analyzed, so no more than 30 μg protein (~10 μg C) of contemporary carbon was added to each fraction. This corresponds to no more than 1 amol of 14C from the protein in any single AMS sample. The contribution of 14C from protein to each fraction is less than the uncertainty of the carrier. We calculated a gross LOQ by adding together the 14C contributions of the carrier baseline (35.0 amol), the maximum protein contribution (1.0 amol), and three times the uncertainty in the baseline (4.2 amol). Any sample containing more than 40.2 amol 14C was above the gross LOQ. This LOQ corresponds to 5.2 amol 14C above carrier baseline.
Protein adduct measurement
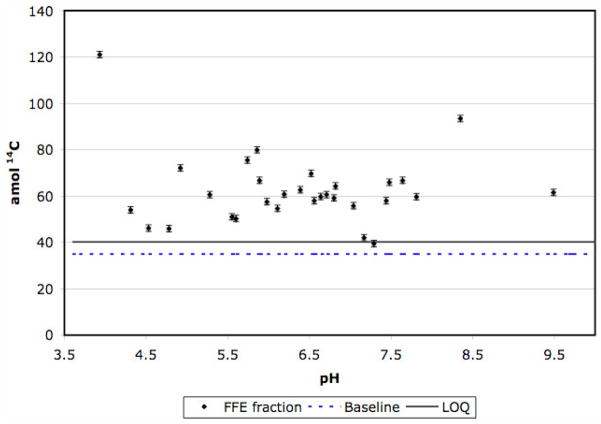
Nearly every fraction between pH 3 and pH 9 had a significant signal above the LOQ. Figure 1 depicts the 14C in each measured fraction as well as the carrier baseline and LOQ. The protein adduct level in any fraction can be calculated from the moles of 14C in the fraction 14Cfr, carrier baseline 14Cb, and protein 14Cpr, and the labeled fraction of naphthalene Lf.
Fig. 1.
Excess 14C in individual FFE fractions. The baseline (35.0 amol) is composed of SDS from dialysis and tributyrin carrier. Dialysis produces a uniform baseline across all fractions. The 14C limit of quantitation (LOQ) is the sum of the baseline, 3x the uncertainty in the baseline (4.2 amol), and the maximum amount of adducted protein in a sample (1.0 amol).
| (1) |
In our case, the labeled fraction was very low (1 in 62,400), so the quantity of naphthalene in individual fractions was large. One could investigate binding of much lower doses of naphthalene simply by increasing the specific activity of the dose material.
Conclusions
FFE is suitable for separating protein-adducts for AMS analysis and, because of the high sensitivity of AMS, appears to be the only practical approach for obtaining quantitative information on the distribution of protein adducts in vivo. Dialysis or some other buffer exchange technique is absolutely necessary prior to AMS analysis, however, due to high carbon content of the FFE buffer. Dialysis accomplished two significant goals, it radically altered the carbon inventory of each fraction and produced a uniform baseline for all fractions. In this study we tackled some basic obstacles in processing FFE fractions for AMS analysis. We chose a simple system with which we had familiarity. FFE offers some distinct advantages over other separation techniques. FFE can separate membrane proteins that are generally difficult to work with due to limited solubility.
Furthermore, FFE can separate different cell organelles, which can be important in cell biology research of drug targeting. We’ve shown here basic approaches to integrating FFE and AMS as complimentary analytical tools.
Acknowledgments
Support was provided by Grant Number P42ES004699 from the National Institute of Environmental Health Sciences and NIH/NCRR (RR13461). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health. This work performed in part under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salam MT, Islam T, Gilliland FD. Curr Opin Pulm Med. 2008;14:3. doi: 10.1097/MCP.0b013e3282f1987a. [DOI] [PubMed] [Google Scholar]
- 2.Lee MG, Phimister A, Morin D, Buckpitt A, Plopper C. J Pharmacol Exp Ther. 2005;314:103. doi: 10.1124/jpet.105.084517. [DOI] [PubMed] [Google Scholar]
- 3.Plopper CG, Suverkropp C, Morin D, Nishio S, Buckpitt A. J Pharmacol Exp Ther. 1992;261:353. [PubMed] [Google Scholar]
- 4.Abdo KM, Grumbein S, Chou BJ, Herbert R. Inhal Toxicol. 2001;13:931. doi: 10.1080/089583701752378179. [DOI] [PubMed] [Google Scholar]
- 5.Preuss R, Drexler H, Bottcher M, Wilhelm M, Bruning T, Angerer J. Int Arch Occup Environ Health. 2005;78:355. doi: 10.1007/s00420-004-0593-3. [DOI] [PubMed] [Google Scholar]
- 6.ATSDR. US Department of Health Services; 2005. Toxicological profile for naphthalene, 1-methylnaphthalene, and 2-methylnaphthalene; pp. 1–292. www.atsdr.cdc.gov/toxprofiles/tp67.html. [Google Scholar]
- 7.Jacob P, III, Wilson M, Benowitz NL. Anal Chem. 2007;79:587. doi: 10.1021/ac060920l. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, Patterson DG., Jr Environ Res. 2008;107:320. doi: 10.1016/j.envres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Lin CY, Isbell MA, Morin D, Boland BC, Salemi MR, Jewell WT, Weir AJ, Fanucchi MV, Baker GL, Plopper CG, Buckpitt AR. Chem Res Toxicol. 2005;18:802. doi: 10.1021/tx049746r. [DOI] [PubMed] [Google Scholar]
- 10.Lin CY, Boland BC, Lee YJ, Salemi MR, Morin D, Miller LA, Plopper CG, Buckpitt AR. Proteomics. 2006;6:972. doi: 10.1002/pmic.200500170. [DOI] [PubMed] [Google Scholar]
- 11.DeStefano Shields CE, Morin D, Buckpitt A. FASEB J. 2008;22:1131.9. [Google Scholar]
- 12.Wheelock AM, Zhang L, Tran MU, Morin D, Penn S, Buckpitt AR, Plopper CG. Am J Physiol Lung Cell Mol Physiol. 2004;286:L399. doi: 10.1152/ajplung.00072.2003. [DOI] [PubMed] [Google Scholar]
- 13.Buchholz BA, Fultz E, Haack KW, Vogel JS, Gilman SD, Gee S, Hammock BD, Hui X, Wester RC, Maibach HI. Anal Chem. 1999;271:3519. doi: 10.1021/ac990152g. [DOI] [PubMed] [Google Scholar]
- 14.Buchholz BA, Dueker SR, Lin Y, Clifford AJ, Vogel JS. Nucl Instr and Meth B. 2000;172:910. [Google Scholar]
- 15.Miyashita M, Presley JM, Buchholz BA, Lam KS, Lee YM, Vogel JS, Hammock BD. PNAS. 2001;98:4403. doi: 10.1073/pnas.071047998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ognibene TJ, Bench G, Vogel JS, Peaslee GF, Murov S. Anal Chem. 2003;75:2192. doi: 10.1021/ac026334j. [DOI] [PubMed] [Google Scholar]
- 17.Vogel JS. Radiocarbon. 1992;34:344. [Google Scholar]
- 18.Ognibene TJ, Bench G, Brown TA, Peaslee GF, Vogel JS. Int J Mass Spectrom. 2002;218:255. [Google Scholar]
- 19.Rozanski K, Stichler W, Gonfiantini R, Scott EM, Beukens RP, Kromer B, van der Plicht J. Radiocarbon. 1992;34:506. [Google Scholar]
- 20.Reimer PJ, Brown TA, Reimer RW. Radiocarbon. 2004;46:1299. [Google Scholar]