Abstract
Pilot studies have suggested that treatment with recombinant human growth hormone (rhGH) is associated with increased T-lymphocyte restoration and enhanced thymic output. We evaluated the immunologic effects of rhGH on HIV+ subjects with incomplete immune reconstitution on antiretroviral therapy (ART). Sixty subjects were randomized to receive rhGH 1.5 mg scqd and ART for 48 weeks (Arm A) or continue ART alone for 24 weeks then add rhGH 3.0 mg scqd for 24 weeks (Arm B). Median baseline CD4 for Arms A and B were 223 and 219, respectively. There was little difference between Arm A and Arm B in change in total or naive CD4 cells or percentage from baseline to week 24. Only one subject in Arm A met the primary endpoint, an increase in naive CD4 percentage of at least 10 percentage points. By week 48 both Arms had statistically significant increases in naive CD4 cell count and percentage and thymus size. Within Arm B, treatment with rhGH was associated with significant increases in naive CD4+ cell count and percentage compared with ART alone. Treatment with rhGH +ART may be associated with modest increases in CD4 lymphocytes over ART alone in subjects with CD4 <350, yet the origin of these naive cells and their impact on immune function require further investigation
Introduction
HIV disease is associated with a decline in naive and memory CD4+ T cells and with the progressive loss of immune response to antigens, including HIV itself.1–3 Following treatment with antiretroviral therapy (ART) significant improvements in naive and memory CD4+ T cells are observed in most patients.4–6 However, in many patients with moderately advanced HIV disease (CD4+ T cells <300 cells/mm3), cellular restoration following ART is incomplete.6,7 Naive and memory CD4+ T cell counts improve but often do not return to the levels seen in HIV-seronegative persons. Furthermore, although the immune response to antigens improves and the incidence of opportunistic diseases declines, the restoration of immune competence against some pathogens is incomplete, and HIV-specific immune responses remain weak or absent in most patients despite long-term therapy.6–8
The lack of complete immune restoration following ART results, at least in part, from a limitation in the ability of the thymus to produce new naive CD4+ T cells.7,9,10 Therapy with growth hormone is associated with increases in thymic mass, improvement in thymic function, and lymphocyte proliferation in animals.11–14 The administration of growth hormone leads to improvements in cell-mediated HIV-specific responses in mice following vaccination with HIV antigens.15 In a pilot study of five HIV-infected subjects, treatment with recombinant human growth hormone (rhGH) increased naive CD4+ T cell percentage and thymus size and volume as measured by computerized tomography (CT) scan.16 The current study sought to test the hypothesis that administration of rhGH to HIV-infected adults with partial immune reconstitution on ART will enhance cellular immune restoration and cell-mediated response to antigens. ACTG 5174 was a randomized, open-label, two-stage pilot study to examine the immunologic effects of rhGH in HIV-infected subjects with incomplete immune restoration on ART, defined as having an absolute CD4+ T cell count <350 cells/mm3 after at least 1 year of ART. Specifically we examined the effect of rhGH on total and naive CD4 lymphocytes, thymic size, recent thymic emigrants as measured by T-lymphocyte recombinant excision circle (TREC) content in peripheral blood mononuclear cells (PBMCs), and response to vaccine antigens and HIV-1.
Materials and Methods
Subjects
Sixty subjects with HIV infection who were treated with ART for ≥1 year, who had plasma HIV-1 RNA <400 copies/ml for ≥6 months prior to entry, and who had CD4+ T cells <350 cells/mm3 were enrolled in the study. Subjects were randomized to receive rhGH 1.5 mg sc once a day plus ART for 48 weeks (Arm A) or continue ART alone for 24 weeks followed by addition of rhGH 3.0 mg sc once a day for 24 weeks (Arm B). After discontinuing rhGH at week 48, subjects in both Arms were followed to week 72. Thirty-one subjects (15 on Arm A and 16 on Arm B) were enrolled into a substudy in which they underwent CT scan of the chest to assess thymus size.
The protocol, the informed consent, and all protocol modifications were reviewed and approved by the institutional review board or ethics committee responsible for oversight of the study at each of the participating sites. A Safety Monitoring Committee reviewed interim safety and efficacy data 1 year after the enrollment of the first subject. This review found that the data did not suggest any significant safety issues with rhGH treatment at either 1.5 mg or 3.0 mg per day and recommended that the study continue without change.
HIV-1 RNA quantification
HIV-1 RNA was performed using the ultrasensitive polymerase chain reaction (PCR)-based assay (detection limit 50 copies/ml) by laboratories certified by the DAIDS Virology Quality Assurance (VQA) program and CLIA certification or its equivalent.
T-Lymphocyte subsets
PBMCs were isolated by density centrifugation. Advanced four-color flow cytometry was performed using antibodies for CD3, CD4, CD45RA, CD45RO, CD62L, CD31, CD28, HLA-DR, Ki67, and CD38 that were conjugated to either fluorescein isothiocyanate, phycoerythrin, peridininchlorophyll-protein, or allophycocyanin (APC) (Becton Dickinson-Pharmingen, San Jose, CA). Naive CD4+ T cell subset was defined as the proportion of CD3+CD4+ T cells coexpressing CD45RA+CD62L+, the memory T cell subset was defined as the proportion of CD45RA–CD45RO+, and the activated T cell subset was defined as the proportion of CD4+ and CD8+ T cells that coexpressed HLA-DR and CD38. In addition, we also determined the frequency of CD4+CD8–CD28+ cells and CD4+CD45RA+CD45RO–CD31+ cells. Participating immunology laboratories were certified by DAIDS.
Pathogen-specific lymphoproliferative assay
CD4+ T cell lymphoproliferative assays (LPA) for pokeweed mitogen (0.1 μg/ml; Sigma Chemical, St. Louis, MO), Candida CASTA antigen (10 μg/ml:, Greer Laboratory, Lenoir, NC), keyhole limpet hemocyanin (KLH, 50 μg/ml, Intracel Corp), and HIV p24 antigen/control (1:400 dilution, National Cancer Institute, Bethesda, MD) were done on fresh PBMCs as described previously.17 Results were expressed as stimulation index (SI), defined as the ratio of the median counts per minute of the wells with antigen to the median counts per minute of the wells without antigen. An SI of >10 was considered positive. A new response was defined as SI >10 that was also a threefold increase.
TREC assay: Measurement of recent thymic emigrants
TRECs were measured on PBMCs using methods that have been described elsewhere.18
Thymus size
Thymus size was assessed via CT scan of the chest as previously described.19,20 Briefly, noncontrast CT scans consisted of contiguous 5-mm sections from the sternal notch through the xiphoid. Radiologists (B.H.G., I.R.F.) scored the CT scan giving it a thymic index from 0 to 5 whereby 0 represents lack of thymic tissue and an organ entirely replaced by fat, 1 represents barely recognizable thymic tissue, 2 represents minimal soft tissue, more obvious, 3 represents obvious thymic tissue, 4 represents moderate thymic tissue, and 5 represents thymic mass of possible concern for thymoma. CT scans were performed before and after subjects in both Arm A and Arm B had received 24 weeks of rhGH, thus Arm A underwent a CT scan at study entry and week 24 and Arm B underwent CT scans at week 24 and week 48. Radiologists were blinded to the subject clinical data and treatment arm.
Vaccinations
Immunocyanin [keyhole limpet hemocyanin (subunit with a molecular weight of 350–390 kDa), KLH, Immucothel] 0.1 mg (0.1 ml) was administered by intradermal injection into the forearm at weeks 16 and 20.
Statistical analysis
The primary endpoint of the study was change from baseline to week 24 in naive CD4+ T cell percent, dichotomized into whether or not subjects had at least a 10 percentage point increase. Fisher's exact test was used to compare the primary endpoint between the two arms. Secondary endpoints included changes in T-lymphocyte subset frequencies and absolute counts, as well as changes in thymus index and TREC frequency over time. The Wilcoxon signed rank test and Spearman rank correlation were used to evaluate the secondary endpoints in each arm. Difference between the two arms was examined using the Wilcoxon rank sum test. Analyses were intent-to-treat, using the available data at each time point, regardless of treatment compliance. Reported p values are two-sided and unadjusted for multiple testing.
Results
Baseline demographics
Table 1 presents the baseline demographic information, CD4+ T cells and plasma HIV-1 RNA by treatment Arm. The median age was 47 years. The majority of subjects (91%) were between 30 and 59 years old. Most subjects were male (92%) and white (78%). The median baseline CD4+ T cells was 219 cells/mm3. All subjects had been on ART for a median of 4.6 years. One subject had missing HIV-1 RNA entry value, but this subject had a plasma HIV-1 RNA <400 copies/ml at screening. Among the remaining 59 subjects, 53 had plasma HIV-1 RNA <50 copies/ml and all 59 had <400 copies/ml. No significant imbalances across treatment Arms were noted at baseline.
Table 1.
Baseline Characteristics by Treatment
| Characteristics | Statistics|categoriesa | Overall (n = 60) | A (n = 30) | B (n = 30) |
|---|---|---|---|---|
| Age (years) | Median (Q1, Q3) | 47 (40, 52) | 47 (41, 52) | 48 (39, 51) |
| Mean (SD) | 46 (8) | 47 (8) | 45 (8) | |
| 13–29 | 2 (3%) | 2 (7%) | 0 (0%) | |
| 30–39 | 11 (18%) | 2 (7%) | 9 (30%) | |
| 40–49 | 26 (43%) | 14 (47%) | 12 (40%) | |
| 50–59 | 18 (30%) | 10 (33%) | 8 (27%) | |
| ≥ 60 | 3 (5%) | 2 (7%) | 1 (3%) | |
| Gender | Male | 55 (92%) | 28 (93%) | 27 (90%) |
| Female | 5 (8%) | 2 (7%) | 3 (10%) | |
| Race/ethnicity | White | 47 (78%) | 23 (77%) | 24 (80%) |
| Black | 6 (10%) | 3 (10%) | 3 (10%) | |
| Hispanic | 7 (12%) | 4 (13%) | 3 (10%) | |
| Baseline CD4 count (cells/mm3) | Median (Q1, Q3) | 219 (161, 300) | 223 (159, 307) | 219 (176, 266) |
| Mean (SD) | 230 (92) | 233 (92) | 228 (93) | |
| HIV-1 RNA at entry (copies/ml) | <50 | 53 (90%) | 26 (87%) | 27 (93%) |
| ≥50 | 6 (10%) | 4 (13%) | 2 (7%) | |
| Missing/unknown | 1 | 0 | 1 |
Q1 and Q3 are 25th and 75th percentiles.
Subject disposition
Six subjects discontinued the study prior to week 48. Among Arm A subjects, two subjects were lost to follow-up and one subject discontinued due to severe debilitation. Among Arm B subjects, two subjects withdrew consent prior to study completion and one subject discontinued due to the inability to make study visits. One additional subject on Arm B went off study before week 48. This subject had a week 43 blood draw, which was carried forward for week 48 analysis.
HIV-1 RNA quantitation
One subject in Arm B experienced a viral rebound (defined as at least two consecutive HIV-1 RNA >1000 copies/ml) and was included in analysis. No subjects in Arm A experienced viral rebound.
CD4 lymphocyte changes
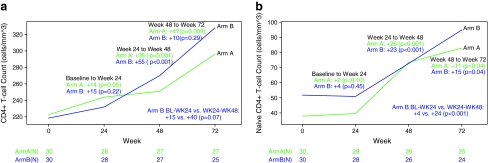
Baseline median total CD4+ T cells for Arms A and B were 223 cells/mm3 and 219 cells/mm3, respectively. Arm A subjects experienced a significant increase in total CD4+ T cells from baseline to week 24 (p = 0.05), whereas Arm B subjects did not have a significant change in total CD4+ T cells (Fig. 1a). However, no significant difference was seen between the arms. Only one Arm A subject reached the study primary endpoint, which was at least a 10 percentage point increase in naive CD4+ T cell percent. No significant change in naive CD4+ T cell count or percentage was observed from baseline to week 24 in either Arm A or Arm B subjects and no significant difference was observed between the arms. However, Arm B subjects trended toward a decrease in naive CD4+ T cell percentage (–2%, p = 0.08) (Fig. 1b and c).
FIG. 1.
Median plot of (a) CD4+ lymphocyte count, (b) naive CD4+ lymphocyte count, and (c) naive CD4+ lymphocyte percentage.
By week 48, both arms had significant increases in total and naive CD4+ T cell counts. From baseline to week 48, the median changes in total and naive CD4+ T cells for Arm A were +36 cells/mm3 and +26 cells/mm3, respectively (p ≤ 0.001). The median changes in total and naive CD4+ T cells in Arm B subjects were +55 cells/mm3 and +23 cells/mm3, respectively (p ≤ 0.001). In Arm B, there was a significant increase in naive CD4+ T cells from week 24 to 48 (ART +rhGH, +24 cells/mm3) compared with from baseline to week 24 (ART alone, +4 cells/mm3) (p < 0.001).
A significant increase in naive CD4+ T cell percentage from baseline to week 48 was seen in both Arms A and B (A: +8%, p < 0.0001; B: +4%, p = 0.002) (Fig. 1c). Ten subjects in Arm A and four subjects in Arm B had a ≥10 percentage point increase by week 48 with Arm A subjects having a greater increase by week 48 compared with Arm B subjects (p = 0.05). In Arm B, there was a significant increase in naive CD4+ T cell percentage from week 24 to 48 (ART +rhGH, +4%) compared to from baseline to week 24 (ART alone, −2%) (p < 0.001).
We also compared T cell changes from baseline to week 24 for Arm A subjects and T cell changes from week 24 to week 48 for Arm B subjects. We found that 24 weeks of rhGH at 3 mg daily was associated with greater increases in naive CD4+ T cell count and percentage than 1.5 mg daily (naive CD4+ count: A +2 vs. B +24, p = 0.0001; naive CD4+%: A 0% vs. B +4%, p = 0.0007).
Other lymphocyte subset analyses
There was no significant change in total or naive CD8+ T-lymphocyte count or percentage following rhGH treatment in either treatment Arm. No significant change in memory CD4 cell count was observed in Arm A following rhGH treatment; however, Arm B had a significant increase at week 48 compared with baseline (+27 cells, p ≤ 0.001).
There were no significant changes in the percentage of CD4+ or CD8+ T cells coexpressing the activation markers HLA-DR and CD38 following treatment with rhGH in either Arm. Both Arms showed significant increases from baseline to week 48 in the percentage of CD4+ and CD8+ T cells coexpressing CD28 following treatment with rhGH (CD4+/CD28+%: A +2%, p = 0.02; B +2%, p = 0.0004; CD8+/CD28+%: A +5%, p < 0.0001; B +6%, p = 0.04).
We evaluated Ki67 expression on naive CD4 lymphocytes to assess the affect of rhGH on cell turnover. Significant decreases in Ki67 expression from baseline to week 24 (median = −1.70%, p = 0.02) and week 48 (median = –1.48%, p = 0.01) were observed in Arm A. Arm A had a significantly greater reduction in Ki67 expression in naive CD4 cells from baseline to week 24 than Arm B during that period (–1.70% vs. +1.00%, p = 0.007) suggesting treatment with rhGH was associated with reduced naive CD4 lymphocyte turnover.
Recent thymic emigrants
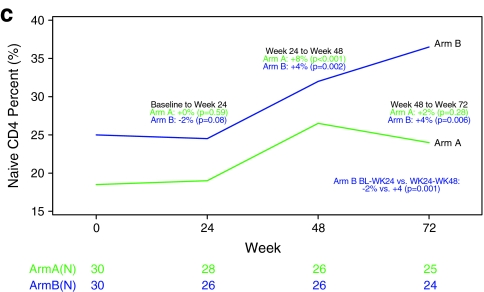
Recent thymic emigrants were quantified by the level of TREC in PBMCs at baseline, week 24, and week 48. There was no significant change in TREC by week 24; however, by week 48 both Arms had significant increases: +1.08 log10 (23%) and +0.47 log10 (9%) for ARM A and B, respectively. When examining Arm B alone, log10 TREC changes were not significantly different before and after rhGH treatment. When we examined both treatment Arms combined, greater increases in TREC were seen in subjects with low baseline TREC (+2.38 log10 vs. −0.09 log10, p < 0.001) (Fig. 2).
FIG. 2.
Scatter plot of change in log10 TREC and change in naive CD4+ T cell count.
We also examined expression of CD31 on naive lymphocyte as a possible marker of recent thymic emigrants.21 Baseline CD31 percent was negatively correlated with age (r = −0.36, p = 0.006) and positively correlated with baseline TREC (r = 0.28, p = 0.03). Of naive CD4 cells 75% and 76% expressed CD31 at baseline in Arm A and Arm B, respectively. No significant difference in the percent of CD31+ naive CD4+ cells was observed before and after rhGH in Arm A. In Arm B, the percent of CD31+ naive CD4+ cells decreased in the 24 weeks before treatment with rhGH (median = –3%, p = 0.002); however, following rhGH treatment these cells increased significantly (median = +4%, p = 0.03).
Thymus size
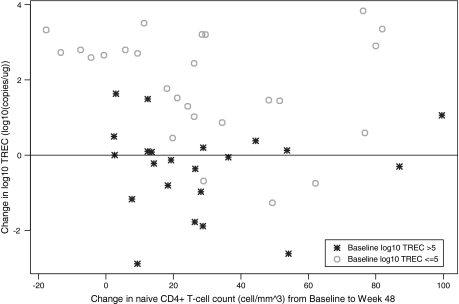
Thymus size was measured prior to treatment with rhGH and after 24 weeks of 1.5 mg rhGH daily in Arm A and after 24 weeks of 3.0 mg of rhGH in Arm B. Subjects in Arm B had significant increase in thymus index (p = 0.02), whereas subjects in Arm A had a marginally significant increase (p = 0.06). Seven of 11 (64%) in Arm A and 7 of 9 (78%) in Arm B had increases in thymus size (Fig. 3a).
FIG. 3.
(a) Thymus index by treatment. (b) Scatter plot of change in IGF-1 and change in thymus size.
Insulin-like growth factor
Many of the biological effects of growth hormone are mediated by insulin-like growth factor type-1 (IGF-1). Significant increases in IGF-1 were observed in both Arms from baseline to week 48 (p < 0.001). IGF-1 change was positively correlated with changes in total CD4+ T cell count (r = 0.29, p = 0.05) and changes in thymus size (r = 0.61, p = 0.001) (Fig. 3b). Unexpectedly, IGF-1 change was negatively correlated with changes in log10 TREC (r = –0.27, p = 0.06).
Response to recall and neoantigens
Recall antigen response
Most subjects had positive LPA responses (stimulation index >10) to Candida and pokeweed mitogen (positive control) at baseline. There was no significant difference in these responses following rhGH treatment in either treatment Arm. Six subjects in Arm A and three subjects in Arm B had positive responses to whole killed HIV-1 at baseline. No new HIV-1 responses were observed at week 24 in either Arm. Two subjects in each Arm developed new whole HIV-1 LPA responses by week 48.
Neoantigen response
Only one subject in Arm A and no subjects in Arm B had positive LPA response to the neoantigen keyhole limpet hemocyanin (KLH) at baseline. Two subjects in Arm A and no subjects in Arm B developed new responses to KLH by week 24 and no significant difference was seen between the Arms. No new KLH LPA responses were observed in either Arm at week 48.
Adverse events
Through week 72, eight subjects discontinued the study due to adverse events. Carpal tunnel syndrome that was probably related to treatment with rhGH was diagnosed or suspected in six subjects. One subject had aseptic meningitis that was unlikely related to treatment with rhGH. One subject experienced progression of a previously diagnosed anal carcinoma that was possibly related to treatment with rhGH. These adverse events were equally distributed in both treatment Arms. Grade 3 or higher symptoms and laboratory events were infrequent and were equally distributed in both treatment arms (Table 2).
Table 2.
Summary of Grade 3 or Higher Symptoms or Higher Laboratory Abnormalities
| Arm A | Arm B | |
|---|---|---|
| Body aches | 1 | 2 |
| Edema | 0 | 3 |
| Any GI | 1 | 1 |
| Headache | 2 | 0 |
| Paresthesia/stiffness | 0 | 1 |
| Fasting glucose | 0 | 1 |
| Nonfasting glucose | 0 | 1 |
| CPK | 1 | 1 |
| Lipase | 1 | 1 |
| Triglycerides | 3 | 0 |
| Any liver | 2 | 1 |
Discussion
We have demonstrated that treatment with rhGH is associated with modest improvements in CD4 lymphocytes over ART alone in subjects with CD4 cell counts below 350 after a minimum of 1 year of ART. It is difficult to ascertain if the increases in CD4 lymphocytes resulted directly from treatment with rhGH or if they would have occurred with more time on ART alone. The fact that the degree of CD4 lymphocyte increase in Arm B during the 6 months of observation on rhGH was significantly greater than that observed on ART alone certainly suggests that rhGH had an effect. This finding is supported by the recently published study by Napolitano et al. in which HIV-infected subjects treated with rhGH demonstrated significant increases in naive and absolute CD4+ lymphocyte count. Notably in that study the CD4 lymphocyte increases were more pronounced, possible as a result of a longer treatment period and larger total dose of rhGH.22 We did not find a difference in the primary study endpoint, the proportion of subjects who achieved at least a 10% increase in naive CD4 percentage at week 24 comparing Arm A (rhGH treated) to Arm B (ART alone). This may have been an overly ambitious expectation giving the low dose of rhGH given to Arm A and the relatively short 24-week window.
We were able to demonstrate increases in thymus size and recent thymic emigrants (as measured by TREC content in PBMCs); however, we should note that the increase in TREC was modest. The observation that subjects with low baseline TREC had the greatest increases in TREC is consistent with observations of other investigators and could suggest that rhGH had the most benefit for those with poor thymopoiesis at baseline.23 This observation warrants further investigation, albeit it may have been influenced in part by regression to the mean. Increased thymus size following ART has been associated with increased naive CD4 cells, increased TREC frequency, and broader T cell repertoire.19,20,24,25 Furthermore, one study demonstrated that the post-ART enlarged thymus of two subjects demonstrated normal thymus histology and normal thymopoiesis, thus suggesting the thymus is capable of contributing to naive CD4 lymphocyte recovery.26
We must consider, however, the possibility that the increase in thymus size following rhGH may represent proliferation of thymic epithelial cells (which have been shown to have growth hormone receptors) rather than proliferation of thymocytes.27 Thus, overall, our findings on the effect of rhGH on thymic output are inconclusive and the origin of the naive lymphocyte expansion is uncertain. It is possible that rhGH led to decreased cellular turnover (as evidenced by reduced KI67 expression on naive lymphocytes), which prolonged their half-life. In the aforementioned paper by Napolitano et al.,22 the proportion of CD4 cells that was activated (HLA-DR and CD38+) decreased significantly following rhGH, which could also suggest prolonged CD4 survival. Alternatively, rhGH may have led to enhanced extrathymic naive lymphocyte proliferation comparable to the effect of interleukin (IL)-7.28
Despite the increases in total and naive CD4 lymphocytes we have not been able to demonstrate significant improvement in immune function as measured by LPA responses to recall antigens, neoantigens, or HIV-1. This is in contrast to data reported by Pires et al., which demonstrated that daily administration of rhGH induced a significant increase in gag- and whole HIV-1 antigen-specific proliferation after 12 weeks in 9 of 12 subjects included in their study.29 The dose of rhGH used in that study was 4 mg/day, which is higher than that used in the present study. In addition, the criteria for positive LPA response (SI >5) was less conservative than that used in the present study (SI >10, which is additionally at least a threefold increase). It is uncertain whether our inability to demonstrate functional improvement results from the fact that no significant changes occur or that the currently available assays for measurement of immune function are unable to identify subtle changes in immune function in subjects with viral suppression on ART.
It is also possible that higher doses of rhGH would lead to greater change in immune function since we demonstrated that 24 weeks of 3.0 mg daily was associated with greater CD4 lymphocyte increase than 24 weeks of 1.5 mg and Napolitano et al.22 had larger increases following larger total rhGH dose. However, other interventions such as IL-2, which were associated with significant CD4+ lymphocyte increases over ART alone, did not consistently lead to improved vaccine responses as measured by LPA.30 Overall, our data indicate that treatment of HIV-infected individuals with rhGH is associated with modest increases in naive CD4+ T-lymphocytes, yet the origin of these naive cells and their impact on immune function require further investigation.
Acknowledgments
We acknowledge the other team members involved in the A5174 study: Chip Lohner, M.A (Field Representative), Monique R. Givens, B.S. (Laboratory Technologist), George Bishopric, M.D., William Strain (CCG Representatives), Amanda Zadzilka (Laboratory Data Coordinator), Lawrence Fox, M.D., Ph.D. (DAIDS Medical Monitor), Jennifer Janik, M.S. (Data Manager), Lynette Purdue, PhArm.D. (DAIDS PhArmacist), Ronald Mitsiyasu (Investigator), Michael Lederman (Investigator), Robert Murphy (Investigator), Donna Mildvan (Investigator), Joe Eron (Investigator), and Jody Lawrence (Investigator). r-hGH for this study was donated by EMD-Serono, Inc., Rockland, MA. The company had no role in the design of the study, its conduct, analysis of data, or interpretation of results other than to provide clarifying answers to questions from the team about rhGH and to offer limited editorial input into the manuscript, subject to final approval of the research team. We would like to add a special thank you to all the patients who participated in the study.
Supported in part by the AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases. Grant numbers: ACTG Central Grant AI38858, ACTG Network Grant AI68636, SDAC: 5 U01 AI38855, UNC: 1U01AI069423-01, Case: 1U01AI069501-01, UCSF: 1U01AI069502-01, Rush: 1U01AI069471-01, UAB: 1U01AI06452-01, UCLA, 1U01AI069424 01, SDAC: 1U01AI068634-01 and AI68634, UCHS: 1U01AI069450.
This study was presented in part at the 3rd International AIDS Society Conference, July 2005, 16th International AIDS Conference, Brazil, 2005.
Author Disclosure Statement
Norma Muurahainen is a full-time salaried employee of Serono, Inc. Hernan Valdez is a full time employee of Pfizer, Inc. Richard B. Pollard is a consultant for Genetic Immunity, Inc.
References
- 1.Ho DD. Neumann AU. Perelson AS, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 2.Wolters KC. Shuitemaker H. Miedema F. Rapid CD4 T-cell turnover in HIV-1 infection: A paradigm revisited. Immunol Today. 1998;19:44–48. doi: 10.1016/s0167-5699(97)01188-2. [DOI] [PubMed] [Google Scholar]
- 3.Conners M. Kovacs JA. Krevat S, et al. HIV infection induces changes in CD4+ T-cell phenotype and depletions within the CD4+ T cell repertoire that are not immediately restored by antiviral or immune based therapies. Nat Med. 1997;3:533–540. doi: 10.1038/nm0597-533. [DOI] [PubMed] [Google Scholar]
- 4.Lederman M. Connick E. Landay A, et al. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: Results of AIDS Clinical Trials Groups Protocol 315. J Infect Dis. 1998;178:70–78. doi: 10.1086/515591. [DOI] [PubMed] [Google Scholar]
- 5.Autran B. Carcelain G. Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T-cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 6.Connick E. Lederman M. Kotzin B, et al. Immune reconstitution in the first year of potent antiretroviral therapy and its relationship to virologic response. J Infect Dis. 2000;181:358–363. doi: 10.1086/315171. [DOI] [PubMed] [Google Scholar]
- 7.Valdez H. Connick E. Smith KY, et al. Limited immune restoration after 3 years' suppression after HIV-1 replication in patients with moderately advanced disease. AIDS. 2002;16:1850–1866. doi: 10.1097/00002030-200209270-00002. [DOI] [PubMed] [Google Scholar]
- 8.Komanduri KV. Feinberg J. Hutchins RK, et al. Loss of cytomegalovirus-specific CD4+ T cell responses in human immunodeficiency virus type 1-infected patients with high CD4+ T cell counts and recurrent retinitis. J Infect Dis. 2001;183(8):1285–1289. doi: 10.1086/319683. [DOI] [PubMed] [Google Scholar]
- 9.Douek DC. McFarland RD. Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–694. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 10.Pakker NG. Notermanns DW. de Boer RJ, et al. Biphasic kinetics of peripheral blood cells after triple combination therapy in HIV infection: A composite of redistribution and proliferation. Nat Med. 1998;4:208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 11.Murphy WJ. Durum SK. Anver MR, et al. Immunologic and hematologic effects of neuroendocrine hormones. Studies on DW/J dwarf mice. J Immunol. 1992;148:3799–3805. [PubMed] [Google Scholar]
- 12.Binz K. Joller P. Froesch P, et al. Repopulation of atrophied thymus in diabetic rats by insulin-like growth factor 1. Proc Natl Acad Sci USA. 1990;87:3690–3694. doi: 10.1073/pnas.87.10.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geffner ME. Bersh N. Lippe BM, et al. Growth hormone mediates the growth of T-lymphoblast cell lines via locally generated insulin-like growth factor 1. J Clin Endocrinol Metab. 1990;71:464–469. doi: 10.1210/jcem-71-2-464. [DOI] [PubMed] [Google Scholar]
- 14.Clark R. Strasser J. McCabe S, et al. Insulin-like growth factor type 1 stimulation of lymphopoiesis. J Clin Invest. 1993;92:540–548. doi: 10.1172/JCI116621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellado M. Llorente M. Rodriguez-Frade M, et al. HIV-1 envelope protein gp 120 triggers a Th2 response in mice that shifts to Th1 in the presence of human growth hormone. Vaccine. 1998;16:1111–1115. doi: 10.1016/s0264-410x(98)80106-0. [DOI] [PubMed] [Google Scholar]
- 16.Napolitano LA. Lo JC. Gotway MB, et al. Increased thymic mass and circulating naive CD4 T cells in HIV-1-infected adults treated with growth hormone. AIDS. 2002;16:1103–1111. doi: 10.1097/00002030-200205240-00003. [DOI] [PubMed] [Google Scholar]
- 17.AIDS Clinical Trials Group Immunology Laboratory Committee Consensus: Methodologies. Bethesda, MD; NIH: 2000. [Google Scholar]
- 18.Al-Harthi L. Marchetti G. Steffens CM, et al. Detection of T cell receptor circles (TRECs) as biomarkers for de novo T cell synthesis using a quantitative polymerase chain reaction-enzyme linked immunosorbent assay (PCR-ELISA) J Immunol Methods. 2000;237:187. doi: 10.1016/s0022-1759(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 19.McCune JM. Loftus R. Schmidt DK, et al. High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J Clin Invest. 1998;101:2301–2308. doi: 10.1172/JCI2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith KY. Valdez H. Landay R, et al. Thymic size and lymphocyte restoration in patients with human immunodeficiency virus infection after 48 weeks of zidovudine, lamivudine, and ritonavir therapy. J Infect Dis. 2000;181(1):141–147. doi: 10.1086/315169. [DOI] [PubMed] [Google Scholar]
- 21.Kimmig S. Przybylski GK. Schmidt CA, et al. Two subsets of naïve T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195:789–794. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Napolitano L. Schmidt D. Gotway MB, et al. Growth hormone enhances thymic function in HIV-1 infected adults. J Clin Invest. 2008;118(3):1085–1098. doi: 10.1172/JCI32830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franco JM. Rubio A. Martínez-Moya M, et al. T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood. 2002;99(10):3702–3706. doi: 10.1182/blood.v99.10.3702. [DOI] [PubMed] [Google Scholar]
- 24.Harris JM. Hazenberg MD. Poulin JF, et al. Multiparameter evaluation of human thymic function: Interpretations and caveats. Clin Immunol. 2005;115(2):138–146. doi: 10.1016/j.clim.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Kolte L. Dreves AM. Ersbøll AK, et al. Association between larger thymic size and higher thymic output in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy. J Infect Dis. 2002;185(11):1578–1585. doi: 10.1086/340418. Epub May 17, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Markert ML. Alvarez-McLeod AP. Sempowski GD, et al. Thymopoiesis in HIV-infected adults after highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2001;17(17):1635–1643. doi: 10.1089/088922201753342040. [DOI] [PubMed] [Google Scholar]
- 27.de Mello-Coelho V. Gagnerault M-C. Souberbielle J-C, et al. Growth hormone and its receptor are expressed in human thymic cells. Endocrinology. 1998;139:3837–3842. doi: 10.1210/endo.139.9.6199. [DOI] [PubMed] [Google Scholar]
- 28.Yu-Waye Chu. Sarfraz A. Memon SO. Sharrow , et al. Exogenous IL-7 increases recent thymic emigrants in peripheral lymphoid tissue without enhanced thymic function. Blood. 2004;104:1110–1119. doi: 10.1182/blood-2003-10-3635. [DOI] [PubMed] [Google Scholar]
- 29.Antonio Pires. Jeffery Pido-Lopez. Graeme Moyle, et al. Enhanced T-cell maturation, differentiation and function in HIV-1 infected individuals after growth hormone and highly active antiretroviral therapy. Antiviral Ther. 2004;9:67–75. [PubMed] [Google Scholar]
- 30.Valdez H. Mitsuyasu R. Landay A, et al. Interleukin-2 increases CD4+ lymphocyte numbers but does not enhance responses to immunization: Results of A5046s. J Infect Dis. 2003;187(2):320–325. doi: 10.1086/346056. [DOI] [PubMed] [Google Scholar]