Abstract
We have studied IgG subclass responses to the HIV-1 proteins gp120, gp41, p24, and Tat in individuals who control their infection without using antiretroviral drugs (HIV-1 controllers; HC) or who progress to disease (chronic progressors; CP). We also measured IgG subclass titers to gp120 in vaccinated individuals. In all cases, the IgG1 subclass dominated the overall response to each antigen. The only IgG titer that differed significantly between the HC and CP groups was to the p24 Gag protein, which was higher in the HC group. IgG1 titers to both p24 and gp120 were significantly higher in the HC group, and IgG3 anti-gp120 antibodies, although rare, were detected more frequently in that group than in CP. Overall, significantly more patients had IgG2 antibodies to gp120 than to gp41. Antibodies to other IgG subclasses were infrequent and their frequency or titers did not differ between the two patient groups. Anti-gp41 and anti-Tat responses also did not correlate with immune control, and anti-Tat antibodies were infrequently detected. Although we found isotypic differences in IgG responses to HIV-1 antigens among vaccinees and the HC and CP individuals, there were no indications of differential TH1:TH2 polarization between the different groups.
Introduction
The design of an effective vaccine against human immunodeficiency virus type 1 (HIV-1 infection) and of immune-based therapies would be facilitated by increased knowledge of the interactions between the virus and the human immune system. Essential information can be derived from studies of individuals who have been able to control their infections in the absence of therapy, presumably as a consequence of developing atypically effective immune responses.1 Such individuals, once termed long-term nonprogressors (LTNP) but now commonly referred to as HIV-1 controllers (HC), naturally suppress plasma viremia, in the cases of “elite” controllers to below the detection limit of standard commercial assays, and maintain their peripheral CD4 T cell counts at normal, or near-normal, levels for multiyear periods.2,3 The course of their infections stands in marked contrast to what is seen in chronic progressors (CP) in whom HIV-1 infection causes inexorable damage to the immune system.
Current HIV-1 vaccine strategies involve harnessing one or both of the humoral (B cell) and cellular (T cell) arms of the immune system. B cell vaccines are usually based on the induction of neutralizing antibodies (NAbs) and T cell vaccines on the activation of cytotoxic T-lymphocytes (CTL). In general, NAbs have the potential to prevent infection and CTLs to help control infection once it has become established in the new host. Other B and T cell effector functions, and aspects of innate immunity, may also be usefully harnessed, at least in principle. The targets for NAbs are the viral envelope glycoproteins, gp120 and gp41. However, only a minor subset of antibodies (Abs) raised to these antigens has neutralizing activity, and Abs to other viral structural and accessory proteins (Gag, Pol, Nef, etc.) have no generally accepted antiviral action. T cell responses can be raised against multiple epitopes in every viral protein, with Gag-targeted CTLs appearing to be the most useful for controlling infection.4–8 Unfortunately, no protection or postinfection viral load reductions were observed in the first large-scale trials of both B and T cell vaccines.9–13 Hence, we need yet more information on how the immune system recognizes critical viral antigens.
What immune parameters, then, correlate with control of HIV-1 infection? In this study, we focus on B cell immunity with specific emphasis on the titer and subclass of the IgG response to various HIV-1 antigens. We have also compared the IgG subclasses in the HC and CP groups with those induced by a gp120 subunit vaccine to determine whether there are qualitative and quantitative differences in the immune responses induced by infection and vaccination.
Materials and Methods
Samples from HIV-1-infected individuals or gp120-vaccinated volunteers
The HC and CP cohorts of nonprogressing and progressing HIV-1-infected individuals, based in Massachusetts General Hospital, Boston, have been described previously.2 Twenty CP and 16 HC samples were randomly chosen for IgG subclass analysis. In the absence of antiretroviral therapy, plasma virus loads in the HCs and CPs are <2000 and >10,000 RNA copies/ml, respectively.2 Of the 16 HC group members, the virus loads in 13 were below the detection levels of an ultrasensitive assay (75 RNA copies/ml); in the other 3, they were <2000 RNA copies/ml. Plasma samples from HC cohort members were obtained within 14–25 years of the date of initial diagnosis of infection (with the exception of five individuals for whom the range was 4–8 years). For the CP group, the range was 1–20 years postdiagnosis.
Plasma samples were initially diluted 1:10 in the TMSS ELISA assay buffer [Tris-buffered saline (TBS; 144 mM NaCl, 25 mM Tris, pH 7.6) containing 5% nonfat milk powder and 20% sheep serum]. The samples were then heat inactivated (56°C for 1 h), allowed to cool to room temperature, and aliquoted for storage at −20°C. On the day of use, samples were further diluted 1:10 to achieve the initial 1:100 start dilution.
Twenty-five serum samples from gp120 vaccine and placebo recipients were obtained, blinded, from the HIV Vaccine Trials Network (HVTN) and the National Institute of Allergy and Infectious Diseases (NIAID). These sera were derived from the HIVNET 026 study initiated in 2001, which involved volunteers from Brazil (Rio de Janeiro), Haiti, Peru, and Trinidad and Tobago.9 Vaccinees received a modified recombinant canarypox virus (ALVAC-HIV vCP1452) expressing HIV-1 env, gag, and pol gene products, followed by a recombinant HIV-1MN gp120 booster in aluminum hydroxide (Alum) adjuvant.9 The placebo was a virus stabilizer mixed with Alum. Of the 25 samples, some were from recipients of a gp120-containing vaccine; the rest were from placebo recipients. The assays were carried out without knowledge of which sample was from which group.
Monoclonal antibodies (MAbs)
MAb b12 (IgG1 isotype) to a binding site (CD4BS)-associated gp120 epitope14–16 and 4E10 and 2F5 to the gp41 membrane-proximal external region (MPER) epitopes17,18 were obtained from the IAVI Neutralizing Antibody Consortium reagent repository. MAbs F425-B4e8 (IgG2 isotype)19 and 447-52D (IgG3 isotype)20 to gp120 V3 epitopes (contributed by L. Cavacini and S. Zolla-Pazner, respectively), and pooled sera from HIV-1-infected individuals (HIVIG), were obtained from the NIH AIDS Research and Reference Reagent Program.
Recombinant antigens for serology studies
A full-length, CHO cell expressed gp120 (HIV-1JR-FL) protein was a gift from Dr. William Olson (Progenics Pharmaceuticals, Tarrytown, NY). A full-length (231 residues) p24 protein, corresponding to Gag residues 132–363 of HIV-1BRU, was purchased from Biodesign International (Cat. #R18301). An 86 residue Tat protein (IIIB) was obtained from Prospec Protein Specialists (Cat. #HIV-129).
The gp41 peptide, N54(L6)C56 (referred to henceforth as gp41-N54) corresponds to HIV-1HXB2 gp160 residues 528–581 and 628–683, connected by a -SGGRGG- linker, but lacks Cluster I epitopes. It was expressed in Escherichia coli BL21(DE3)/pLysS using a modified pET3a vector (Novagen, EMD Chemicals, Inc., Gibbstown, NJ). Cells were lysed using glacial acetic acid and centrifuged to separate the soluble fraction from inclusion bodies. The soluble fraction was subsequently dialyzed into 5% acetic acid overnight at 4°C. The N54(L6)C56 peptide was purified to homogeneity from the soluble fraction by reverse-phase high-performance liquid chromatography (HPLC) (Waters Corp, Milford, MA) on a C18 preparative column (Vydac, Hesperia, CA), and then lyophilized.
A second gp41-based peptide, corresponding to the immunodominant region RVLAVERYLRDQQLLGIWGCSGKLICTTAVPWNASWSNKSLNKI, was also used. This peptide, gp41-SP400, was synthesized by Primm Biotech Inc., Cambridge, MA and provided as a gift by Dr. Georgia D. Tomaras (Duke University School of Medicine, Durham, NC). Its previous use to detect anti-gp41 antibodies in plasma from HIV-1-infected donors has been described elsewhere.21
The gp41-N54 peptide was dissolved in 10 mM Tris buffer (pH 8.0) containing 0.1% sodium dodecyl sulfate (SDS) and the gp41-SP400 peptide was dissolved in dimethyl sulfoxide (DMSO). Reconstituted peptides were stored at −20°C until used for coating.
Determination of IgG subclasses in human serum
The gp120-capture ELISA procedure has been described previously.22,23 Briefly, the plates were coated with 10 μg/ml of sheep Ab D7324 to the gp120 C-terminus (Cliniqa Corp, CA, Cat. #6205). Three-fold dilution of plasma or serum starting at 1:100 dilution was added to wells that did (in duplicate) or did not (single) receive gp120 [300 ng/ml in Tris-buffered saline (TBS)]. In other ELISAs, the antigens were coated directly to wells (5 μg/ml of gp41-N54, 2 μg/ml of gp41-SP400, and 1 μg/ml of p24 or Tat) by an overnight incubation in a 0.1 M sodium carbonate buffer (pH 9.5). In initial experiments, MAbs 4E10, 2F5 (for gp41-N54), and HIVIG (for p24 and gp41-SP400) were used to confirm binding of the respective antigens to the ELISA plate wells.
The specific horseradish peroxidase (HRP)-conjugated polyclonal detection antibodies were goat antihuman IgG (AHP1323P, AbD Serotec, Oxford, UK) and sheep antihuman IgG 1–4 (AP006, 007, 008, 009, The Binding Site, Birmingham, UK). A tetramethylbenzidine (TMB) substrate (BD Pharmingen), followed by 2 N H2SO4, was used for the colorimetric endpoint. Plates were read at 450 nm on an E max precision microplate reader (Molecular Devices, Sunnyvale, CA). The above conjugates were selected based on their lack of reactivity with the D7324 capture Ab (some others we tested did cross-react significantly with this sheep Ab, creating unacceptably high backgrounds). In initial experiments, the intensity of reaction with plate-bound HIVIG (10 μg/ml input concentration) was used to determine a series of conjugate dilutions before we used the MAb-based assay to arrive at the final working dilution (see below). The specificity and sensitivity of each antisubclass detection antibody were evaluated using 3-fold dilutions of human MAbs of known subclasses: b12 (IgG1),24 F425-B4e8 (IgG2),19 and 447-52D (IgG3).20 Starting concentrations of the MAbs were 1 and 0.75 μg/ml for b12 and F425- B4e8 and 10 μl/ml for 447-52D. We are unaware of any gp120-binding IgG4 human MAb.
Calculation of endpoint titers
To calculate endpoint titers, background OD values (wells without gp120) were subtracted from ones derived from test wells containing both antigen and serum. The corrected OD values were fitted to a four-parameter sigmoid function with the bottom plateau constrained to 0 (Graphpad, Prism). The cut-off was set to 5 SD above the mean OD value derived from four background wells. Sera with extrapolated reciprocal titers <1.0 × 102 (1:100 serum dilution), or ones generating curve fits with poor R2 values (<0.7), were considered negative. Only samples yielding a background-corrected OD450 value >0.10 at the starting dilution of 1:100 were considered to have a correctly calculated endpoint titer; if not, the sample was deemed negative for the Ab specificity.
Statistical analyses
Statistical significance was assessed using a one-tailed Mann–Whitney U test and a one-tailed Fisher's exact test. As noted above, a value of 10 was assigned whenever a subclass titer was <1.0 × 102. We performed a total of nine Mann–Whitney U tests and we used Fisher's exact test seven times, which raises the problem of mass significance. However, Bonferroni's and other corrections for multiple testing rest on controversial assumptions and risk introducing errors of their own.25 We therefore present all p values for critical assessment against an unmodified α level of 0.05. Spearman's rank correlation was used to calculate the r coefficients.
Results
IgG subclass assays for human anti-gp120, gp41, p24, and Tat antibodies
To measure the titers of specific IgG subclasses in sera, we used ELISA-based methods.22,23 To measure gp120-specific titers, we used Ab D7324 to capture gp120 to the solid phase, while for the gp41, p24, and Tat ELISAs the antigen was directly coated onto the plate wells. The specificities of the various IgG subclass ELISAs were assessed using human anti-gp120 MAbs of known isotypes: b12 for IgG1, F425-B4e8 for IgG2, and 447-52D for IgG3. We were unable to identify a human anti-gp120 MAb of the (relatively rare) IgG4 isotype.
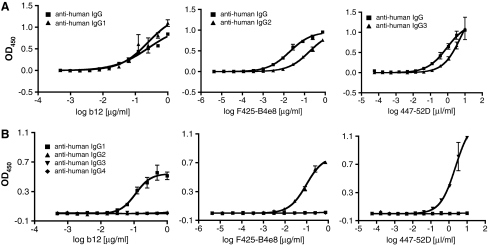
We conducted preliminary studies to identify the most suitable dilutions of IgG subclass-specific detection antibody conjugates. We sought assay conditions that balanced sensitivity and specificity. Our goal was to detect a gp120-bound MAb approximately as sensitively with the appropriate subclass-specific conjugate as with a polyclonal antihuman IgG conjugate, but without significant cross-reactivity with MAbs of a different subclass. The resulting anti-gp120 IgG1, IgG2, and IgG3 subclass assays were compared with the total anti-gp120 IgG assay (Fig. 1A). A 1:15,000 dilution of the antihuman IgG conjugate allowed detection of the appropriate anti-gp120 MAb with sensitivities comparable to the use of 1:1000, 1:500, and 1:1000 dilutions of the IgG1-, IgG2-, and IgG3-specific detection antibody conjugates (note that the latter are used at concentrations ∼6- to 25-fold higher than recommended by the manufacturer). At these dilutions, the antihuman IgG1, IgG2, and IgG3 conjugates detected only the cognate MAbs b12, F425-B4e8, and 447-52D, respectively, with negligible cross-reactivity with MAbs from other subclasses (Fig. 1B). Although we had no positive control for the specificity of the antihuman IgG4 conjugate, we confirmed that it did not cross-react with MAbs from other subclasses when used at a 1:500 dilution (Fig. 1B). Under the conditions of the various assays, there was no significant background signal when sera from HIV-1-uninfected humans were tested at a 1:100 dilution (data not shown).
FIG. 1.
Specificity, cross-reactivity, and sensitivity tests for mouse antihuman IgG1, G2, G3, and G4 MAb conjugates. (A) gp120-binding MAbs of known IgG subclasses (IgG1-b12, IgG2-F425-B4e8, IgG3-447-52D) were used to compare the detection sensitivities of HRP-conjugated sheep antihuman IgG1 (1:1000), IgG2 (1:500), and IgG3 (1:1000) antibodies with that of an HRP-conjugated goat antihuman IgG polyclonal antibody (1:15,000) using a D7324-based gp120-capture ELISA. (B) HRP-conjugated sheep antihuman IgG1, IgG2, IgG3 [each at the same dilutions as in (A)], and IgG4 (1:500 dilution) antibodies were assessed for specificity and cross-reactivity using the same MAbs and ELISA format as in (A).
The assays for anti-gp120 IgG1, IgG2, IgG3, and IgG4 antibodies are therefore specific. Moreover, although there is some loss of sensitivity compared to when a subclass-independent conjugate is used to detect total IgG, at least some degree of cross-comparison is possible; the assays are semiquantitative for comparing subclass-specific responses to the same antigen (e.g., gp120). We also used the same conjugate dilutions when studying the IgG subclass responses to gp41, p24, and Tat, so again the resulting subclass titers can be cross-compared on a semiquantitative basis. The titers cannot, however, be meaningfully compared between the various antigens, as the antigen-coating conditions used in the different assays are different.
The anti-gp120 response to an Env-containing vaccine is predominantly IgG1
We first studied the gp120-specific IgG subclass distribution in 25 volunteers in the HVTN 026 trial, a subset of 160 participants.9
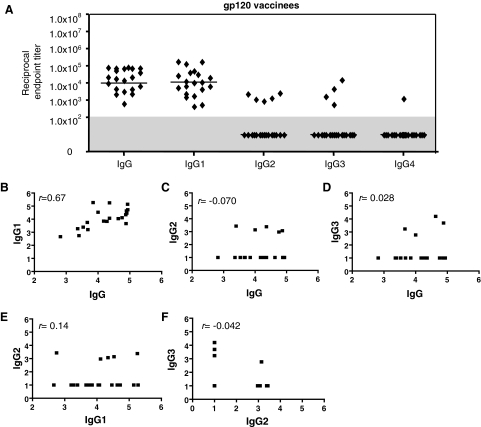
Twenty of the 25 serum samples contained detectable levels of anti-gp120 IgG, with titers ranging from 6.0 × 102 to 9.0 × 104 and a median of 1.0 × 104 (Fig. 2). No gp120-specific IgG was detectable in the other five samples, which where later confirmed to be from placebo recipients (data not shown). No subclass-specific assays were carried out on these five placebo samples.
FIG. 2.
Anti-gp120 binding IgG subclass profiles in vaccinated individuals. (A) The D7324-based gp120-capture ELISA was used to assess anti-gp120 IgG endpoint titers in sera from 25 vaccinees or placebo recipients. The 20 samples in which anti-gp120 IgG could be detected and quantified were further assessed for IgG subclass titers. Each symbol represents the endpoint titer for one vaccinee; the horizontal bar is the median for the group. Nonresponders (titers <1.0 × 102) are shown in the gray shaded area of the plot. (B–F) Correlations between IgG and IgG subclasses: r coefficients were calculated by Spearman's rank correlation.
All 20 individuals from the vaccine group had quantifiable levels of IgG1-specific anti-gp120 antibodies, with a median titer similar to that for the total IgG response (Fig. 2A). There was a strong correlation between the total IgG and IgG1 anti-gp120 titers among the group (r = 0.67) (Fig. 2B). In contrast to the uniform IgG1 response, only five and four individuals had detectable IgG2 and IgG3 responses to gp120, respectively, and only a single sample was positive for IgG4, and then only very weakly (Fig. 2A). There was no correlation between the total IgG and either the IgG2 (r = −0.070) or IgG3 (r = 0.028) subclass-specific anti-gp120 titers (Fig. 2C,D) between the IgG1 and IgG2 titers (r = 0.14) or between the IgG2 and IgG3 (r = −0.042) titers (Fig. 2E and F). (The IgG2 and IgG3 responses were too few to correlate with each other.) Thus, IgG1 was the predominant gp120-binding IgG subclass in gp120-vaccinated individuals in terms of both prevalence and titer.
Anti-gp120 IgG subclass responses in HIV-1 controllers and chronic progressors
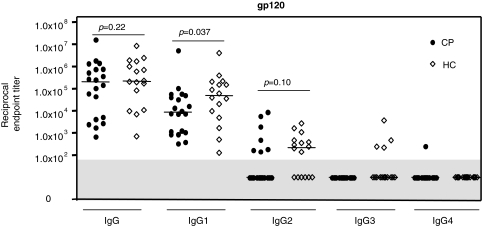
We used the same assays to quantify anti-gp120 subclass responses in HIV-1-infected people who have been categorized as members of the HC and CP cohorts based on plasma virus load and disease progression profiles.2 There was no difference between the two groups in respect of the range of gp120-specific IgG titer responses or the median values (p = 0.22) (Fig. 3). The median IgG titers in both groups (2.2 × 105 for HC, 2.1 × 105 for CP) were significantly greater (by ∼ 10-fold) than in the gp120 vaccinees (2.1 × 104) (p = 0.00070 vs. HC and p = 0.012 vs. CP) (Figs. 2A and 3). The median IgG1 titer for the HC group was ∼7-fold higher than for the CP group (p = 0.037).
FIG. 3.
Anti-gp120 IgG subclass profiles in the HC and CP groups. Endpoint titers of anti-gp120 IgG and IgG subclasses were assessed in plasma from 16 HC and 20 CP group members. Each symbol represents the titer for one individual; the horizontal bar is the median value for the group. Nonresponders (titers <1.0 × 102) are shown in the gray shaded area of the plot.
Quantifiable IgG2 responses were moderately more frequent in the HC group (10 of 16, 62.5%) than in the CP group (7 of 20, 35%), and the median titer for the HC group was also moderately higher. However, neither difference was statistically significant (p = 0.096 for the frequency of response, by Fisher's exact test; p = 0.10 for the titer difference, by Mann–Whitney U test). The detection of IgG2 was somewhat more frequent in sera from the HIV-1-infected individuals than the vaccinees (17 of 36 vs. 5 of 20, p = 0.088, Fisher's exact test). IgG3 responses were infrequent, although they were more often detected in the HC group (4 of 16) than in the CP group (0 of 20) (p = 0.031), while a gp120-specific IgG4 titer was quantifiable in only a single individual, a member of the CP group (Fig. 3).
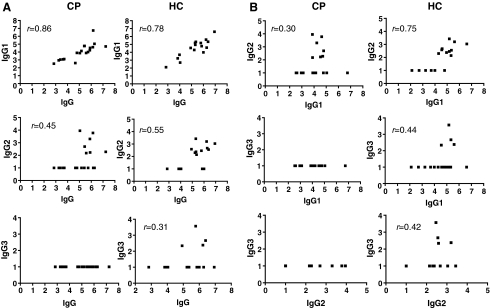
The gp120-specific IgG1 titers were significantly correlated with the corresponding IgG responses for both groups (r = 0.86 and r = 0.78 for CP and HC, respectively) (Fig. 4A, top panel). The IgG2 and total IgG titers were also correlated (r = 0.45 and r = 0.55 for CP and HC, respectively) (Fig. 4A, middle panel), but there was no correlation between the infrequent IgG3 and IgG4 responses and the total IgG titers (Fig. 4A, bottom panel and data not shown).
FIG. 4.
Correlations between anti-gp120 IgG and IgG subclasses (A) and among individual subclasses (B) for the HC and CP groups. The r coefficients were calculated by Spearman's rank correlation.
The IgG1 titers in the HC group were significantly correlated with both the IgG2 (r = 0.75) and the IgG3 (r = 0.44) titers, but this was not the case for the CP group (Fig. 4B, upper and middle panel). However, the IgG2 and IgG3 titers in the HC group were not correlated (Fig. 4B, bottom panel). Thus, the TH1-associated IgG subclasses, IgG2 and to some extent IgG3 (see Discussion), were more frequently detected in the HC group. Overall, the IgG subclass profile was broader in these individuals than in the CP group or in the gp120 vaccine recipients.
Anti-gp41 responses of HIV-1 controllers and chronic progressors
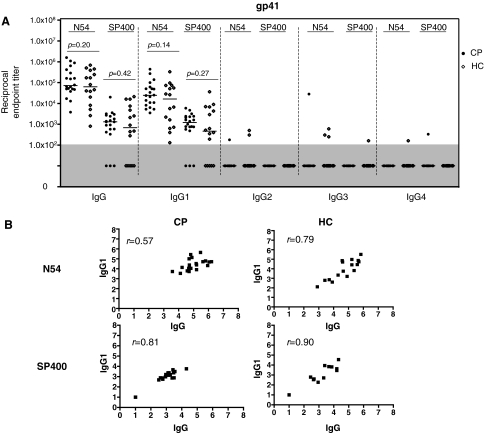
All the HC and CP plasma samples were positive for IgG antibodies reactive with the gp41-N54 peptide, albeit over a wide range of titers (8.0 × 102 to 1.0 × 107). The median IgG titers of ∼1.0 × 105 did not differ between the two groups (p = 0.20) and were comparable to the median anti-gp120 IgG titers (Fig. 5A, compare with Fig. 3).
FIG. 5.
Anti-gp41 IgG subclass profiles in the HC and CP groups. (A) The endpoint titers of IgG and IgG subclass antibodies to the gp41-N54 and gp41-SP400 peptides were assessed using plasma from 16 HC and 20 CP group members. Each symbol represents the titer for one individual; the horizontal bar is the median value for the group. Nonresponders (titers <1.0 × 102) are shown in the gray shaded area of the plot. (B) Correlations between anti-gp41 IgG and IgG subclasses were investigated by Spearman's rank correlation.
The IgG titers against the second gp41-based peptide, gp41-SP400, were lower than those to gp41-N54 (medians of 1.3 × 103 and 6.9 × 102 for the HC and CP groups, respectively). In addition, 5 of the 16 HC samples and 3 of the 20 CP samples had no detectable IgG antibodies against gp41-SP400 (Fig. 5A). However, as was observed using gp41-N54, the median IgG titers against gp41-SP400 did not differ between the two groups (p = 0.42).
IgG1 was the dominant subclass in both patient groups against each of the gp41 peptides, with IgG2, IgG3, and IgG4 antibodies either completely absent (0 of 36 combined samples were positive using gp41-SP400) or rare (3 of 36 positive using gp41-N54) (Fig. 5A). Thus, IgG2, IgG3, and IgG4 antibodies to gp41 were much less frequently detected than to gp120, despite the similarities in the median IgG titers to gp120 and gp41. The deficit of IgG2 antibodies to gp41-N54 (3 of 36 positive) and to gp41-SP400 (0 of 36), compared to gp120 (17 of 36 positive), was particularly notable (p = 0.00022 and p = 6.7 × 10−7, Fisher's exact test) (Figs. 4A and 5A). At least for gp41-N54 this was not a mere reflection of lower IgG titers than against gp120, although with gp41-SP400 that may have been the case (Figs. 3 and 5A). The IgG1 titers against both gp41-N54 and gp41-SP400 correlated strongly with the total IgG titers for both patient groups (Fig. 5B).
Anti-p24 responses of HIV-1 controllers and chronic progressors
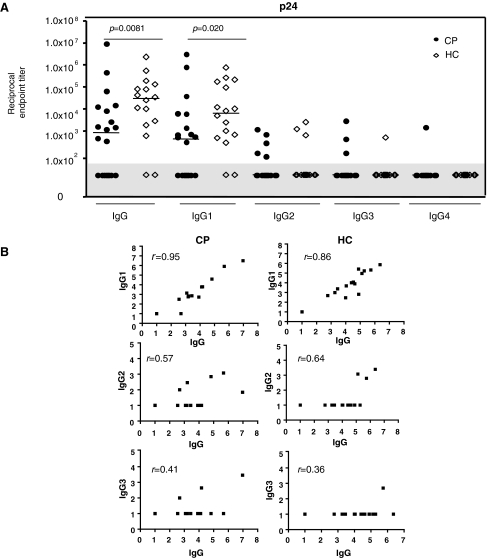
The IgG and IgG1 anti-p24 titers in the HC group were significantly greater than in the CP group (Fig. 6A). For total anti-p24 IgG, the median titers of 3.0 × 104 compared to 8.5 × 102 for the HC and CP groups, respectively, differed by ∼35-fold (p = 0.0081); for IgG1, the corresponding titer differential was ∼15-fold (6.4 × 103 compared to 4.3 × 102; p = 0.020). Moreover, a smaller proportion of the CP group had anti-p24 antibodies (12/20 or 60% compared to 14/16 or 87% in the HC group, for the total IgG response to p24). This difference was not, however, statistically significant (p = 0.071 by Fisher's exact test). Only a minority of samples (4 of 16 HC, 9 of 20 CP) contained quantifiable titers of IgG2, IgG3, or IgG4 antibodies to p24, with no statistically significant difference in prevalence between the HC and CP groups (p = 0.19 by Fisher's exact test) (Fig. 6A).
FIG. 6.
Anti-p24 IgG subclass profiles in the HC and CP groups. (A) Endpoint titers of anti-p24 IgG and IgG subclasses were assessed in plasma from 16 HC and 20 CP group members. Each symbol represents the titer for one individual; the horizontal bar is the median value for the group. Nonresponders (titers <1.0 × 102) are shown in the gray shaded area of the plot. (B) Correlations between anti-p24 IgG and IgG subclasses were investigated by Spearman's rank correlation.
For both groups, the IgG and IgG1 responses were strongly correlated (Fig. 6B, upper panel) while there was a moderate correlation between the IgG and IgG2 responses (middle panel) but weaker between IgG and IgG3 (bottom panel).
Anti-Tat responses of HIV-1 controllers and chronic progressors
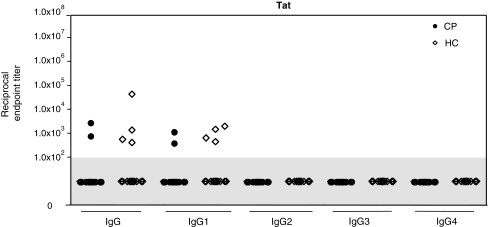
Very few samples from either patient group contained quantifiable anti-Tat IgG and IgG1 antibodies, and no IgG2, IgG3, and IgG4 antibodies could be detected in either group (Fig. 7). The prevalence of IgG and IgG1 anti-Tat antibodies was, however, moderately greater for the HC group (4/16 or 25%) than for the CP group (2/20 or 10%), although the difference was not statistically significant (p = 0.23, Fisher's exact test). There was also no significant difference in the median titers between the HC and CP groups (p = 0.23).
FIG. 7.
Anti-Tat IgG subclass profiles in the HC and CP groups. Endpoint titers of anti-Tat IgG and IgG subclasses were assessed in plasma from 16 HC and 20 CP group members. Each symbol represents the titer for one individual; the horizontal bar is the median value for the group. Nonresponders (titers <1.0 × 102) are shown in the gray shaded area of the plot.
Discussion
Multiple studies of several cohorts over the past 20 years have shown that anti-gp120 IgG titers are not predictive of the rate of disease progression despite the antiviral effects that can be attributed to such antibodies.2,26–28 In contrast, anti-p24 IgG titers are consistently higher in nonprogressing individuals, and the loss of this response correlates with disease progression.29–34 Our own results confirm these earlier findings; anti-p24 IgG titers were higher, and to some extent more frequent, in the HC cohort than in the CP group. As anti-p24 Abs lack any well-documented antiviral activities, the generally accepted explanation is that their absence, or a decline in their titers during progressive HIV-1 infection, reflects the loss of T-helper responses. An alternative possibility is that p24 antibodies are removed from circulation by Gag antigens, which are more abundant in progressing patients because of their higher viral loads. However, anti-p24 antibodies are present in plasma at concentrations (10–1000 μg/ml) that are several orders of magnitude greater than p24 proteins (10–1000 pg/ml), so it seems unlikely that sufficient viral antigens would be produced to consume enough antibodies by forming immune complexes. Moreover, Env proteins are produced in amounts roughly comparable with Gag proteins and yet anti-gp120 antibody titers usually do not decline during progressive infection.26,35
The relationship between antibody responses and disease progression is less clear-cut for other HIV-1 antigens. Studies on the Amsterdam cohort indicated that progressors had significantly higher anti-gp120 and anti-gp41 IgG titers than nonprogressors at 6 months after seroconversion.34 Later in infection, however, no such titer differences could be identified.34 Our results on cohorts of HC and CP individuals who had been infected for several years are consistent with the latter finding that anti-gp120 and anti-gp41 antibody titers are not a correlate of progression. Lambotte et al.36 recently concluded that no single anti-HIV-1 antibody specificity (gp120, p66, p55, gp41, p31, p24, p17) was a clear correlate of effective immunity in a cohort of 22 French virus controllers.
Anti-Tat antibodies have been suggested to delay progression to AIDS, based on reports that these antibodies are more frequently detected in individuals who are nonprogressing or who have low viral loads.33,37–39 However, no such association was observed in another study.40 In agreement with the latter report, we found that only a few individuals in either the HC or the CP group had quantifiable anti-Tat antibodies. Although IgG and IgG1 anti-Tat antibodies were detected slightly more frequently in the HC group (25%) than in the CP group (10%), the difference was not statistically significant, and the median titers were also not different between the two groups. This contrasts with our observations on the anti-p24 response. In general, anti-Tat antibodies are detected less frequently and at lower titers than anti-p24 antibodies.26,33,40–43 It has been argued that because anti-Tat antibodies are sometimes more frequent in nonprogressing individuals than in progressors, they must have an antiviral effect in vivo.33,37,38,44 The same argument could also be made, and on much stronger statistical grounds, for anti-p24 antibodies. However, as noted above, the general accepted explanation is that high anti-p24 antibody titers are merely a surrogate marker for well-preserved T-helper functions. We suggest that the same applies to anti-Tat antibodies, although their lower prevalence makes them a less reliable marker for nonprogression.
The subclass of the IgG response to various HIV-1 antigens has also been reported to vary with progression status.45,46 Thus, in the Sydney Blood Bank Cohort, nonprogressors had more broadly based IgG subclass responses than progressors; more specifically, a few nonprogressors had anti-p24 IgG3 antibodies that were not detected in progressors. A more extensive analysis of IgG subclass responses to HIV-1 proteins was carried out on the French Asymptomatic Long-Term (ALT) cohort.47 The ALT cohort members generally had strong IgG1 responses to Env and Pol antigens and, as usually observed with nonprogressors, most of them also had strong anti-p24 IgG responses; these were distributed across all four subclasses, IgG1, IgG2, IgG3, and IgG4.47 A striking and unusual observation, however, was that Env-specific IgG2 antibodies, particularly to gp41 epitopes, were more prevalent in the nonprogressors.47 The slowest decline in CD4+ TH1 cells was seen in individuals who had a combination of gp41-specific IgG2 antibodies and p24-reactive CD4 TH1 cells, suggesting that a TH1-biased response to HIV-1 infection might be more beneficial than one skewed toward TH2.48
In our study, there were no features of the anti-gp41 peptide response that distinguished the HC and CP groups from each other, and the anti-gp41 peptide response was strongly IgG1 dominated in both groups. Whereas 20–40% of the ALT cohort members (divided into two categories based on viral load) had IgG2 antibodies to gp41,47,48 we very rarely detected these antibodies. Indeed, in the HC and CP cohorts combined, the deficit of IgG2 antibodies to gp41-N54 (3 of 36) and to gp41-SP400 (0 of 36), compared to gp120 (17 of 36 positive), was particularly notable (p = 0.00022 and p = 6.7 × 10−7, Fisher's exact test). Why this should be so is not clear, but is worth further investigation.
Comparing the outcome of studies on different cohorts is difficult, because there are varying definitions of nonprogression and because different methodologies are used to study Ab responses to different HIV-1 antigens. Thus, in the ALT cohort study a semiquantitative Western blot assay was used to detect Abs to virus-derived, full-length gp41.47 In contrast, we used an ELISA to quantify Abs to two different gp41 peptides that contain various antibody epitopes, in one case (gp41-SP400) including the immunodominant cluster I sites.
As noted above, a conclusion of the ALT cohort study was that a TH1-biased immune response to HIV-1 infection might be more favorable than one with a TH2 bias.48 We were unable to confirm this finding, while not being able to formally refute it either. Human IgG1 can be associated with both TH1 and TH2 responses, IgG2 and IgG4 are unambiguously associated with TH1 and TH2 responses respectively, while IgG3 may be predominantly linked to TH1 responses.49–56 Unfortunately, IgG2, IgG3, and IgG4 antibodies to all the HIV-1 antigens were detected too inconsistently in our cohorts for us to be able to carry out any meaningful analysis of TH polarization. Hence, we also could not confirm the report that 60–80% of ALT cohort members had anti-p24 antibodies from the IgG2, IgG3, and IgG4 subclasses.47 In our HC cohort, the prevalence of these antibodies was only 25%, less than in the CP group (45%).
We detected antibodies from the IgG2, TH1-associated, subclass more frequently with gp120 than with the gp41 peptide, although neither the prevalence nor titer of anti-gp120 IgG2 antibodies was correlated with HC or CP status. The antibody responses to the two Env subunits also differ in longevity: the half-lives of anti-gp120 and anti-gp41 titers were recently reported to be ∼80 weeks and ∼30 weeks respectively, although both are shorter than the anti-Gag titer half-life (∼650 weeks). Whether there are causal links between the IgG subclass response and factors such as the glycosylation and T cell help dependence of different antigens, or B cell memory and plasma cell conversion, remains to be investigated.
Individuals immunized with the gp120-containing vaccine had markedly lower titers of anti-gp120 antibodies than infected people, which is again consistent with previous reports.26,57–59 We observed that the anti-gp120 response was IgG1 dominated in individuals who were primed with a canarypox vaccine vector expressing Gag, Pol, and Env proteins, and then boosted with recombinant gp120 in Alum, just as it was in both groups of infected people. We note, however, that anti-gp120 IgG2 antibodies were less frequently detected in vaccinees than in both groups of infected individuals. In a previous study, in which vaccinees received four doses of gp120 in Alum, TH2-associated IgG4 antibodies to gp120 were observed frequently and at relatively high titers.58 This finding contrasts with our observations that IgG4 antibodies to gp120 were very rare in the prime-boost vaccine group. Indeed, IgG4 antibodies are generally the least common of the four subclasses in both healthy and HIV-1-infected individuals.56,60–62 Hence, responses to multiple immunizations with a gp120 protein-only immunogen may be more TH2 biased than the ones we have measured, explaining the increased frequency of IgG4 antibodies reported by Gorse et al.58 Although this would need to be confirmed by a direct comparison, a synthesis of our results and those of Gorse et al.58 would create the following rank order for the TH2 bias of the anti-gp120 response: gp120 only > canarypox plus gp120 > natural infection.
The TH2 bias in the human anti-gp120 response is consistent with observations made in mice63 and may be based, at least in part, on the properties of the antigen itself.64,65 It may be relevant that the TH2-associated IgG4 and IgE responses to HIV-1 antigens are more frequently detected among hemophiliacs, who may have been more repeatedly exposed both to infectious and partly inactivated virus than other infected individuals.66,67 The TH2-biased response to a protein-only immunization is also consistent with HA-specific antibody responses induced by different vaccines in mice. Thus, recombinant HA elicits TH2-biased antibody responses (although not as pronounced as those to gp120 seen in mice64,68), whereas a whole-inactivated influenza immunogen elicits HA-specific responses that are more TH1 biased.69 The difference arises because of TLR activation by other components in the inactivated virus vaccine that are absent from Alum-formulated recombinant proteins.69 Similar mechanisms are likely to play a role during the shaping of gp120-specific antibody responses during natural HIV-1 infection and in different vaccination settings.
It was recently reported that the HIV-1 Nef protein can selectively suppress IgG2 and IgA responses to the virus.70 It seems possible that HIV-1 counters these isotypes because they are particularly effective, in relative terms, at inhibiting the replication or transmission of the virus in certain circumstances. For example, IgA is potentially important for preventing mucosal transmission, but IgA responses to HIV-1 antigens are unusually deficient in HIV-1-infected people.71 Larger scale studies of the IgG subclass responses to various HIV-1 antigens are probably justified, to increase our understanding of the correlates of nonprogressive infection. This type of information might also be useful for the design of preventative vaccines, including the use of TH-polarizing adjuvants.
Acknowledgments
We appreciate the advice on antihuman subclass conjugates that we received from Dr. Bharat S. Parekh (Chief, Serology/Incidence and Diagnostics Team) and Ms. Debra B. Kuehl of the Center for Disease Control and Prevention, Atlanta. We thank Dr. Andrea Cerutti (Weill Cornell Medical College) for helpful discussions on the TH polarization of human IgG subclasses. We are grateful to Dr. Georgia Tomaras for providing the gp41-SP400 peptide. This work was supported by NIH Grants AI 36082, AI 45463, and AI 30914 and by an Unrestricted Grant for Infectious Diseases Research from the Bristol Myers Squibb Foundation. We thank the HIV Vaccine Trials Network (HVTN) and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIAID-NIH) for plasma samples from vaccinee samples, and in particular David Montefiori for facilitating this interaction.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Deeks SG. Walker BD. Human immunodeficiency virus controllers: Mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Pereyra F. Addo MM. Kaufmann DE. Liu Y. Miura T. Rathod A. Baker B. Trocha A. Rosenberg R. Mackey E. Ueda P. Lu Z. Cohen D. Wrin T. Petropoulos CJ. Rosenberg ES. Walker BD. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197:563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 3.Baker BM. Block BL. Rothchild AC. Walker BD. Elite control of HIV infection: Implications for vaccine design. Expert Opin Biol Ther. 2009;9:55–69. doi: 10.1517/14712590802571928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards BH. Bansal A. Sabbaj S. Bakari J. Mulligan MJ. Goepfert PA. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol. 2002;76:2298–2305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geldmacher C. Currier JR. Herrmann E. Haule A. Kuta E. McCutchan F. Njovu L. Geis S. Hoffmann O. Maboko L. Williamson C. Birx D. Meyerhans A. Cox J. Hoelscher M. CD8 T-cell recognition of multiple epitopes within specific Gag regions is associated with maintenance of a low steady-state viremia in human immunodeficiency virus type 1-seropositive patients. J Virol. 2007;81:2440–2448. doi: 10.1128/JVI.01847-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honeyborne I. Prendergast A. Pereyra F. Leslie A. Crawford H. Payne R. Reddy S. Bishop K. Moodley E. Nair K. van der Stok M. McCarthy N. Rousseau CM. Addo M. Mullins JI. Brander C. Kiepiela P. Walker BD. Goulder PJ. Control of human immunodeficiency virus type 1 is associated with HLA-B*13 and targeting of multiple gag-specific CD8+ T-cell epitopes. J Virol. 2007;81:3667–3672. doi: 10.1128/JVI.02689-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiepiela P. Ngumbela K. Thobakgale C. Ramduth D. Honeyborne I. Moodley E. Reddy S. de Pierres C. Mncube Z. Mkhwanazi N. Bishop K. van der Stok M. Nair K. Khan N. Crawford H. Payne R. Leslie A. Prado J. Prendergast A. Frater J. McCarthy N. Brander C. Learn GH. Nickle D. Rousseau C. Coovadia H. Mullins JI. Heckerman D. Walker BD. Goulder P. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 8.Streeck H. Lichterfeld M. Alter G. Meier A. Teigen N. Yassine-Diab B. Sidhu HK. Little S. Kelleher A. Routy JP. Rosenberg ES. Sekaly RP. Walker BD. Altfeld M. Recognition of a defined region within p24 gag by CD8+ T cells during primary human immunodeficiency virus type 1 infection in individuals expressing protective HLA class I alleles. J Virol. 2007;81:7725–7731. doi: 10.1128/JVI.00708-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleghorn F. Pape JW. Schechter M. Bartholomew C. Sanchez J. Jack N. Metch BJ. Hansen M. Allen M. Cao H. Montefiori DC. Tomaras GD. Gurunathan S. Eastman DJ. do Lago RF. Jean S. Lama JR. Lawrence DN. Wright PF. Lessons from a multisite international trial in the Caribbean and South America of an HIV-1 Canarypox vaccine (ALVAC-HIV vCP1452) with or without boosting with MN rgp120. J Acquir Immune Defic Syndr. 2007;46:222–230. doi: 10.1097/QAI.0b013e318149297d. [DOI] [PubMed] [Google Scholar]
- 10.Flynn NM. Forthal DN. Harro CD. Judson FN. Mayer KH. Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 11.Mascola JR. Snyder SW. Weislow OS. Belay SM. Belshe RB. Schwartz DH. Clements ML. Dolin R. Graham BS. Gorse GJ. Keefer MC. McElrath MJ. Walker MC. Wagner KF. McNeil JG. McCutchan FE. Burke DS. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 12.Pitisuttithum P. Gilbert P. Gurwith M. Heyward W. Martin M. van Griensven F. Hu D. Tappero JW. Choopanya K. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 13.Moore JP. Klasse PJ. Dolan MJ. Ahuja SK. AIDS/HIV: A STEP into darkness or light? Science. 2008;320:753–755. doi: 10.1126/science.1154258. [DOI] [PubMed] [Google Scholar]
- 14.Buchacher A. Predl R. Strutzenberger K. Steinfellner W. Trkola A. Purtscher M. Gruber G. Tauer C. Steindl F. Jungbauer A, et al. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 15.Scanlan CN. Pantophlet R. Wormald MR. Ollmann Saphire E. Stanfield R. Wilson IA. Katinger H. Dwek RA. Rudd PM. Burton DR. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1 → 2 mannose residues on the outer face of gp120. J Virol. 2002;76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trkola A. Purtscher M. Muster T. Ballaun C. Buchacher A. Sullivan N. Srinivasan K. Sodroski J. Moore JP. Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardoso RM. Zwick MB. Stanfield RL. Kunert R. Binley JM. Katinger H. Burton DR. Wilson IA. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22:163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Parker CE. Deterding LJ. Hager-Braun C. Binley JM. Schulke N. Katinger H. Moore JP. Tomer KB. Fine definition of the epitope on the gp41 glycoprotein of human immunodeficiency virus type 1 for the neutralizing monoclonal antibody 2F5. J Virol. 2001;75:10906–10911. doi: 10.1128/JVI.75.22.10906-10911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavacini L. Duval M. Song L. Sangster R. Xiang SH. Sodroski J. Posner M. Conformational changes in env oligomer induced by an antibody dependent on the V3 loop base. AIDS. 2003;17:685–689. doi: 10.1097/00002030-200303280-00006. [DOI] [PubMed] [Google Scholar]
- 20.Gorny MK. Conley AJ. Karwowska S. Buchbinder A. Xu JY. Emini EA. Koenig S. Zolla-Pazner S. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J Virol. 1992;66:7538–7542. doi: 10.1128/jvi.66.12.7538-7542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomaras GD. Yates NL. Liu P. Qin L. Fouda GG. Chavez LL. Decamp AC. Parks RJ. Ashley VC. Lucas JT. Cohen M. Eron J. Hicks CB. Liao HX. Self SG. Landucci G. Forthal DN. Weinhold KJ. Keele BF. Hahn BH. Greenberg ML. Morris L. Karim SS. Blattner WA. Montefiori DC. Shaw GM. Perelson AS. Haynes BF. Initial B-cell responses to transmitted human immunodeficiency virus type 1: Virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore JP. Jarrett RF. Sensitive ELISA for the gp120 and gp160 surface glycoproteins of HIV-1. AIDS Res Hum Retroviruses. 1988;4:369–379. doi: 10.1089/aid.1988.4.369. [DOI] [PubMed] [Google Scholar]
- 23.Moore JP. Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70:1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burton DR. Barbas CF., 3rd Persson MA. Koenig S. Chanock RM. Lerner RA. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci USA. 1991;88:10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binley JM. Klasse PJ. Cao Y. Jones I. Markowitz M. Ho DD. Moore JP. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J Virol. 1997;71:2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groopman JE. Benz PM. Ferriani R. Mayer K. Allan JD. Weymouth LA. Characterization of serum neutralization response to the human immunodeficiency virus (HIV) AIDS Res Hum Retroviruses. 1987;3:71–85. doi: 10.1089/aid.1987.3.71. [DOI] [PubMed] [Google Scholar]
- 28.Bailey JR. Lassen KG. Yang HC. Quinn TC. Ray SC. Blankson JN. Siliciano RF. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J Virol. 2006;80:4758–4770. doi: 10.1128/JVI.80.10.4758-4770.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheingsong-Popov R. Panagiotidi C. Bowcock S. Aronstam A. Wadsworth J. Weber J. Relation between humoral responses to HIV gag and env proteins at seroconversion and clinical outcome of HIV infection. BMJ. 1991;302:23–26. doi: 10.1136/bmj.302.6767.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown AE. Vahey MT. Zhou SY. Chung RC. Ruiz NM. Hofheinz D. Lane JR. Mayers DL. Quantitative relationship of circulating p24 antigen with human immunodeficiency virus (HIV) RNA and specific antibody in HIV-infected subjects receiving antiretroviral therapy. The RV43 Study Group. J Infect Dis. 1995;172:1091–1095. doi: 10.1093/infdis/172.4.1091. [DOI] [PubMed] [Google Scholar]
- 31.Hogervorst E. Jurriaans S. de Wolf F. van Wijk A. Wiersma A. Valk M. Roos M. van Gemen B. Coutinho R. Miedema F, et al. Predictors for non- and slow progression in human immunodeficiency virus (HIV) type 1 infection: Low viral RNA copy numbers in serum and maintenance of high HIV-1 p24-specific but not V3-specific antibody levels. J Infect Dis. 1995;171:811–821. doi: 10.1093/infdis/171.4.811. [DOI] [PubMed] [Google Scholar]
- 32.Chargelegue D. Stanley CM. O'Toole CM. Colvin BT. Steward MW. The affinity of IgG antibodies to gag p24 and p17 in HIV-1-infected patients correlates with disease progression. Clin Exp Immunol. 1995;99:175–181. doi: 10.1111/j.1365-2249.1995.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zagury JF. Sill A. Blattner W. Lachgar A. Le Buanec H. Richardson M. Rappaport J. Hendel H. Bizzini B. Gringeri A. Carcagno M. Criscuolo M. Burny A. Gallo RC. Zagury D. Antibodies to the HIV-1 Tat protein correlated with nonprogression to AIDS: A rationale for the use of Tat toxoid as an HIV-1 vaccine. J Hum Virol. 1998;1:282–292. [PubMed] [Google Scholar]
- 34.Zwart G. van der Hoek L. Valk M. Cornelissen MT. Baan E. Dekker J. Koot M. Kuiken CL. Goudsmit J. Antibody responses to HIV-1 envelope and gag epitopes in HIV-1 seroconverters with rapid versus slow disease progression. Virology. 1994;201:285–293. doi: 10.1006/viro.1994.1293. [DOI] [PubMed] [Google Scholar]
- 35.Klasse PJ. Moore JP. Is there enough gp120 in the body fluids of HIV-1-infected individuals to have biologically significant effects? Virology. 2004;323:1–8. doi: 10.1016/j.virol.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Lambotte O. Ferrari G. Moog C. Yates NL. Liao HX. Parks RJ. Hicks CB. Owzar K. Tomaras GD. Montefiori DC. Haynes BF. Delfraissy JF. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009;23:897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Re MC. Furlini G. Vignoli M. Ramazzotti E. Zauli G. La Placa M. Antibody against human immunodeficiency virus type 1 (HIV-1) Tat protein may have influenced the progression of AIDS in HIV-1-infected hemophiliac patients. Clin Diagn Lab Immunol. 1996;3:230–232. doi: 10.1128/cdli.3.2.230-232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Re MC. Vignoli M. Furlini G. Gibellini D. Colangeli V. Vitone F. La Placa M. Antibodies against full-length Tat protein and some low-molecular-weight Tat-peptides correlate with low or undetectable viral load in HIV-1 seropositive patients. J Clin Virol. 2001;21:81–89. doi: 10.1016/s1386-6532(00)00189-x. [DOI] [PubMed] [Google Scholar]
- 39.Richardson MW. Mirchandani J. Duong J. Grimaldo S. Kocieda V. Hendel H. Khalili K. Zagury JF. Rappaport J. Antibodies to Tat and Vpr in the GRIV cohort: Differential association with maintenance of long-term non-progression status in HIV-1 infection. Biomed Pharmacother. 2003;57:4–14. doi: 10.1016/s0753-3322(02)00327-x. [DOI] [PubMed] [Google Scholar]
- 40.Senkaali D. Kebba A. Shafer LA. Campbell GR. Loret EP. Van Der Paal L. Grosskurth H. Yirrell D. Kaleebu P. Tat-specific binding IgG and disease progression in HIV type 1-infected Ugandans. AIDS Res Hum Retroviruses. 2008;24:587–594. doi: 10.1089/aid.2007.0171. [DOI] [PubMed] [Google Scholar]
- 41.Binley JM. Jin X. Huang Y. Zhang L. Cao Y. Ho DD. Moore JP. Persistent antibody responses but declining cytotoxic T-lymphocyte responses to multiple human immunodeficiency virus type 1 antigens in a long-term nonprogressing individual with a defective p17 proviral sequence and no detectable viral RNA expression. J Virol. 1998;72:3472–3474. doi: 10.1128/jvi.72.4.3472-3474.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butto S. Fiorelli V. Tripiciano A. Ruiz-Alvarez MJ. Scoglio A. Ensoli F. Ciccozzi M. Collacchi B. Sabbatucci M. Cafaro A. Guzman CA. Borsetti A. Caputo A. Vardas E. Colvin M. Lukwiya M. Rezza G. Ensoli B. Sequence conservation and antibody cross-recognition of clade B human immunodeficiency virus (HIV) type 1 Tat protein in HIV-1-infected Italians, Ugandans, and South Africans. J Infect Dis. 2003;188:1171–1180. doi: 10.1086/378412. [DOI] [PubMed] [Google Scholar]
- 43.Krone WJ. Debouck C. Epstein LG. Heutink P. Meloen R. Goudsmit J. Natural antibodies to HIV-tat epitopes and expression of HIV-1 genes in vivo. J Med Virol. 1988;26:261–270. doi: 10.1002/jmv.1890260306. [DOI] [PubMed] [Google Scholar]
- 44.Reiss P. de Wolf F. Kuiken CL. de Ronde A. Dekker J. Boucher CA. Debouck C. Lange JM. Goudsmit J. Contribution of antibody response to recombinant HIV-1 gene-encoded products nef, rev, tat, and protease in predicting development of AIDS in HIV-1-infected individuals. J Acquir Immune Defic Syndr. 1991;4:165–172. [PubMed] [Google Scholar]
- 45.Gorry PR. McPhee DA. Verity E. Dyer WB. Wesselingh SL. Learmont J. Sullivan JS. Roche M. Zaunders JJ. Gabuzda D. Crowe SM. Mills J. Lewin SR. Brew BJ. Cunningham AL. Churchill MJ. Pathogenicity and immunogenicity of attenuated, nef-deleted HIV-1 strains in vivo. Retrovirology. 2007;4:66. doi: 10.1186/1742-4690-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verity EE. Zotos D. Wilson K. Chatfield C. Lawson VA. Dwyer DE. Cunningham A. Learmont J. Dyer W. Sullivan J. Churchill M. Wesselingh SL. Gabuzda D. Gorry PR. McPhee DA. Viral phenotypes and antibody responses in long-term survivors infected with attenuated human immunodeficiency virus type 1 containing deletions in the nef and long terminal repeat regions. J Virol. 2007;81:9268–9278. doi: 10.1128/JVI.00650-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ngo-Giang-Huong N. Candotti D. Goubar A. Autran B. Maynart M. Sicard D. Clauvel JP. Agut H. Costagliola D. Rouzioux C. HIV type 1-specific IgG2 antibodies: Markers of helper T cell type 1 response and prognostic marker of long-term nonprogression. AIDS Res Hum Retroviruses. 2001;17:1435–1446. doi: 10.1089/088922201753197105. [DOI] [PubMed] [Google Scholar]
- 48.Martinez V. Costagliola D. Bonduelle O. N'go N. Schnuriger A. Theodorou I. Clauvel JP. Sicard D. Agut H. Debre P. Rouzioux C. Autran B. Combination of HIV-1-specific CD4 Th1 cell responses and IgG2 antibodies is the best predictor for persistence of long-term nonprogression. J Infect Dis. 2005;191:2053–2063. doi: 10.1086/430320. [DOI] [PubMed] [Google Scholar]
- 49.Jabara HH. Loh R. Ramesh N. Vercelli D. Geha RS. Sequential switching from mu to epsilon via gamma 4 in human B cells stimulated with IL-4 and hydrocortisone. J Immunol. 1993;151:4528–4533. [PubMed] [Google Scholar]
- 50.Punnonen J. Aversa G. Cocks BG. McKenzie AN. Menon S. Zurawski G. de Waal Malefyt R. de Vries JE. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Darmaki S. Knightshead K. Ishihara Y. Best A. Schenkein HA. Tew JG. Barbour SE. Delineation of the role of platelet-activating factor in the immunoglobulin G2 antibody response. Clin Diagn Lab Immunol. 2004;11:720–728. doi: 10.1128/CDLI.11.4.720-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawano Y. Noma T. Yata J. Regulation of human IgG subclass production by cytokines. IFN-gamma and IL-6 act antagonistically in the induction of human IgG1 but additively in the induction of IgG2. J Immunol. 1994;153:4948–4958. [PubMed] [Google Scholar]
- 53.Kitani A. Strober W. Regulation of C gamma subclass germ-line transcripts in human peripheral blood B cells. J Immunol. 1993;151:3478–3488. [PubMed] [Google Scholar]
- 54.Kilpinen S. Laine S. Hulkkonen J. Hurme M. Immunoglobulin G3 and immunoglobulin M isotype plasma levels are influenced by interleukin-1alpha genotype. Scand J Immunol. 2003;57:296–302. doi: 10.1046/j.1365-3083.2003.01231.x. [DOI] [PubMed] [Google Scholar]
- 55.Kotowicz K. Callard RE. Human immunoglobulin class and IgG subclass regulation: Dual action of interleukin-4. Eur J Immunol. 1993;23:2250–2256. doi: 10.1002/eji.1830230930. [DOI] [PubMed] [Google Scholar]
- 56.Aalberse RC. Stapel SO. Schuurman J. Rispens T. Immunoglobulin G4: An odd antibody. Clin Exp Allergy. 2009;39:469–477. doi: 10.1111/j.1365-2222.2009.03207.x. [DOI] [PubMed] [Google Scholar]
- 57.Connor RI. Korber BT. Graham BS. Hahn BH. Ho DD. Walker BD. Neumann AU. Vermund SH. Mestecky J. Jackson S. Fenamore E. Cao Y. Gao F. Kalams S. Kunstman KJ. McDonald D. McWilliams N. Trkola A. Moore JP. Wolinsky SM. Immunological and virological analyses of persons infected by human immunodeficiency virus type 1 while participating in trials of recombinant gp120 subunit vaccines. J Virol. 1998;72:1552–1576. doi: 10.1128/jvi.72.2.1552-1576.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorse GJ. Corey L. Patel GB. Mandava M. Hsieh RH. Matthews TJ. Walker MC. McElrath MJ. Berman PW. Eibl MM. Belshe RB. HIV-1MN recombinant glycoprotein 160 vaccine-induced cellular and humoral immunity boosted by HIV-1MN recombinant glycoprotein 120 vaccine. National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. AIDS Res Hum Retroviruses. 1999;15:115–132. doi: 10.1089/088922299311547. [DOI] [PubMed] [Google Scholar]
- 59.Gilbert PB. Peterson ML. Follmann D. Hudgens MG. Francis DP. Gurwith M. Heyward WL. Jobes DV. Popovic V. Self SG. Sinangil F. Burke D. Berman PW. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis. 2005;191:666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 60.Binley JM. Lybarger EA. Crooks ET. Seaman MS. Gray E. Davis KL. Decker JM. Wycuff D. Harris L. Hawkins N. Wood B. Nathe C. Richman D. Tomaras GD. Bibollet-Ruche F. Robinson JE. Morris L. Shaw GM. Montefiori DC. Mascola JR. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J Virol. 2008;82:11651–11668. doi: 10.1128/JVI.01762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klasse J. Blomberg J. Patterns of antibodies to human immunodeficiency virus proteins in different subclasses of IgG. J Infect Dis. 1987;156:1026–1030. doi: 10.1093/infdis/156.6.1026. [DOI] [PubMed] [Google Scholar]
- 62.Raux M. Finkielsztejn L. Salmon-Ceron D. Bouchez H. Excler JL. Dulioust E. Grouin JM. Sicard D. Blondeau C. IgG subclass distribution in serum and various mucosal fluids of HIV type 1-infected subjects. AIDS Res Hum Retroviruses. 2000;16:583–594. doi: 10.1089/088922200309007. [DOI] [PubMed] [Google Scholar]
- 63.Daly LM. Johnson PA. Donnelly G. Nicolson C. Robertson J. Mills KH. Innate IL-10 promotes the induction of Th2 responses with plasmid DNA expressing HIV gp120. Vaccine. 2005;23:963–974. doi: 10.1016/j.vaccine.2004.03.072. [DOI] [PubMed] [Google Scholar]
- 64.Banerjee K. Andjelic S. Klasse PJ. Kang Y. Sanders RW. Michael E. Durso RJ. Ketas TJ. Olson WC. Moore JP. Enzymatic removal of mannose moieties can increase the immune response to HIV-1 gp120 in vivo. Virology. 2009;389:108–121. doi: 10.1016/j.virol.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shan M. Klasse PJ. Banerjee K. Dey AK. Iyer SP. Dionisio R. Charles D. Campbell-Gardener L. Olson WC. Sanders RW. Moore JP. HIV-1 gp120 mannoses induce immunosuppressive responses from dendritic cells. PLoS Pathog. 2007;3:e169. doi: 10.1371/journal.ppat.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klasse PJ. Berntorp E. Hansson BG. An aberrant subclass pattern of HIV-specific immunoglobulin G in sera from haemophiliacs. AIDS. 1988;2:311–313. [PubMed] [Google Scholar]
- 67.Khalife J. Guy B. Capron M. Kieny MP. Ameisen JC. Montagnier L. Lecocq JP. Capron A. Isotypic restriction of the antibody response to human immunodeficiency virus. AIDS Res Hum Retroviruses. 1988;4:3–9. doi: 10.1089/aid.1988.4.3. [DOI] [PubMed] [Google Scholar]
- 68.Jankovic D. Caspar P. Zweig M. Garcia-Moll M. Showalter SD. Vogel FR. Sher A. Adsorption to aluminum hydroxide promotes the activity of IL-12 as an adjuvant for antibody as well as type 1 cytokine responses to HIV-1 gp120. J Immunol. 1997;159:2409–2417. [PubMed] [Google Scholar]
- 69.Geeraedts F. Goutagny N. Hornung V. Severa M. de Haan A. Pool J. Wilschut J. Fitzgerald KA. Huckriede A. Superior immunogenicity of inactivated whole virus H5N1 influenza vaccine is primarily controlled by Toll-like receptor signalling. PLoS Pathog. 2008;4:e1000138. doi: 10.1371/journal.ppat.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu W. Santini PA. Sullivan JS. He B. Shan M. Ball SC. Dyer WB. Ketas TJ. Chadburn A. Cohen-Gould L. Knowles DM. Chiu A. Sanders RW. Chen K. Cerutti A. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10:1008–1017. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mestecky J. Jackson S. Moldoveanu Z. Nesbit LR. Kulhavy R. Prince SJ. Sabbaj S. Mulligan MJ. Goepfert PA. Paucity of antigen-specific IgA responses in sera and external secretions of HIV-type 1-infected individuals. AIDS Res Hum Retroviruses. 2004;20:972–988. doi: 10.1089/aid.2004.20.972. [DOI] [PubMed] [Google Scholar]