Abstract
Bacterial lipopolysaccharide (endotoxin) is a frequent contaminant of biological specimens and is also known to be a potent inducer of β-chemokines and other soluble factors that inhibit HIV-1 infection in vitro. Though lipopolysaccharide (LPS) has been shown to stimulate the production of soluble HIV-1 inhibitors in cultures of monocyte-derived macrophages, the ability of LPS to induce similar inhibitors in other cell types is poorly characterized. Here we show that LPS exhibits potent anti-HIV activity in phytohemagglutinin-stimulated peripheral blood mononuclear cells (PBMCs) but has no detectable anti-HIV-1 activity in TZM-bl cells. The anti-HIV-1 activity of LPS in PBMCs was strongly associated with the production of β-chemokines from CD14-positive monocytes. Culture supernatants from LPS-stimulated PBMCs exhibited potent anti-HIV-1 activity when added to TZM-bl cells but, in this case, the antiviral activity appeared to be related to IFN-γ rather than to β-chemokines. These observations indicate that LPS stimulates PBMCs to produce a complex array of soluble HIV-1 inhibitors, including β-chemokines and IFN-γ, that differentially inhibit HIV-1 depending on the target cell type. The results also highlight the need to use endotoxin-free specimens to avoid artifacts when assessing HIV-1-specific neutralizing antibodies in PBMC-based assays.
Introduction
Neutralizing antibody (Ab) assays for HIV-1 are widely utilized to study the immune response in infected individuals, to quantify monoclonal Ab performance, to explore viral antigenic diversity, and to evaluate vaccine immunogens in preclinical and clinical trials. For many years, neutralizing Ab assays quantified a reduction in HIV-1 infection in mitogen-stimulated peripheral blood mononuclear cells (PBMCs); however, this system is both resource intensive and difficult to standardize and validate because of substantial genetic and phenotypic variations in uncloned virus stocks and wide donor–donor variability in PBMCs for detecting neutralization.1,2 To address these concerns, and to further maximize assay performance, substantial resources have been invested in the development of a new neutralizing Ab assay technology that utilizes molecularly cloned, Env-pseudotyped viruses and a genetically engineered cell line (TZM-bl) that contains a stable Tat-inducible luciferase (Luc) reporter gene.3–6 This latter assay has been highly standardized, optimized, and validated, and it allows for genetic analysis and manipulation of clonal viruses to probe neutralization determinants in greater detail than before. As a result, the TZM-bl assay and other similar assay technologies7 have been widely implemented as the primary quantitative tool to assess neutralizing Abs against HIV-1.
Recent studies have shown that several neutralizing Abs exhibit much greater potency in the PBMC assay than in the TZM-bl assay.2,8–12 This has raised concern that the TZM-bl assay falsely underestimates the neutralizing activity of some Abs. Without a clinically established and measureable humoral immune correlate of HIV-1 protection, these differences create uncertainty as to which assay provides a more meaningful assessment of Ab-mediated HIV-1 neutralization.2,5,13
Multiple factors could account for the discrepancy in Ab potency between the PBMCs and TZM-bl assays. Some of these factors include differences in CCR5 density,14 CD4:CCR5 ratio,2 Fcγ receptor expression,15 effects of cellular proteins incorporated into the viral membrane,16–19 and viral entry mechanisms20 between the two cell types. Additionally, chemokine and cytokine production may play a substantial role in cases in which samples are contaminated with endotoxin. Bacterial lipopolysaccharide (LPS), also known as endotoxin, is an essential structural component of the outer membrane of gram-negative bacteria that when released can associate with serum carrier proteins and bind to a CD14/TLR4/MD-2 receptor complex on monocytes and macrophages.21–25 This binding activates an innate inflammatory response that leads to the production of chemokines, cytokines, and other unidentified soluble factors in cultures of monocyte-derived macrophages that inhibit R5 and X4 strains of HIV-1 in vitro.26–29 Macrophage inflammatory protein-1α (MIP-1α), MIP-1β, and regulated on activation, normal T cell expressed and secreted factor (RANTES) have been identified as the primary chemokines responsible for R5 HIV-1 inhibition by LPS.28 These CCR5 ligands have been shown to block HIV-1 infection by a combination of steric hindrance and CCR5 downregulation.29–32 LPS-induced soluble factors responsible for X4 HIV-1 inhibition remain to be identified and appear to be independent of stromal cell-derived factor-1 (SDF-1), interferon (IFN)-α, IFN-β, macrophage-derived chemokine, leukemia inhibitory factor, and tumor necrosis factor (TNF)-α.29,33
Epithelial HeLa cell lines, such as TZM-bl, express TLR4 but lack CD14 and MD-234,35 and thus should be resistant to LPS activation. Here we show that LPS exhibits potent anti-HIV activity in PBMCs but not in TZM-bl cells. Moreover, we show that β-chemokine production from CD14-positive monocytes accounts for the antiviral effect of LPS in PBMCs and we demonstrate that these β-chemokines have little or no anti-HIV-1 activity in TZM-bl cells. Finally, we provide evidence that LPS stimulates PBMCs to produce IFN-γ and that this cytokine is capable of inhibiting HIV-1 in TZM-bl cells but not in PBMCs.
Materials and Methods
Cells
TZM-bl (JC53-BL) cells were obtained from the NIH AIDS Research and Reference Reagent Program (ARRRP) as contributed by John Kappes and Xiaoyun Wu. These cells are a genetically engineered HeLa cell clone that expresses CD4, CXCR4, and CCR5 and contains Tat-responsive reporter genes for firefly luciferase (Luc) and Escherichia coli β-galactosidase under regulatory control of an HIV-1 long terminal repeat.36,37 TZM-bl cells were confirmed to be CD14 negative by flow cytometry (data not shown). 293T/17 cells were obtained from the American Type Culture Collection (catalog no. 11268). TZM-bl cells and 293T/17 cells were maintained in Dulbecco's modified Eagle's medium (Gibco) containing 10% heat-inactivated fetal bovine serum, 25 mM HEPES, and 50 μg gentamicin/ml in vented T-75 culture flasks (Corning-Costar). Cultures were incubated at 37°C in a humidified 5% CO2–95% air environment. TZM-bl cell monolayers were split 1:10 at confluence by treatment with 0.25% trypsin, 1 mM EDTA (Invitrogen). The JC.6, JC.10, and JC.37 engineered HeLa cell lines36 were a generous gift of Drs. David Kabat and Emily Platt and were maintained in the same manner as TZM-bl cells.
PBMCs were obtained via leukopheresis of healthy donors and cryopreserved in 1-ml aliquots at a density of 2 × 107 cells/ml. Before use, aliquots of PBMCs were thawed in a room temperature water bath and incubated in growth medium (RPMI 1640, 20% fetal bovine serum) containing 5 μg/ml phytohemagglutinin-P (PHA-P), 5% human interleukin-2 (IL-2), and 50 μg gentamicin/ml for 1 day at 37°C in upright T-75 flasks placed in a humidified 5% CO2/95% air environment. The cells were suspended by vigorous pipetting to dislodge adherent cells, washed, and placed in fresh growth medium containing IL-2 but no PHA-P prior to use. Unless indicated otherwise, PBMCs were used for neutralization assays and for virus propagation immediately after this 1-day stimulation with PHA-P.
Viruses
This study utilized replication-competent infectious molecular clones (IMC) of HIV-1 in which a Renilla luciferase reporter gene was inserted between env and nef, without abrogating nef expression. Furthermore, viruses were engineered such that env genes of choice can be inserted in cis in an isogenic backbone in which all other viral proteins were expressed, and in which the reporter gene is genetically stable38 (T.G. Edmonds et al., unpublished observations); this type of construct is referred to as Env-IMC-LucR reporter virus. All Env-IMC-LucR viruses included in this study are based on NL4-3 and expressed the respective ectodomains (i.e., all of gp120 and the ectodomain and membrane-spanning domain of gp41) of the Env of choice. The cytoplasmic domain of gp41 was derived from NL4-3, thereby avoiding chimerisms in tat, rev, and vpu. These proviral plasmids are designated pNL-LucR.T2A-Env.ecto (wherein “Env” is placed by the name of the respective inserted env gene). Details on the construction and characteristics of the viruses will be reported separately39 (T.G. Edmonds et al., unpublished observations). The Env-IMC-LucR viruses were generated by transfection in 293T cells of proviral DNA. Where noted, several experiments utilized Env-IMC-LucR virus that was propagated by a subsequent passage in PBMCs. All viruses were titrated to achieve an empirically determined tissue culture infectious dose (TCID) that produced optimal relative luminescence units (RLU) in virus control wells in the neutralization assay (>10 × background RLU of cell control wells without evidence of virus-induced cytopathic effects). All viruses except those expressing WEAU env utilize CCR5 (R5) as coreceptor; WEAU Env utilizes both CCR5 and CXCR4 (R5/X4). BaL and SF162 Env are considered Tier 1 Envs for possessing a high neutralization-sensitive phenotype when assayed with HIV-1-positive serum samples, whereas WITO, WEAU, CH040, CH058, and CH077 Env are considerably less sensitive to neutralization and are considered Tier 2 Envs.4 The WITO, WEAU, CH040, CH058, and CH077 envs represent the env genes of the transmitted/founder HIV-1 strains from sexual transmission in the respective patients40–42 (C. Ochsenbauer et al., unpublished observations).
LPS standards
The primary source of endotoxin used in this study was a “smooth” phenotype LPS from E. coli O55:B5 (3.8 EU/ng) (N185, Lonza, Switzerland). This preparation was produced as Control Standard Endotoxin and is referenced against the USP Reference Standard Endotoxin. Three other smooth LPS preparations were used for comparison: E. coli O55:B5 (3.9 EU/ng) (L6529, Sigma); E. coli 0127:B8 (1.9 EU/ng) (L4516, Sigma); and Salmonella enterica serotype typhimurium (2.2 EU/ng) (L6143, Sigma, St. Louis, MO). An Re mutant rough strain variant of S. enterica serotype typhimurium (1.7 EU/ng) (L9516, Sigma) was also tested to determine if bacterial colony phenotype (i.e., smooth or rough) had any effect on antiviral activity. Prototypical LPS consists of lipid A, an oligosaccharide core component, and a highly variable polysaccharide O-antigen. Rough strains of LPS have truncated, or nonexistent, O-antigen and are present in varying proportions among different species of gram-negative bacteria.41 The smooth vs. rough nomenclature refers to the appearance of the bacterial colonies. Because lipid A is the bioactive moiety, both smooth and rough versions of LPS are strongly immunogenic; however, there is recent evidence that rough LPS may potentiate a broader immune response since it requires only a TLR-4/MD-2 complex and can signal in the absence of CD14.43,44 All endotoxin preparations were stored in glass vials, used within 4 weeks of reconstitution, and vortexed vigorously for ≥15 min prior to use. Polymyxin B sulfate was purchased from EMD Biosciences (San Diego, CA).
Recombinant chemokines and Abs
Human recombinant MIP-1α (CCL3), MIP-1α (LD78β isoform, CCL3L1), MIP-1β (CCL4), RANTES (CCL5), IFN-γ, macrophage-derived chemokine (MDC, CCL22), and affinity purified Abs to human MIP-1α, MIP-1β, RANTES, and IFN-γ were purchased from R&D Systems (Minneapolis, MN). Quantikine ELISA kits for the detection of human MIP-1α, MIP-1β, RANTES, MDC, IFN-γ, and SDF-1 (CXCL12) were also purchased from R&D Systems. Monoclonal Ab IgG1b12 was a gift from Dennis Burton (Scripps Research Institute, La Jolla, CA). Monoclonal Abs 2G12, 2F5, and 4E10 were purchased from Polymun Scientific GmbH (Vienna, Austria). HIVIG (human immunodeficiency virus immune globulin) is a purified IgG fraction prepared from pooled plasma of asymptomatic, HIV-1 Ab-positive donors with CD4+ counts above 400 cells/μl; this product is available from the NIH AIDS Research and Reference Reagent Program.
Monocyte depletion
Following 1-day PHA stimulation, PBMCs from a single donor were depleted of monocytes by using a Dynabeads CD14+ depletion kit (Invitrogen, Carlsbad, CA) per the manufacturer's instructions. Prior to depletion, ∼17% of PBMCs were CD14+; following depletion, <1% CD14+ cells remained by flow cytometry (data not shown). Cells were used immediately following depletion.
Endotoxin detection
Endotoxin levels were measured by using a commercially available limulus amoebocyte lysate (LAL) kinetic chromogenic assay (Kinetic-QCL, Lonza, Switzerland). In kinetic LAL assays, endotoxin activates a proenzyme in LAL that cleaves p-nitroaniline (pNA) from a colorless substrate. The amount of endotoxin present determines the rate of pNA release. The resulting color change was continuously measured photometrically at 405 nm and compared to a standard curve of known endotoxin amounts.
Neutralization assays
Neutralization in the TZM-bl cell assay was measured as a reduction in firefly luciferase (Luc) reporter gene expression after a single round of infection with Env-pseudotyped viruses as described previously.3,6,45 Briefly, virus was incubated with serial threefold dilutions of test sample (eight dilutions total) in duplicate in a total volume of 150 μl for 1 h at 37°C in 96-well flat-bottom culture plates. Freshly trypsinized cells (10,000 cells in 100 μl of growth medium containing 10 μg/ml DEAE dextran) were added to each well. One set of control wells received cells plus virus (virus control) and another set received cells only (background control). After a 48-h incubation, 100 μl of cells was transferred to a 96-well black solid plate (Costar) for measurement of luminescence using the Britelite Plus Luminescence Reporter Gene Assay System (PerkinElmer Life Sciences).
Neutralization of HIV-1 in the PBMC assay was measured as a reduction in LucR reporter gene expression after multiple rounds of virus replication. Virus was incubated with serial 3-fold dilutions of test sample (eight dilutions total) in duplicate in a total volume of 150 μl of IL-2-containing growth medium for 1 h at 37°C in a 96-well U-bottom culture plate. One-day-old PHA-PBMCs (2 × 105 cells in 50 μl of IL-2-containing growth medium) were added to each well. One set of control wells received cells plus virus (virus control) and another set received cells only (background control). After a 4-day incubation, 100 μl of cells was transferred to a 96-well white solid plates (Costar) for measurements of Renilla luciferase luminescence using the ViviRen Live Cell Substrate (Promega). For β-chemokine blocking experiments, a final concentration of 3750 EU/ml of LPS was added to all wells containing test samples.
For both assays, the inhibitory concentration (IC50 and IC80) of test samples was defined as the sample concentration at which RLUs were reduced by either 50% or 80% compared to virus control wells after subtraction of background RLUs in cell control wells. IC50 was used in the TZM-bl assay because it provides optimal sensitivity and precision of measurements. IC80 was used as the more reliable measure in the PBMC assay.46
Supernatant transfer experiments
To test the antiviral activity of LPS-conditioned culture supernatants, PBMCs were incubated overnight in the presence of serial dilutions of LPS in 96-well U-bottom culture plates. Conditioned culture supernatants (80 μl) were transferred to corresponding wells of fresh 96-well flat-bottom culture plates to which virus and TZM-bl cells were added as described above for neutralization assays. RLUs were measured 48 h later.
Endotoxin spike experiments
Monoclonal Abs and HIVIG were spiked with a relatively high concentration of LPS (30,000 EU/ml, 7.89 μg) immediately prior to testing for neutralizing activity in TZM-bl cells and PBMCs. Nonspiked samples were assayed in parallel as controls.
Results
LPS inhibits HIV-1 in PBMCs but not in TZM-bl cells
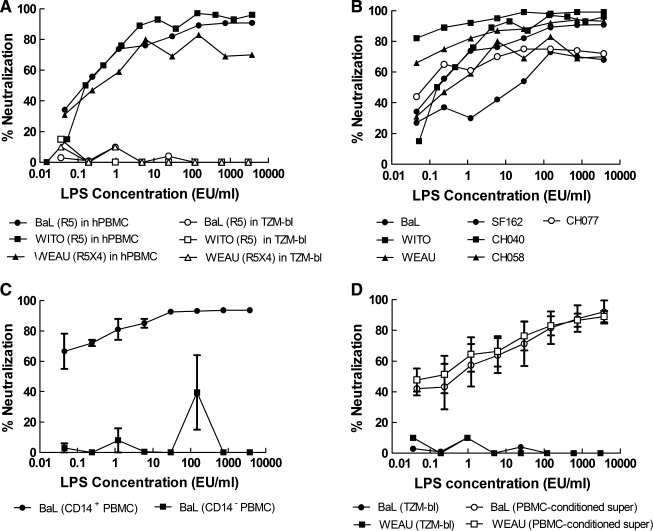
Previous studies of the anti-HIV-1 activity of LPS utilized fractionated monocyte-derived macrophages that were allowed to differentiate in culture for 5–14 days prior to use.26–29 Because neutralizing Ab assays with HIV-1 are often performed with fresh, unfractionated PBMCs that are cultured for 1 day in the presence of a mitogen, it was necessary to determine whether LPS would inhibit HIV-1 under this condition. Indeed, LPS inhibited a wide range of clade B primary isolates under standard conditions of the PBMC assay, including one R5X4-tropic virus and several R5 viruses with a Tier 2 neutralization phenotype (Fig. 1A and B). 293T-grown and PBMC-grown Env-IMC-LucR virus had equivalent sensitivities to lipopolysaccharide in the PBMC assay (data not shown). Addition of 25 μg/ml of Polymyxin B sulfate, an endotoxin-binding antibiotic, completely abrogated the HIV-1 inhibitory activity of LPS (data not shown). LPS-mediated inhibition of HIV-1 in PBMCs was prevented by removing CD14+ cells, thus confirming that monocytes were the primary cells involved in the LPS effect (Fig. 1C). LPS did not inhibit HIV-1 in the TZM-bl assay (Fig. 1A); however, supernatants from PBMCs that were incubated overnight with LPS exhibited potent HIV-1 inhibition activity in the TZM-bl assay (Fig. 1D). This latter observation is consistent with previous reports showing that LPS inhibits HIV-1 by stimulating the production of soluble inhibitory factors.28,29
FIG. 1.
LPS inhibits HIV-1 infection in PBMCs but not in TZM-bl cells. LPS was tested for inhibitory activity against several different strains of HIV-1 as Env-IMC-LucR viruses expressing the indicated env genes. (A) Inhibitory activity of LPS against three different strains of PBMC-grown HIV-1 as assayed in either PBMCs (filled symbols) or TZM-bl cells (open symbols). Results are shown as the percent reduction in RLU. (B) Inhibitory activity of LPS against several additional strains of PBMC-grown HIV-1 as assayed in PBMCs. (C) Inhibitory activity of LPS in PBMCs depleted and not depleted of CD14+ cells, respectively. Results are the mean ± range in two independent experiments. (D) Inhibition of HIV-1 in TZM-bl cells by culture supernatants from PBMCs that were conditioned overnight with LPS. Results are the mean ± SEM of three independent experiments. In all figures, endotoxin concentrations correspond to the final volume after the addition of cells. For supernatant transfer experiments, endotoxin concentrations correspond to the concentration of LPS in the initial PBMC plate.
LPS stimulates PBMCs to produce β-chemokines and IFN-γ
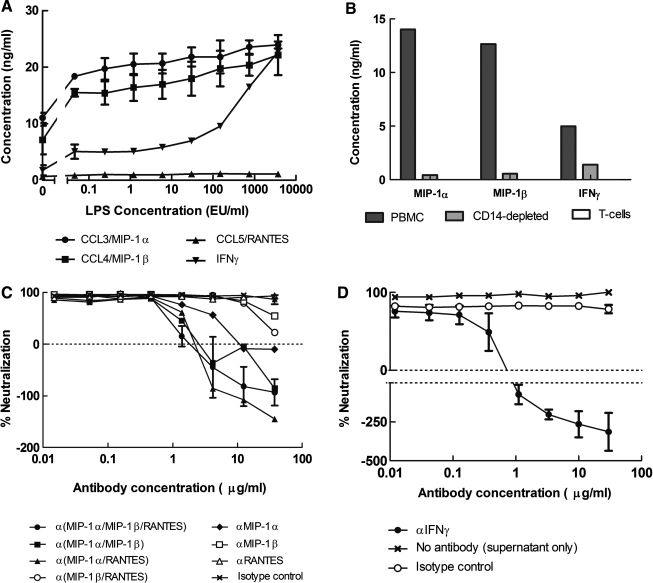
Culture supernatants from PBMCs, before and after overnight stimulation with LPS, were evaluated for the presence of chemokines and cytokines. As shown in Fig. 2A, LPS stimulated the secretion of MIP-1α, MIP-1β, and IFN-γ into the culture medium. Removal of CD14+ cells from PBMCs prevented the secretion of β-chemokines and reduced the amount of IFN-γ produced (Fig. 2B). LPS-induced secretion of MIP-1α, MIP-1β, or IFN-γ was not detected in a culture of isolated T cells from PBMCs (Fig. 2B). This suggests that a CD14-negative population other than T cells is partly responsible for IFN-γ secretion in response to LPS. Although suggested to have a role in X4-tropic HIV-1 inhibition,47–49 MDC expression was not affected by LPS stimulation (data not shown); SDF-1α was not detected in PBMC supernatants (data not shown).
FIG. 2.
Identification of LPS-induced soluble factors that inhibit HIV-1 in PBMCs and TZM-bl cells. (A) LPS-induced upregulation of β-chemokine and IFN-( production in PBMCs. Results are expressed as the mean ± range in two sets of assays in PBMCs from a single donor. Positive values in the absence of LPS are due to the constitutive expression of cytokines after overnight stimulation with PHA-P. (B) Depletion of CD14+ cells from PBMC culture prevented the secretion of MIP-1α and MIP-1β after overnight incubation with 750 EU/ml LPS. CD14 depletion also resulted in lower levels of IFN-γ. No secretion of MIP-1α, MIP-1β, or IFN-γ was detected in isolated T cells. Graphs show concentration of cytokines above an LPS-free baseline. (C) Ability of antichemokine Abs to block the antiviral activity of LPS against PBMC-grown LucR reporter HIV-1 encoding BaL Env in PBMCs. Serial dilutions of antibodies were incubated with virus and cell culture media containing LPS at 5000 EU/ml for 1 h prior to the addition of cells. Results for the MIP-1(/MIP-1(/RANTES combination and the isotype control Ab are expressed as the mean ± range from two experiments; other points are from a single experiment. (D) Ability of anti-IFN-γ Abs to block the antiviral activity of LPS against BaL env expressing Env-IMC-LucR reporter HIV-1 in TZM-bl cells. PBMCs were incubated with LPS at 5000 EU/ml overnight. Culture supernatants were transferred into a new 96-well plate and serial dilutions of anti-IFN-γ Abs were added, followed by virus. TZM-bl cells were added after a 1-h incubation. Results are the mean ± range of two experiments. All concentrations are based on the final volume after addition of cells.
HIV-1 is differentially inhibited by β-chemokines and IFN-γ in PBMCs and TZM-bl cells
To determine a possible link between the LPS-induced upregulation of chemokines and cytokines and the anti-HIV-1 activity of LPS, we tested whether the chemokines and cytokines as pure proteins were inhibitory toward HIV-1 in our assays. As shown in Table 1, recombinant human MIP-1α, MIP-1α/LD78β, MIP-β, and RANTES were all inhibitory toward multiple R5 stains HIV-1 in the PBMC assay. In particular, we confirmed previous reports30,31,50–54 that the LD78β isoform of MIP-1α (CCL3L1) is the most potent inhibitory β-chemokine against HIV-1. This LD78β isoform of MIP-1α is likely the primary chemokine responsible for LPS-mediated inhibition of R5 viruses in PBMCs, as its IC80 values (Table 1) were well within the levels of MIP-1α detected in PBMC culture (Fig. 2A). RANTES also exhibited a potent inhibitory effect against multiple R5 viruses, followed in potency by MIP-1α. MIP-β was the least active chemokine, inhibiting only two of the six R5 viruses tested and requiring relatively high concentrations for inhibition. None of the β-chemokines inhibited the reporter virus encoding the R5/X4-tropic WEAU Env. IFN-γ and MDC at concentrations as high as 1250 ng/ml had no inhibitory effect on HIV-1 infection in PBMCs.
Table 1.
Inhibition of HIV-1 by β-Chemokines in the PBMC Assay
| |
IC80(ng/ml) in PBMCsa |
||||||
|---|---|---|---|---|---|---|---|
| Recombinant protein | Bal R5 | SF162 R5 | WITO R5 | CH040 R5 | CH058 R5 | CH077 R5 | WEAU R5X4 |
| MIP-1α | 400 | >1250 | 120 | 126 | >1250 | >1250 | >1250 |
| MIP-1α/LD78β | 13 | 43 | 10 | 7 | 18 | 109 | >1250 |
| MIP-1β | >1250 | >1250 | 1160 | 615 | >1250 | >1250 | >1250 |
| RANTES | 66 | 171 | 36 | 37 | 75 | >1250 | >1250 |
| IFN-γ | >5000 | ND | ND | ND | ND | ND | >5000 |
| MDC | >1250 | ND | >1250 | ND | ND | ND | >1250 |
Chemokines and IFN-γ were assayed in PBMCs against PBMC-grown NL-LucR.T2A-Env.ecto reporter HIV-1 expressing the indicated envs. Values are the concentration at which RLU were reduced 80% compared to virus control wells after subtraction of background RLU in cell control wells. ND, inhibition experiments were not done with these viruses.
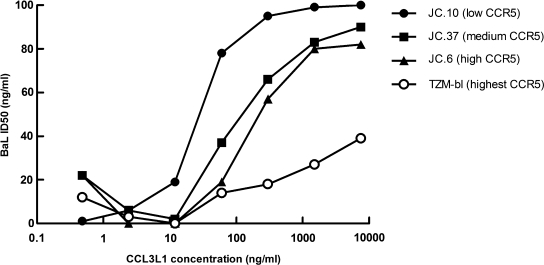
None of the β-chemokines inhibited the reporter viruses encoding the Envs for BaL, WITO, and WEAU or the R5 Env-pseudovirus PVO.4 in the TZM-bl assay at well above physiologic concentrations (≤1250 ng/ml), with one exception: NL-LucR.T2A-WITO.ecto infection was inhibited by MIP-1α LD78β/CCL3L1 at an IC50 of 845 ng/ml. To understand the apparent resistance of TZM-bl cells to β-chemokine-mediated viral inhibition, further tests were conducted using three additional HeLa cell lines that were engineered similarly to the TZM-bl line, but with lower densities of CCR5 on their surface.36 As shown in Fig. 3, sensitivity to β-chemokine-mediated viral inhibition was directly related to cellular coreceptor density, with lower density associated with more potent inhibition. This outcome strongly suggests that TZM-bl cells are resistant to β-chemokines because of their much higher levels of CCR5 on the surface. In contrast, IFN-γ potently inhibited BaL(R5) and WEAU (R5X4) in TZM-bl cells, with IC50 values of 580 pg/ml and 150 pg/ml, respectively (data not shown). These effective concentrations are well within the IFN-γ levels in LPS-stimulated PBMC culture supernatants (Fig. 2A).
FIG. 3.
The potency of the recombinant human β-chemokine CCL3L1 against HIV-1 NL-LucR.T2A-BaL.ecto was evaluated in four different HeLa cell lines with previously established levels of CCR5: JC.10 cells (2.0 × 103 molecules CCR5/cell), JC.37 cells (1.5 × 104 molecules CCR5/cell), JC.10 cells (2.7 × 104 molecules CCR5/cell), and TZM-bl/JC.53 cells (1.3 × 105 molecules CCR5/cell).36 Viral inhibition was measured by a reduction in Renilla luciferase expression after 48 h incubation.
To further confirm a role for β-chemokines and IFN-γ in the LPS-mediated anti-HIV activity observed here, additional experiments were performed in which we attempted to block the antiviral activity of LPS by adding antichemokine and anti-IFN-γ Abs. As shown in Fig. 2C, anti-MIP-1α Abs completely abolished the antiviral activity of LPS in the PBMC assay at antibody concentrations above 10 μg/ml. High concentrations of anti-MIP-1β Ab only partially reversed the antiviral activity of LPS, whereas anti-RANTES Ab had no measurable effect. These results support the notion that MIP-1α was the dominant LPS-induced chemokine responsible for HIV-1 inhibition in the PBMC assay. Nonetheless, our results indicate that MIP-1β and RANTES contribute to this inhibition in so much as combinations of antichemokine Abs were more effective than any single antichemokine Ab alone (Fig. 2C). In particular, anti-MIP-1α, when combined with either anti-MIP-1β or anti-RANTES, was more effective than when tested alone. Finally, anti-IFN-γ Abs completely abolished the HIV-1 inhibitory activity of LPS-conditioned PBMC culture supernatants in the TZM-bl assay (Fig. 2D), whereas anti-β-chemokine antibodies had no effect (data not shown), strongly suggesting that IFN-γ is responsible for the anti-HIV-1 activity of LPS-conditioned PBMC supernatant in this assay.
It should be noted that in some cases, the addition of antichemokine and anti-IFN-γ Abs appeared to enhance virus infection, resulting in negative inhibition values (i.e., RLU in test wells were higher than those in the virus control wells that were used as our measure for zero inhibition). We attribute this effect to constitutive levels of β-chemokine and IFN-γ production in PHA-P-stimulated PBMCs (Fig. 1A), which would partially inhibit infection in the virus control wells but not in wells containing antichemokine and anti-IFN-γ Abs.
Variables that affect the HIV-1-inhibitory activity of LPS
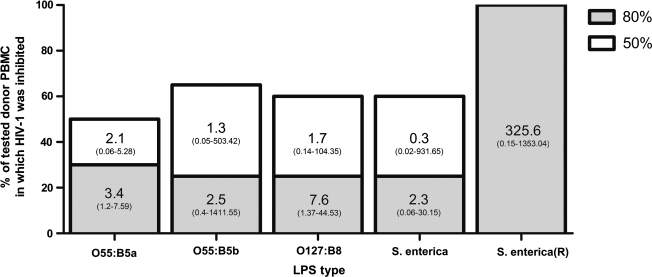
We tested the HIV-1 inhibitory activity of both smooth and rough LPS from three different strains of bacteria (E. coli O55:B5, E. coli 0127:B8, and S. enterica) by using PBMCs from different donors. There was little difference in antiviral activity among the four smooth LPS strains; however, large differences were seen between smooth and rough LPS strains and in the sensitivity of donor PBMCs to LPS-mediated inhibition of HIV-1 (Fig. 4). The activity of LPS differed in each donor PBMCs. The tested smooth strains of LPS produced 50% inhibition of Bal env expressing LucR reporter HIV-1 in only 50–65% of randomly selected donor PBMCs, with large variations in IC50 values. Eighty percent inhibition, with similar IC80 variation, was achieved only in 25–30% of tested PBMCs. In contrast, the rough LPS strain inhibited HIV-1 > 80% in all (100%) tested donor PBMCs (Fig. 4), although with substantial variation among IC80 values. These findings suggest that there are genetic differences in donor PBMCs that affect the anti-HIV-1 activity of LPS in vitro. Additionally, they support the current hypothesis that rough LPS, through its ability to mediate CD14-independent signaling, may potentiate immune responses in a broader range of cell types.43,44 However, these demonstrated differences in PBMC response to LPS cannot be solely attributed to variations in CD14 expression or function, as a range of LPS-mediated inhibitory responses was seen among PBMC donors even with the rough strain of LPS (Fig. 4).
FIG. 4.
PBMC donors have differential sensitivity to LPS-mediated inhibition of HIV-1. Bars show the percentage of tested PBMC donors in which LPS inhibited HIV-1 NL-LucR.T2A-BaL.ecto ≥50% (white bars) or ≥80% (gray bars). The 80% bars are superimposed on the 50% bars, as any PBMCs with 80% inhibition also showed 50% inhibition. Values inset within vertical bars represent median IC50 or IC80 values with ranges in parentheses to show variation; all values are in EU/ml. Twenty-one different PBMCs were tested for O55:B5b, O127:B8, and S. enterica; 10 different PBMCs were tested for O55:B5a and S. enterica (R). O55:B5a and O55:B5b are LPS preparations manufactured by Lonza and Sigma, respectively.
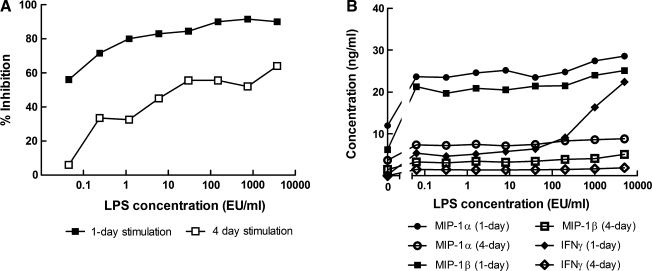
Because of variation between laboratories in the length of time that PBMCs are stimulated with PHA-P prior to use in neutralization assays, we examined how differences in the length of time of stimulation affects LPS-mediated HIV-1 inhibition. PBMCs that had been stimulated for 4 days showed a substantial decrease in sensitivity to LPS inhibition (Fig. 5A). Also, PBMC infectivity was markedly decreased by longer stimulation times: luminescence values from virus control wells 4 days after addition of the same amount of HIV-1 NL-LucR.T2A-BaL.ecto were ∼75,000 with 1-day PHA-stimulated PBMCs and only ∼2000 with 4-day stimulated PBMCs. This phenomenon was repeatable with different donor PBMCs. Longer stimulation times also reduced the amount of MIP-1α and MIP-1β produced by the PBMCs in response to LPS by approximately 3- and 6-fold, respectively. Interestingly, 4-day stimulated PBMCs did not produce any INF-γ in response to LPS (Fig. 5B).
FIG. 5.
Prolonged stimulation of PBMCs with PHA-P diminishes the antiviral effect of LPS against HIV-1 NL-LucR.T2A-BaL.ecto (A) and the amount of cytokines released (B). PBMCs from two different donors were stimulated with PHA-P either overnight (filled symbols) or for 4 days (open symbols) prior to use in a neutralization assay with LPS. Data represent mean values from two independent experiments.
Endotoxin contamination increases monoclonal Ab potency in the PBMC assay
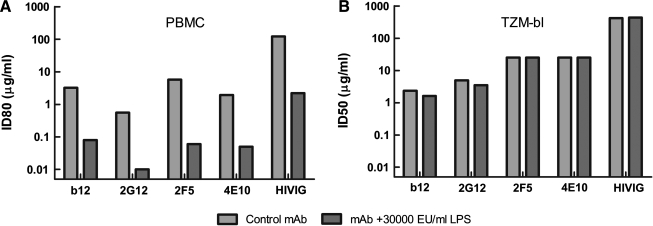
We tested the effect of endotoxin contamination on several human monoclonal antibodies spanning a diverse range of epitope specificities: IgG1b12 recognizes a complex epitope overlapping the CD4-binding domain,55–57 2G12 binds a mannose cluster on the outer domain of gp120 involving multiple N-linked glycans,58–60 and the epitopes for 2F561–63 and 4E1064,65 are adjacent to each other in the membrane-proximal ectodomain of gp41. The effect of endotoxin on an IgG fraction of pooled sera from HIV-1 Ab-positive donors (HIVIG) was also tested. All reagents were spiked with a large (30,000 EU/ml) amount of endotoxin and evaluated for antiviral activity in both the TZM-bl and PBMC assay. LPS contamination increased the potency of the samples by ∼100-fold in the PBMC assay but had no measureable effect in the TZM-bl assay (Fig. 6).
FIG. 6.
High concentrations of LPS have no effect on the potency of neutralizing Abs when assayed in TZM-bl cells. Neutralizing monoclonal Abs (b12, 2G12, 2F5, 4E10) and a neutralizing polyclonal antiserum (HIVIG) were spiked with 30,000 EU/ml of E. coli O55:B5 LPS prior to assay. Neutralizing activity of the spiked samples and corresponding nonspikes samples was assayed against PBMC-grown NL-LucR.T2A-BaL.ecto in PBMCs (A) and in TZM-bl cells (B). Gray bars, LPS absent; black bars, LPS present. For comparison, the median IC80 of E. coli O55:B5 LPS in identical donor PBMCs was 1.56 EU/ml.
Discussion
Our results confirm and extend previous reports26–29,66 that LPS has potent and broad inhibitory activity against both R5 and R5X4 strains of HIV-1. Previous studies examined the anti-HIV-1 activity of LPS by using cultures of fractionated monocyte-derived macrophages for LPS stimulation. Here we show that LPS inhibits HIV-1 by stimulating the production of chemokines in the monocyte population of cryopreserved PBMCs that were thawed and used after 1 day in culture in the presence of PHA-P and IL-2. We also demonstrate that LPS has no direct anti-HIV-1 activity in TZM-bl cells, probably because these cells lack both CD14 and MD-2.34,35 The differential effect of LPS in the two cell types could cause discrepancies in the measured neutralization potency of endotoxin-contaminated reagents. It is not known to what extent, if any, endotoxin contamination contributed to recent reports of Abs possessing greater neutralization potency in PBMCs compared to TZM-bl cells.2,8–12 Indeed, several other factors might contribute to an apparent diminished sensitivity of the TZM-bl assay for detecting neutralizing Abs.2,14,15 At a minimum, any reagent (mAb, Ig fraction, human/animal sera, or antiviral compound) that demonstrates, or has demonstrated, potent neutralization only in a PBMC or macrophage-based assay should be tested for endotoxin contamination to rule out possible artifactual results. Additionally, it seems prudent to ensure that in the future, only endotoxin-free reagents are used in the PBMC assay.
Our results indicate that β-chemokines MIP-1α and, to a lesser extent, MIP-1β and RANTES are the major effectors of LPS-mediated inhibition of HIV-1 in PBMCs. We also confirmed prior reports that the LD78β (CCL3L1) isoform of MIP-1α is the most potent anti-HIV-1 chemokine,30,31,51–54 and we successfully demonstrated that blocking β-chemokines abrogates LPS-mediated HIV-1 inhibition. HIV-1 inhibition by β-chemokines is thought to occur through a combination of CCR5 steric hindrance, downregulation, and/or dimerization mechanisms.31 HIV-1 and β-chemokines have been shown to compete for CCR5 binding; the antifusion activity of β-chemokines decreases with increased CCR5 expression.67 Because TZM-bl cells express ∼100 × more CCR5 on their surface than PBMCs,2 high CCR5 density could explain the lack of HIV-1 inhibitory activity of β-chemokines in the TZM-bl assay. This hypothesis was tested and confirmed using additional engineered HeLa cell lines that were developed along with the parental TZM-bl cell line, JC.53, but that express lower CCR5 densities on their surface.36 Engineered HeLa cells with lower CCR5 densities were more sensitive to β-chemokine-mediated viral inhibition, and receptor density was directly related to the potency of β-chemokine-mediated viral inhibition. (Fig. 3)
Interestingly, the anti-HIV-1 activity of LPS-conditioned PBMC culture supernatants in the TZM-bl assay appeared to be mediated by IFN-γ. IFN-γ has been shown to inhibit HIV-1 replication in cultured macrophages27,68 and in other cell lines.27 However, the apparent inhibitory effect of IFN-γ on HIV-1 may be due to its actions on TZM-bl cells. IFN-γ has also been shown to retard cellular growth, inhibit DNA synthesis, and be moderately toxic to HeLa cell culture at concentrations >5 ng/ml) 69,70; the combination of these effects could potentially interfere with the TZM-bl assay and result in a reduction of RLU. Some toxicity and growth inhibition were seen at higher tested concentrations of IFN-γ; however, HIV-1 inhibition was detected at concentrations permissive to monolayer formation. The wide variability of endotoxin-mediated inhibition of HIV-1 across PBMC donors is not unexpected as LPS is known to cause disparate cytokine responses across individuals.71 The reasons behind this variation are likely multifactorial and may include known differences in gene copy number of CCL3L1,50 the most potent anti-HIV β-chemokine, as well as possible desensitizing mutations in the gene encoding TLR472 and/or the downstream signaling protein IRAK-4.73
The soluble factor responsible for LPS-mediated inhibition of the R5X4-tropic virus expressing WEAU Env in the PBMC assay remains unknown. WEAU Env was completely resistant to inhibition by β-chemokines and IFN-γ. Although SDF-1α is a natural ligand for CXCR4 and blocks X4 HIV-1 virus entry,75,76 it is produced in stromal cells rather than PBMCs.29 Several studies have reported that antibodies to MDC/CCL22 can block soluble factor-mediated inhibition of X4 viruses47–49; however, we, and others,29,76 did not detect any inhibition of R5 or R5/X4 HIV-1 with MDC, nor did we detect an increase in MDC production after LPS stimulation of PBMCs (data not shown). The type I interferons, IFN-α and IFN-β, have been shown to inhibit X4 viruses in PBMCs,77,78 but well above physiologic concentrations.29 Additionally, we did not detect an increase in IFN-α production following LPS stimulation of PBMCs. LPS has been shown to downregulate the surface expression of CD4, CCR5, and CXCR4 on monocyte-derived macrophages.29 Recent reports have suggested that CXCR4 binds LPS and has a role as a complementary LPS receptor to the CD14/TLR4/MD-2 main sensing complex.79,80 As such, LPS may competitively inhibit X4-tropic HIV-1 binding to CXCR4. A combination of β-chemokines, receptor downregulation, and competitive inhibition by LPS might explain dual-tropic viral inhibition by endotoxin.
Detection of endotoxin is relatively straightforward by any of several commercially available LAL assay kits. Samples containing serum or high protein content should be checked for assay inhibition per the manufacturer's instructions, as serum/protein can inhibit LPS detection and cause false-negative readings. Care should be taken in the manufacture and/or purification of any reagent used in neutralization assays as downstream endotoxin removal is very difficult in small sample volumes. Bacterial LPSs strongly associate with proteins in solution and are stable at a wide range of temperature and pH. An excellent review of endotoxin removal methods is available.81 However, because both PBMC response to endotoxin and HIV-1 sensitivity to β-chemokines vary, there is no generic “safe” threshold of endotoxin. In our experience, LPS concentrations of ≤0.1 EU/ml (∼29 pg/ml) are permissible with negligible effects on HIV-1 infection in vitro. Removal of CD14+ cells from PBMC culture is also an effective means of abrogating LPS-mediated HIV-1 inhibition in cases in which use of contaminated reagents is unavoidable; however, note that CD14− PBMCs have been shown to have reduced Ab-dependent cell-mediated virus inhibition (ADCVI) activity.82
Finally, we note that our recommended safe level of endotoxin (∼29 pg/ml) for avoiding artifacts in HIV-1 neutralization assays compares well with recent measurements of the physiologic LPS concentration in plasma from normal, HIV-1-negative individuals.83–85 However, recent studies have revealed that HIV-1-infected persons have increased levels of plasma LPS due to microbial translocation of bacteria through the gut mucosa.83–86 Based on our results, these increased plasma LPS levels may affect measurements of the neutralizing ability of HIV-1-positive serum and plasma samples in some PBMC-based assays by inducing antiviral chemokine release. Additionally, our results document a robust immunological cascade in primary cells after exposure to low concentrations of LPS and thereby add support to the hypothesis that LPS introduced via microbial translocation in vivo may contribute to immune activation in HIV-1-infected individuals.82–89 However, although LPS has profound inhibitory effects on HIV-1 in vitro, the effect, if any, of LPS-induced immunostim-ulation and subsequent antiviral chemokine release on viral replication, diversity, and/or pathogenesis in vivo is yet undeter-mined. Finally, it is interesting to speculate as to whether variations in the sensitivity of donor PBMCs to in vitro LPS stimulation would translate to in vivo variations in the immunostimulatory response to HIV-1-induced microbial translocation.
In summary, endotoxin contamination of serological samples mediates a release of antiviral chemokines in susceptible PBMCs and can cause false-positive results in neutralizing antibody assays done in PBMCs. Although this phenomenon is variable depending on donor PBMCs and LPS phenotype, it can occur at very low concentrations of endotoxin. Therefore, to ensure the correct assessment of tested samples all reagents used in the PBMC assay should be endotoxin free. This recommendation should also be followed when assessing HIV-1 neutralization in any cell type known to secrete β-chemokines in response to LPS stimulation, such as macrophages. HIV-1 neutralization assays in TZM-bl cells were unaffected by endotoxin contamination; the effect of endotoxin on other pseudovirus-based assay technologies utilizing engineered cell targets was not evaluated. Our study reinforces the need for continued standardization and validation of a variety of assay technologies to test the HIV-1 neutralizing ability of serologic reagents.
Acknowledgments
We gratefully acknowledge support from the HIV Vaccine Clinical Trials Network (HVTN) (NIH AI46705), the Center for HIV/AIDS Vaccine Immunology (CHAVI) (NIH AI067854), the Bill and Melinda Gates Foundation Collaboration for Vaccine Discovery (Grant 38619), and the Preclinical Branch, Divisions of AIDS, U.S. National Institutes of Health (AI30034). We appreciate the advice on experimental design and data analysis provided by the Duke Center for AIDS Research (CFAR) Flow Cytometry Core, an NIH-funded program (P30 AI 64518). We also thank Drs. Carl Alving and Gabriel Perez for helpful insights, Dr. Thomas Denny for providing PBMCs, and Drs. David Kabat and Emily Platt for providing the additional engineered HeLa cell lines.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Montefiori DC. Morris L. Ferrari G. Mascola JR. Neutralizing and other antiviral antibodies in HIV-1 infection and vaccination. Curr Opin HIV AIDS. 2007;2:169–176. doi: 10.1097/COH.0b013e3280ef691e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polonis VR. Brown BK. Borges AR, et al. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology. 2008;375:315–320. doi: 10.1016/j.virol.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Li M. Gao F. Mascola JR, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascola JR. D'Souza P. Gilbert P, et al. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J Virol. 2005;79:10103–10107. doi: 10.1128/JVI.79.16.10103-10107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montefiori D. Sattentau Q. Flores J. Esparza J. Mascola J. Antibody-based HIV-1 vaccines: Recent developments and future directions. PLoS Med. 2007;4:e348. doi: 10.1371/journal.pmed.0040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol. 2009;485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 7.Richman DD. Wrin T. Little SJ. Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci USA. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binley JM. Wrin T. Korber B, et al. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78:13232–13252. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown BK. Karasavvas N. Beck Z, et al. Monoclonal antibodies to phosphatidylinositol phosphate neutralize human immunodeficiency virus type 1: Role of phosphate-binding subsites. J Virol. 2007;81:2087–2091. doi: 10.1128/JVI.02011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choudhry V. Zhang M-Y. Sidorov IA, et al. Cross-reactive HIV-1 neutralizing monoclonal antibodies selected by screening of an immune human phage library against an envelope glycoprotein (gp140) isolated from a patient (R2) with broadly HIV-1 neutralizing antibodies. Virology. 2007;363:79–90. doi: 10.1016/j.virol.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M-Y. Vu BK. Choudhary A, et al. Cross-reactive human immunodeficiency virus type 1-neutralizing human monoclonal antibody that recognizes a novel conformational epitope on gp41 and lacks reactivity against self-antigens. J Virol. 2008;82:6869–6879. doi: 10.1128/JVI.00033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann AM. Rusert P. Berlinger L. Kuster H. Gunthard HF. Trkola A. HIV sensitivity to neutralization is determined by target and virus producer cell properties. AIDS. 2009;23:1659–1667. doi: 10.1097/QAD.0b013e32832e9408. [DOI] [PubMed] [Google Scholar]
- 13.Fenyo EM. Heath A. Dispinseri S, et al. International network for comparison of HIV neutralization assays: The NeutNet report. PLoS ONE. 2009;4:e4505. doi: 10.1371/journal.pone.0004505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choudhry V. Zhang M-Y. Harris I, et al. Increased efficacy of HIV-1 neutralization by antibodies at low CCR5 surface concentration. Biochem Biophys Res Commun. 2006;348:1107–1115. doi: 10.1016/j.bbrc.2006.07.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez LG. Costa MR. Todd CA. Haynes BF. Montefiori DC. Utilization of IgG Fc receptors by human immunodeficiency virus type 1: A specific role for antibodies against the membrane proximal external region of gp41. J Virol. 2009;83:7397–7410. doi: 10.1128/JVI.00656-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beausejour Y. Tremblay MJ. Susceptibility of HIV type 1 to the fusion inhibitor T-20 is reduced on insertion of host intercellular adhesion molecule 1 in the virus membrane. J Infect Dis. 2004;190:894–902. doi: 10.1086/422698. [DOI] [PubMed] [Google Scholar]
- 17.Losier M. Fortin JF. Cantin R. Bergeron MG. Tremblay MJ. Virion-bound ICAM-1 and activated LFA-1: A combination of factors conferring resistance to neutralization by sera from human immunodeficiency virus type 1-infected individuals independently of the disease status and phase. Clin Immunol. 2003;108:111–118. doi: 10.1016/s1521-6616(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 18.Rizzuto CD. Sodroski JG. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J Virol. 1997;71:4847–4851. doi: 10.1128/jvi.71.6.4847-4851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louder MK. Sambor A. Chertova E, et al. HIV-1 envelope pseudotyped viral vectors and infectious molecular clones expressing the same envelope glycoprotein have a similar neutralization phenotype, but culture in peripheral blood mononuclear cells is associated with decreased neutralization sensitivity. Virology. 2005;339:226–238. doi: 10.1016/j.virol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Miyauchi K. Kim Y. Latinovic O. Morozov V. Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009;137:433–444. doi: 10.1016/j.cell.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoshino K. Takeuchi O. Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 22.Nagai Y. Akashi S. Nagafuku M, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 23.Poltorak A. He X. Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 24.Wright SD. CD14 and innate recognition of bacteria. J Immunol. 1995;155:6–8. [PubMed] [Google Scholar]
- 25.Wright SD. Ramos RA. Tobias PS. Ulevitch RJ. Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein MS. Tong-Starksen SE. Locksley RM. Activation of human monocyte-derived macrophages with lipopolysaccharide decreases human immunodeficiency virus replication in vitro at the level of gene expression. J Clin Invest. 1991;88:540–545. doi: 10.1172/JCI115337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kornbluth RS. Oh PS. Munis JR. Cleveland PH. Richman DD. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J Exp Med. 1989;169:1137–1151. doi: 10.1084/jem.169.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verani A. Scarlatti G. Comar M, et al. C-C chemokines released by lipopolysaccharide (LPS)-stimulated human macrophages suppress HIV-1 infection in both macrophages and T cells. J Exp Med. 1997;185:805–816. doi: 10.1084/jem.185.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verani A. Sironi F. Siccardi AG. Lusso P. Vercelli D. Inhibition of CXCR4-tropic HIV-1 infection by lipopolysaccharide: Evidence of different mechanisms in macrophages and T lymphocytes. J Immunol. 2002;168:6388–6395. doi: 10.4049/jimmunol.168.12.6388. [DOI] [PubMed] [Google Scholar]
- 30.Aquaro S. Menten P. Struyf S, et al. The LD78β isoform of MIP-1a is the most potent CC-chemokine in inhibiting CCR5-dependent human immunodeficiency virus type 1 replication in human macrophages. J Virol. 2001;75:4402–4406. doi: 10.1128/JVI.75.9.4402-4406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menten P. Wuyts A. Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13:455–481. doi: 10.1016/s1359-6101(02)00045-x. [DOI] [PubMed] [Google Scholar]
- 32.Worgall S. Connor R. Kaner RJ, et al. Expression and use of human immunodeficiency virus type 1 coreceptors by human alveolar macrophages. J Virol. 1999;73:5865–5874. doi: 10.1128/jvi.73.7.5865-5874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikulak J. Gianolini M. Versmisse P. Pancino G. Lusso P. Verani A. Biological and physical characterization of the X4 HIV-1 suppressive factor secreted by LPS-stimulated human macrophages. Virology. 2009;390:37–44. doi: 10.1016/j.virol.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Thibault S. Tardif MR. Barat C. Tremblay MJ. TLR2 signaling renders quiescent naive and memory CD4+ T cells more susceptible to productive infection with X4 and R5 HIV-Type 1. J Immunol. 2007;179:4357–4366. doi: 10.4049/jimmunol.179.7.4357. [DOI] [PubMed] [Google Scholar]
- 35.Wyllie DH. Kiss-Toth E. Visintin A, et al. Evidence for an accessory protein function for toll-like receptor 1 in anti-bacterial responses. J Immunol. 2000;165:7125–7132. doi: 10.4049/jimmunol.165.12.7125. [DOI] [PubMed] [Google Scholar]
- 36.Platt EJ. Wehrly K. Kuhmann SE. Chesebro B. Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophage tropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei X. Decker JM. Liu H, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edmonds T. Ding D. Conway J, et al. Rapid, quantitative analysis of HIV-1 neutralization in primary cells via Renilla luciferase-expressing infectious molecular clones. Presented at the Conference on Retroviruses and Opportunistic Infections; Montreal, Quebec, Canada: 2009. [Google Scholar]
- 39.Ochesenbauer C. Kappes JC. New virologic reagents for neutralizing antibody assays. Curr Opin HIV AIDS. 2009;4:418–425. doi: 10.1097/COH.0b013e32832f011e. [DOI] [PubMed] [Google Scholar]
- 40.Keele BF. Giorgi EE. Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salazar-Gonzalez JF. Salazar MG. Keele BF, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206:1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ochsenbauer-Jambor C. Ding H. Keele BF, et al. Generation, biological characterization of infectious molecular clones derived from clade B HIV-1 transmitted/founder viruses. Presented at the Conference on Retroviruses and Opportunistic Infections; Montreal, Quebec, Canada: 2009. [Google Scholar]
- 43.Godowski PJ. A smooth operator for LPS responses. Nat Immunol. 2005;6:544–546. doi: 10.1038/ni0605-544. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Z. Georgel P. Du X, et al. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 45.Montefiori DC. Coligan JE. Kruisbeek AM. Shevach EM. Strober W. Coico R. Current Protocols in Immunology. John Wiley & Sons; New York: 2004. Evaluating neutralizing antibodies against HIV, SIV, SHIV in luciferase reporter gene assays; pp. 12.11.1–12.11.15. [DOI] [PubMed] [Google Scholar]
- 46.Bures R. Gaitan A. Zhu T, et al. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 2000;16:2019–2035. doi: 10.1089/088922200750054756. [DOI] [PubMed] [Google Scholar]
- 47.Abdelwahab SF. Cocchi F. Bagley KC, et al. HIV-1-suppressive factors are secreted by CD4+ T cells during primary immune responses. Proc Natl Acad Sci USA. 2003;100:15006–15010. doi: 10.1073/pnas.2035075100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cocchi F. DeVico AL. Garzino-Demo A. Arya SK. Gallo RC. Lusso P. Identification of RANTES, MIP-1a, and MIP-1b as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 49.Pal R. Garzino-Demo A. Markham PD, et al. Inhibition of HIV-1 infection by the β-chemokine MDC. Science. 1997;278:695–698. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 50.Urban TJ. Weintrob AC. Fellay J, et al. CCL3L1 and HIV/AIDS susceptibility. Nat Med. 2009;15:1110–1112. doi: 10.1038/nm1009-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menten P. Struyf S. Schutyser E, et al. The LD78beta isoform of MIP-1alpha is the most potent CCR5 agonist and HIV-1-inhibiting chemokine. J Clin Invest. 1999;104:R1–5. doi: 10.1172/JCI7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakao M. Nomiyama H. Shimada K. Structures of human genes coding for cytokine LD78 and their expression. Mol Cell Biol. 1990;10:3646–3658. doi: 10.1128/mcb.10.7.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nibbs RJ. Yang J. Landau NR. Mao JH. Graham GJ. LD78β, a non-allelic variant of human MIP-1alpha (LD78alpha), has enhanced receptor interactions and potent HIV suppressive activity. J Biol Chem. 1999;274:17478–17483. doi: 10.1074/jbc.274.25.17478. [DOI] [PubMed] [Google Scholar]
- 54.Struyf S. Menten P. Lenaerts JP, et al. Diverging binding capacities of natural LD78b isoforms of macrophage inflammatory protein-1a to the CC chemokine receptors 1, 3 and 5 affect their anti-HIV-1 activity and chemotactic potencies for neutrophils and eosinophils. Eur J Immunol. 2001;31:2170–2178. doi: 10.1002/1521-4141(200107)31:7<2170::aid-immu2170>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 55.Burton DR. Pyati J. Koduri R, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 56.Mo H. Stamatatos L. Ip JE, et al. Human immunodeficiency virus type 1 mutants that escape neutralization by human monoclonal antibody IgG1b12. J Virol. 1997;71:6869–6874. doi: 10.1128/jvi.71.9.6869-6874.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pantophlet R. Ollmann Saphire E. Poignard P. Parren PW. Wilson IA. Burton DR. Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120. J Virol. 2003;77:642–658. doi: 10.1128/JVI.77.1.642-658.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calarese DA. Scanlan CN. Zwick MB, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 59.Sanders RW. Venturi M. Schiffner L, et al. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J Virol. 2002;76:7293–7305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scanlan CN. Pantophlet R. Wormald MR, et al. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1–2 mannose residues on the outer face of gp120. J Virol. 2002;76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barbato G. Bianchi E. Ingallinella P, et al. Structural analysis of the epitope of the anti-HIV antibody 2F5 sheds light into its mechanism of neutralization and HIV fusion. J Mol Biol. 2003;330:1101–1115. doi: 10.1016/s0022-2836(03)00611-9. [DOI] [PubMed] [Google Scholar]
- 62.Muster T. Steindl F. Purtscher M, et al. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Purtscher M. Trkola A. Gruber G, et al. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1994;10:1651–1658. doi: 10.1089/aid.1994.10.1651. [DOI] [PubMed] [Google Scholar]
- 64.Stiegler G. Kunert R. Purtscher M, et al. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 2001;17:1757–1765. doi: 10.1089/08892220152741450. [DOI] [PubMed] [Google Scholar]
- 65.Zwick MB. Labrijn AF. Wang M, et al. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmidtmayerova H. Sherry B. Bukrinsky M. Chemokines and HIV replication. Nature. 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- 67.Dragic T. Litwin V. Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 68.Moriuchi H. Moriuchi M. Combadiere C. Murphy PM. Fauci AS. CD8+ T-cell-derived soluble factor(s), but not beta-chemokines RANTES, MIP-1 alpha, and MIP-1 beta, suppress HIV-1 replication in monocyte/macrophages. Proc Natl Acad Sci USA. 1996;93:15341–15345. doi: 10.1073/pnas.93.26.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morrison RP. Differential sensitivities of Chlamydia trachomatis strains to inhibitory effects of gamma interferon. Infect Immun. 2000;68:6038–6040. doi: 10.1128/iai.68.10.6038-6040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Um S-J. Kim E-J. Hwang E-S. Kim S-J. Namkoong S-E. Park J-S. Antiproliferative effects of retinoic acid/interferon in cervical carcinoma cell lines: Cooperative growth suppression of IRF-1 and p53. Int J Cancer. 2000;85:416–423. [PubMed] [Google Scholar]
- 71.Miller SI. Ernst RK. Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 72.Arbour NC. Lorenz E. Schutte BC, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 73.Picard C. Puel A. Bonnet M, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 74.Bleul CC. Farzan M. Choe H, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 75.Oberlin E. Amara A. Bachelerie F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 76.Lee B. Rucker J. Doms RW, et al. β-Chemokine MDC and HIV-1 infection. Science. 1998;281:487a. [Google Scholar]
- 77.Ho DD. Hartshorn KL. Rota TR, et al. Recombinant human interferon alpha-A suppresses HTLV-III replication in vitro. Lancet. 1985;1:602–604. doi: 10.1016/s0140-6736(85)92144-0. [DOI] [PubMed] [Google Scholar]
- 78.Yamamoto JK. Barre-Sinoussi F. Bolton V. Pedersen NC. Gardner MB. Human alpha- and beta-interferon but not gamma- suppress the in vitro replication of LAV, HTLV-III, and ARV-2. J Interferon Res. 1986;6:143–152. doi: 10.1089/jir.1986.6.143. [DOI] [PubMed] [Google Scholar]
- 79.Triantafilou K. Triantafilou M. Dedrick RL. A CD14-independent LPS receptor cluster. Nat Immunol. 2001;2:338–345. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- 80.Triantafilou M. Lepper PM. Briault CD, et al. Chemokine receptor 4 (CXCR4) is part of the lipopolysaccharide “sensing apparatus.”. Eur J Immunol. 2008;38:192–203. doi: 10.1002/eji.200636821. [DOI] [PubMed] [Google Scholar]
- 81.Magalhaes PO. Lopes AM. Mazzola PG. Rangel-Yagui C. Penna TC. Pessoa A., Jr Methods of endotoxin removal from biological preparations: A review. J Pharm Sci. 2007;10:388–404. [PubMed] [Google Scholar]
- 82.Forthal DN. Landucci G. Cole KS. Marthas M. Becerra JC. Van Rompay K. Rhesus macaque polyclonal and monoclonal antibodies inhibit simian immunodeficiency virus in the presence of human or autologous Rhesus effector cells. J Virol. 2006;80:9217–9225. doi: 10.1128/JVI.02746-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ancuta P. Kamat A. Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brenchley JM. Price DA. Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 85.Lester RT. Yao X-D. Ball TB, et al. HIV-1 RNA dysregulates the natural TLR response to subclinical endotoxemia in Kenyan female sex-workers. PLoS ONE. 2009;4:e5644. doi: 10.1371/journal.pone.0005644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang W. Lederman MM. Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mandl JN. Barry AP. Vanderford TH, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 88.Pandrea I. Gaufin T. Brenchley JM, et al. Cutting edge: Experimentally induced immune activation in natural hosts of simian immunodeficiency virus induces significant increases in viral replication and CD4+ T cell depletion. J Immunol. 2008;181:6687–6691. doi: 10.4049/jimmunol.181.10.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marchetti G. Bellistri GM. Borghi E, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22:2035–2038. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]