Abstract
Depo-Provera® (medroxyprogesterone acetate), a long-acting derivative of progesterone, is utilized during many nonhuman primate microbicide studies to facilitate simian immunodeficiency virus (SIV) infection by thinning the vaginal epithelium. To date, the systemic effects of this steroid hormone in regard to SIV/HIV pathogenesis are not well understood, but an increase in infection rates and lymphoproliferation following progesterone application has been reported. Therefore, a proactive study using 20 Chinese rhesus macaques was designed to investigate the effect of a single Depo-Provera injection on SIV disease progression. Group 1 (n = 10) was treated with 30 mg Depo-Provera intramuscularly 30 days prior to intravenous challenge with 50 TCID50 SIVmac251, while Group 2 (n = 10) remained untreated, but received the same amount of SIV. Blood samples were taken at predetermined intervals to measure RNA viral loads, CD4+, CD8+, and CD20+ lymphocyte counts and percentages and absolute numbers of naive and memory T lymphocytes. Upon statistical endpoint data analysis, none of the parameters measured were shown to be significantly different between the groups. One animal in the Depo-Provera-treated group and two macaques in the control group were euthanized prior to study end due to the development of clinical signs (in weeks 43 and 51, respectively). All other animals were euthanized between weeks 68 and 71 post-SIV infection. Histopathological evaluations revealed that 5 of 10 animals in each group had developed simian AIDS (SAIDS). In summary, this prospective study demonstrated that a single injection of 30 mg Depo-Provera did not have a significant influence on SIV disease progression.
Introduction
This year marks the twenty-seventh anniversary of the identification of the first HIV-1 infection, which resulted in a worldwide HIV pandemic and the associated disease of acquired immunodeficiency syndrome (AIDS).1 Despite multinational efforts, no effective HIV vaccine has been developed to date.2 In recent years, the scientific community has shifted some of its focus from preventive vaccine to topical microbicide development in order to broaden the strategies for combating HIV/AIDS.3 Topical microbicides are chemical or biological agents, sometimes in combination with a device, which can be self-administered prior to sexual intercourse and are specifically designed to prevent sexually transmitted diseases, in particular HIV.4,5 Although some anti-HIV microbicides advanced into clinical trials, most are still in the preclinical development stage.
To facilitate preclinical topical microbicide efficacy and toxicity evaluations, numerous animal models have been developed.6,7 Rodents and rabbits are frequently used for toxicity studies,8–10 and the rabbit vaginal irritation model is required by the Food and Drug Administration (FDA) for vaginal product licensure. Microbicide efficacy testing is performed in nonhuman primates, which represent the only animal species capable of productive infection with simian immunodeficiency virus (SIV) or SIV-HIV hybrids (SHIV), two close genetic relatives of HIV. However, atraumatic introduction of SIV/SHIV into the primate vaginal tract usually requires large amounts of virus (in the 105 TCID50 range) and/or multiple challenges for successful infection. Variability is also increased by the fact that at the time of infection, nonhuman primates are in different stages of their menstrual cycle, resulting in different thicknesses of the vaginal epithelium. Indeed, during the follicular phase, increased estrogen levels lead to thickening and cornifying of the vaginal epithelium making it very difficult for SIV to penetrate.11,12 However, during the luteal phase, progesterone diminishes the number of vaginal epithelial layers,13 making them more susceptible to penetration by viruses, including HIV/SIV. Taking these physiological changes into account, vaginal SIV or SHIV transmission is by far more inefficient and variable than intravenous SIV infection.14
To establish a more uniform vaginal infection procedure, the epithelium thinning effect of progesterone has been recognized and employed in initial studies for nonhuman primate microbicide development. Subsequently, Depo-Provera (an FDA-approved long-acting progestin for human use) was selected as the progesterone formulation of choice at a dose of 30 mg to facilitate virus infection after vaginal challenge. This dose of Depo-Provera is equivalent, on a per kilogram body weight basis, to that administered to women for contraceptive purposes.13 In rhesus macaques, serum levels of medroxyprogesterone acetate were maximal on day 14 and nondetectable by day 70 following a single intramuscular injection.13
In addition to the effects of Depo-Provera on the menstrual cycle and vaginal epithelium, progesterone and its derivatives have been described as immunosuppressive via various mechanisms. Progesterone, a C-21 steroid hormone, can be converted to 17-hydroxyprogesterone, which is the substrate for cortisol (glucocorticoid). Of seven previous prospective studies examining depot-medroxyprogesterone acetate use and HIV acquisition among women, two have found statistically significant increased HIV risk.15 Ehring et al.16 proposed that progesterone acts as an endogenous immunosuppressant by directly and reversibly blocking K+ channels. It has also previously been indicated that progesterone increased the expression of the CCR5 receptor on CD14+ monocytes and the CXCR4 receptor expression on CD14+ monocytes and CD4+ and CD8+ lymphocytes in vitro.17 Another study demonstrated that cell surface expression of CCR5 increased with oral contraceptive use on cervical CD4 T cells but not CD8 T cells. This upregulation of CCR5 on CD4 T cells was associated with the early activation marker CD69+.18 In another context, it has been reported that progesterone enhanced susceptibility and decreased immune responses to vaginal herpes virus infection in mice.19,20
To obtain additional data on the effects of Depo-Provera on SIV infection and the resulting consequences for microbicide model development, a prospective study was planned and conducted in female Chinese macaques, a nonhuman primate species commonly used for this type of research. Twenty macaques were divided into two groups; Group 1 animals were injected with 30 mg Depo-Provera intramuscularly 30 days prior to intravenous SIVmac251 challenge, whereas Group 2 animals did not receive hormonal treatment. The intravenous challenge route was chosen to allow all untreated control animals (Group 2) to become infected with SIV.
Materials and Methods
Study animals and treatment
Nonhuman primate housing, care, and treatments were performed in accordance with the standards of the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC), the Animal Welfare Act as amended, and the Public Health Service Policy on Humane Care and Use of Laboratory Animals, 2002. Prior to study start, the study protocol was approved by the Southern Research Institutional Animal Care and Use Committee (IACUC). All animals tested negative for simian type D retrovirus (SRV) type 1, 2, 3, and 5 and simian T cell lymphotropic virus type 1 and 2 (STLV-1 and −2) prior to and during the study. SIV antibody tests were negative before study start.
Two groups of 10 female Chinese rhesus macaques were used in this study (Table 1). The ages were between 4 years 7 months and 6 years 4 months, and the body weights were between 5.3 kg and 9.1 kg. Animal randomization was performed using the Student's t test based on prestudy body weights and blood CD3+CD4+ cell counts. Group 1 animals received a single intramuscular injection of 30 mg Depo-Provera, whereas Group 2 animals remained untreated. Thirty days after Depo-Provera treatment of Group 1, all macaques were challenged intravenously with 50 TCID50 of SIVmac251 produced in CEMx174 cells. The virus stock had an SIV Gag p27 content of 145 ng/ml. The in vitro infectious titer was determined to be 5.9 × 104 TCID50/ml in CEMx174 cells and 2.5 × 103 TCID50/ml in interleukin (IL)-2-stimulated peripheral blood mononuclear cells (PBMCs) from rhesus macaques. All macaques were followed up for virological and immunological parameters for a period of 64 weeks.
Table 1.
Animal Enrollmenta
| Animal ID | Origin | Sex | Age at SIV challenge | Weight (kg)1 | Absolute CD3+CD4+cells (/μl)b | Depo-Provera®administration |
|---|---|---|---|---|---|---|
| Group 1 | ||||||
| 3771 | Ch | F | 5 yr 11 mo | 7.4 | 1417 | Yes |
| 3772 | Ch | F | 6 yr 4 mo | 5.3 | 730 | Yes |
| 3777 | Ch | F | 5 yr 1 mo | 5.7 | 722 | Yes |
| 3779 | Ch | F | 5 yr 0 mo | 6.9 | 1129 | Yes |
| 3784 | Ch | F | 4 yr 12 mo | 6.7 | 932 | Yes |
| 3786 | Ch | F | 4 yr 11 mo | 6.1 | 1388 | Yes |
| 3788 | Ch | F | 5 yr 7 mo | 5.5 | 628 | Yes |
| 3790 | Ch | F | 5 yr 10 mo | 8.0 | 1129 | Yes |
| 3794 | Ch | F | 4 yr 11 mo | 9.1 | 504 | Yes |
| 3819 | Ch | F | 4 yr 7 mo | 7.0 | 936 | Yes |
| Mean | 5 yr 4 mo | 6.8 | 951 | |||
| Group 2 | ||||||
| 3774 | Ch | F | 5 yr 1 mo | 5.9 | 947 | No |
| 3776 | Ch | F | 5 yr 1 mo | 6.1 | 1109 | No |
| 3781 | Ch | F | 5 yr 8 mo | 6.9 | 551 | No |
| 3782 | Ch | F | 5 yr 6 mo | 5.7 | 602 | No |
| 3798 | Ch | F | 4 yr 10 mo | 6.4 | 1767 | No |
| 3809 | Ch | F | 4 yr 9 mo | 6.5 | 758 | No |
| 3812 | Ch | F | 5 yr 9 mo | 6.9 | 637 | No |
| 3818 | Ch | F | 4 yr 9 mo | 7.8 | 810 | No |
| 3821 | Ch | F | 4 yr 10 mo | 7.6 | 1196 | No |
| 3826 | Ch | F | 4 yr 8 mo | 6.4 | 953 | No |
| Mean | 5 yr 1 mo | 6.6 | 933 |
Ch, Chinese origin rhesus; F, female; yr, year; mo, month.
Before study start.
SIV-specific TaqMan real time reverse transcriptase polymerase chain reaction (RT-PCR)
Plasma SIV RNA loads were determined by quantitative TaqMan RT-PCR on an ABI 7900HT sequence detector at weeks 0, 1, 2, 3, 4, 6, 8, 10, and 12 post-SIV infection and every 4 weeks thereafter. Standard RNA templates have previously been created by DNA PCR from the p239SpSp5′ plasmid DNA (NIH AIDS Research and Reference Reagent Program) using SIV gag-specific primers 5′-AGA TAG AGT GGG AGA TGG GCG TGA GA-3′ and 5′-GTC TAC TTG TTT TTG GCA TAG TT-3′. The resultant PCR product (384 bp) was cloned directly into pCR-Script II (Stratagene, La Jolla, CA). The plasmid was then linearized with EcoRI (New England BioLabs Inc., Ipswich, MA) and was used to perform an in vitro transcription with T3 RNA polymerase (Promega, Madison, WI). After digestion with RNase-free DNase (Promega), the resulting RNA transcripts were purified (Qiagen, Valencia, CA). The concentration of the standard RNA templates was determined by optical density (OD) measurement. A standard curve for the quantitative TaqMan RT-PCR was prepared composed of duplicate half log10 serial dilutions ranging from 107 to 101 copies.
Five hundred microliters of plasma separated from EDTA blood was diluted with 1 ml phosphate-buffered saline (PBS) and then centrifuged at 7500 × g for 1 h at 4°C. Viral RNA was extracted from the pellet with RNA-STAT-60 reagent (Tel-Test, Inc., Gainesville, FL) according to the manufacturer's instructions and resuspended in 30 μl of RNase-free water. Six microliters of viral RNA were added to a mix of One-Step RT-PCR Master Mix (Applied Biosystems, Foster, City, CA) with primers and probe designed to amplify a conserved region of the SIVmac239 gag gene: (1) forward primer, SIV-F 5′-AGT ATG GGC AGC AAA TGA AT-3′; (2) reverse primer, SIV-R 5′-TTC TCT TCT GCG TGA ATG C-3′; and (3) the probe, SIV-P 6FAM-AGA TTT GGA TTA GCA GAA AGC CTG TTG GA-TAMRA. On an ABI 7900HT sequence detector, the RNA test samples were reverse transcribed at 48°C for 30 min and 95°C for 10 min, and then a cDNA-PCR reaction was run for 40 cycles at 95°C for 15 s and 60°C for 1 min. All test sample reactions were performed in triplicate. The viral RNA copy numbers of each test sample were extrapolated from the SIV RNA standard curve.
Blood lymphocyte phenotyping, complete blood counts (CBCs), and serum chemistries
CBCs were determined using a VetScan HMT Hematology Analyzer (Abaxis, Inc., Union City, CA), and serum chemistries were monitored using a VetScan HMT Chemistry Analyzer (Abaxis, Inc., Union City, CA).
Lymphocyte subsets determinations were performed during weeks –6, –4, –2, 0, 2, 3, 4, 6, 8, 10, and 12 relative to the day of SIV infection and every 4 weeks thereafter. The following CD markers were analyzed from whole blood: CD3-FITC, CD4-PE, CD8-PerCP, CD2-FITC (BD Biosciences Pharmingen, San Jose, CA), and CD20-PE (Beckman Coulter, Fullerton, CA). Briefly, 110 μl of whole blood was stained with antibody for 15 min at room temperature. Subsequently, the red blood cells were lysed, and the white blood cells fixed using the appropriate solutions provided in the Whole Blood Lysing Reagent Kit (Beckman Coulter). After washing the white blood cells three times with PBS, they were resuspended in PBS, and their surface CD marker expression was measured and analyzed with CellQuest software on a BD FACSCalibur Flow Cytometer (BD Biosciences).
Naive and memory T lymphocytes were monitored by flow cytometry from Histopaque −1077 (Sigma, St. Louis, MO) separated lymphocytes at weeks –6, –4, –2, 0, 2, 4, 6, 8, 10, and 12 and every 4 weeks thereafter. The following surface markers combinations were used in this assay: (1) CD4-PerCP/CD28-FITC/CD95-APC/Mouse IgG2a-PE [FMO control], (2) CD4-PerCP/CD28-PE/CD95-APC/Mouse IgG2a-FITC [FMO control], (3) CD4-PerCP/CD28-FITC/CD95-APC/CCR5-PE, (4) CD4-PerCP/CD28-PE/CD95-APC/CCR7-FITC, (5) CD8β-PE/CD28-APC/ D95-PECy5/Mouse Ig2a-FITC [FMO control], and (6) CD8β-PE/CD28-APC/CD95-PECy5/CCR7-FITC. Anti-CD2-FITC, anti-CD3-FITC, anti-CD4-PE, anti-CD4-PerCP, anti-CD8-FITC, anti-CD28-FITC, anti-CD28-PE, anti-CD28-APC, anti-CD95-APC, anti-CD95-PE-Cy5, anti-CCR5 PE, mouse IgG1-PE, mouse IgG1 PerCP, mouse IgG1-APC, mouse IgG2A-FITC, and mouse IgG2a-PE antibodies were obtained from BD Biosciences (San Jose, CA). Anti-CD8β-PE, anti-CD20-PE, and mouse IgG1-FITC antibodies were purchased from Beckman Coulter (Fullerton, CA). Anti-CCR7-FITC antibody was obtained from R&D systems (Minneapolis, MN). Briefly, 1–2 × 106 PBMCs were incubated with the combinations of antibodies described above in RPMI 1640 supplemented with 10% fetal calf serum (FCS) for 20 min on ice (protected from light), washed twice with PBS, and fixed with 1% paraformaldehyde. Samples were acquired on a BD FACSCalibur Flow Cytometer using CellQuest software and analyzed using FlowJo analysis software (TreeStar, Inc., Ashland, OR).
Euthanasia, necropsy, and pathology
Study animals were euthanized when they became moribund or at the end of the study. For early terminations, quantitative and qualitative study endpoint criteria based on weight loss, diarrhea, cardiac and pulmonary disease, progressive neurological signs, opportunistic infections, and laboratory findings were established by the Southern Research Institute IACUC prior to study start. All euthanasia procedures were carried out in accordance with the 2000 Report of the American Veterinary Medical Association (AVMA) Panel on Euthanasia and the 2007 AVMA Guidelines on Euthanasia. Gross pathological lesions were recorded at necropsy. The following tissues were collected and placed in 10% buffered formalin: gastroesophageal junction, stomach, gastroduodenal junction, pancreas, duodenum, ileocecal junction, ileum, colon, rectum, skin, noticeably enlarged lymph nodes, tongue, submandibular lymph node, any atrophied muscles, thymus, esophagus, trachea, thyroid, lung, heart, liver, spleen, two mesenteric lymph nodes, adrenal glands, kidneys, gonads, urinary bladder, femur, brain, pituitary gland, and spinal cord. Fixed tissues were paraffin embedded, and the sections were stained with hematoxylin and eosin for histopathological investigations.
Statistical analyses
Plasma SIV RNA viral loads, absolute CD4, CD8, and B lymphocyte counts, and percentages of naive/memory CD4 and CD8 T cells were compared statistically between Group 1 and 2. The Inequality Test for Two Means in a Repeated Measures Design function in the Power Analysis and Sample Size (PASS) System software21 was utilized to calculate expected power across the entire study or defined ranges of the study. Differences in means between Depo-Provera-treated and control groups were calculated. The mean of each group was calculated as the mean value across all time points in a given range, where the value measure at each time point was calculated as the mean value across all macaques at the specified time point. Mixed models repeated measures (MMRM) analyses (PROC MIXED in SAS) were implemented to test if there was a difference in the measured value between animals treated with Depo-Provera versus those that remained untreated. The significance threshold chosen was α = 0.2.
Results
SIV infection did not cause differences in standard clinical pathology measures between Depo-Provera-treated and control Chinese rhesus macaques
The CBC values were relatively stable throughout the study (data not shown). Intermittent increases in relative and absolute numbers of white blood cells were observed in some animals probably due to bacterial infections, which returned to normal ranges after therapeutic treatment. Periodic lymphopenia and polycythemia were experienced by some animals showing signs of progressive SIV infection.
Blood chemistry values were unremarkable throughout the study period except for some mild persistent abnormalities, such as hyperglobulinemia, elevated ALT, and decreased calcium and phosphorus levels throughout SIV infection but were not life threatening and did not have any effect on the overall stable clinical status of the animals (data not shown). Albumin concentrations were terminally decreased in 4 out of 10 macaques indicating progressive signs of SIV disease, a finding that was described recently.22 There were no statistically significant differences between Groups 1 and 2 animals in regard to CBC or clinical chemistry parameters.
No significant differences in plasma viral loads between Depo-Provera-treated and control Chinese rhesus macaques
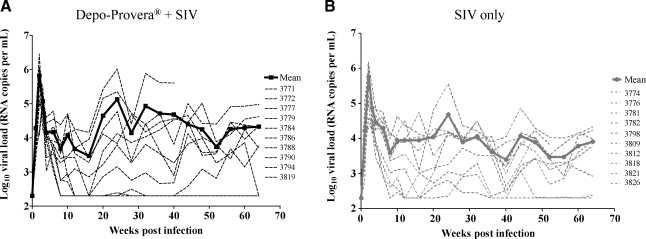
All macaques inoculated intravenously with 50 TCID50 of SIVmac251/CEMx174 established infection evidenced by the detection of the initial peak of viremia within 2–3 weeks postchallenge (Fig. 1). The initial peak of viremia ranging from 1 × 105 to 2.8 × 106 copies/ml in the Depo-Provera-treated group and from 2.4 × 104 to 1.4 × 106 copies/ml in the untreated group was comparable. In contrast, the mean peak viral load in the animals diagnosed terminally with simian AIDS (SAIDS) was higher than in the nonprogressor animals (3.3 × 105 vs. 9.4 × 105 RNA copies/ml) irrespective of group assignment. As expected for SIV infection, the viral load declined in all macaques after the initial peak and reached its set point between weeks 8 and 12 postchallenge. The set point ranged from 2 × 102 to 5 × 104copies/ml for the Group 1 animals and 2 × 102 to 4.4 × 104 copies/ml for the Group 2 animals. Two animals in Group 1 and two animals in Group 2 controlled virus replication. Statistical analyses comparing the two groups clearly revealed that there was no significant difference in viral loads between the two groups at α = 0.2 (Fig. 1).
FIG. 1.
Viral loads were determined with TaqMan RT-PCR as described in the Materials and Methods section. (A) The thick black line represents the mean RNA viral load of the Depo-Provera-treated macaques after SIV infection. (B) The thick gray line shows the mean RNA viral load of the control group macaques after SIV infection. The dashed lines represent RNA viral loads of individual monkeys. There was no statistically significant difference between the two groups (p = 0.39, power 22%).
Lymphocyte subsets showed characteristic effects of SIV infection, but no differences in response to Depo-Provera administration were observed
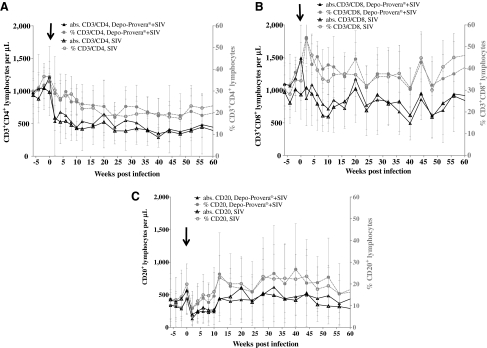
Blood lymphocyte subsets were monitored throughout SIV infection in both groups by flow cytometry and percentages and absolute counts are shown in Fig. 2. Percentages and absolute CD3+CD4+ (CD4) lymphocyte counts declined precipitously within 2 weeks following SIV challenge in all macaques as illustrated in Fig. 2A. Initial recovery coincided with the decline in viral load in all animals (Fig. 1). Statistical analysis of the absolute CD4 counts did not show any significant differences between the Depo-Provera-treated group and the untreated group at α = 0.2. Also, percentages and absolute CD3+CD8+ (CD8) T cell counts were analyzed by flow cytometry throughout the study (Fig. 2B). Overall, there were fluctuations in the CD8 T cell counts throughout infection suggesting continuous replenishment of this population in the infected Chinese rhesus macaques. Statistical analysis did not show any significant difference in CD8 T cell counts between the Depo-Provera-treated group and untreated group. In addition to T lymphocyte markers, percentages and absolute B cell counts were analyzed throughout the study by flow cytometry using the surface marker CD20 (Fig. 2C). Following SIV infection, B cell numbers decreased and then gradually increased as a result of B cell hyperactivation previously described in HIV-infected individuals and SIV-infected macaques.23–25 This increase in B cell numbers persisted longer in six animals (three in each group), which may suggest either a strong and persistent antigen stimulation or a lymphoproliferative disorder often associated with progressive SIV infection.
FIG. 2.
Mean and standard deviations of percentages and absolute CD3+CD4+ lymphocyte counts (A), CD3+CD8+ lymphocyte counts (B), and CD20+ lymphocyte counts are shown from week –6 to week 60 post-SIVmac251 infection. Absolute lymphocyte counts were determined with a VetScan HMT Hematology Analyzer, and the absolute numbers of lymphocyte populations were determined by the following equation: (Percent CD marker expression × absolute lymphocyte count)/100. There were no statistically significant differences between the groups [(A) p = 0.83, power = 14%; (B) p = 0.77, power = 17%; (C) p = 0.66, power = 26%]. The arrow indicates the day of SIV infection.
Comparison of naive and memory T cell subsets showed no effect of Depo-Provera administration
CD4 and CD8 T cell memory immunophenotyping was performed by flow cytometry throughout SIV infection using CD28, CD95, CCR5, and CCR7 markers.
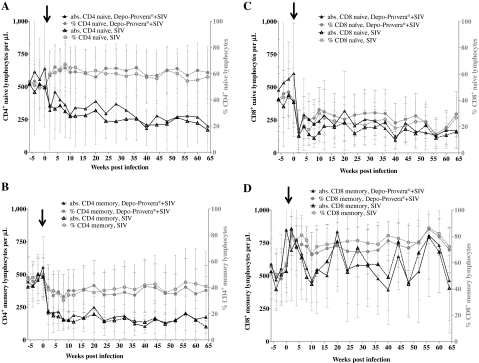
Average percentages of naive CD4 T cells (CD4+, CD95–) increased after SIV infection in both groups (Fig. 3A), whereas average percentages of memory CD4 T cells (CD4+, CD95+) decreased (Fig. 3B). Absolute numbers of naive CD4 T cells decreased slowly after infection in both groups (Fig. 3A), whereas absolute numbers of memory CD4 T cells decreased rapidly in the first weeks after infection and then stabilized (Fig. 3B). Both average percentages and absolute numbers of naive CD8 T cells (CD8+, CD95–) decreased in the first weeks after SIV infection and then stabilized (Fig. 3C). The average percentages of memory CD8 T cells (CD8+, CD95+) increased in the first week after SIV infection and then stabilized (Fig. 3D). The absolute numbers of memory CD8 T cells remained constant after SIV infection and throughout the observation period (Fig. 3D). There were no statistically significant differences between the Depo-Provera-treated and control group in the naive or memory CD4 or CD8 T cells (Fig. 3).
FIG. 3.
Mean and standard deviations of percentages and absolute CD4+CD95– (naive) cells (A), CD4+CD95+ (memory) cells (B), CD8+CD95– (naive) cells (C), and CD8+CD95+ (memory) cells (D) are shown from week –6 to week 64 post-SIVmac251 infection. Absolute lymphocyte counts were determined with a VetScan HMT Hematology Analyzer and the absolute numbers of lymphocyte populations were determined by the following equation: (Percent CD marker expression × absolute CD4 or CD8 lymphocyte count)/100. There were no statistically significant differences between the groups [(A) p = 0.65, power = 20%; (B) p = 0.89, power = 15%; (C) p = 0.47, power = 40%; (D) p = 0.52, power = 37%]. The arrow indicates the day of SIV infection.
Depo-Provera administration did not result in any significant immunological changes
Neutralizing antibodies were measured in the first 20 weeks after infection in the laboratory of Dr. Montefiori.26 No neutralizing antibodies were detected against the pseudotyped virus SIVmac251CS.41 in TZM-bl cells, whose envelope protein has a neutralization pattern similar to the uncloned parental SIVmac251 strain (data not shown). It has been described as difficult to neutralize, and neutralizing antibodies in the range of 1:10–1:100 are usually expected only after a long period of infection. In contrast, high titers of antibodies against the neutralization-sensitive control strain SIVmac251-TCLA in M7 Luc cells were detected as early as 4 weeks after infection. Overall, there was no difference between Group 1 and 2 in the neutralizing antibody titers (data not shown).
Interferon (IFN)-γ-expressing cells in response to SIV peptide stimulation were measured by ELISPOT in weeks 6, 8, 10, and 12 postinfection after stimulation with two Gag and three Env overlapping peptide pools.27 A low number of IFN-γ-producing spot-forming colonies per 106 PBMC (mean number per group ≤ 27) was detected with no apparent difference between the Depo-Provera-treated and control groups (data not shown).
SAIDS symptoms develop in 50% of the macaques after > 1 year of SIV infection
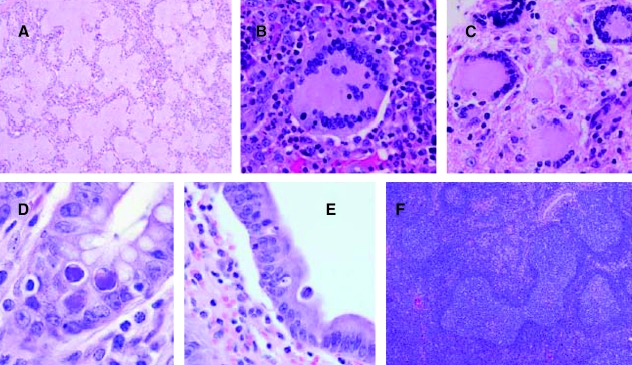
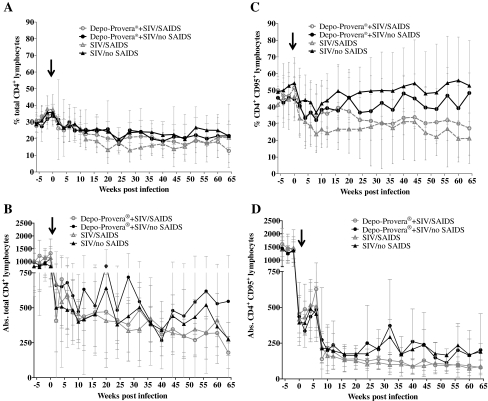
Three macaques became moribund before the end of the study (animal 3779 in Group 1 and animals 3781 and 3821 in Group 2) at weeks 43 and 51, respectively (Table 2). Animal 3779 was presented prior to euthanasia with a history of diarrhea, anorexia, lethargy, and dehydration. Animals 3781 and 3821 had a history of diarrhea and weight loss. The other 17 animals were euthanized between week 68 and 72. Generally, the animals that were diagnosed with SAIDS had a higher number of clinical signs, mainly treatment-responsive diarrhea, weight loss, and dehydration, compared to the nonprogressor animals, but there were no differences in the severity of clinical signs between the Depo-Provera-treated and control groups. Gross pathological lesions in the SAIDS-diagnosed animals mainly consisted of enlarged lymph nodes, distended/thickened large intestine, icteric liver, and enlarged or contracted spleen. The histopathological findings of the animals diagnosed with SAIDS are listed in Table 2. They were represented by typical diagnoses associated with SAIDS, SIV encephalitis and myelitis, SIV giant cell pneumonia and lymphadenitis, adenovirus enteritis, SIV arteriopathy, Enterocytoon bieneusi cholecystitis and cholangeohepatitis, lymphoproliferative disorders, and Pneumocystis carinii pneumonia (Table 2 and Fig. 4). There were no statistically significant differences in organ weights (heart, kidney, spleen, liver, brain) measured in percent body weight between the two investigational groups. Five out of 10 monkeys in each group were diagnosed with SAIDS, showing that SIV disease progression between both groups was similar. There was only a small difference in peak viral load between the Depo-Provera and non-Depo-Provera-treated animals (6.9 × 106 vs. 5.8 × 106 RNA copies per ml), whereas the difference in peak viral load between the SAIDS and nonprogressor animals was threefold (3.3 × 106 vs. 9.9 ×106 RNA copies per ml). Also, as shown in Fig. 5, percentages of total CD4+ and CD4+ memory (CD95+) T lymphocytes declined more rapidly in the macaques diagnosed terminally with SAIDS than in the nonprogressor animals, independent of Depo-Provera treatment.
Table 2.
Survival and SAIDS-Defining Pathology
| Animal ID | Depo-Provera®administration | SAIDS defining pathology | Survival after SIV infection (weeks) | Viral load (RNA copies/ml) | Terminal CD4 count (/μl) | SAIDS |
|---|---|---|---|---|---|---|
| Group 1 | ||||||
| 3771 | Yes | None | 70 | 133,013 | 247 | No |
| 3772 | Yes | Adenovirus enteritis; giant cell lymphadenitis | 68 | 174,636 | 207 | Yes |
| 3777 | Yes | None | 71 | 121,067 | 310 | No |
| 3779 | Yes | SIV encephalitis and myelitis; SIV giant cell pneumonia and lymphadenitis; adenovirus enteritis | 43 | 1,068,658 | 171 | Yes |
| 3784 | Yes | None | 72 | 640,690 | 214 | No |
| 3786 | Yes | SIV encephalitis and meningitis; SIV giant cell pneumonia | 69 | 151,276 | 284 | Yes |
| 3788 | Yes | None | 71 | 99,546 | 184 | No |
| 3790 | Yes | SIV arteriopathy; SIV encephalitis | 70 | 2,847,848 | 230 | Yes |
| 3794 | Yes | Enterocytozoon bieneusi cholecystitis | 72 | 1,142,649 | 177 | Yes |
| 3819 | Yes | None | 73 | 537,041 | 584 | No |
| Group 2 | ||||||
| 3774 | No | None | 68 | 170,119 | 482 | No |
| 3776 | No | None | 71 | 34,572 | 548 | No |
| 3781 | No | Adenovirus enteritis; lymphoproliferative disorder | 51 | 1,154,261 | 316 | Yes |
| 3782 | No | None | 72 | 24,018 | 228 | No |
| 3798 | No | Adenovirus enteritis | 69 | 539,519 | 423 | Yes |
| 3809 | No | E. bieneusi cholecystitis and cholangiohepatitis | 70 | 829,080 | 103 | Yes |
| 3812 | No | None | 68 | 37,557 | 296 | No |
| 3818 | No | None | 73 | 1,515,619 | 78 | No |
| 3821 | No | SIV encephalitis; adenovirus enteritis | 51 | 116,176 | 199 | Yes |
| 3826 | No | Pneumocystis carinii pneumonia; E. bieneusi cholecystitis | 69 | 1,352,781 | 168 | Yes |
FIG. 4.
AIDS-defining pathology in SIVmac251-infected Chinese rhesus macaques. Typical opportunistic and viral-associated lesions were observed in both experimental and control groups. (A) Lung, Pneumocystis carinii pneumonia (3826). (B) Lung, SIV giant cell pneumonia (3786). (C) Brain, SIV encephalitis (3786). (D) Small intestine, adenovirus enteritis (3779). (E) Gallbladder, Enterocytozoon bieneusi cholecystitis (3826). (F) Spleen, follicular lymphocytic hyperplasia with dysplasia (3781).
FIG. 5.
(A–D) Comparison of percentages and absolute CD4+ T lymphocytes and CD4+ T memory cells between animals diagnosed with SAIDS and nonprogressor animals. Mean and standard deviations are shown from week –6 to week 64 post-SIVmac251 infection. The arrow indicates the day of infection.
Discussion
This study was designed to investigate the effect of a single administration of 30 mg Depo-Provera on disease progression in female Chinese rhesus macaques after intravenous SIV infection. The study's sole intent was to evaluate the systemic effect of Depo-Provera administration as the only variable between two SIV-infected study groups. Chinese rhesus macaques were chosen because they are frequently utilized for vaginal SIV or SHIV microbicide studies, given the limited availability of female Indian rhesus macaques. An observation from Marx et al.28 more than a decade ago showed that subcutaneous progesterone implants thinned the vaginal epithelium of macaques and enhanced SIV vaginal transmission 7.7-fold over that observed in macaques treated with placebo implants and exposed to SIV in the follicular phase of the menstrual cycle. Progesterone treatment also increased the number of SIV DNA-positive cells in the vaginal lamina propria.28 Moreover, plasma viral RNA was increased for the first 3 months in macaques with progesterone implants, and three of the progesterone-treated macaques developed relatively rapid disease courses. The dose of 30 mg as single intramuscular (im) injection was first established by Mascola et al.29 to suppress the macaque menstrual cycle and enable successful vaginal SHIV infection to test the protective efficacy of anti-HIV-1 neutralizing monoclonal antibodies 2F5 and 2G12 and HIV immune globulin passive transfer. Since then, Depo-Provera has been administered in the same dosage regimen in the course of many nonhuman primate microbicide studies to enable consistent SIV or SHIV vaginal challenge.30–33 However, whether Depo-Provera administration results in systemic effects on SIV/SHIV disease progression has always remained under discussion. In humans, at least one study has suggested an increased rate of HIV infection in Depo-Provera users,34 but a number of other studies found no such association.35–37 However, it appears that women do not respond to exogenous progestins with the dramatic vaginal thinning seen in rhesus macaques,38 and thus it is not surprising if a different effect is seen in humans compared to nonhuman primates.
From the data presented here, it can be concluded that Depo-Provera did not affect disease progression of Chinese rhesus macaques after intravenous SIV challenge. None of the parameters measured (viral loads, lymphocyte subsets, naive and memory T lymphocytes, neutralizing antibodies, and ELISPOT responses) showed a significant difference between the Depo-Provera-treated and control groups. With respect to disease progression, five animals in each group progressed to SAIDS within 15 months post-SIV infection, whereas the five other animals in each group were classified as remaining healthy on euthanasia. In addition, Chinese rhesus macaques diagnosed with SAIDS had a more rapid total CD4 T lymphocyte loss and CD4 T memory lymphocyte decline compared to the nonprogressor SIV-infected macaques, in agreement with pathogenesis studies reported for Indian rhesus macaques.39,40
Recently, three other studies reporting the systemic effect of Depo-Provera on SIV or SHIV infection were published. In contrast to the presented study, all three investigators described a negative effect of Depo-Provera treatment on the study outcome. Genesca et al.41 determined if progesterone alters the efficacy of live attenuated vaccines through local or systemic effects. Seven male rhesus macaques were immunized with SHIV89.6 and then challenged intravenously with SIVmac239 in addition to four naive controls. Three of the SHIV-immunized animals were treated with Depo-Provera 30 days prior to SIV challenge. The SHIV-immunized, untreated animals showed statistically significantly lower plasma viral RNA levels than the unimmunized control animals, whereas the Depo-Provera-treated, SHIV-immunized animals did not. Genesca et al.41 concluded that Depo-Provera eliminated live-attenuated lentivirus vaccine efficacy in male rhesus macaques through systemic effects on antiviral immunity and/or viral replication. Although the challenge was carried out intravenously as well, the experiment is not directly comparable to the presented study since the animals were preimmunized with SHIV89.6 and the challenge virus was SIVmac239, not SIVmac251 as in this study. In addition, the animals were of Indian, Chinese, or mixed-breed origin.
A similar study was conducted by Abel et al.42 testing the effect of Depo-Provera treatment on SHIV89.6-immunized, SIVmac239-challenged macaques. The main result was that the rate of protection after intravaginal challenge with SIVmac239 was significantly lower (p < 0.05) and the acute postchallenge plasma viral RNA levels significantly higher (p < 0.006) in Depo-Provera-treated, SHIV89.6-immunized macaques than in Depo-Provera-naive, SHIV89.6-immunized macaques. Similar to the study performed by Genesca et al.,41 the experiment by Abel et al. was designed as a vaccine study using SHIV89.6 immunization and SIVmac239 challenge. In addition, the SIV challenge was performed intravaginally rather than intravenously as in the study presented here.
Trunova et al.43 followed the pathogenesis of Indian rhesus macaques with and without Depo-Provera treatment after intravaginal infection with a mixture of the pathogenic CXCR4 (X4)-SHIVSF33A and CCR5 (R5)-SHIVSF162P3 viruses. The authors observed a statistically significant higher mean peak as well as higher acute viral loads in animals treated with Depo-Provera (p < 0.05). In contrast, in the presented study, higher viral loads were also observed in the Depo-Provera-treated, intravenously SIV-infected Chinese rhesus macaques, but the difference to the untreated group was not statistically significant (p = 0.05). Overall, in all three previous publications,41–43 there were differences in the animal study design employing Depo-Provera and SIV/SHIV and, thus, it is impossible to compare the published studies to the study presented here.
In summary, the systemic effects of Depo-Provera administration on SIV disease progression were investigated in the Chinese rhesus macaque model. No statistically significant differences between the two groups (Depo-Provera-treated and SIV-infected and SIV-infected only groups) concerning viral loads, lymphocyte counts, naive/memory phenotype, and immune responses were found. In addition, no enhancement of SIV disease progression after a single injection of Depo-Provera was detected. Thus, if Depo-Provera is used for the facilitation of vaginal SIV infection, any observed effects are likely due to changes of the vaginal epithelium and not to a systemic pharmacological effect.
Acknowledgments
We would like to thank Drs. Preston Marx and Ron Veazey for guidance in the design of the study. Furthermore, we would like to thank Dr. Ron Desrosiers for providing the SIVmac251/CEMx174 challenge stock and Dr. David Montefiori for performing the neutralizing antibody assays (NIAID contract N01-AI-30034). The statistical analyses were performed by BioStat Solutions, Inc., Mount Airy, MD. This project has been funded with Federal funds from the NIAID, National Institutes of Health, under contract N01-AI-15451 (Project Officer Dr. Frosso Voulgaropoulou).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Fauci AS. 25 years of HIV/AIDS science: Reaching the poor with research advances. Cell. 2007;131:429–432. doi: 10.1016/j.cell.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Flores J. Seeking new pathways for HIV vaccine discovery. Future Microbiol. 2009;4:1–7. doi: 10.2217/17460913.4.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Turpin JA. Schito ML. Jenkins LM. Inman JK. Appella E. Topical microbicides: A promising approach for controlling the AIDS pandemic via retroviral zinc finger inhibitors. Adv Pharmacol. 2008;56:229–256. doi: 10.1016/S1054-3589(07)56008-4. [DOI] [PubMed] [Google Scholar]
- 4.Klasse PJ. Shattock R. Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med. 2008;59:455–471. doi: 10.1146/annurev.med.59.061206.112737. [DOI] [PubMed] [Google Scholar]
- 5.McGowan I. Microbicides: A new frontier in HIV prevention. Biologicals. 2006;34:241–255. doi: 10.1016/j.biologicals.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Denton PW. Garcia JV. Novel humanized murine models for HIV research. Curr HIV/AIDS Rep. 2009;6:13–19. doi: 10.1007/s11904-009-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Rompay KK. Antiretroviral drug studies in nonhuman primates: A valid animal model for innovative drug efficacy and pathogenesis experiments. AIDS Rev. 2005;7:67–83. [PubMed] [Google Scholar]
- 8.Catalone BJ. Kish-Catalone TM. Budgeon LR, et al. Mouse model of cervicovaginal toxicity and inflammation for preclinical evaluation of topical vaginal microbicides. Antimicrob Agents Chemother. 2004;48:1837–1847. doi: 10.1128/AAC.48.5.1837-1847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckstein P. Jackson MC. Millman N. Sobrero AJ. Comparison of vaginal tolerance tests of spermicidal preparations in rabbits and monkeys. J Reprod Fertil. 1969;20:85–93. doi: 10.1530/jrf.0.0200085. [DOI] [PubMed] [Google Scholar]
- 10.Galen BT. Martin AP. Hazrati E, et al. A comprehensive murine model to evaluate topical vaginal microbicides: Mucosal inflammation and susceptibility to genital herpes as surrogate markers of safety. J Infect Dis. 2007;195:1332–1339. doi: 10.1086/513279. [DOI] [PubMed] [Google Scholar]
- 11.Smith SM. Baskin GB. Marx PA. Estrogen protects against vaginal transmission of simian immunodeficiency virus. J Infect Dis. 2000;182:708–715. doi: 10.1086/315776. [DOI] [PubMed] [Google Scholar]
- 12.Smith SM. Mefford M. Sodora D. Klase Z. Singh M. Alexander N, et al. Topical estrogen protects against SIV vaginal transmission without evidence of systemic effect. AIDS. 2004;18:1637–1643. doi: 10.1097/01.aids.0000131393.76221.cc. [DOI] [PubMed] [Google Scholar]
- 13.Hild-Petito S. Veazey RS. Larner JM. Reel JR. Blye RP. Effects of two progestin-only contraceptives, Depo-Provera and Norplant-II, on the vaginal epithelium of rhesus monkeys. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S125–S130. [PubMed] [Google Scholar]
- 14.Sodora DL. Gettie A. Miller CJ. Marx PA. Vaginal transmission of SIV: Assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S119–S123. [PubMed] [Google Scholar]
- 15.Morrison CS. Commentary: Hormonal contraception and HIV acquisition—current evidence and ongoing research needs. Int J Epidemiol. 2007;36:175–177. doi: 10.1093/ije/dyl304. [DOI] [PubMed] [Google Scholar]
- 16.Ehring GR. Kerschbaum HH. Eder C, et al. A nongenomic mechanism for progesterone-mediated immunosuppression: Inhibition of K+ channels, Ca2+ signaling, and gene expression in T lymphocytes. J Exp Med. 1998;188:1593–1602. doi: 10.1084/jem.188.9.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patterson BK. Landay A. Andersson J, et al. Repertoire of chemokine receptor expression in the female genital tract: Implications for human immunodeficiency virus transmission. Am J Pathol. 1998;153:481–490. doi: 10.1016/S0002-9440(10)65591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prakash M. Kapembwa MS. Gotch F. Patterson S. Oral contraceptive use induces upregulation of the CCR5 chemokine receptor on CD4(+) T cells in the cervical epithelium of healthy women. J Reprod Immunol. 2002;54:117–131. doi: 10.1016/s0165-0378(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 19.Gillgrass AE. Ashkar AA. Rosenthal KL. Kaushic C. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J Virol. 2003;77:9845–9851. doi: 10.1128/JVI.77.18.9845-9851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaushic C. Ashkar AA. Reid LA. Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol. 2003;77:4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hintze J PASS 2008. NCSS,LLC.; Kaysville, Utah: 2008. [Google Scholar]
- 22.Graham SM. Holte S. Kimata JT. Wener MH. Overbaugh J. A decrease in albumin in early SIV infection is related to viral pathogenicity. AIDS Res Hum Retroviruses. 2009;25:433–440. doi: 10.1089/aid.2008.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezikyan S. Kaaya EE. Ekman M, et al. B-cell lymphomagenesis in SIV-immunosuppressed cynomolgus monkeys. Int J Cancer. 1995;61:574–579. doi: 10.1002/ijc.2910610423. [DOI] [PubMed] [Google Scholar]
- 24.Swingler S. Zhou J. Swingler C. Dauphin A. Greenough T. Jolicoeur P. Stevenson M. Evidence for a pathogenic determinant in HIV-1 Nef involved in B cell dysfunction in HIV/AIDS. Cell Host Microbe. 2008;4:63–76. doi: 10.1016/j.chom.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Maza O. Crabb E. Mitsuyasu RT. Fahey JL. Giorgi JV. Infection with the human immunodeficiency virus (HIV) is associated with an in vivo increase in B lymphocyte activation and immaturity. J Immunol. 1987;138:3720–3724. [PubMed] [Google Scholar]
- 26.Montefiori DC. Current Protocols in Immunology. 2004:12.11.1–12.11.17. doi: 10.1002/0471142735.im1211s64. [DOI] [PubMed] [Google Scholar]
- 27.Silvera P. Savary JR. Livingston V, et al. Vaccination with gp120-depleted HIV-1 plus immunostimulatory CpG oligodeoxynucleotides in incomplete Freund's adjuvant stimulates cellular and humoral immunity in rhesus macaques. Vaccine. 2004;23:827–839. doi: 10.1016/j.vaccine.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 28.Marx PA. Spira AI. Gettie A, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 29.Mascola JR. Stiegler G. VanCott TC. Katinger H. Carpenter CB. Hanson CE, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 30.Veazey RS. Ling B. Green LC, et al. Topically applied recombinant chemokine analogues fully protect macaques from vaginal simian-human immunodeficiency virus challenge. J Infect Dis. 2009;199:1525–1527. doi: 10.1086/598685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veazey RS. Ketas TA. Klasse PJ, et al. Tropism-independent protection of macaques against vaginal transmission of three SHIVs by the HIV-1 fusion inhibitor T-1249. Proc Natl Acad Sci USA. 2008;105:10531–10536. doi: 10.1073/pnas.0802666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pal R. Nuttall J. Galmin L. Weiss D. Chung HK. Romano J. Characterization of vaginal transmission of a simian human immunodeficiency virus (SHIV) encoding the reverse transcriptase gene from HIV-1 in Chinese rhesus macaques. Virology. 2009;386:102–108. doi: 10.1016/j.virol.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Turville SG. Aravantinou M. Miller T, et al. Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity. PLoS One. 2008;3:e3162. doi: 10.1371/journal.pone.0003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin HL., Jr. Nyange PM. Richardson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178:1053–1059. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]
- 35.Bulterys M. Chao A. Habimana P. Dushimimana A. Nawrocki P. Saah A. Incident HIV-1 infection in a cohort of young women in Butare, Rwanda. AIDS. 1994;8:1585–1591. doi: 10.1097/00002030-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Myer L. Denny L. Wright TC. Kuhn L. Prospective study of hormonal contraception and women's risk of HIV infection in South Africa. Int J Epidemiol. 2007;36:166–174. doi: 10.1093/ije/dyl251. [DOI] [PubMed] [Google Scholar]
- 37.Kiddugavu M. Makumbi F. Wawer MJ, et al. Hormonal contraceptive use and HIV-1 infection in a population-based cohort in Rakai, Uganda. AIDS. 2003;17:233–240. doi: 10.1097/00002030-200301240-00014. [DOI] [PubMed] [Google Scholar]
- 38.Mauck CK. Callahan MM. Baker J, et al. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999;60:15–24. doi: 10.1016/s0010-7824(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 39.Mattapallil JJ. Douek DC. Hill B. Nishimura Y. Martin M. Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 40.Veazey RS. Tham IC. Mansfield KG, et al. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: Highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Genesca M. Li J. Fritts L, et al. Depo-Provera abrogates attenuated lentivirus-induced protection in male rhesus macaques challenged intravenously with pathogenic SIVmac239. J Med Primatol. 2007;36:266–275. doi: 10.1111/j.1600-0684.2007.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abel K. Rourke T. Lu D. Bost K. McChesney MB. Miller CJ. Abrogation of attenuated lentivirus-induced protection in rhesus macaques by administration of Depo-Provera before intravaginal challenge with simian immunodeficiency virus mac239. J Infect Dis. 2004;190:1697–1705. doi: 10.1086/424600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trunova N. Tsai L. Tung S, et al. Progestin-based contraceptive suppresses cellular immune responses in SHIV-infected rhesus macaques. Virology. 2006;352:169–177. doi: 10.1016/j.virol.2006.04.004. [DOI] [PubMed] [Google Scholar]