Abstract
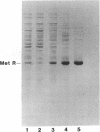
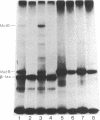
A plasmid (pRSE562) containing the metE and metR genes of Escherichia coli was used to study the expression of these genes and the role of the MetR protein in regulating metE expression. DNA sequence analysis of the 236-base-pair region separating these genes showed the presence of seven putative met boxes. When this plasmid was used to transform either wild-type E. coli, metE mutant, or metR mutant, MetE enzyme activity increased 5- to 7-fold over wild-type levels. The metR gene was subcloned from pRSE562, and this plasmid, pMRIII, relieved the methionine auxotrophy of a metR mutant after transformation. The metR gene was also cloned into a vector containing the lambda PL promoter, and the MetR protein was overexpressed and purified to near homogeneity. This protein, when added to an in vitro DNA-dependent protein synthesis system in which the MetE and/or MetR proteins were synthesized, caused a large increase in the expression of the metE gene but a decrease in the expression of the metR gene. The in vitro expression of both genes was inhibited by the MetJ protein and S-adenosylmethionine in the presence or absence of MetR protein. These results provide evidence that the product of the metR gene is a trans-activator of the expression of the metE gene and that the expression of the metR gene is under autogenous regulation and is repressed by the MetJ protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. Mechanism of repression of methionine biosynthesis in Escherichia coli. I. The role of methionine, s-adenosylmethionine, and methionyl-transfer ribonucleic acid in repression. Mol Gen Genet. 1973 Jul 16;123(4):299–324. doi: 10.1007/BF00433648. [DOI] [PubMed] [Google Scholar]
- Belfaiza J., Guillou Y., Margarita D., Perrin D., Saint Girons I. Operator-constitutive mutations of the Escherichia coli metF gene. J Bacteriol. 1987 Feb;169(2):670–674. doi: 10.1128/jb.169.2.670-674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfaiza J., Parsot C., Martel A., de la Tour C. B., Margarita D., Cohen G. N., Saint-Girons I. Evolution in biosynthetic pathways: two enzymes catalyzing consecutive steps in methionine biosynthesis originate from a common ancestor and possess a similar regulatory region. Proc Natl Acad Sci U S A. 1986 Feb;83(4):867–871. doi: 10.1073/pnas.83.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chu J., Shoeman R., Hart J., Coleman T., Mazaitis A., Kelker N., Brot N., Weissbach H. Cloning and expression of the metE gene in Escherichia coli. Arch Biochem Biophys. 1985 Jun;239(2):467–474. doi: 10.1016/0003-9861(85)90713-1. [DOI] [PubMed] [Google Scholar]
- Crowl R., Seamans C., Lomedico P., McAndrew S. Versatile expression vectors for high-level synthesis of cloned gene products in Escherichia coli. Gene. 1985;38(1-3):31–38. doi: 10.1016/0378-1119(85)90200-8. [DOI] [PubMed] [Google Scholar]
- Dawes J., Foster M. A. Vitamin B 12 and methionine synthesis in Escherichia coli. Biochim Biophys Acta. 1971 Jun 22;237(3):455–464. doi: 10.1016/0304-4165(71)90263-7. [DOI] [PubMed] [Google Scholar]
- Greene R. C., Williams R. D., Kung H. F., Spears C., Weissbach H. Effect of methionine and vitamin B-12 on the activities of methionine biosynthetic enzymes in metJ mutants of Escherichia coli K12. Arch Biochem Biophys. 1973 Sep;158(1):249–256. doi: 10.1016/0003-9861(73)90619-x. [DOI] [PubMed] [Google Scholar]
- KERESZTESY J. C., DONALDSON K. P. Synthetic prefolic A. Biochem Biophys Res Commun. 1961 Jul 26;5:286–288. doi: 10.1016/0006-291x(61)90164-4. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Spears C., Greene R. C., Weissbach H. Regulation of the terminal reactions in methionine biosynthesis by vitamin B 12 and methionine. Arch Biochem Biophys. 1972 May;150(1):23–31. doi: 10.1016/0003-9861(72)90005-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner L., Whitfield C., Weissbach H. Effect of L-methionine and vitamin B 12 on methionine biosynthesis in Escherichia coli. Arch Biochem Biophys. 1969 Sep;133(2):413–419. doi: 10.1016/0003-9861(69)90470-6. [DOI] [PubMed] [Google Scholar]
- Mulligan J. T., Margolin W., Krueger J. H., Walker G. C. Mutations affecting regulation of methionine biosynthetic genes isolated by use of met-lac fusions. J Bacteriol. 1982 Aug;151(2):609–619. doi: 10.1128/jb.151.2.609-619.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamann L. S., Stauffer G. V. Nucleotide sequence of the Salmonella typhimurium metR gene and the metR-metE control region. J Bacteriol. 1987 Sep;169(9):3932–3937. doi: 10.1128/jb.169.9.3932-3937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Girons I., Belfaiza J., Guillou Y., Perrin D., Guiso N., Bârzu O., Cohen G. N. Interactions of the Escherichia coli methionine repressor with the metF operator and with its corepressor, S-adenosylmethionine. J Biol Chem. 1986 Aug 15;261(23):10936–10940. [PubMed] [Google Scholar]
- Saint-Girons I., Duchange N., Cohen G. N., Zakin M. M. Structure and autoregulation of the metJ regulatory gene in Escherichia coli. J Biol Chem. 1984 Nov 25;259(22):14282–14285. [PubMed] [Google Scholar]
- Sanger F. Determination of nucleotide sequences in DNA. Science. 1981 Dec 11;214(4526):1205–1210. doi: 10.1126/science.7302589. [DOI] [PubMed] [Google Scholar]
- Schulte L. L., Stauffer L. T., Stauffer G. V. Cloning and characterization of the Salmonella typhimurium metE gene. J Bacteriol. 1984 Jun;158(3):928–933. doi: 10.1128/jb.158.3.928-933.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. K., Ehninger D. J. Methods for the analysis and preparation of adenosylmethionine and adenosylhomocysteine. Anal Biochem. 1966 May;15(2):323–333. doi: 10.1016/0003-2697(66)90038-8. [DOI] [PubMed] [Google Scholar]
- Shoeman R., Coleman T., Redfield B., Greene R. C., Smith A. A., Saint-Girons I., Brot N., Weissbach H. Regulation of methionine synthesis in Escherichia coli: effect of metJ gene product and S-adenosylmethionine on the in vitro expression of the metB, metL and metJ genes. Biochem Biophys Res Commun. 1985 Dec 17;133(2):731–739. doi: 10.1016/0006-291x(85)90965-9. [DOI] [PubMed] [Google Scholar]
- Shoeman R., Redfield B., Coleman T., Greene R. C., Smith A. A., Brot N., Weissbach H. Regulation of methionine synthesis in Escherichia coli: Effect of metJ gene product and S-adenosylmethionine on the expression of the metF gene. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3601–3605. doi: 10.1073/pnas.82.11.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. A., Greene R. C., Kirby T. W., Hindenach B. R. Isolation and characterization of the product of the methionine-regulatory gene metJ of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6104–6108. doi: 10.1073/pnas.82.18.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. Regulation of the metR gene of Salmonella typhimurium. J Bacteriol. 1987 Dec;169(12):5841–5844. doi: 10.1128/jb.169.12.5841-5844.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer L. T., Plamann L. S., Stauffer G. V. A new methionine locus, metR, that encodes a trans-acting protein required for activation of metE and metH in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1987 Apr;169(4):1391–1397. doi: 10.1128/jb.169.4.1391-1397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C. D., Weissbach H. Binding of the folate substrate to 5-methyltetrahydropteroyltriglutamate-homocysteine transmethylase. J Biol Chem. 1970 Jan 25;245(2):402–409. [PubMed] [Google Scholar]