Abstract
H2O2-producing commensal lactobacilli inhibit N. gonorrhoeae in vitro and clinical data suggest they are associated with reduced risk of gonorrhea. Here we pre-colonized mice with Lactobacillus crispatus and then challenged them with N. gonorrhoeae to measure the effects of H2O2-producing lactobacilli on gonococcal infection. We found no difference in the duration of infection or number of gonococci recovered from untreated mice and mice colonized with L. crispatus. A gonococcal catalase mutant and a catalase, cytochrome C peroxidase mutant exhibited greater susceptibility to L. crispatus in vitro than wild type bacteria; however, recovery of these mutants from mice was not affected by L. crispatus. We also found no evidence that utilization of lactobacillus-produced lactate by N. gonorrhoeae balances the detrimental effects of H2O2 during infection. We conclude the relationship between lactobacilli and gonococci is complex and may be subject to factors that have not been reproduced in vitro.
Keywords: Neisseria gonorrhoeae, lactobacilli, H2O2, catalase, cytochrome C peroxidase, lactate, mouse
INTRODUCTION
Neisseria gonorrhoeae is a sexually transmitted pathogen that has impressively evaded human eradication for thousands of years. Gonorrhea has maintained its ranking as the second most commonly reported infectious disease in the United States for decades [1] with an estimated 62 million annual cases worldwide [2]. N. gonorrhoeae causes significant morbidity and mortality particularly in women, with estimated annual healthcare costs in excess of one billion dollars [3]. Gonorrhea is also a risk factor for human immunodeficiency virus (HIV) [4–6], and the alarming emergence of antibiotic resistant strains threatens current control measures. For these reasons, aggressive attempts to develop a vaccine and other prophylactic strategies remain active.
There has been much interest in the prophylactic potential of vaginal Lactobacillus spp. against sexually transmitted pathogens [7–10] and in preventing or eradicating bacterial vaginosis [11, 12]. Lactobacilli are the most populous, and arguably most important, of the indigenous vaginal flora of healthy pre-menopausal females [13–16]. Several clinical studies have reported an inverse relationship between gonorrhea and vaginal colonization by lactobacilli [7, 17–19] or H2O2-producing Lactobacillus strains in particular [11, 12]. The hypothesis that H2O2-producing lactobacilli are an innate defense against N. gonorrhoeae is consistent with reports that H2O2-producing Lactobacillus sp. commonly isolated from the vagina and cervix inhibit gonococci in vitro [11, 20]. Lactobacillus-mediated inhibition of N. gonorrhoeae can be neutralized with exogenous catalase, which implicates H2O2 as the significant inhibitory factor [20, 21]; acidity, proteases, and the production of a bacteriocin-like compound may also participate in the inhibition [21].
The role of lactobacilli in defending against gonococcal infection warrants further testing based on the likelihood that interactions between gonococci and lactobacilli are influenced by physiological factors such as oxygen tension, pH, iron, glucose, and lactate. Additionally, the gonococcus produces a battery of anti-oxidant factors including catalase [22, 23] and cytochrome C peroxidase (Ccp) [24] that are regulated by environmental stimuli [24]. Therefore, to investigate the effect of commensal lactobacilli on N. gonorrhoeae in the context of a physiologically relevant system, we developed a mouse model to test the effect of pre-colonization with L. crispatus on gonococcal colonization. We also tested gonococcal mutants that lacked catalase, Ccp, and, or the capacity to uptake lactate to better define the interactions between N. gonorrhoeae and L. crispatus during infection.
METHODS
Bacterial strains and culture conditions
The bacterial strains utilized in this study are described in Table 1. The FA1090 kat,ccp (GP507) and kat,lctP mutants (GP508) were constructed in the FA1090 kat mutant GP500 by transformation of insertionally inactivated ccp and lctP alleles from strains GP302 (H. Wu, A.A. Soler-Garcia, AE. Jerse, submitted) and GP900 [25]. Transformants were selected on GC agar with 10 μg/ml Cm. All mutations were confirmed by PCR and sequence analysis. Streptomycin resistant (SmR) mutants of L. crispatus strain ATCC 33197 and L jensenii strain ATCC 25258 were used for all in vitro and in vivo studies, and were obtained by serial passage of early log cultures in Lactobacillus Mannose-Rogosa-Sharpe (L-MRS) broth with increasing concentrations of Sm (10–50 μg/ml). All lactobacillus strains were resistant to >400 μg/ml trimethoprim. Gonococci were cultured on supplemented GC agar or GC agar with vancomycin, colistin, nystatin, trimethoprim sulfate and streptomycin sulfate (GC-VCNTS) as described [26]. Lactobacilli were cultured on L-MRS agar or in L-MRS broth at 37°C in 5% CO2. All media were from Difco.
Table 1.
Bacterial strains used in this study
| Strain | Description | Reference |
|---|---|---|
| N. gonorrhoeae FA1090 | Wild type strain, SmR | [45] |
| N. gonorrhoeae GP500 | FA1090 with a kat47–1384::aphA3 mutation | [23] |
| N. gonorrhoeae GP506 | GP500 with an intact kat gene expressed from an ectopic chromosomal site | (H.Wu, AA.Soler- Garcia and AE Jerse, submitted). |
| N. gonorrhoeae GP507 | GP500 with a ccp::CmR mutation | This study |
| N. gonorrhoeae GP 508 | GP500 with an lctP::CmR mutation | This study |
| L. crispatus ATCC 33197 | H2O2-producing; human urine isolate | [20] |
| L. jensenii ATCC 25258 | H2O2-producing; human vaginal isolate | [20] |
| L. murinus 10A | Non-H2O2-producing; murine vaginal isolate; SmR | [27] |
Agar overlay inhibition assay
Lactobacillus inhibition of N. gonorrhoeae was measured using an agar overlay assay as described [20]. In some experiments, NaNO2 (2mM) was included in the overlay to allow anaerobic growth of N. gonorrhoeae, and the second incubation was performed in an anaerobic jar.
Animal studies
To promote long-term colonization by N. gonorrhoeae, female BALB/c mice (National Cancer Institute) (6–8 weeks old, not specific pathogen-free) were implanted with 21 day slow-release 17-β estradiol pellets (5 mg), treated with streptomycin and trimethoprim sulfate [27], and housed in autoclaved cages with autoclaved water, litter, and food as described [28]. For pilot studies with human Lactobacillus sp., mice were inoculated intravaginally with 107 or 109 cfu of L. crispatus, L. jensenii, or L. murinus suspended in 20 μl of 28 mM 2[N-Morpholino] ethanesulfonic acid (MES) buffer (pH 6.7) (Sigma) or MES buffer alone two days after estradiol treatment. Inocula were prepared from mid-logarithmic phase L-MRS broth cultures (OD600 0.7–1.0) incubated at 37°C in 5% CO2; bacteria were washed twice with sterile 28 mM MES, and resuspended in MES to the desired concentration. For in vivo challenge studies, estradiol-treated mice were inoculated with 5 × 106 or 107 cfu of L. crispatus (test mice) or MES buffer alone (control mice) as above with the modification that L. crispatus was harvested directly from L-MRS agar plates after 18–22 hrs of growth and washed twice with sterile MES buffer before inoculation. Test and control mice were challenged three to four hours later with 20 μl of wild type or mutant gonococci (4 × 105 or 1 × 106 cfu) prepared in PBS as described [28]. Test mice received a booster inoculum containing106 cfu of L. crispatus on days four or five post-inoculation. All bacterial doses were confirmed by quantitative culture. Recovery of N. gonorrhoeae or Lactobacillus sp. from vaginal mucus was measured by quantitative culture on GC-VCNTS and L-MRS agar, respectively. Vaginal mucus was collected with a Dacron swab and suspended in 100 μl of saline for 14 days (pilot studies) or every even-numbered day for 10–12 days (challenge studies). Stained vaginal smears were examined for polymorphonuclear leukocyte (PMNs) as described [29]. In pilot studies, the average number of lactobacilli per epithelial cell was determined after viewing 100 consecutive epithelial cells under a light microscope. All animal experiments were conducted in the laboratory animal facility at the Uniformed Services University, which is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care under a protocol approved by the University's Institutional Animal Care and Use Committee.
Identification of L. crispatus
L. crispatus was identified among vaginal isolates by colony morphology and H2O2 phenotype as described [30]. The identity of two representative colonies on primary culture plates from each mouse was confirmed by PCR analysis using the method of Song, et al [31]. The specificity of the PCR reaction was confirmed by testing our laboratory collection of six lactobacillus strains that represent four Lactobacillus sp. and two different strains of L. murinus.
pH determination and lactate concentration
Vaginal mucosal pH was measured in duplicate for each mouse one hr prior to culture using a 16-gauge MI-414 combination pH microelectrode (Microelectrode, Inc). Care was taken to minimize disruption of the mucosa during insertion. Lactate concentrations were measured in pooled vaginal swab suspensions from mice within the same experimental groups using Sigma Diagnostics procedure 826-UV. The pH and lactate concentration of supernatants from L-MRS broth cultures of L. crispatus, L. jensenii, and L. murinus were measured similarly.
Statistical analysis
Differences in the in vitro sensitivity of gonococci to H2O2-producing lactobacilli and in the average duration of colonization by N. gonorrhoeae or L. crispatus were analyzed using an unpaired t test. The average number of gonococci or lactobacilli recovered from test and control mice was compared using a repeated measures analysis of variance (ANOVA). p values <0.05 were considered significant.
RESULTS
Catalase and Ccp protect against H2O2-producing lactobacilli in vitro
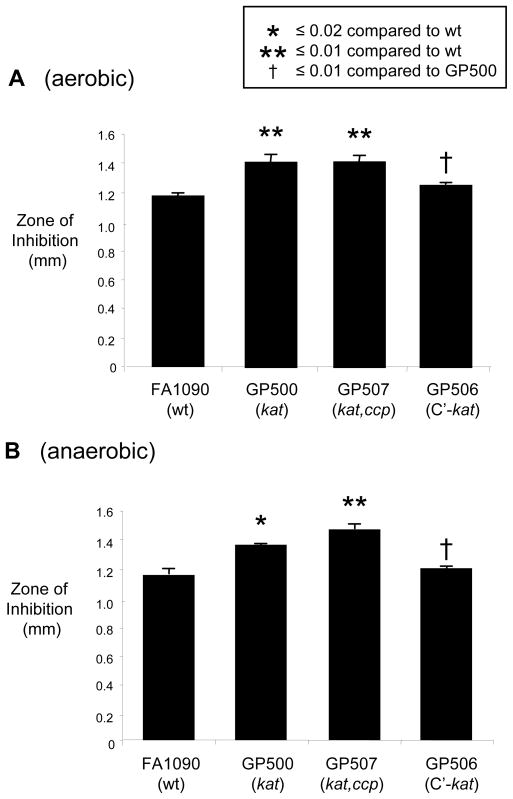
We previously reported that N. gonorrhoeae was susceptible to H2O2-producing L. crispatus strain ATCC 33197 and that bovine catalase neutralized the inhibition [20]. To determine if gonococcal catalase partially protected N. gonorrhoeae from H2O2-producing lactobacilli, here we tested the susceptibility of a genetically defined kat mutant (GP500) to L. crispatus. Mutant GP500 was more sensitive to L. crispatus than the parent strain, and resistance could be restored to wild type levels via genetic complementation with an intact kat gene (strain GP506) (Fig. 1A). Inhibition of wild type bacteria by L. crispatus was neutralized by incorporating bovine catalase (5 U/ml) into the overlay assay; ten-fold more catalase was required to neutralize the inhibition of catalase-deficient gonococci (data not shown). Ccp also protects gonococci from H2O2 in vitro [32]. We therefore tested a kat,ccp mutant to determine if the absence of Ccp further reduces resistance to H2O2-producing lactobacilli. Ccp is produced under anaerobic conditions, and as expected, there was no difference in the size of the inhibition zones around the kat mutant GP500 compared to kat,ccp double mutant GP507 when tested under aerobic conditions. However, when the overlay culture was incubated anaerobically, the zones of inhibition around the kat,ccp mutant and wild type strain showed a significantly greater difference (p 0.005) than the difference between the kat mutant and wild type strain (p 0.015). L. murinus strain 10A, which does not produce H2O2, did not inhibit wild type or kat mutant gonococci (data not shown). We conclude that both catalase and Ccp contribute detectable levels of protection to N. gonorrhoeae under oxygen-limited conditions. Based on the relatively low oxygen tension of the female lower genital tract [33] we hypothesized both these factors may defend against H2O2-producing lactobacilli during infection.
Figure 1. Catalase and Ccp-deficient mutants are more sensitive to H2O2-producing lactobacilli.
An agar overlay assay [20] was utilized to measure the inhibition of wild type strain FA1090, kat mutant GP500, and the kat,ccp double mutant GP507 under A) aerobic and B) anaerobic conditions. Briefly, lactobacilli [ca. 7 × 105 colony forming units (cfu)] were cultured on heart infusion agar for 20–24 hrs. Supplemented GC agar with or without 2 mM NaNO2 (for anaerobic conditions) was pipetted over the surface of the plates, allowed to solidify, and 100 μl of a saline suspension containing 106 cfu of the gonococcal strain to be tested were spread onto the overlay. Plates were incubated at 37°C in 7% CO2 (aerobic conditions) or in an anaerobic jar for 20–24 hrs. The diameters of the lactobacillus growth and inhibition zone were measured, and a ratio of the two diameters was calculated to allow for standardization despite differing diameters of lactobacillus growth. The average diameters of the normalized inhibition zones were calculated from 3–4 plates and are shown with standard error bars.
Vaginal colonization of mice with human lactobacillus strains
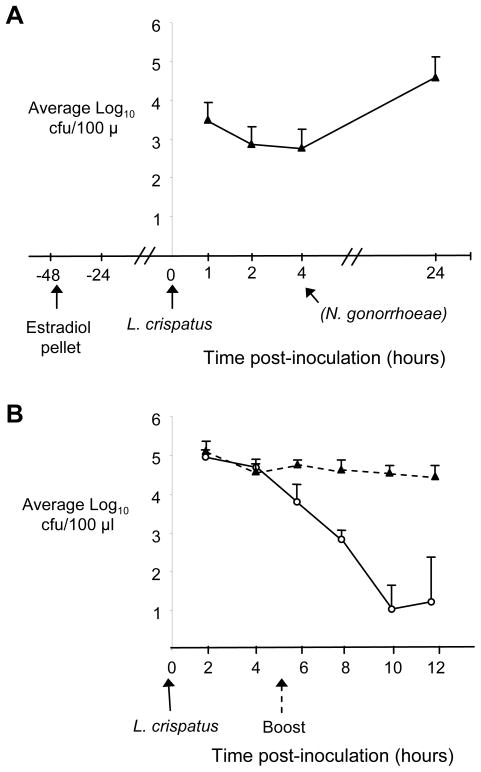
We next performed pilot studies to test the capacity of L. crispatus and L. jensenii to colonize female mice. We were unable to colonize BALB/c mice in the different stages of the estrous cycle for longer than 2–3 days. Therefore, we tested estradiol-treated mice using a protocol similar to that used to promote susceptibility to N. gonorrhoeae and found mice were susceptible to long-term colonization by human lactobacillus strains only when inoculated 2 days after estradiol treatment and not at earlier time points. Intravaginal inoculation of estradiol-treated mice with 107 cfu of L. murinus, L. crispatus, or L. jensenii resulted in average durations of colonization of 12.2, 8.2, and 9.8 days, respectively with no statistical difference between strains. The average number of lactobacilli recovered was also similar for each strain. In a separate group of mice, we showed the recovery of L. crispatus was stable when tested between 1 and 4 hrs post-inoculation (Fig. 2A), and therefore, chose 4 hrs after inoculation as the time point at which we would challenge with N. gonorrhoeae since mice are significantly less susceptible to N. gonorrhoeae 3 days after estradiol pellet implantation (A.E. Jerse, unpublished observation). In general, after five days, a decrease in colonization load occurred in a majority of mice. Therefore, in subsequent challenge studies, a lactobacillus “booster” dose was given at day 5 to sustain high levels of colonization during the in vivo challenge experiments (Fig. 2B). Occasional mice were colonized by both human strains and their own commensal lactobacilli, the latter of which did not produce H2O2. Bacteria with a morphology similar to that of L. crispatus and L. jensenii, which are longer and thinner than L. murinus, were observed adherent to squamous and nucleated epithelial cells in mice inoculated with the human strains but not in mice inoculated L. murinus or buffer. The average number of lactobacilli adherent to each epithelial cell was between 1.49–1.93, with no significant difference detected between the three strains. There was no influx of vaginal PMNs in response to lactobacillus colonization.
Figure 2. Kinetics of L. crispatus colonization of estradiol-treated mice.
Female BALB/c mice were inoculated with 1 × 107 cfu of L. crispatus 44 hrs after implantation of a 5 mg slow-release estradiol pellet and vaginal mucus was quantitatively cultured for L. crispatus over time. A) The average number of L. crispatus recovered at 1, 2, 4, and 24 hrs post-inoculation from vaginal swab suspensions is shown (n = 5 mice/group). The time point (4 hrs) at which N. gonorrhoeae was inoculated in the challenge experiments is indicated, although no N. gonorrhoeae was used here. This time point corresponds to a period of stable recovery; the number of L. crispatus recovered 20 hrs later was 1.5 logs higher, however estradiol-treated mice not highly susceptible to N. gonorrhoeae at this time point. B) Recovery of L. crispatus from mice given 107 cfu of L. crispatus with (solid triangles, dashed line) and without (open circles, solid line) a booster dose of 106 cfu on day 5 post-inoculation (n = 8 mice/group).
In humans, lactobacilli are the main factor that contributes to a low vaginal pH [34], and therefore, we measured the effect of Lactobacillus colonization on murine vaginal pH. Inoculation of mice with 107 cfu of the human lactobacillus strains did not result in a decreased vaginal pH compared to that of estradiol-treated mice inoculated with buffer. To try and increase the colonization load, we also inoculated groups of estradiol-treated mice with 109 cfu of L. murinus, L. crispatus, or L. jensenii. A fourth group received MES buffer (estradiol-treated control) and a fifth group received no estrogen, antibiotics, or bacteria (untreated control). The average recovery of lactobacilli from each test group was not statistically higher than that recovered when a dose of 107 was used, and ranged from 104 to >107 cfu/100 μl vaginal swab suspension through day five (Fig. 3A). Although not statistically significant, the estradiol-treated control group had the lowest average vaginal pH on days 1 and 3 after inoculation. Test groups had a lower pH than the untreated control group (Fig. 3B) and pooled vaginal washes from all groups contained increasing levels of lactate over time (Fig. 3C). Multiple assessments of the data showed no clear association with number of lactobacilli recovered, vaginal pH, or lactate concentration. In comparison, we observed a direct relationship between the number of lactobacilli, pH, and lactate concentration when the three strains of lactobacilli were cultured in L-MRS broth (Fig. 3D-F), and the drop in pH was paralleled by an increase in lactate. The concentration of bacteria in the broth cultures was 10–100 times higher [108 cfu/100 μl (log phase) and 1010 cfu/100 μl (stationary phase)] than that recovered from mice. We conclude vaginal pH is influenced by estradiol and murine vaginal pH and lactate levels are not directly related to the number of lactobacilli, at least not at the colonization levels we achieved.
Figure 3. Relationship between the number of lactobacilli, pH, and lactate in vitro and in vivo.
Female BALB/c mice were treated with estradiol and antibiotics and inoculated with MES buffer (estradiol-treated control), or 109 cfu of L. crispatus, L. jensenii, or L. murinus (n = 5 mice) as described in the Methods. A fifth group of mice in the diestrus stage of the estrous cycle were left untreated (n = 3) (untreated control). A) Average log10 cfu recovered of each strain. A decrease in the average number of lactobacilli recovered occurred on day 7 for all species, including the murine commensal L. murinus. For these reasons, a booster dose of L. crispatus (106 cfu) was given on day for subsequent in vivo challenge experiments. B) Average vaginal pH on days 1, 3, 5, 7, and 9 post-inoculation with bacteria or buffer. C) Concentration of lactate in pooled vaginal washes from mice in each group at the same time points. The day 7 vaginal wash sample was not measured for the L. crispatus or untreated control groups. Standard error bars are shown. For in vitro conditions, L. crispatus, L. jensenii, and L. murinus were inoculated into 100 ml of L-MRS broth (starting pH 5.6), and 25, 3 ml aliquots of each suspension were prepared and incubated in a CO2 incubator at 37°C. One tube was removed at the time points indicated and the D) optical density at 600 nm (A600), E) culture pH, and F) lactate concentration were determined as described in the Methods. The highest number of viable lactobacilli for each strain (1011 CFU/ml) was recovered at the 11 hr time point.
In vivo protection studies
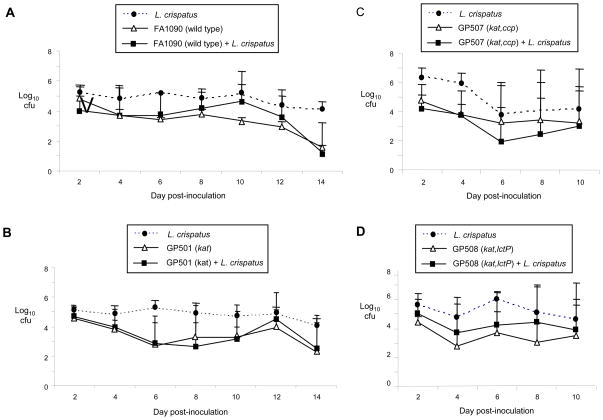
After characterizing the vaginal colonization of mice with human lactobacillus strains, we next examined the effect of H2O2-producing L. crispatus on experimental gonococcal infection. Mice were inoculated with 5 × 106 cfu of L. crispatus (test group) or buffer (control group). Four hours later, all mice were challenged with either wild type N. gonorrhoeae strain FA1090 or the catalase-deficient strain GP500 at a dose known to infect ca. 80% of mice. Mice in all four groups were colonized with N. gonorrhoeae for an average of 12.4 days (range 2–14 days), and there was no difference in the number of wild type or catalase-deficient gonococci recovered from test versus control groups over time (Fig. 4A and 4B). A repeat experiment with a slightly higher dose of L. crispatus (107 cfu) also showed no significant difference in gonococcal colonization between any of the groups. In both experiments, all test mice were colonized with high numbers of L. crispatus (average, 104–105 cfu/100 μl suspension) (Fig. 4A and 4B; dashed lines), and the identity of the isolates as L. crispatus was confirmed by PCR (Fig. 5A). L. crispatus isolates retained the H2O2-positive phenotype over the course of the experiment, and both lactobacilli and gonococci were associated with epithelial cells in stained vaginal smears. Gonococci were associated with PMNs in mice with a PMN influx (Fig. 5B).
Figure 4. L. crispatus does not inhibit N. gonorrhoeae in vivo.
Mice were inoculated with 5 × 106 cfu of L. crispatus (test group) or the suspension buffer (control group) and challenged 4 hours later with A) wild type N. gonorrhoeae strain FA1090 (1.8 × 106 cfu); B) kat mutant GP500 (4.2 × 105 cfu); C) kat,ccp mutant GP507 (1.0 × 106 cfu); D) kat,lctP mutant GP508 (1.2 × 106 cfu) (n = 7–8 mice per group). Results are shown as average number of N. gonorrhoeae cfu isolated from test and control groups (solid lines) or L. crispatus from test groups only (dashed lines) recovered per 100 μl vaginal swab suspension over time. Standard error bars are shown. The limit of detection was 4 cfu. There was no statistical difference in the recovery of wild type N. gonorrhoeae or any of the mutant strains from mice that were or were not colonized with L. crispatus.
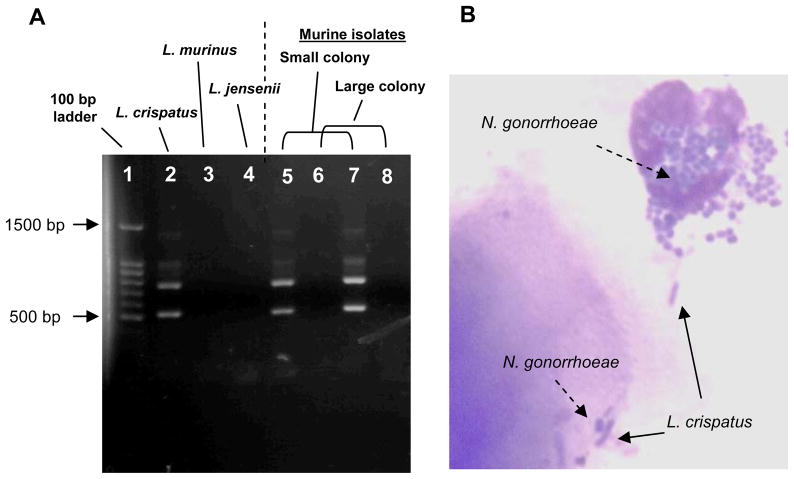
Figure 5. Confirmation of L. crispatus colonization.
L. crispatus produced distinct colony morphology and H2O2 compared to native murine lactobacilli, which produced larger colonies on L-MRS agar and were uniformly H2O2-negative. PCR analysis was used to confirm the identity of putative L. crispatus colonies in mixed cultures using primers Lcri-3 and Lcri-2, which correspond to distinct 16S–23S rRNA intergenic spacer regions in L. crispatus [31]. PCR reaction conditions consisted of 3.0 μl of colony lysate supernatant, Taq DNA polymerase, reaction buffer (Promega Corporation), 200 μM each of dATP, dCTP, dGTP, and dTTP and 5 μl of 10-pmol primer mix that contained each primer described in the Methods (final volume 60 μl). Reaction conditions were 20 sec at 95°C, 2 min at 55°C, and 5 min at 74° C for 35 cycles followed by a final 5 min extension step at 74°C. A) PCR analysis of colony lysates for small (L. crispatus-like) and large colonies (probable L. murinus) from test mice shows the presence of the 522 bp PCR product. A second product of ca. 770 bp was also consistently amplified from L. crispatus but no other human or murine lactobacillus strains. The identity of this product is not known. B) Stained vaginal smear from a mouse colonized with L. crispatus and also infected with wild type N. gonorrhoeae. The sample was taken 8 days after intra-vaginal inoculation with bacteria and shows lactobacilli adherent to epithelial cells (solid arrows) and a PMN with intracellular gonococci (arrow with broken line).
To test whether Ccp can protect the kat mutant from L. crispatus in vivo, we performed similar experiments with the kat,ccp double mutant GP507. Again there was no significant difference in the duration of recovery or colonization load of GP507 bacteria in mice colonized with L. crispatus versus control mice (Fig. 4C). Based on the importance of lactate in neisserial pathogenesis [35] [25], we hypothesized that H2O2-mediated inhibition of N. gonorrhoeae may be balanced by the advantage conferred to N. gonorrhoeae by lactobacillus-produced lactate. To test this hypothesis, we tested a kat mutant that was also unable to utilize lactate due to a mutation in the lctP gene. Recovery of this strain, GP508, from test versus control mice was not significantly different (Fig. 4D). We conclude L. crispatus does not challenge N. gonorrhoeae in this surrogate model of gonococcal genital tract infection, and gonococcal resistance to L. crispatus in vivo is not due to the production of two well-characterized anti-oxidant factors or the beneficial effects of lactate utilization.
DISCUSSION
Several clinical studies have shown inverse relationships between gonorrhea and vaginal lactobacilli [8, 10–12, 18, 19]. Among these reports, only one showed a trend towards significance colonization with H2O2-producing lactobacilli and increased risk of gonorrhea [12]. Two other reports found an inverse correlation between gonorrhea and vaginal lactobacilli, but the lactobacilli were not analyzed for H2O2 production [8, 10, 19] or an inverse relationship was identified regardless if lactobacilli produced H2O2 or not [11, 18]. Here we confirmed that lactobacillus-produced H2O2 inhibits gonococci in vitro and we also showed that gonococcal catalase and Ccp increase resistance to this inhibition. Interestingly, however, we did not observe the same relationship between L. crispatus and N. gonorrhoeae in vivo. It is possible that the reduced oxygen tension of the female genital tract may limit the amount of H2O2 produced by lactobacilli to subinhibitory levels. Alternatively, several other factors protect N. gonorrhoeae from H2O2 in vitro, one or more of which might be protective against lactobacillus-produced H2O2 in vivo [36] [37] [38] [39] [10], including and newly described peroxidase-induced genes [40]. Whether mutants in one or more of these additional factors would be less able to co-exist with L. crispatus in the murine genital tract is not known. We hypothesize biofilm formation by N. gonorrhoeae [41], which could be mixed with lactobacilli, may also protect against lactobacillus-produced H2O2 later in infection.
While there are many similarities between the murine and human vaginal tracts, the difference in vaginal pH should be considered with respect to our findings. We did not find a significant association between vaginal pH and numbers of lactobacilli, and we could not achieve murine vaginal acidity to that of normal humans. Acidity stabilizes H2O2 and may increase the production of bacteriocin-like products [21], L. crispatus is more inhibitory to N. gonorrhoeae when tested at pH 5.8 versus neutral pH [20]. Therefore, lactobacilli may be less toxic in the less acidic pH of murine vagina. We note, however, that H2O2-producing lactobacilli are also isolated from the human cervix, the primary site of infection in women of reproductive age, and human cervical pH is similar to that of mice [human cervical mucus: average pH 6.8, range 5.5–8.0 (proliferative stage); average 6.1, range 5.1–8.4 (secretory stage) [42]]. We therefore do not believe a less acidic pH entirely accounts for our findings. Another limitation in our study was being restricted to challenging the mice the same day they were inoculated with L. crispatus. It is conceivable that allowing L. crispatus to colonize and replicate overnight (as shown in Fig. 2A) may have posed a greater challenge to N. gonorrhoeae, although vaginal pH did reflect this higher colonization load, even in mice given a booster dose of L. crispatus (data not shown). Another difference between women and female mice is the low to absent numbers of anaerobes in the murine vagina [43]. This difference combined with the use of antibiotics in the mouse model may eliminate potential contributions of other flora to lactobacillus-mediated inhibition of N. gonorrhoeae, or interfere with the results if lactobacilli are a marker for some unidentified organism or substance that is absent in female mice. Finally, commensal lactobacilli in the control groups to which all data were compared may have also affected our data, although we have never isolated H2O2-producing lactobacilli from mice and therefore believe this system allows one to test whether lactobacillus-produced H2O2 challenges N. gonorrhoeae during infection. Examination of the role of lactobacillus-produced bacteriocins and competition for adherence receptors in challenging gonococci in vivo may require the use of germ-free mice since L. murinus may also have these properties.
N. gonorrhoeae has no animal or environmental reservoir, and thus has evolved many sophisticated adaptation mechanisms to ensure its survival on human mucosal surfaces. We are therefore intrigued with the hypothesis that lactobacilli may produce factors that enhance gonococcal growth or survival, and that the balance between hostile and supportive factors allows gonococci to co-exist with H2O2-producing lactobacilli in vivo. We have repeatedly observed over the years that mice with high numbers of L. murinus are often heavily colonized with N. gonorrhoeae compared to mice with undetectable levels of L. murinus [27]. Of particular interest was the possibility that lactobacillus-produced lactate may enhance growth or survival of N. gonorrhoeae during infection. The gonococcus has a preference for lactate, the primary metabolite of lactobacillus fermentation, which leads to changes in its metabolic pathways [44], and the inability to utilize lactate during infection confers a survival or growth disadvantage to gonococci in the mouse model [25]. We did not find evidence, however, that lactate utilization tipped the balance in favor of the gonococcus when L. crispatus was present, even when the a catalase-deficient challenge strain was used. We previously demonstrated that L. murinus enables N. gonorrhoeae strain FA1090 to grow on solid agar plates that otherwise do not support its growth [27]. Unfortunately these results were dependant on the specific lot of media used and we have not been able to find a defined liquid media that is suitable for these studies. Other lactobacillus-produced factors that may play a role in growth enhancement or be detrimental to N. gonorrhoeae are described in Fig. 6.
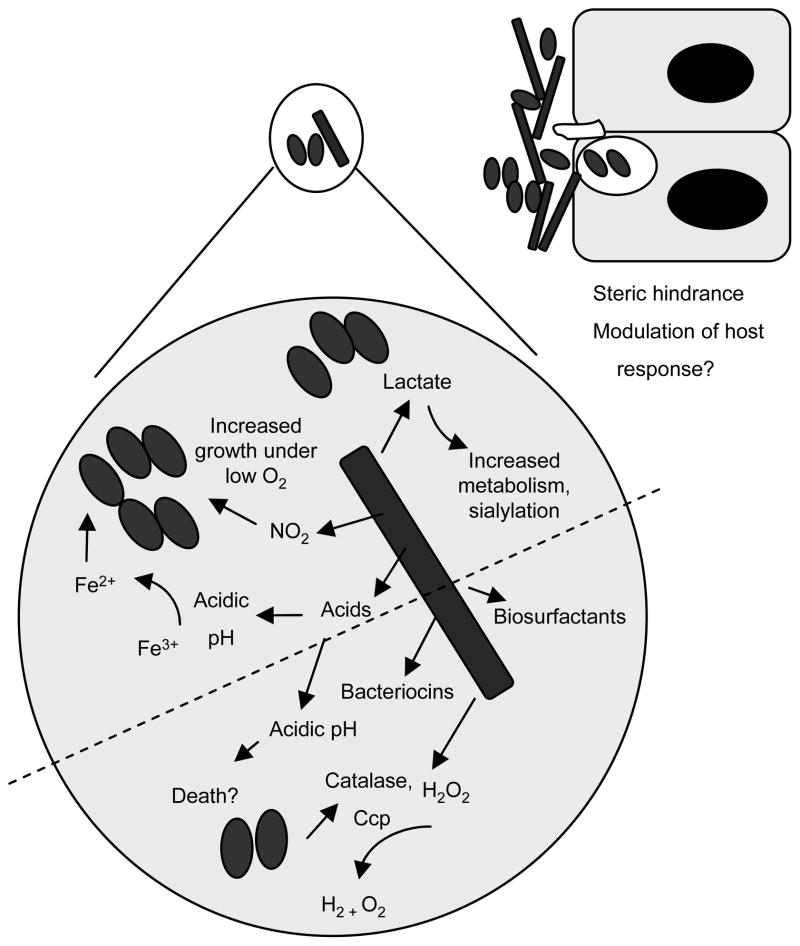
Figure 6. Supportive and inhibitory interactions between N. gonorrhoeae and H2O2-producing lactobacilli.
The microenvironment of the female lower genital tract is a complex milieu that is affected by the metabolic by-products of commensal flora. Commensal flora may also form part of the innate defense via the production of bacteriocins and competition for nutrients and colonization receptors. Potential inhibitory factors that are produced by lactobacilli, the predominant flora of the healthy female genital tract, are shown below the dotted line. Factors that may reduce N. gonorrhoeae colonization include biosurfactants, steric hindrance, direct toxicity through the action of H2O2, bacteriocins, and low pH. These inhibitory aspects of lactobacilli may be balanced by one or more factors that could support gonococcal growth or survival, which are illustrated below the dotted line. Positive factors include the production of lactate, which in the presence of glucose, increases gonococcal metabolism [44] and resistance to the bactericidal activity of normal human serum [25, 35], nitrite, which the gonococcus can use as an electron acceptor for anaerobic growth [46], and increased iron solubility via a reduction in pH. Lactobacilli may also play an immunoprotective role via modulation of the host response [47].
In summary, the continued prevalence of gonorrhea and the recent increase in incidence [1] speak to the vital need for continued diligent efforts to understand gonococcal adaptation mechanisms and develop innovative and effective prophylactic measures. Our results emphasize the need to further investigate the association between H2O2-producing lactobacilli and the risk of gonorrhea. The differences between our in vitro and in vivo results suggest a complex relationship exists between lactobacilli and N. gonorrhoeae in vivo, the intricacies of which may perhaps be dissected through the testing of genetic mutants in the murine challenge model.
Acknowledgments
We thank Dr. Cara Olsen for assistance with statistical analyses and Lotisha Garvin for her excellent assistance with manuscript preparation.
Financial support was through NIH grant R01-AI42053 (to A.E. Jerse).
Footnotes
None of the authors have a conflict of interest.
Portions of this work were presented at the 13th International Pathogenic Neisseria Conference in Oslo, Norway, September, 2002.
References
- 1.Services DoHaH. Gonococcal Isolate Surveillance Project (GISP) Annual Report 2005. Atlanta: Centers for Disease Control and Prevention, Division of STD Prevention; 2005. [Google Scholar]
- 2.Organization WH. Global Prevalence and Incidence of selected curable Sexually Transmitted Infections Overview and Estimates. Geneva: World Health Organization; 2001. pp. 15–20. [Google Scholar]
- 3.JE S. The economic burden of sexually transmitted diseases in the United States. In: Holmes K, Sparling PF, Mardh P-A, editors. Sexually transmitted diseases. 3. New York (NY): McGraw-Hill; 1999. pp. 1367–79. [Google Scholar]
- 4.McClelland RS, Lavreys L, Katingima C, et al. Contribution of HIV-1 infection to acquisition of sexually transmitted disease: a 10-year prospective study. J Infect Dis. 2005;191:333–8. doi: 10.1086/427262. [DOI] [PubMed] [Google Scholar]
- 5.Dunkle KL, Beksinska ME, Rees VH, Ballard RC, Htun Y, Wilson ML. Risk factors for HIV infection among sex workers in Johannesburg, South Africa. Int J STD AIDS. 2005;16:256–61. doi: 10.1258/0956462053420220. [DOI] [PubMed] [Google Scholar]
- 6.Scott KC, Philip S, Ahrens K, Kent CK, Klausner JD. High Prevalence of Gonococcal and Chlamydial Infection in Men Who Have Sex With Men With Newly Diagnosed HIV Infection: An Opportunity for Same-Day Presumptive Treatment. J Acquir Immune Defic Syndr. 2008 doi: 10.1097/QAI.0b013e318165dc0b. [DOI] [PubMed] [Google Scholar]
- 7.Plante M, Cadieux N, Rioux CR, Hamel J, Brodeur BR, Martin D. Antigenic and molecular conservation of the gonococcal NspA protein. Infect Immun. 1999;67:2855–61. doi: 10.1128/iai.67.6.2855-2861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newton ER, Piper JM, Shain RN, Perdue ST, Peairs W. Predictors of the vaginal microflora. Am J Obstet Gynecol. 2001;184:845–53. doi: 10.1067/mob.2001.113848. discussion 853–5. [DOI] [PubMed] [Google Scholar]
- 9.Gray RH, Wawer MJ, Sewankambo N, Serwadda D. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:1780. doi: 10.1016/s0140-6736(05)63612-4. [DOI] [PubMed] [Google Scholar]
- 10.Hillier SL, Krohn MA, Klebanoff SJ, Eschenbach DA. The relationship of hydrogen peroxide-producing lactobacilli to bacterial vaginosis and genital microflora in pregnant women. Obstet Gynecol. 1992;79:369–73. doi: 10.1097/00006250-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Antonio MA, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis. 1999;180:1950–6. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- 12.Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663–8. doi: 10.1086/367658. [DOI] [PubMed] [Google Scholar]
- 13.Redondo-Lopez V, Cook RL, Sobel JD. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 1990;12:856–72. doi: 10.1093/clinids/12.5.856. [DOI] [PubMed] [Google Scholar]
- 14.Pascual LM, Daniele MB, Pajaro C, Barberis L. Lactobacillus species isolated from the vagina: identification, hydrogen peroxide production and nonoxynol-9 resistance. Contraception. 2006;73:78–81. doi: 10.1016/j.contraception.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 15.Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology. 2004;150:2565–73. doi: 10.1099/mic.0.26905-0. [DOI] [PubMed] [Google Scholar]
- 16.Vallor AC, Antonio MA, Hawes SE, Hillier SL. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J Infect Dis. 2001;184:1431–6. doi: 10.1086/324445. [DOI] [PubMed] [Google Scholar]
- 17.Hillier SL, Krohn MA, Nugent RP, Gibbs RS. Characteristics of three vaginal flora patterns assessed by gram stain among pregnant women. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1992;166:938–44. doi: 10.1016/0002-9378(92)91368-k. [DOI] [PubMed] [Google Scholar]
- 18.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–8. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 19.Saigh JH, Sanders CC, Sanders WE., Jr Inhibition of Neisseria gonorrhoeae by aerobic and facultatively anaerobic components of the endocervical flora: evidence for a protective effect against infection. Infect Immun. 1978;19:704–10. doi: 10.1128/iai.19.2.704-710.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St Amant DC, Valentin-Bon IE, Jerse AE. Inhibition of Neisseria gonorrhoeae by Lactobacillus species that are commonly isolated from the female genital tract. Infect Immun. 2002;70:7169–71. doi: 10.1128/IAI.70.12.7169-7171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng HY, Alcorn TM, Cohen MS. Effects of H2O2-producing lactobacilli on Neisseria gonorrhoeae growth and catalase activity. J Infect Dis. 1994;170:1209–15. doi: 10.1093/infdis/170.5.1209. [DOI] [PubMed] [Google Scholar]
- 22.Johnson SR, Steiner BM, Cruce DD, Perkins GH, Arko RJ. Characterization of a catalase-deficient strain of Neisseria gonorrhoeae: evidence for the significance of catalase in the biology of N. gonorrhoeae. Infect Immun. 1993;61:1232–8. doi: 10.1128/iai.61.4.1232-1238.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soler-Garcia AA, Jerse AE. A Neisseria gonorrhoeae catalase mutant is more sensitive to hydrogen peroxide and paraquat, an inducer of toxic oxygen radicals. Microb Pathog. 2004;37:55–63. doi: 10.1016/j.micpath.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Seib KL, Wu HJ, Kidd SP, Apicella MA, Jennings MP, McEwan AG. Defenses against oxidative stress in Neisseria gonorrhoeae: a system tailored for a challenging environment. Microbiol Mol Biol Rev. 2006;70:344–61. doi: 10.1128/MMBR.00044-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Exley RM, Wu H, Shaw J, et al. Lactate acquisition promotes successful colonization of the murine genital tract by Neisseria gonorrhoeae. Infect Immun. 2007;75:1318–24. doi: 10.1128/IAI.01530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerse AE, Cohen MS, Drown PM, et al. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med. 1994;179:911–20. doi: 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jerse AE, Crow ET, Bordner AN, et al. Growth of Neisseria gonorrhoeae in the female mouse genital tract does not require the gonococcal transferrin or hemoglobin receptors and may be enhanced by commensal lactobacilli. Infect Immun. 2002;70:2549–58. doi: 10.1128/IAI.70.5.2549-2558.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jerse AE. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect Immun. 1999;67:5699–708. doi: 10.1128/iai.67.11.5699-5708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soler-Garcia AA, Jerse AE. Neisseria gonorrhoeae Catalase Is Not Required for Experimental Genital Tract Infection despite the Induction of a Localized Neutrophil Response. Infect Immun. 2007 doi: 10.1128/IAI.01513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGroarty JA, Tomeczek L, Pond DG, Reid G, Bruce AW. Hydrogen peroxide production by Lactobacillus species: correlation with susceptibility to the spermicidal compound nonoxynol-9. J Infect Dis. 1992;165:1142–4. doi: 10.1093/infdis/165.6.1142. [DOI] [PubMed] [Google Scholar]
- 31.Song Y, Kato N, Liu C, Matsumiya Y, Kato H, Watanabe K. Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group- and species-specific primers derived from the 16S–23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol Lett. 2000;187:167–73. doi: 10.1111/j.1574-6968.2000.tb09155.x. [DOI] [PubMed] [Google Scholar]
- 32.Turner S, Reid E, Smith H, Cole J. A novel cytochrome c peroxidase from Neisseria gonorrhoeae: a lipoprotein from a Gram-negative bacterium. Biochem J. 2003;373:865–73. doi: 10.1042/BJ20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rashad AL, Toffler WL, Wolf N, et al. Vaginal PO2 in healthy women and in women infected with Trichomonas vaginalis: potential implications for metronidazole therapy. Am J Obstet Gynecol. 1992;166:620–4. doi: 10.1016/0002-9378(92)91687-6. [DOI] [PubMed] [Google Scholar]
- 34.Boskey ER, Cone RA, Whaley KJ, Moench TR. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod. 2001;16:1809–13. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 35.Smith H, Tang CM, Exley RM. Effect of host lactate on gonococci and meningococci: new concepts on the role of metabolites in pathogenicity. Infect Immun. 2007;75:4190–8. doi: 10.1128/IAI.00117-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skaar EP, Tobiason DM, Quick J, et al. The outer membrane localization of the Neisseria gonorrhoeae MsrA/B is involved in survival against reactive oxygen species. Proc Natl Acad Sci U S A. 2002;99:10108–13. doi: 10.1073/pnas.152334799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tseng HJ, Srikhanta Y, McEwan AG, Jennings MP. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol Microbiol. 2001;40:1175–86. doi: 10.1046/j.1365-2958.2001.02460.x. [DOI] [PubMed] [Google Scholar]
- 38.Seib KL, Jennings MP, McEwan AG. A Sco homologue plays a role in defence against oxidative stress in pathogenic Neisseria. FEBS Lett. 2003;546:411–5. doi: 10.1016/s0014-5793(03)00632-x. [DOI] [PubMed] [Google Scholar]
- 39.Wu HJ, Seib KL, Edwards JL, Apicella MA, McEwan AG, Jennings MP. Azurin of pathogenic Neisseria spp. is involved in defense against hydrogen peroxide and survival within cervical epithelial cells. Infect Immun. 2005;73:8444–8. doi: 10.1128/IAI.73.12.8444-8448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stohl EA, Criss AK, Seifert HS. The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection. Mol Microbiol. 2005;58:520–32. doi: 10.1111/j.1365-2958.2005.04839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greiner LL, Edwards JL, Shao J, Rabinak C, Entz D, Apicella MA. Biofilm Formation by Neisseria gonorrhoeae. Infect Immun. 2005;73:1964–70. doi: 10.1128/IAI.73.4.1964-1970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer A. The uterine cervix from adolescence to the menopause. Br J Obstet Gynaecol. 1975;82:81–99. doi: 10.1111/j.1471-0528.1975.tb02204.x. [DOI] [PubMed] [Google Scholar]
- 43.Noguchi K, Tsukumi K, Urano T. Qualitative and quantitative differences in normal vaginal flora of conventionally reared mice, rats, hamsters, rabbits, and dogs. Comp Med. 2003;53:404–12. [PubMed] [Google Scholar]
- 44.Smith H, Yates EA, Cole JA, Parsons NJ. Lactate stimulation of gonococcal metabolism in media containing glucose: mechanism, impact on pathogenicity, and wider implications for other pathogens. Infect Immun. 2001;69:6565–72. doi: 10.1128/IAI.69.11.6565-6572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen MS, Cannon JG, Jerse AE, Charniga LM, Isbey SF, Whicker LG. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J Infect Dis. 1994;169:532–7. doi: 10.1093/infdis/169.3.532. [DOI] [PubMed] [Google Scholar]
- 46.Knapp JS, Clark VL. Anaerobic growth of Neisseria gonorrhoeae coupled to nitrite reduction. Infect Immun. 1984;46:176–81. doi: 10.1128/iai.46.1.176-181.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tien MT, Girardin SE, Regnault B, et al. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J Immunol. 2006;176:1228–37. doi: 10.4049/jimmunol.176.2.1228. [DOI] [PubMed] [Google Scholar]