Abstract
Study Design
Randomized, controlled trial.
Objectives
To assess the believability of a novel sham intervention for a neurodynamic technique (NDT) in participants with signs and symptoms of carpal tunnel syndrome (CTS). Additionally, we wished to assess a potential mechanism of NDT (hypoalgesia) and to compare outcomes related to clinical pain and upper extremity disability between NDT and a sham intervention.
Background
Preliminary evidence suggests that NDT is effective in the treatment of CTS. A sham-controlled study is lacking from the literature and could provide insight to the efficacy of NDT, as well as the corresponding mechanisms.
Methods
Participants with signs and symptoms consistent with CTS provided baseline measures of expectation, clinical pain intensity, upper extremity disability, and experimental pain sensitivity. Participants were then randomly assigned to receive either a NDT known to anatomically stress the median nerve or a sham technique intended to minimize stress to the median nerve. Following brief exposure to the assigned technique, expectation was reassessed to observe for group-dependent changes. Participants received the assigned intervention over 3 weeks. Additionally, all participants received a prefabricated wrist splint for their involved hands, with instructions to sleep in the splint and to wear it during painful activities when awake. Following 3 weeks of the assigned intervention and splint wear, baseline measures were reassessed and participants were asked which intervention they believed they had received.
Results
Forty females agreed to participate. Expectations for pain relief and perceived group assignment were similar between the groups. Within-session decreases in clinical pain intensity and pressure pain sensitivity were observed independent of group assignment. Reduction of temporal summation was observed only in participants receiving NDT. Significant improvements in clinical pain intensity and upper extremity disability were observed at 3 weeks, independent of group assignment.
Conclusion
The sham intervention was successful in blinding the participants. Immediate changes in pain sensitivity and intensity and 3-week changes in clinical pain intensity and upper extremity disability associated with NDT were equivalent to a sham intervention to which the participants were adequately blinded. Conversely, reduction of temporal summation was only observed in participants receiving the NDT, suggesting the potential of a favorable neurophysiological effect.
Keywords: central sensitization, manual therapy, musculoskeletal pain, placebo
Manual therapy is a complementary and alternative medicine encompassing numerous techniques in which a force is directed towards a given structure of the body. Neurodynamic techniques (NDT) are a form of manual therapy in which forces are intended to be directed to the neural structures through positioning and movement of multiple joints.13 Physical therapists may include NDT in the treatment of musculoskeletal pain and, specific to the present study, these techniques have shown promise in the treatment of carpal tunnel syndrome (CTS).66,72,81,92 However, a limitation of the current literature is the lack of a validated sham comparison in randomized controlled trials of the effectiveness of NDT. An appropriate sham could provide further evidence as to the efficacy of NDT and insight into the underlying mechanisms of NDT in the treatment of musculoskeletal pain.
A prerequisite of a valid sham for NDT is to provide adequate blinding of participants to the type of intervention they are receiving.48,49,98 To our knowledge, a sham-controlled trial of NDT has not previously been performed; however, prior studies of manual therapy have included sham interventions such as light touch,52 light massage,49 subtherapeutic ultrasound,24 and detuned laser.73 A limitation of these studies is the failure to assess whether participants were adequately blinded to which intervention they received24,73 or whether participant expectation of benefit for the comparison groups were similar.24,49,52,73 The results of sham-controlled studies could be confounded by a significant number of participants receiving the sham intervention, believing they were not receiving an active intervention. Additionally, participants receiving a sham intervention may believe they received an active intervention. However, the sham may provoke lower expectations for treatment effectiveness than the studied intervention. For example, while detuned ultrasound or light touch may be believable as active interventions, they may produce lower expectations among participants for their ability to lessen musculoskeletal pain than a comparative manual therapy intervention. Consequently, both the sham intervention and the studied manual therapy technique may be believable as active interventions, but produce different expectations for their ability to help the individual. These points are reinforced by the observations of placebo-controlled studies unrelated to manual therapy, in which outcomes were not dependent on the intervention received but, rather, on the intervention the participant thought he or she received.2,65 Furthermore, studies have observed expectation as influential in the outcomes associated with manual therapy.7,57 Consequently, a valid sham intervention should blind participants to the intervention group to which they are assigned and should provide similar expectations for treatment success as the studied intervention.
In addition to believability, a negligible treatment effect is suggested as a prerequisite for a sham intervention for manual therapy.48,49,98 As the literature suggests a powerful effect of placebo on pain,75,96,97 sham interventions with negligible treatment effects may be inappropriate for manual therapy studies. The current placebo literature suggests, “The focus has shifted from the ‘inert’ content of the placebo agent (eg, starch capsules) to the concept of a simulation of an active therapy within a psychosocial context.”76 Consequently, a believable sham intervention for manual therapy is likely to produce some positive treatment effect.
A valid sham for NDT could provide important clues to the mechanisms through which these techniques produce clinical outcomes. Both biomechanical22,36 and neurophysiological effects20,69,71 are associated with manual therapy and are potentially pertinent to NDT. A biomechanical mechanism of NDT is plausible, as adhesions and edema accompany CTS27,28,33,70 and may be responsible for the subsequent signs and symptoms. Furthermore, individuals with CTS may present with limited mobility of the median nerve.26,53 Both in vivo and in vitro studies have demonstrated movement and stress localized to the median nerve with the performance of specific NDT.21,22 Consequently, NDT may be of benefit to individuals experiencing CTS due to biomechanical mechanism such as restoration of mobility to the median nerve13 or the mobilization of edema.12 A neurophysiological mechanism of NDT is also possible, as manual therapy has been theorized to directly mediate neuroplastic changes associated with pain.10 Neuroplastic changes are changes in neurons within the central nervous system in response to stimulation and are suggestive of an adaptive rather than fixed nervous system. Specific to CTS, prior studies have observed altered central processing of pain31,94,95,104 associated with CTS suggestive of neuroplastic changes resulting from prolonged irritation of the median nerve. For example, Fernandez-de-las-Penas et al31 observed generalized decreased threshold to pressure pain in individuals with CTS as compared to healthy controls, suggesting a centrally mediated response to pain. Consequently, NDT may be effective in the treatment of CTS by inducing neuroplastic changes. Support for this theory is provided by immediate hypoalgesia associated with other forms of manual therapy.34,38,91,102 A validated sham control for NDT would allow insight into whether treatment effects are dependent upon a specific stress across the median nerve for individuals with CTS or more general effects of treatment. Additionally, a validated sham could provide a means to test the magnitude of the placebo effect of NDT through a comparison of the outcomes associated with the sham to those associated with NDT and natural history.
This randomized trial of NDT in the treatment of participants with CTS had 3 primary purposes. First, we tested the believability of a novel sham for NDT in study participants. We have reported on a potential sham technique in healthy participants exposed to experimental pain5 and wished to study the same technique in a clinical sample. We hypothesized that participants in our study would have similar beliefs for which intervention they received and similar expectations for treatment success, regardless of treatment group assignment.
Second, we studied immediate changes in clinical pain intensity and experimental pain sensitivity as an indirect measurement of a potential mechanism of NDT. Immediate hypoalgesia to clinical pain and increases in mechanical pain threshold have been associated with other forms of manual therapy18,91,99,100 and suggests a neurophysiological mechanism of action. Additionally, we have previously observed hypoalgesia of temporal summation associated with spinal manipulation in healthy participant,38 in those experiencing low back pain,8 and associated with NDT5 in healthy participants. Temporal summation is a behavioral measure of central sensitization, characterized by the perception of increasing pain intensity to repetitive heat pulses of unchanging temperature provided at a frequency of less than 3 seconds. Temporal summation has not previously been assessed in individuals with CTS to our knowledge; however, individuals with musculoskeletal pain conditions such as fibromyalgia77,88 and temporomandibular joint dysfunction78,84 demonstrate greater magnitude of temporal summation than healthy controls. Additionally, temporal summation has been observed to significantly contribute to pain-related disability in individuals with chronic low back pain.39 Consequently, temporal summation appears to be a valid measure of pain sensitivity in other musculoskeletal pain conditions worthy of assessment in individuals with CTS. Temporal summation is a proximal measure of dorsal horn excitability,23,42,45,67 and inhibition of temporal summation from NDT could represent a mechanism of action for pain relief. We hypothesized that participants receiving NDT would experience greater immediate within-session inhibition of clinical pain, temporal summation, thermal pain threshold, and pressure pain threshold in comparison to those receiving a sham technique. Confirmation of this hypothesis would suggest that NDT elicits a neurophysiological response beyond that of a sham related to hypoalgesia and, in the case of temporal summation, specific to inhibition of dorsal horn excitability.
Our third purpose was to compare outcomes related to pain intensity, upper extremity disability, and neurological status in participants with CTS randomly assigned to receive an NDT specific to the median nerve14,21,22,103 and a sham technique intended to lessen the biomechanical stresses to the median nerve. We hypothesized that participants receiving the NDT would demonstrate greater improvements in measures of clinical pain and disability than those receiving the sham intervention. Additionally, concern has been raised that a direct stretch across a nerve, as implied by some methods of NDT, has the potential for detrimental effects.21,22,40,87 Consequently, we monitored neurological status in participants and hypothesized that worsening of neurological status would not be observed in either treatment group.
Methods
Participants
The study was approved by the Institutional Review Board of the University of Florida. Participants were recruited from the clinics of orthopaedic surgeons at the University of Florida and from the general public through posted flyers and electronic distribution. Participants were included if they were between the ages of 18 and 70 years and had signs and symptoms consistent with CTS, as defined by pain or paresthesia in the median nerve distribution and/or clinical examination findings consistent with CTS. Additionally, participants were required to have CTS symptoms present for greater than 12 weeks and a rating of their CTS pain intensity or symptom intensity of at least 4/10 on a numeric rating scale (range of 0 to 10, with 0 indicating no pain at all and 10 indicating the worst pain imaginable over the past 24 hours). Individuals were excluded from participation for the following reasons: non-English speaking, prior surgery for CTS, prior treatment with the studied NDT, pregnancy, diagnosed with a systemic disease known to cause peripheral neuropathy, current or history of chronic pain conditions, or CTS as the result of an upper extremity fracture.
Measures
Demographic and Clinical Characteristics
A demographic questionnaire was used to gather information related to age, sex, ethnicity, racial group, employment status, marital status, education level, household income, hand dominance, CTS-affected side, whether CTS was work related, and duration of CTS (weeks). In cases of bilateral CTS, participants were further asked to identify the more symptomatic side or to indicate that both sides were equally symptomatic.
Patient Expectation
Individual expectation for outcome was assessed using the Patient-Centered Outcome Questionnaire (PCOQ).80 The PCOQ is a 5-item questionnaire that uses individual 101-point numeric rating scales (NRSs) to quantify the usual, desired, and expected levels of pain, fatigue, emotional distress, and interference with daily activities associated with a pain condition. Additionally, the PCOQ uses the same NRS to quantify the ratings of pain, fatigue, emotional distress, and interference with daily activities the participant would consider to be a successful treatment and the self-perceived importance of improvement in pain, fatigue, emotional distress, and interference with daily activities. Individual NRSs were anchored with 0 (none) and 100 (the worst imaginable). Participants were asked to answer these questions regarding their CTS-related symptoms. We used ratings for expected level of pain following treatment to quantify expectation for our data analyses.
Participants were asked at the 3-week follow-up visit to indicate whether they believed they had been assigned to receive the “direct” NDT or the “indirect” NDT.
Mechanisms of NDT
Within-Session Changes in Clinical Pain Intensity
Immediate decreases in clinical pain have been reported in prior studies of manual therapy,18,91,101 and we wished to assess similar changes associated with NDT. Clinical pain intensity was assessed for immediate within-session changes using a 100-mm mechanical visual analog scale (MVAS), anchored with “no pain” and “the most intense pain sensation imaginable,” to quantify current clinical pain. An MVAS is a white slide ruler covering a 100-mm red area. Participants were asked to pull the ruler to uncover the red area to correspond to their pain intensity. No pain was indicated by no red showing, while the most intense pain sensation imaginable was indicated by all the red showing. A 100-mm ruler on the back of the MVAS allowed quantification of pain rating by the distance (mm) of red area uncovered. The participant was blinded to the markings on the ruler while using the MVAS. The MVAS is commonly used in the assessment of pain and has demonstrated sound psychometric properties, including the characteristics of a ratio scale.74 Within-session MVAS assessment of current clinical pain intensity was obtained prior to and immediately following the assigned intervention at the initial session and at 3 weeks.
Pressure Pain Assessment
An immediate increase in pain pressure threshold has been observed in prior studies of manual therapy,29,91,101 and we wished to observe whether similar changes occurred in response to NDT. A pressure algometer (Pain Diagnostics & Thermography, Great Neck, NY) was used to determine mechanical pain. We wished to observe pain response to a standard painful stimulus. Accordingly, 2.3 kg of force was applied to all participants at a rate of 1 kg/s through a 1-cm2 application tip at the thenar eminence, and the participants were asked to rate associated pain on a 100-mm MVAS.
Thermal Pain Assessment
Thermal pain sensitivity was assessed in 2 ways: for threshold and as a means to assess temporal summation. Prior studies of manual therapy have assessed pain response to thermal stimuli following manual therapy,29,38,91,100 and we wished to observe for changes related to NDT. Participants underwent thermal pain assessment using a neurosensory analyzer (TSA-2001; Medoc, Ramat Yishai, Israel) with a handheld, peltier element-based stimulator. Thermal pain threshold was assessed with the stimulator applied to the thenar surface of the palm at a baseline temperature of 35.0°C, and increased at a rate of 0.5°C/s. Participants were instructed to verbally indicate “as soon as the sensation changed from warm to painful,” and the stimulus was then immediately terminated manually by the examiner. Pain was then quantified using a 100-mm MVAS. Similar to our prior studies, threshold was assessed 2 times and the average calculated.5,8,38 Temporal summation assessment used previously established protocols77,89 to observe pain perception to 10 heat pulses at 51°C, applied to the thenar surface of the palm of the hand with an interstimulus interval of 0.33 seconds. A 101-point NRS, anchored with “no pain” and “the most intense pain sensation imaginable,” quantified the pain experienced with each heat pulse, and participants were instructed to rate their “second pain.” Temporal summation was calculated by the average pain rating of the first 5 heat pulses for purposes of our data analyses.77
Clinical Outcomes
Clinical Ratings of Pain Intensity
Clinical pain intensity was assessed for between-session changes from baseline to 3 weeks using an NRS from the PCOQ to quantify “usual pain.” NRS are commonly used in the assessment of pain. While lacking the ratio properties of MVAS,74 the NRS has demonstrated good reliability and validity35,54 and is a common measure of clinical pain intensity.
Disability of the Arm, Shoulder, and Hand Questionnaire (DASH)
The Quick DASH is a self-report measure of upper extremity disability that contains 19 items, ranging from 1 (“no difficulty”) to 5 (“unable”). The DASH contains 3 modules that are scored independently and include an 11-question general module, a 4-item work module, and a 4-item sports/performing arts module. The DASH is used in assessment of general upper extremity disorders, has sound reliability and validity,3,41,43,44,56 and has similar responsiveness as the carpal tunnel-specific Boston Questionnaire.41 We report only the 11-question general module of the Quick DASH, providing a range of 11 to 55 possible points, with lower values indicating less disability for descriptive statistics and in our analyses.
Neurological Status
Grip Strength
Grip strength is a common measure of motor performance in studies of CTS37,55 and was assessed at baseline and at 3 weeks using a Jamar hydraulic hand dynamometer. A significant decline in grip strength could indicate a deleterious effect of NDT. We chose to monitor grip strength as an indicator of motor performance rather than a clinical outcome measure. Participants were seated with their arm at their side, elbows flexed to 90°, and forearm in neutral, and were instructed to squeeze the dynamometer as hard as they could for 3 separate, consecutive trials. The average of the 3 trials was recorded in kilograms as grip strength.
Sensation
Semmes-Weinstein monofilament testing is a common measure of sensation in CTS50,63 and was assessed at baseline and at 3 weeks at the tip of the thumb, index finger, and middle finger. Participants closed their eyes and were instructed to verbally indicate when they felt the monofilament. Pressure was applied to the thumb, index finger, and middle finger to just bend the monofilament and each individual stimulus was applied up to 3 times. The monofilament at which the participant indicated sensation was recorded.
Electrodiagnostic Testing
Median nerve function was assessed through a nerve conduction study (NCS), composed of both motor and sensory testing of the median nerve using standard techniques.9,47,61,79 The NCS was performed at baseline and at 3 weeks on a subset of participants, based on their willingness to consent to this procedure and availability of testing equipment. NCS was performed by a medical doctor (K.R.V.) experienced in this assessment, using a TECASynergy N2 ultraportable 2-channel NCS system (VIASYS Healthcare, Inc, San Diego, CA). Distal onset latency (8 cm) and peak amplitude were measured at the abductor pollicis brevis muscle for the motor portion of the NCS. The sensory portion of the NCS was obtained through the combined sensory index (CSI), a standard test for evaluating CTS.61,79 In brief, the CSI measures the sensory nerve action potentials peak latency and amplitude from the median, ulnar, and radial nerves as they cross the wrist. The differences measured between the nerves are combined into an overall score with a value greater than 1 indicative of slowing of median nerve conduction through the carpal tunnel. Further details of the CSI can be found elsewhere.61,79
Procedures
Interested individuals were informed that they would be randomly assigned to receive either an NDT that “directly stresses the median nerve through shoulder, elbow, and wrist movements” or that “indirectly stresses the median nerve through shoulder, elbow, and wrist movements.” Individuals agreeing to participate signed an informed consent form approved by the University of Florida Institutional Review Board and then completed an intake demographic form, the PCOQ, and the DASH. As appropriate, certain participants underwent an NCS. Next, a standardized physical examination was performed on all participants, which included grip strength and sensation assessment. After the examination, all participants were provided with splints for their involved hands. Finally, all participants underwent baseline pressure and thermal pain testing. Participants were then randomly assigned to receive either NDT or a sham intervention. A licensed physical therapist (J.E.B.) performed all baseline assessments (with the exception of the NCS, which was performed by K.R.V.) and all postrandomization assessments until the final session at 3 weeks.
Randomization
Randomization was computer generated, with group assignment maintained in sealed, sequentially numbered, opaque envelopes. The envelopes were opened in sequential order based on entry in the study and after all baseline measures were completed for the participant.
Intervention
Similar to prior studies of the effectiveness of NDT in the treatment of CTS,1,72,81 all participants in the current study received splints for their involved wrist(s) (Wristoform; Orthorehab, Inc, Tempe, AZ). Standard splint instructions were used for all participants and included directions to sleep with the splints and to use them during daytime activities that worsened their CTS complaints. After splint instruction, participants were randomly assigned to 1 of 2 intervention groups (FIGURE 1). Group 1 received a specific NDT intended to provide anatomical stress across the median nerve, including contralateral cervical sidebending, shoulder depression, shoulder abduction and external rotation to 90°, full elbow extension, and forearm supination.21,22 Group 2 received a sham technique that minimized anatomical stress across the median nerve and included neutral cervical spine position (ie, no sidebending), no shoulder depression, shoulder abduction and external rotation to 45°, 45° of elbow extension, and forearm pronation. This sham technique was used in healthy subjects and demonstrated good potential for use in clinical trials.5 Repeated passive wrist and finger flexion and extension through the available range of motion following appropriate positioning of the cervical spine and upper extremity were used for each intervention. Each repetition was performed for a period of 6 seconds from full wrist and finger flexion to full wrist and finger extension and back to standardize the dosage. The participants received 5 sets of 10 cycles for the first 3 sessions and 7 sets of 10 cycles for sessions 4 through 6. The assigned intervention was applied bilaterally to all participants, regardless of whether their CTS complaints were bilateral. A licensed physical therapist applied the intervention at each session.
FIGURE 1.
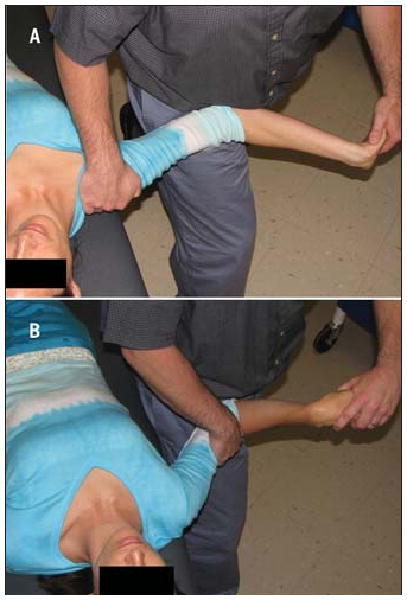
Illustration of the neurodynamic technique (NDT) and the sham intervention. The NDT (A) included positioning and range of motion known to cause significant stress across the median nerve.20,21 Specifically, the NDT included 25° of contralateral cervical sidebending, ipsilateral shoulder depression and abduction to 90°, shoulder external rotation to 90°, 45° of elbow extension, forearm supination, and repetitive wrist and finger flexion and extension through the available range of motion. The sham technique (B) was intended to minimize stress across the median nerve and consisted of the cervical spine maintained in neutral sidebending, no ipsilateral shoulder depression and abduction to 45°, shoulder external rotation to 45°, 45° of elbow extension, and forearm pronation, and repetitive wrist and finger flexion and extension applied passively by the examiner through the available range of motion.
Immediately following group assignment, participants were positioned for their assigned intervention (NDT or sham) and received 2 repetitions of passive wrist and finger flexion and extension range of motion. Participants were then asked to complete the expectation portion of the PCOQ for a second time. We chose 2 repetitions of wrist and finger flexion and extension range of motion to provide exposure to the intervention at a dosage not expected to cause a treatment effect, but sufficient enough to provide experience with the treatment technique prior to the assessment of expectation.
Next, participants completed a MVAS of current CTS pain intensity. Participants underwent the first session of their assigned intervention, followed immediately by a within-session follow-up MVAS of current CTS pain. Pressure and thermal pain assessment were again performed to observe for an immediate within-session treatment effect. Participants were followed over the next 3 weeks for application of the randomly assigned intervention. Subsequent sessions included only the application of the assigned intervention. Total number of sessions for the study was preset at 6 in a pattern of 2 times per week for 3 weeks; however, not all participants were able to attend all 6 sessions. Approximately 3 weeks following randomization, participants were seen for a final visit, consisting of assessment of which intervention the participant believed they had received (NDT versus sham), follow-up questionnaires, follow-up NCS (if appropriate), physical examination, preintervention and postintervention pressure and thermal pain testing, and measurement of clinical pain intensity. Assessment at the 3-week follow-up session was performed by an examiner blinded to group assignment.
Data Analysis
We analyzed data from the involved extremity for participants reporting unilateral CTS and the extremity indicated as “more symptomatic” for participants with bilateral CTS. In cases of bilateral CTS, where the participant reported no difference in pain or symptoms between the hands, we used the dominant extremity for analysis. Individual t tests and chi-square tests were used to assess for postrandomization group differences in parametric and nonparametric variables, respectively. Alpha levels were set at .05, and all analyses were performed using the SPSS statistical package, Version 14.0 (SPSS Inc, Chicago, IL).
Sample Size Determination
Sample size estimate was based upon a reduction in temporal summation of pain with NDT by using effect sizes from a prior study involving fentanyl.77 To generate a conservative estimate of power, we used the smaller analgesic effect size of measures of temporal summation (η2 = 0.20), a 2-tailed null hypothesis, and an alpha of .01 (to account for the multiple comparisons). A sample of 20 subjects per treatment group was determined to provide greater than 95% power to detect a group-by-time interaction in the proposed analysis of variance (ANOVA) model.
Believability of Sham Technique
A chi-square analysis was used to compare assessment of group assignment (NDT versus sham). Random assignment was compared to perceived assignment. A mixed-model ANOVA was used to test for an interaction of group (NDT versus sham) by time (immediately following the informed consent form versus following brief exposure to the randomly assigned intervention) for measures of expectation.
Mechanisms of NDT
Mixed-model ANOVAs were used to test for an interaction of group (NDT versus sham) by time (preintervention to postintervention) for measures of clinical, pressure, and thermal pain. Separate models were used to assess immediate within-session differences at baseline and at discharge. In the event of a statistically significant group-by-time interaction, numbers needed to treat were calculated to provide an estimate of the treatment effect.
Clinical Treatment Effects
Separate mixed-model ANOVAs were used to test for an interaction of group (NDT versus sham) by time (baseline to 3 weeks) for measures of pain and disability occurring over the 3-week period of the study.
Neurological Status
A mixed-model ANOVA was used to test for an interaction of group (NDT versus sham) by time (baseline to 3 weeks) for measures of grip strength. Chi-square was used to assess for group-dependent categorical changes in sensation to the Semmes-Weinstein monofilaments over the 3-week period of the study. Sensation was categorized both at baseline and 3-week follow-up as normal (perception of 2.83 monofilament), minimal loss of sensation (perception of 3.61 or 4.31 monofilament), and severe loss of sensation (lack of perception or perception of 4.56 or 6.65 monofilament). Analysis was performed following further categorization, based on whether participants improved in sensation category, worsened in sensation category, or stayed the same over the 3-week period of the study. Mixed-model ANOVAs were used to test for a group-by-time interaction for NCS measures of distal latency of the median motor to the abductor pollicis brevis (in milliseconds) and the combined sensory index.
Results
Eighty-three individuals were screened for the study, and 40 females agreed to participate (FIGURE 2). Twenty-seven participants (68%) reported bilateral CTS. Of the participants reporting bilateral CTS, 22 reported one side as more symptomatic, and this side was used in the analysis. Five participants reporting bilateral complaints were unable to identify one side as worse than the other, and the data for the dominant arm were analyzed. Visual observation of the data indicated a nonnormal distribution of duration with a right skew. Consequently, a Mann-Whitney U test was performed and indicated a significant difference between the groups (U = 81.50, P = .02). Upon further analysis, duration was not included as a covariate in our models due to small, nonsignificant correlations between duration and our outcome variables (r between −0.27 and 0.22, P>.05). Postrandomization baseline measures did not otherwise differ between the groups (P>.05) (TABLE 1). Thirty-nine of the 40 (98%) enrolled participants completed both a baseline and 3-week session. The participant not returning for the 3-week follow-up was assigned to the NDT group providing 3-week analysis for 19/20 (95%) participants assigned to receive NDT and 20/20 (100%) participants assigned to receive the sham intervention. Range of attended sessions was between 2 and 6 in individuals completing the study, with a mean number of sessions attended of 4.6 (SD, 1.6) for the individuals assigned to receive NDT, 4.9 (SD, 1.2) for individuals assigned to receive the sham intervention, and 4.7 (SD, 1.4) for the total sample. Number of sessions attended did not differ by intervention group (P = .63).
FIGURE 2.
Summary of recruitment, enrollment, randomization, allocation, follow-up, and analysis for the study.
TABLE 1. Baseline Comparison of Intervention Groups*.
| NDT | Sham | Total Sample | P Value for Difference | |
|---|---|---|---|---|
| Age (y) | 44.30 (6.97) | 49.50 (12.35) | 46.90 (10.25) | .11 |
| Duration of CTS (wk)† | 104 (30-221) | 364 (153-520) | 156 (52-520) | |
| Work related (number [percent] yes) | 14/19 (74%) | 11/19 (58%) | 25/38 (66%) | .31 |
| Expectation of pain (PCOQ)‡ | 26.1 (19.5) | 27.5 (20.5) | 26.8 (19.8) | .82 |
| Usual pain (PCOQ)‡ | 51.3 (28.0) | 45.0 (28.5) | 48.1 (28.1) | .49 |
| Current pain (MVAS)§ | 22.7 (16.3) | 14.9 (15.8) | 18.8 (16.3) | .13 |
| Temporal summation (NRS) | 36.9 (22.6) | 33.3 (29.9) | 35.1 (26.2) | .66 |
| DASH questionnaire‖ | 35.3 (15.4) | 40.7 (18.7) | 38.0 (17.1) | .34 |
| Grip (kg) | 21.76 (10.88) | 22.25 (7.84) | 22.00 (9.35) | .88 |
| NCS distal latency (ms)¶ | 4.64 (1.95) | 4.61 (1.38) | 4.63 (1.64) | .97 |
| NCS CSI (ms)¶ | 1.89 (1.81) | 1.92 (1.38) | 1.90 (1.53) | .98 |
| Sensation: thumb# | 1.00 | |||
| WNL | 5 (28%) | 5 (28%) | 10 (28%) | |
| Min | 10 (56%) | 10 (56%) | 20 (56%) | |
| >Min | 3 (17%) | 3 (17%) | 6 (17%) | |
| Sensation: index finger# | .74 | |||
| WNL | 6 (33%) | 4 (22%) | 9 (25%) | |
| Min | 9 (50%) | 11 (61%) | 20 (56%) | |
| >Min | 3 (17%) | 3 (17%) | 6 (17%) | |
| Sensation: middle finger# | .80 | |||
| WNL | 4 (24%) | 5 (28%) | 9 (26%) | |
| Min | 11 (65%) | 12 (67%) | 23 (66%) | |
| >Min | 2 (12%) | 1 (6%) | 3 (9%) | |
| Sensation: ulnar border, ring finger# | .77 | |||
| WNL | 7 (39%) | 5 (28%) | 12 (33%) | |
| Min | 10 (56%) | 12 (67%) | 22 (61%) | |
| >Min | 1 (6%) | 1 (6%) | 2 (6%) | |
| Sensation: small finger# | .42 | |||
| WNL | 8 (44%) | 6 (33%) | 14 (39%) | |
| Min | 9 (50%) | 12 (67%) | 21 (58%) | |
| >Min | 1 (6%) | 0 (0%) | 1 (3%) |
Abbreviations: CSI, combined sensory index; CTS, carpal tunnel syndrome; DASH, Disability of Arm, Shoulder, and Hand Questionnaire; MVAS, mechanical visual analog scale; NCS, nerve conduction study; NDT, neurodynamic technique; NRS, numeric rating scale; PCOQ, Patient-Centered Outcome Questionnaire; WNL, within normal limits.
All data are reported as mean (SD) ratings unless otherwise noted.
Presented as median (interquartile range) due to nonnormal distribution.
PCOQ possible response range from 0 (expect no pain following 3 weeks of participation in the study) to 100 (expect worst pain imaginable following 3 weeks of participation in the study).
MVAS possible response range from 0 (“no pain at all”) to 100 (“worst pain imaginable”).
DASH questionnaire possible response range 11 to 55, with smaller numbers indicating less disability.
NCS performed on 12 of 40 participants. NCS distal latency median motor to abductor pollicis brevis.
Sensation as measured by Semmes-Weinstein monofilament: normal, ≤2.83; min, minimal loss of sensation (3.61-4.31); >min, moderate to severe loss of sensation (≥4.56).
Believability of Sham Technique
Perceived Group Assignment
The frequencies of perceived group assignment did not differ by actual group assignment (χ2 [;1, n = 37] = 2.10 [;P = .15]) for 37 of 40 participants. These data were missing in 3 participants due to incomplete forms. Of the 37 participants for whom we were able to collect these data, 11/18 participants (61%) receiving the sham intervention and 7/19 participants (37%) receiving the NDT perceived having received the NDT.
Expectation for Intervention Effectiveness
An interaction of group (NDT versus sham) by time (immediately post-consent versus following brief exposure to assigned intervention) was not observed for expected pain intensity following the study (P = .87), suggesting that the groups did not differ in their expectation of pain relief following brief exposure to the assigned intervention. Additionally, a main treatment effect for time was not observed (P = .13), suggesting that expectation for treatment did not differ at baseline and following brief exposure to the randomly assigned intervention.
Mechanisms of NDT
Immediate Within-Session Change in Clinical Pain Intensity
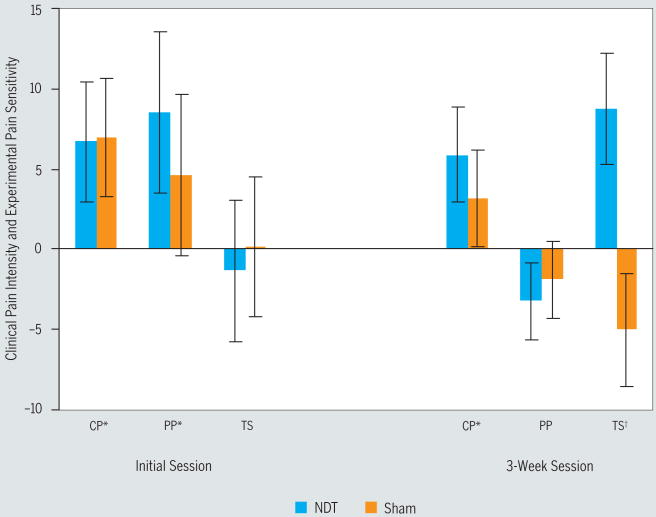
An interaction of group (NDT versus sham) by time (preintervention to immediately postintervention) was not observed for self-report of current pain intensity at either baseline (P = .96) or 3-week follow-up (P = .45). However, a main treatment effect for time was observed at both baseline (P = .01, partial η2 = 0.17) and 3-week follow-up (P = .02, partial η2 = 0.15), suggesting an immediate within-session improvement in pain intensity that was not dependent upon group assignment. Mean change in current pain intensity during the initial session was 6.8 (SD, 15.1; Cohen's d = 0.45). Mean change in pain at the 3-week session was 4.5 (SD, 11.1, Cohen's d = 0.25) (FIGURE 3, TABLE 2).
FIGURE 3.
The immediate within-session change in clinical pain intensity, pressure pain sensitivity, and temporal summation at both the initial visit and at 3 weeks. Pain was quantified using a mechanical visual analog scale (MVAS), anchored with “no pain” at all and “worst pain imaginable” for clinical pain intensity and pressure pain sensitivity, and a numeric rating scale (NRS) anchored with 0 (“no pain at all”) and 100 (“worst pain imaginable”) for temporal summation. Positive numbers indicate hypoalgesia, while negative numbers indicate hyperalgesia. *Significant (P<.05) main treatment effect over time (preintervention to postintervention). †Significant (P<.05) interaction of group (neurodynamic technique [NDT] versus sham) by time (preintervention to postintervention). Abbreviations: CP, clinical pain intensity; PP, pressure pain sensitivity; TS, temporal summation. Error bars indicate 1 standard error of the mean.
TABLE 2. Summary of Findings for Potential Mechanisms of Neurodynamic Interventions and 3-Week Clinical Efficacy*.
| Complete Data | Preintervention | Postintervention | Group-by-Time Interaction P Value (Effect Size) | Main Effect of Time P Value (Effect Size) | |
|---|---|---|---|---|---|
| Mechanisms | |||||
| Clinical pain (MVAS)† | |||||
| Baseline | .96 (<.01) | .01 (.17) | |||
| NDT | 20/20 | 22.7 (16.3) | 16.0 (15.0) | ||
| Sham | 20/20 | 14.9 (15.8) | 7.9 (12.1) | ||
| Total | 40/40 | 18.8 (16.3) | 11.9 (14.0) | ||
| 3-week | .45 (.02) | .02 (.15) | |||
| NDT | 19/20 | 17.3 (21.0) | 11.4 (14.8) | ||
| Sham | 20/20 | 11.5 (19.4) | 8.4 (17.7) | ||
| Total | 39/40 | 14.3 (20.1) | 9.8 (16.2) | ||
| Pressure pain (MVAS) | |||||
| Baseline | .49 (.01) | .03 (.13) | |||
| NDT | 19/20 | 20.6 (26.3) | 12.1 (14.7) | ||
| Sham | 19/20 | 21.3 (31.1) | 16.7 (31.3) | ||
| Total | 38/40 | 20.9 (28.4) | 14.4 (24.2) | ||
| 3-week | .67 (.01) | .10 (.08) | |||
| NDT | 18/20 | 10.4 (15.0) | 13.6 (18.6) | ||
| Sham | 19/20 | 13.8 (22.1) | 15.7 (21.5) | ||
| Total | 37/40 | 12.2 (18.8) | 14.7 (19.9) | ||
| Thermal pain | |||||
| Threshold (MVAS) | |||||
| Baseline | .37 (.02) | .63 (.01) | |||
| NDT | 20/20 | 43.3 (2.4) | 43.4 (3.2) | ||
| Sham | 20/20 | 44.1 (3.0) | 43.6 (3.5) | ||
| Total | 40/40 | 43.7 (2.7) | 43.5 (3.3) | ||
| 3-week | .28 (.03) | .83 (<.01) | |||
| NDT | 19/20 | 44.0 (2.1) | 44.3 (3.1) | ||
| Sham | 20/20 | 43.3 (3.4) | 43.7 (3.8) | ||
| Total | 39/40 | 43.6 (2.8) | 44.0 (3.4) | ||
| Temporal summation (NRS) | |||||
| Baseline | |||||
| NDT | 20/20 | 36.9 (22.6) | 38.2 (27.9) | .83 (<.01) | .79 (<.01) |
| Sham | 20/20 | 33.3 (29.9) | 33.1 (28.7) | ||
| Total | 40/40 | 35.1 (26.2) | 35.7 (28.1) | ||
| 3-week | |||||
| NDT | 19/20 | 43.6 (25.8) | 34.9 (24.9) | .01 (.16) | .36 (.02) |
| Sham | 20/20 | 43.0 (28.7) | 47.2 (32.1) | ||
| Total | 39/40 | 43.3 (26.9) | 41.2 (29.1) | ||
| Clinical efficacy | |||||
| Pain (NRS) | .73 (<.01) | .01 (.17) | |||
| NDT | 19/20 | 51.3 (28.8) | 34.7 (27.5) | ||
| Sham | 20/20 | 45.0 (28.5) | 37.9 (29.5) | ||
| Total | 39/40 | 48.1 (28.4) | 36.2 (28.1) | ||
| Disability (DASH)‡ | >.99 (<.01) | .01 (.21) | |||
| NDT | 18/20 | 36.0 (15.5) | 30.6 (19.4) | ||
| Sham | 18/20 | 41.3 (19.0) | 35.9 (17.9) | ||
| Total | 36/40 | 38.1 (17.3) | 33.2 (18.5) |
Abbreviations: DASH, Disability of Arm, Shoulder, and Hand Questionnaire; MVAS, mechanical visual analog scale; NDT, neurodynamic technique; NRS, numeric rating scale.
Potential mechanisms for NDTs are indicated by immediate within-session changes in pain intensity and sensitivity when assessed at baseline and at 3 weeks. Effect sizes are provided in partial η2.
MVAS possible response range from 0 (no pain at all) to (100, worst pain imaginable).
DASH questionnaire possible response range 11 to 55, with smaller numbers indicating less disability.
Pressure Pain Sensitivity
An interaction of group (NDT versus sham) by time (preintervention to postintervention) was not present during the initial session for mechanical pain sensitivity (P = .49). However, a main treatment effect was present for time (P = .03, partial η2 = 13), suggesting hypoalgesia regardless of group assignment. Mean reduction in self-report of mechanical pain sensitivity at the initial visit was 6.6 (SD, 17.4; Cohen's d = 0.25). Neither a group-by-time interaction (P = .67) nor a main treatment effect for time (P = .10) was present for a within-session change in mechanical pain sensitivity at the 3-week session.
Thermal Pain Sensitivity
Neither an interaction for group (NDT versus sham) by time (preintervention to postintervention), nor a main treatment effect for time, was observed for heat threshold during the initial session nor the 3-week session (P>.05). Neither a group-by-time interaction (P = .79) nor a main treatment effect for time (P = .83) was observed for temporal summation during the initial session. A significant interaction for group by time (P = .01, partial η2 = 0.16) was observed for temporal summation at the 3-week follow-up session. Paired t tests indicated a mean decrease of self-report of temporal summation pain of −8.8 (SD, 14.7; P = .02; Cohen's d = 0.35) in participants receiving the NDT and a mean increase of temporal summation pain of +4.2 (SD, 16.0; P = .26; Cohen's d = 0.13) in participants receiving the sham (FIGURE 3, TABLE 2).
Clinical Pain and Disability Outcomes
Three-Week Change in Clinical Pain Intensity
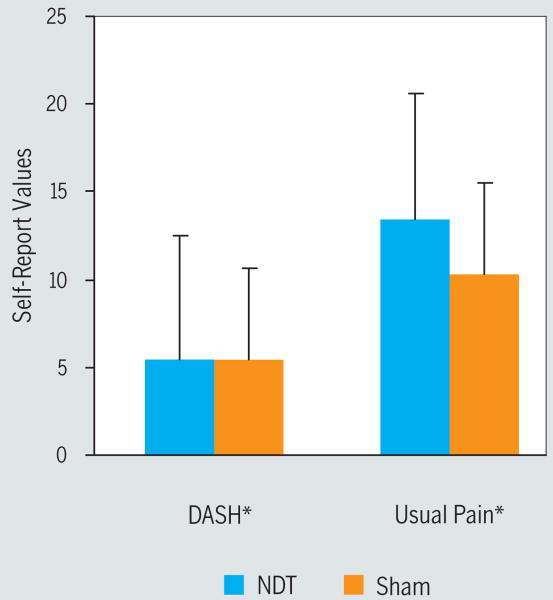
For self-report of pain intensity, an interaction of group (NDT versus sham) by time (initial session versus 3-week follow-up) was not present (P = .73); however, a main treatment effect for time (P = .01, partial η2 = 0.17) existed. Mean self-report of usual pain at the initial visit was 48.1 (SD, 28.4) and 36.2 (SD, 28.2) at 3-week follow-up (Cohen's d = 0.42) (FIGURE 4, TABLE 2).
FIGURE 4.
Changes in clinical pain and disability over 3 weeks. Usual pain was quantified using a numeric rating scale (NRS) anchored with 0 (no pain at all) and 100 (worst pain imaginable). Disability was measured using the 11-question general module of the Quick Disability of the Arm, Shoulder, and Hand Questionnaire (Quick DASH), providing a range of 11 to 55 possible points, with lower values indicating less disability. Positive numbers on the y-axis indicate improvement in the respective outcome over the 3 weeks of the study. *Significant (P<.05) main treatment effect over time (baseline to 3 weeks). Note: group-related differences were not observed in changes in pain or disability over the 3 weeks of the study. Error bars indicate 1 standard error of the mean. Abbreviation: NDT, neurodynamic technique.
Three-Week Change in Disability
An interaction of group (NDT versus sham) by time (initial session versus 3-week follow-up) was not present for the DASH Questionnaire (P>.99). However, a main treatment effect for time was observed (P = .01, partial η2 = 0.21), suggesting an improvement in the DASH over time, regardless of the assigned intervention. Mean DASH score at the initial visit was 38.6 (SD, 17.3) and 33.2 (SD, 18.6) at 3-week follow-up (Cohen's d = 0.30) (FIGURE 4, TABLE 2).
Neurological Status
An interaction of group (NDT versus sham) by time (baseline to 3 weeks) was not present for grip strength (P = .56). However, a significant main treatment effect for time was present (P = .02, partial η2 = 0.16), suggesting a change in grip strength over time independent of the assigned intervention. Mean baseline grip strength was 22.0 kg (SD, 9.6 kg), while 3-week mean grip strength was 24.9 kg (SD, 10.0 kg), corresponding to a Cohen's d of 0.29. Baseline to 3-week changes in sensation, as assessed by Semmes-Weinstein monofilaments, did not differ by group when assessed for the thumb (P = .85), index finger (P = .68), and middle finger (P = .76). A subgroup of 12 participants agreed to undergo baseline and 3-week NCS. The 12 participants did not differ significantly from those not undergoing an NCS in terms of age, duration of symptoms, baseline pain, or baseline expectation for treatment (P>.05). A group-by-time interaction was not observed for median motor onset latency measured at the abductor pollicis brevis (P = .09, partial η2 = 0.26), or for the combined sensory index (P = .21, partial η2 = 0.15), suggesting that the groups did not differ in changes related to the NCS over the 3-week period of the study. A main treatment effect for time was not observed for measures of distal latency from median motor to abductor pollicis brevis (P = .88, partial η2<0.01) or the combined sensory index (P = .33, partial η2 = 0.10), suggesting no changes in nerve conduction over time.
Discussion
Believability of Sham Intervention
A valid sham intervention for comparison to NDT is necessary to better design and interpret efficacy studies of NDT and to better understand the mechanisms through which these techniques produce their clinical effects. The participants in our study had similar expectations and beliefs regardless of group assignment. These findings suggest the validity of our sham intervention in providing adequate blinding and creating similar expectations for pain outcomes as the NDT in participants presenting with CTS. Additionally, we observed similar outcomes from NDT and the sham intervention in terms of immediate and 3-week outcomes related to pain and disability. The design of our study does not control for the common use of splints by the groups or natural history. Consequently, we are unable to determine whether NDT and a sham intervention are both equally ineffective or to what extent they are equally more effective than the use of splints alone or natural history alone. Our findings of similar outcomes between interventions (NDT and sham) in providing similar expectations are consistent with the current placebo literature suggesting expectation as an important factor in the magnitude of placebo analgesia.4,6,25,60,86 Consequently, the placebo effect could represent a primary mechanism in the outcomes associated with NDT in individuals with CTS. Future studies should control for factors such as cointerventions (eg, night splints) and natural history to determine the magnitude of the treatment effect of both NDT and the sham intervention in individual with CTS.
Mechanisms of NDT
Prior studies have observed immediate hypoalgesia to pressure pain threshold,29,30,91,99,101 temporal summation,7,38 and clinical pain ratings,18,91,101 suggesting a neurophysiological mechanism for manual therapy. Interestingly, in the current study the immediate hypoalgesia to pressure pain and clinical pain did not differ significantly in participants receiving NDT or the sham technique. Our findings differ from prior studies that observed hypoalgesia specific to manual therapy in comparison to manual contact,18,29,30,91,101 oscillatory mobilization,99 or no contact controls.91,101 A plausible explanation for this discrepancy is differences in participant expectations for the studied and sham interventions, for which prior studies did not account. For example, participant expectation for pain relief from the studied manual therapy technique might have been significantly greater than for the comparison group. We ascertained adequate blinding to the assigned intervention and similar group-specific expectations for pain relief between our studied intervention and sham control, while others have not. The influence of expectation on musculoskeletal pain has been observed in both manual therapy outcomes7,57 and on musculoskeletal pain in general,51,58,62 and the sham interventions included in previous studies of manual therapy might have provided inadequate blinding or failed to establish adequate expectation. Our findings suggest that with similar expectations and blinding, NDT does not differ from a sham for outcomes related to hypoalgesia of pressure pain sensitivity and immediate, within-session changes in clinical pain intensity in individuals with signs and symptoms consistent with CTS
In contrast, we did observe inhibition of temporal summation specific to NDT. The effect of NDT on temporal summation might have promising clinical implications that the length of our study was insufficient to observe. Temporal summation is mediated by the c-fibers, which have been implicated in the progression of acute pain to chronic pain and in the maintenance of chronic pain.83 Consequently, interventions effective in inhibiting temporal summation have potential benefits in these processes, because they decrease excitability of dorsal horn cells. Our findings suggest that NDT specific to the median nerve may be more effective in inhibiting temporal summation in individuals with signs and symptoms consistent with CTS than a sham intervention. Prior studies have observed experimental pain to be predictive of future clinical pain response.46,82,85,90 For example, Rudin et al82 observed that preoperative pain sensitivity to experimental pain was predictive of postoperative pain intensity. Similarly, the 3-week group-dependent differences in temporal summation that we observed might have been indicative of forthcoming group-dependent changes in clinical pain and disability. This reasoning is speculative, and longer follow-up than the 3 weeks in the current study may be necessary to determine if changes in clinical outcomes, such as pain and function, correspond to the group-specific changes in temporal summation that we observed.
Effects on Clinical Pain Intensity and Disability
The participants in our study demonstrated significant improvement in measures of clinical pain intensity and upper extremity disability, and these findings are consistent with prior studies of NDT in the treatment of CTS.72,81,92 Similar to the findings for pressure-pain sensitivity and immediate within-session changes in clinical pain intensity, the 3-week improvements in clinical pain and disability observed in the current study occurred regardless of whether the participants received the NDT or the sham. These results suggest that the clinical outcomes related to NDT are independent of a mechanical force specific to the median nerve. Our findings are consistent with the manual therapy literature, suggesting that outcomes are related to receiving manual therapy rather than a specific manual therapy intervention.16,19,59 For example, similar outcomes have been observed to different spinal manipulation techniques in individuals positive on a clinical prediction rule that suggested that they would respond favorably to spinal manipulation.19 Specific to CTS, similar improvements in symptoms and function have been reported in participants randomly assigned to receive a carpal tunnel massage protocol or general massage treatment.68 Additionally, similar improvements in pain related to CTS have been reported in participants randomly assigned to receive either NDT or joint mobilization to the carpal bones,92 with both intervention groups outperforming a control group. Collectively, the findings of prior studies68,92 and our own preliminary findings suggest that the short-term effectiveness of NDT in the treatment of CTS may be unrelated to a specific mechanical stress across the median nerve. Additional sham-controlled NDT studies with control groups and longer follow-up times are needed to confirm these findings.
Safety Issues in the Use of NDT
Safety concerns have been expressed regarding the use of forceful NDT.21,22,87 However, the current study observed no group differences in neurological status for NDT or a sham technique. These results suggest that, as applied in this particular study, NDT known to specifically provide stress across the median nerve is as safe as a sham technique intended to minimize the stress across the median nerve when neurological outcomes are monitored over 3 weeks.
Limitations
The current study has several limitations. While not intentional, our sample consisted only of women. CTS is more common in females,11,64,93 so a higher frequency of women to men was expected. However, with no males represented, the results may not be generalizable to men. A second limitation is the lack of a true control group. We were able to compare the findings of the NDT to a sham technique yet were unable to compare either to natural history. Consequently, we can make no conclusions about the absolute benefit of NDT or a sham intended to mimic NDT in comparison to what would have occurred with no intervention. Additionally, both groups in our study received wrist splints. The changes in outcomes observed independent of group assignment could have resulted from the use of splints and not the NDT or sham intervention. The design of our study does not allow us to differentiate treatment effects resulting from the splints and those due to the studied interventions. A third limitation of our study was the 3-week follow-up period. The 3-week period might have been inadequate to detect clinical changes in response to treatment in a chronic sample of participants with CTS and did not allow us to monitor whether the observed changes were lasting. A further limitation of our study was that our sham technique was based on biomechanical principles to minimize the force and movement through the median nerve in comparison to NDT specific to the median nerve.21,22 We did not specifically measure the movement or forces associated with either technique and, consequently, we could not quantify the extent to which they differed. Additionally, the mobilization component of both techniques involved repetitive wrist and finger flexion and extension. While our sham technique was based upon lessening stress to the median nerve as a whole, we are unable to comment upon the specific local biomechanical effects occurring with the repetitive wrist and finger range of motion. A further limitation of our study was the relatively small sample size. Our study on temporal summation was powered for the primary purpose of exploring potential mechanisms of NDT; however, we have previously observed changes in temporal summation specific to other forms of manual therapy38 and NDT.5 Clinical outcome measures were included in this study to determine any clinical effects over the 3-week course of the study. Consequently, we might have been underpowered to observe group-dependent differences in other variables. The magnitude of the observed effects suggests that small effects sizes for any group-related differences and larger sample sizes will be needed for future clinical studies. A final limitation of our study was the inclusion of all individuals fitting the clinical picture of CTS. Recent studies of manual therapy have suggested better outcomes if more homogeneous participants are included.15,17,32 As validated guidelines are currently lacking to identify those individuals with CTS most likely to benefit from NDT, our study may offer insight into these techniques as they are frequently applied clinically.
Conclusion
We presented a novel sham intervention for NDT in the treatment of CTS that was successful in blinding participants to their assigned intervention and produced similar expectation for treatment success as the studied NDT. Similar immediate, within-session changes were observed for the NDT and the sham intervention in measures of pressure pain sensitivity and clinical pain. Longitudinal changes in clinical pain and disability were similar between the groups over 3 weeks. Collectively, these findings suggest that NDT specific to the median nerve in individuals with CTS is no more effective than a sham technique that produces adequate blinding and similar expectations for treatment effect over a 3-week period. Furthermore, these findings suggest that the mechanisms of action behind NDT in the treatment of individuals with CTS are independent of the specific mechanical stress across the median nerve. Conversely, favorable changes in temporal summation were only observed in participants receiving the NDT. These changes in temporal summation are encouraging and may reflect group-dependent changes in clinical outcomes beyond 3 weeks; however, larger studies with longer follow-up times are required to confirm this.
Key Points.
Findings
The sham intervention in the current study was not distinguishable from the studied NDT and produced similar expectations for pain relief as the NDT. Similar immediate within-session reduction in clinical pain intensity and experimental pain sensitivity and 3-week improvements in clinical outcomes were observed in participants receiving both the NDT and the sham intervention, suggesting a nonspecific, neurophysiological mechanism of action. Hypoalgesia to temporal summation was observed only in participants receiving the NDT, suggesting a potential specific mechanism of action.
Implication
The sham intervention is valid in providing suitable blinding and may be a viable option in future sham-controlled studies of NDT. With the exception of altering temporal summation, the mechanisms of NDT in the treatment of individuals with signs and symptoms consistent with CTS appear to be neurophysiological in nature and occur independently of a mechanical force across the median nerve. The clinical implications of a direct effect of NDT on temporal summation are not currently established.
Caution
The current study did not include a no-treatment control group. Additionally, all participants received splints for the study, and the NDT and sham intervention each included passive range of motion to the wrist and fingers. Consequently, we were unable to determine the influence of natural history, splint wear, and passive wrist and finger range of motion on the observed non–group-dependent changes in clinical outcomes. Finally, the observed changes occurred over a 3-week period. Longer follow-up may be necessary for NDT to differentiate itself from a sham intervention in mechanisms and clinical outcomes.
Acknowledgments
This study was approved by The Institutional Review Board of The University of Florida. The project was supported by grant number AT002796 from the National Institutes of Health, National Center for Complementary and Alternative Medicine. This manuscript was written while J.E.B. received support from the National Institutes of Health T-32 Neural Plasticity Research Training Fellowship (T32HD043730). This study was also registered at www.clinicaltrials.gov under the identifier of NCT00929123.
Footnotes
Level of Evidence: Therapy, level 1b. J Orthop Sports Phys Ther 2009;39(10):709-723. doi:10.2519/jospt.2009.3117
References
- 1.Akalin E, El O, Peker O, et al. Treatment of carpal tunnel syndrome with nerve and tendon gliding exercises. Am J Phys Med Rehabil. 2002;81:108–113. doi: 10.1097/00002060-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Bausell RB, Lao L, Bergman S, Lee WL, Berman BM. Is acupuncture analgesia an expectancy effect? Preliminary evidence based on participants' perceived assignments in two placebo-controlled trials. Eval Health Prof. 2005;28:9–26. doi: 10.1177/0163278704273081. http://dx.doi.org/10.1177/0163278704273081. [DOI] [PubMed]
- 3.Beaton DE, Wright JG, Katz JN. Development of the QuickDASH: comparison of three item-reduction approaches. J Bone Joint Surg Am. 2005;87:1038–1046. doi: 10.2106/JBJS.D.02060. http://dx.doi.org/10.2106/JBJS.D.02060. [DOI] [PubMed]
- 4.Beauregard M. Mind does really matter: evidence from neuroimaging studies of emotional self-regulation, psychotherapy, and placebo effect. Prog Neurobiol. 2007;81:218–236. doi: 10.1016/j.pneurobio.2007.01.005. http://dx.doi.org/10.1016/j.pneurobio.2007.01.005. [DOI] [PubMed]
- 5.Beneciuk JM, Bishop MD, George SZ. Effects of upper extremity neural mobilization on thermal pain sensitivity: a sham-controlled study in asymptomatic participants. J Orthop Sports Phys Ther. 2009;39:428–438. doi: 10.2519/jospt.2009.2954. http://dx.doi.org/10.2519/jospt.2009.2954. [DOI] [PubMed]
- 6.Benedetti F. Placebo analgesia. Neurol Sci. 2006;27 2:S100–102. doi: 10.1007/s10072-006-0580-4. http://dx.doi.org/10.1007/s10072-006-0580-4. [DOI] [PubMed]
- 7.Bialosky JE, Bishop MD, Robinson ME, Barabas JA, George SZ. The influence of expectation on spinal manipulation induced hypoalgesia: an experimental study in normal subjects. BMC Musculoskelet Disord. 2008;9:19. doi: 10.1186/1471-2474-9-19. http://dx.doi.org/10.1186/1471-2474-9-19. [DOI] [PMC free article] [PubMed]
- 8.Bialosky JE, Bishop MD, Robinson ME, Zeppieri G, George SZ. The immediate effects of spinal manipulative therapy on thermal pain sensitivity in participants with low back pain. Phys Ther. 2009 doi: 10.2522/ptj.20090058. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bland JD. A neurophysiological grading scale for carpal tunnel syndrome. Muscle Nerve. 2000;23:1280–1283. doi: 10.1002/1097-4598(200008)23:8<1280::aid-mus20>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 10.Boal RW, Gillette RG. Central neuronal plasticity, low back pain and spinal manipulative therapy. J Manipulative Physiol Ther. 2004;27:314–326. doi: 10.1016/j.jmpt.2004.04.005. http://dx.doi.org/10.1016/j.jmpt.2004.04.005. [DOI] [PubMed]
- 11.Bongers FJ, Schellevis FG, van den Bosch WJ, van der Zee J. Carpal tunnel syndrome in general practice (1987 and 2001): incidence and the role of occupational and non-occupational factors. Br J Gen Pract. 2007;57:36–39. [PMC free article] [PubMed] [Google Scholar]
- 12.Burke FD, Ellis J, McKenna H, Bradley MJ. Primary care management of carpal tunnel syndrome. Postgrad Med J. 2003;79:433–437. doi: 10.1136/pmj.79.934.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler DS, Jones MA. Mobilisation of the Nervous System. London, UK: Churchill Livingstone; 1991. [Google Scholar]
- 14.Byl C, Puttlitz C, Byl N, Lotz J, Topp K. Strain in the median and ulnar nerves during upper-extremity positioning. J Hand Surg [Am] 2002;27:1032–1040. doi: 10.1053/jhsu.2002.35886. http://dx.doi.org/10.1053/jhsu.2002.35886. [DOI] [PubMed]
- 15.Childs JD, Fritz JM, Flynn TW, et al. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: a validation study. Ann Intern Med. 2004;141:920–928. doi: 10.7326/0003-4819-141-12-200412210-00008. [DOI] [PubMed] [Google Scholar]
- 16.Chiradejnant A, Maher CG, Latimer J, Stepkovitch N. Efficacy of “therapist-selected” versus “randomly selected” mobilisation techniques for the treatment of low back pain: a randomised controlled trial. Aust J Physiother. 2003;49:233–241. doi: 10.1016/s0004-9514(14)60139-2. [DOI] [PubMed] [Google Scholar]
- 17.Cleland JA, Childs JD, Fritz JM, Whitman JM, Eberhart SL. Development of a clinical prediction rule for guiding treatment of a subgroup of patients with neck pain: use of thoracic spine manipulation, exercise, and patient education. Phys Ther. 2007;87:9–23. doi: 10.2522/ptj.20060155. http://dx.doi.org/10.2522/ptj.20060155. [DOI] [PubMed]
- 18.Cleland JA, Childs JD, McRae M, Palmer JA, Stowell T. Immediate effects of thoracic manipulation in patients with neck pain: a randomized clinical trial. Man Ther. 2005;10:127–135. doi: 10.1016/j.math.2004.08.005. http://dx.doi.org/10.1016/j.math.2004.08.005. [DOI] [PubMed]
- 19.Cleland JA, Fritz JM, Whitman JM, Childs JD, Palmer JA. The use of a lumbar spine manipulation technique by physical therapists in patients who satisfy a clinical prediction rule: a case series. J Orthop Sports Phys Ther. 2006;36:209–214. doi: 10.2519/jospt.2006.36.4.209. http://dx.doi.org/10.2519/jospt.2006.2163. [DOI] [PubMed]
- 20.Colloca CJ, Keller TS, Gunzburg R. Neuromechanical characterization of in vivo lumbar spinal manipulation. Part II. Neurophysiological response. J Manipulative Physiol Ther. 2003;26:579–591. doi: 10.1016/j.jmpt.2003.08.004. http://dx.doi.org/10.1016/j.jmpt.2003.08.004. [DOI] [PubMed]
- 21.Coppieters MW, Alshami AM. Longitudinal excursion and strain in the median nerve during novel nerve gliding exercises for carpal tunnel syndrome. J Orthop Res. 2007;25:972–980. doi: 10.1002/jor.20310. http://dx.doi.org/10.1002/jor.20310. [DOI] [PubMed]
- 22.Coppieters MW, Butler DS. Do ‘sliders’ slide and ‘tensioners’ tension? An analysis of neurodynamic techniques and considerations regarding their application. Man Ther. 2008;13:213–221. doi: 10.1016/j.math.2006.12.008. http://dx.doi.org/10.1016/j.math.2006.12.008. [DOI] [PubMed]
- 23.Cuellar JM, Dutton RC, Antognini JF, Carstens E. Differential effects of halothane and isoflurane on lumbar dorsal horn neuronal windup and excitability. Br J Anaesth. 2005;94:617–625. doi: 10.1093/bja/aei107. http://dx.doi.org/10.1093/bja/aei107. [DOI] [PubMed]
- 24.Deyle GD, Henderson NE, Matekel RL, Ryder MG, Garber MB, Allison SC. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med. 2000;132:173–181. doi: 10.7326/0003-4819-132-3-200002010-00002. [DOI] [PubMed] [Google Scholar]
- 25.Enck P, Benedetti F, Schedlowski M. New insights into the placebo and nocebo responses. Neuron. 2008;59:195–206. doi: 10.1016/j.neuron.2008.06.030. http://dx.doi.org/10.1016/j.neuron.2008.06.030. [DOI] [PubMed]
- 26.Erel E, Dilley A, Greening J, Morris V, Cohen B, Lynn B. Longitudinal sliding of the median nerve in patients with carpal tunnel syndrome. J Hand Surg Br. 2003;28:439–443. doi: 10.1016/s0266-7681(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 27.Ettema AM, Amadio PC, Zhao C, Wold LE, An KN. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am. 2004;86-A:1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Ettema AM, Amadio PC, Zhao C, et al. Changes in the functional structure of the tenosynovium in idiopathic carpal tunnel syndrome: a scanning electron microscope study. Plast Reconstr Surg. 2006;118:1413–1422. doi: 10.1097/01.prs.0000239593.55293.c7. http://dx.doi.org/10.1097/01.prs.0000239593.55293.c7. [DOI] [PubMed]
- 29.Fernandez-Carnero J, Fernandez-de-las-Penas C, Cleland JA. Immediate hypoalgesic and motor effects after a single cervical spine manipulation in subjects with lateral epicondylalgia. J Manipulative Physiol Ther. 2008;31:675–681. doi: 10.1016/j.jmpt.2008.10.005. http://dx.doi.org/10.1016/j.jmpt.2008.10.005. [DOI] [PubMed]
- 30.Fernandez-de-las-Penas C, Alonso-Blanco C, Cleland JA, Rodriguez-Blanco C, Alburquerque-Sendin F. Changes in pressure pain thresholds over C5-C6 zygapophyseal joint after a cervicothoracic junction manipulation in healthy subjects. J Manipulative Physiol Ther. 2008;31:332–337. doi: 10.1016/j.jmpt.2008.04.006. http://dx.doi.org/10.1016/j.jmpt.2008.04.006. [DOI] [PubMed]
- 31.Fernandez-de-las-Penas C, de la Llave-Rincon AI, Fernandez-Carnero J, Cuadrado ML, Arendt-Nielsen L, Pareja JA. Bilateral widespread mechanical pain sensitivity in carpal tunnel syndrome: evidence of central processing in unilateral neuropathy. Brain. 2009;132:1472–1479. doi: 10.1093/brain/awp050. http://dx.doi.org/10.1093/brain/awp050. [DOI] [PubMed]
- 32.Flynn T, Fritz J, Whitman J, et al. A clinical prediction rule for classifying patients with low back pain who demonstrate short-term improvement with spinal manipulation. Spine. 2002;27:2835–2843. doi: 10.1097/00007632-200212150-00021. [DOI] [PubMed] [Google Scholar]
- 33.Freeland AE, Tucci MA, Barbieri RA, Angel MF, Nick TG. Biochemical evaluation of serum and flexor tenosynovium in carpal tunnel syndrome. Microsurgery. 2002;22:378–385. doi: 10.1002/micr.10065. http://dx.doi.org/10.1002/micr.10065. [DOI] [PubMed]
- 34.Frey Law LA, Evans S, Knudtson J, Nus S, Scholl K, Sluka KA. Massage reduces pain perception and hyperalgesia in experimental muscle pain: a randomized, controlled trial. J Pain. 2008;9:714–721. doi: 10.1016/j.jpain.2008.03.009. http://dx.doi.org/10.1016/j.jpain.2008.03.009. [DOI] [PubMed]
- 35.Gagliese L, Weizblit N, Ellis W, Chan VW. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain. 2005;117:412–420. doi: 10.1016/j.pain.2005.07.004. http://dx.doi.org/10.1016/j.pain.2005.07.004. [DOI] [PubMed]
- 36.Gal J, Herzog W, Kawchuk G, Conway PJ, Zhang YT. Movements of vertebrae during manipulative thrusts to unembalmed human cadavers. J Manipulative Physiol Ther. 1997;20:30–40. [PubMed] [Google Scholar]
- 37.Geere J, Chester R, Kale S, Jerosch-Herold C. Power grip, pinch grip, manual muscle testing or thenar atrophy - which should be assessed as a motor outcome after carpal tunnel decompression? A systematic review. BMC Musculoskelet Disord. 2007;8:114. doi: 10.1186/1471-2474-8-114. http://dx.doi.org/10.1186/1471-2474-8-114. [DOI] [PMC free article] [PubMed]
- 38.George SZ, Bishop MD, Bialosky JE, Zeppieri G, Jr, Robinson ME. Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study. BMC Musculoskelet Disord. 2006;7:68. doi: 10.1186/1471-2474-7-68. http://dx.doi.org/10.1186/1471-2474-7-68. [DOI] [PMC free article] [PubMed]
- 39.George SZ, Wittmer VT, Fillingim RB, Robinson ME. Fear-avoidance beliefs and temporal summation of evoked thermal pain influence self-report of disability in patients with chronic low back pain. J Occup Rehabil. 2006;16:95–108. doi: 10.1007/s10926-005-9007-y. http://dx.doi.org/10.1007/s10926-005-9007-y. [DOI] [PubMed]
- 40.Greening J, Leary R. Re: Improving application of neurodynamic (neural tension) and treatments: a message to researchers and clinicians. Man Ther. 2007;12:e2. doi: 10.1016/j.math.2006.01.004. author reply e3-6. http://dx.doi.org/10.1016/j.math.2006.01.004. [DOI] [PubMed]
- 41.Greenslade JR, Mehta RL, Belward P, Warwick DJ. Dash and Boston questionnaire assessment of carpal tunnel syndrome outcome: what is the responsiveness of an outcome questionnaire? J Hand Surg Br. 2004;29:159–164. doi: 10.1016/j.jhsb.2003.10.010. http://dx.doi.org/10.1016/j.jhsb.2003.10.010. [DOI] [PubMed]
- 42.Guan Y, Borzan J, Meyer RA, Raja SN. Windup in dorsal horn neurons is modulated by endogenous spinal mu-opioid mechanisms. J Neurosci. 2006;26:4298–4307. doi: 10.1523/JNEUROSCI.0960-06.2006. http://dx.doi.org/10.1523/JNEUROSCI.0960-06.2006. [DOI] [PMC free article] [PubMed]
- 43.Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11. doi: 10.1186/1471-2474-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gummesson C, Ward MM, Atroshi I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord. 2006;7:44. doi: 10.1186/1471-2474-7-44. http://dx.doi.org/10.1186/1471-2474-7-44. [DOI] [PMC free article] [PubMed]
- 45.Hanai F. C fiber responses of wide dynamic range neurons in the spinal dorsal horn. Clin Orthop Relat Res. 1998:256–267. doi: 10.1097/00003086-199804000-00032. [DOI] [PubMed] [Google Scholar]
- 46.Hartrick CT, Kovan JP, Naismith P. Outcome prediction following sympathetic block for complex regional pain syndrome. Pain Pract. 2004;4:222–228. doi: 10.1111/j.1533-2500.2004.04306.x. http://dx.doi.org/10.1111/j.1533-2500.2004.04306.x. [DOI] [PubMed]
- 47.Havton LA, Hotson JR, Kellerth JO. Correlation of median forearm conduction velocity with carpal tunnel syndrome severity. Clin Neurophysiol. 2007;118:781–785. doi: 10.1016/j.clinph.2006.12.011. http://dx.doi.org/10.1016/j.clinph.2006.12.011. [DOI] [PubMed]
- 48.Hawk C, Long CR, Reiter R, Davis CS, Cambron JA, Evans R. Issues in planning a placebo-controlled trial of manual methods: results of a pilot study. J Altern Complement Med. 2002;8:21–32. doi: 10.1089/107555302753507159. http://dx.doi.org/10.1089/107555302753507159. [DOI] [PubMed]
- 49.Hawk C, Long CR, Rowell RM, Gudavalli MR, Jedlicka J. A randomized trial investigating a chiropractic manual placebo: a novel design using standardized forces in the delivery of active and control treatments. J Altern Complement Med. 2005;11:109–117. doi: 10.1089/acm.2005.11.109. http://dx.doi.org/10.1089/acm.2005.11.109. [DOI] [PubMed]
- 50.Hentz VR, Lalonde DH. MOC-PS(SM) CME article: self-assessment and performance in practice: the carpal tunnel. Plast Reconstr Surg. 2008;121:1–10. doi: 10.1097/01.prs.0000305930.24851.26. http://dx.doi.org/10.1097/01.prs.0000305930.24851.26. [DOI] [PubMed]
- 51.Heymans MW, de Vet HC, Knol DL, Bongers PM, Koes BW, van Mechelen W. Workers' beliefs and expectations affect return to work over 12 months. J Occup Rehabil. 2006;16:685–695. doi: 10.1007/s10926-006-9058-8. http://dx.doi.org/10.1007/s10926-006-9058-8. [DOI] [PubMed]
- 52.Hoiriis KT, Pfleger B, McDuffie FC, et al. A randomized clinical trial comparing chiropractic adjustments to muscle relaxants for subacute low back pain. J Manipulative Physiol Ther. 2004;27:388–398. doi: 10.1016/j.jmpt.2004.05.003. http://dx.doi.org/10.1016/j.jmpt.2004.05.003. [DOI] [PubMed]
- 53.Hough AD, Moore AP, Jones MP. Reduced longitudinal excursion of the median nerve in carpal tunnel syndrome. Arch Phys Med Rehabil. 2007;88:569–576. doi: 10.1016/j.apmr.2007.02.015. http://dx.doi.org/10.1016/j.apmr.2007.02.015. [DOI] [PubMed]
- 54.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 55.Jerosch-Herold C, Leite JC, Song F. A systematic review of outcomes assessed in randomized controlled trials of surgical interventions for carpal tunnel syndrome using the International Classification of Functioning, Disability and Health (ICF) as a reference tool. BMC Musculoskelet Disord. 2006;7:96. doi: 10.1186/1471-2474-7-96. http://dx.doi.org/10.1186/1471-2474-7-96. [DOI] [PMC free article] [PubMed]
- 56.Jester A, Harth A, Wind G, Germann G, Sauerbier M. Disabilities of the arm, shoulder and hand (DASH) questionnaire: Determining functional activity profiles in patients with upper extremity disorders. J Hand Surg Br. 2005;30:23–28. doi: 10.1016/j.jhsb.2004.08.008. http://dx.doi.org/10.1016/j.jhsb.2004.08.008. [DOI] [PubMed]
- 57.Kalauokalani D, Cherkin DC, Sherman KJ, Koepsell TD, Deyo RA. Lessons from a trial of acupuncture and massage for low back pain: patient expectations and treatment effects. Spine. 2001;26:1418–1424. doi: 10.1097/00007632-200107010-00005. [DOI] [PubMed] [Google Scholar]
- 58.Kapoor S, Shaw WS, Pransky G, Patterson W. Initial patient and clinician expectations of return to work after acute onset of work-related low back pain. J Occup Environ Med. 2006;48:1173–1180. doi: 10.1097/01.jom.0000243401.22301.5e. http://dx.doi.org/10.1097/01.jom.0000243401.22301.5e. [DOI] [PubMed]
- 59.Kent P, Marks D, Pearson W, Keating J. Does clinician treatment choice improve the outcomes of manual therapy for nonspecific low back pain? A metaanalysis. J Manipulative Physiol Ther. 2005;28:312–322. doi: 10.1016/j.jmpt.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 60.Kong J, Kaptchuk TJ, Polich G, Kirsch I, Gollub RL. Placebo analgesia: findings from brain imaging studies and emerging hypotheses. Rev Neurosci. 2007;18:173–190. doi: 10.1515/revneuro.2007.18.3-4.173. [DOI] [PubMed] [Google Scholar]
- 61.Lew HL, Wang L, Robinson LR. Test-retest reliability of combined sensory index: implications for diagnosing carpal tunnel syndrome. Muscle Nerve. 2000;23:1261–1264. doi: 10.1002/1097-4598(200008)23:8<1261::aid-mus16>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 62.Linde K, Witt CM, Streng A, et al. The impact of patient expectations on outcomes in four randomized controlled trials of acupuncture in patients with chronic pain. Pain. 2007;128:264–271. doi: 10.1016/j.pain.2006.12.006. http://dx.doi.org/10.1016/j.pain.2006.12.006. [DOI] [PubMed]
- 63.MacDermid JC, Doherty T. Clinical and electrodiagnostic testing of carpal tunnel syndrome: a narrative review. J Orthop Sports Phys Ther. 2004;34:565–588. doi: 10.2519/jospt.2004.34.10.565. http://dx.doi.org/10.2519/jospt.2004.1505. [DOI] [PubMed]
- 64.McDiarmid M, Oliver M, Ruser J, Gucer P. Male and female rate differences in carpal tunnel syndrome injuries: personal attributes or job tasks? Environ Res. 2000;83:23–32. doi: 10.1006/enrs.2000.4042. http://dx.doi.org/10.1006/enrs.2000.4042. [DOI] [PubMed]
- 65.McRae C, Cherin E, Yamazaki TG, et al. Effects of perceived treatment on quality of life and medical outcomes in a double-blind placebo surgery trial. Arch Gen Psychiatry. 2004;61:412–420. doi: 10.1001/archpsyc.61.4.412. http://dx.doi.org/10.1001/archpsyc.61.4.412. [DOI] [PubMed]
- 66.Medina McKeon JM, Yancosek KE. Neural gliding techniques for the treatment of carpal tunnel syndrome: a systematic review. J Sport Rehabil. 2008;17:324–341. doi: 10.1123/jsr.17.3.324. [DOI] [PubMed] [Google Scholar]
- 67.Mitsuyo T, Dutton RC, Antognini JF, Carstens E. The differential effects of halothane and isoflurane on windup of dorsal horn neurons selected in unanesthetized decerebrated rats. Anesth Analg. 2006;103:753–760. doi: 10.1213/01.ane.0000230605.22930.52. http://dx.doi.org/10.1213/01.ane.0000230605.22930.52. [DOI] [PubMed]
- 68.Moraska A, Chandler C, Edmiston-Schaetzel A, Franklin G, Calenda EL, Enebo B. Comparison of a targeted and general massage protocol on strength, function, and symptoms associated with carpal tunnel syndrome: a randomized pilot study. J Altern Complement Med. 2008;14:259–267. doi: 10.1089/acm.2007.0647. http://dx.doi.org/10.1089/acm.2007.0647. [DOI] [PubMed]
- 69.Morelli M, Sullivan SJ, Chapman CE. Inhibitory influence of soleus massage onto the medial gastrocnemius H-reflex. Electromyogr Clin Neurophysiol. 1998;38:87–93. [PubMed] [Google Scholar]
- 70.Oh J, Zhao C, Zobitz ME, Wold LE, An KN, Amadio PC. Morphological changes of collagen fibrils in the subsynovial connective tissue in carpal tunnel syndrome. J Bone Joint Surg Am. 2006;88:824–831. doi: 10.2106/JBJS.E.00377. http://dx.doi.org/10.2106/JBJS.E.00377. [DOI] [PubMed]
- 71.Pickar JG, Kang YM. Paraspinal muscle spindle responses to the duration of a spinal manipulation under force control. J Manipulative Physiol Ther. 2006;29:22–31. doi: 10.1016/j.jmpt.2005.11.014. http://dx.doi.org/10.1016/j.jmpt.2005.11.014. [DOI] [PubMed]
- 72.Pinar L, Enhos A, Ada S, Gungor N. Can we use nerve gliding exercises in women with carpal tunnel syndrome? Adv Ther. 2005;22:467–475. doi: 10.1007/BF02849867. [DOI] [PubMed] [Google Scholar]
- 73.Preyde M. Effectiveness of massage therapy for subacute low-back pain: a randomized controlled trial. CMAJ. 2000;162:1815–1820. [PMC free article] [PubMed] [Google Scholar]
- 74.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56:217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 75.Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. http://dx.doi.org/10.1016/j.pain.2006.08.001. [DOI] [PubMed]
- 76.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. http://dx.doi.org/10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed]
- 77.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 78.Raphael KG. Temporal summation of heat pain in temporomandibular disorder patients. J Orofac Pain. 2009;23:54–64. [PMC free article] [PubMed] [Google Scholar]
- 79.Robinson LR, Micklesen PJ, Wang L. Strategies for analyzing nerve conduction data: superiority of a summary index over single tests. Muscle Nerve. 1998;21:1166–1171. doi: 10.1002/(sici)1097-4598(199809)21:9<1166::aid-mus7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 80.Robinson ME, Brown JL, George SZ, et al. Multidimensional success criteria and expectations for treatment of chronic pain: the patient perspective. Pain Med. 2005;6:336–345. doi: 10.1111/j.1526-4637.2005.00059.x. http://dx.doi.org/10.1111/j.1526-4637.2005.00059.x. [DOI] [PubMed]
- 81.Rozmaryn LM, Dovelle S, Rothman ER, Gorman K, Olvey KM, Bartko JJ. Nerve and tendon gliding exercises and the conservative management of carpal tunnel syndrome. J Hand Ther. 1998;11:171–179. doi: 10.1016/s0894-1130(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 82.Rudin A, Wolner-Hanssen P, Hellbom M, Werner MU. Prediction of post-operative pain after a laparoscopic tubal ligation procedure. Acta Anaesthesiol Scand. 2008;52:938–945. doi: 10.1111/j.1399-6576.2008.01641.x. http://dx.doi.org/10.1111/j.1399-6576.2008.01641.x. [DOI] [PubMed]
- 83.Rygh LJ, Svendsen F, Fiska A, Haugan F, Hole K, Tjolsen A. Long-term potentiation in spinal nociceptive systems--how acute pain may become chronic. Psychoneuroendocrinology. 2005;30:959–964. doi: 10.1016/j.psyneuen.2005.04.007. http://dx.doi.org/10.1016/j.psyneuen.2005.04.007. [DOI] [PubMed]
- 84.Sarlani E, Garrett PH, Grace EG, Greenspan JD. Temporal summation of pain characterizes women but not men with temporomandibular disorders. J Orofac Pain. 2007;21:309–317. [PMC free article] [PubMed] [Google Scholar]
- 85.Schiff E, Eisenberg E. Can quantitative sensory testing predict the outcome of epidural steroid injections in sciatica? A preliminary study. Anesth Analg. 2003;97:828–832. doi: 10.1213/01.ANE.0000078583.47735.69. [DOI] [PubMed] [Google Scholar]
- 86.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. http://dx.doi.org/10.1016/j.neuron.2007.06.028. [DOI] [PubMed]
- 87.Shacklock M. Improving application of neurodynamic (neural tension) testing and treatments: a message to researchers and clinicians. Man Ther. 2005;10:175–179. doi: 10.1016/j.math.2005.03.001. http://dx.doi.org/10.1016/j.math.2005.03.001. [DOI] [PubMed]
- 88.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007;8:893–901. doi: 10.1016/j.jpain.2007.06.006. http://dx.doi.org/10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed]
- 89.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 90.Sterling M, Jull G, Vicenzino B, Kenardy J, Darnell R. Physical and psychological factors predict outcome following whiplash injury. Pain. 2005;114:141–148. doi: 10.1016/j.pain.2004.12.005. http://dx.doi.org/10.1016/j.pain.2004.12.005. [DOI] [PubMed]
- 91.Sterling M, Jull G, Wright A. Cervical mobilisation: concurrent effects on pain, sympathetic nervous system activity and motor activity. Man Ther. 2001;6:72–81. doi: 10.1054/math.2000.0378. [DOI] [PubMed] [Google Scholar]
- 92.Tal-Akabi A, Rushton A. An investigation to compare the effectiveness of carpal bone mobilisation and neurodynamic mobilisation as methods of treatment for carpal tunnel syndrome. Man Ther. 2000;5:214–222. doi: 10.1054/math.2000.0355. http://dx.doi.org/10.1054/math.2000.0355. [DOI] [PubMed]
- 93.Tanaka S, Wild DK, Seligman PJ, Behrens V, Cameron L, Putz-Anderson V. The US prevalence of self-reported carpal tunnel syndrome: 1988 National Health Interview Survey data. Am J Public Health. 1994;84:1846–1848. doi: 10.2105/ajph.84.11.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tecchio F, Padua L, Aprile I, Rossini PM. Carpal tunnel syndrome modifies sensory hand cortical somatotopy: a MEG study. Hum Brain Mapp. 2002;17:28–36. doi: 10.1002/hbm.10049. http://dx.doi.org/10.1002/hbm.10049. [DOI] [PMC free article] [PubMed]
- 95.Tinazzi M, Zanette G, Volpato D, et al. Neurophysiological evidence of neuroplasticity at multiple levels of the somatosensory system in patients with carpal tunnel syndrome. Brain. 1998;121(Pt 9):1785–1794. doi: 10.1093/brain/121.9.1785. [DOI] [PubMed] [Google Scholar]
- 96.Vase L, Riley JL, 3rd, Price DD. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain. 2002;99:443–452. doi: 10.1016/S0304-3959(02)00205-1. [DOI] [PubMed] [Google Scholar]
- 97.Vase L, Robinson ME, Verne GN, Price DD. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain. 2003;105:17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 98.Vernon H, MacAdam K, Marshall V, Pion M, Sadowska M. Validation of a sham manipulative procedure for the cervical spine for use in clinical trials. J Manipulative Physiol Ther. 2005;28:662–666. doi: 10.1016/j.jmpt.2005.07.020. http://dx.doi.org/10.1016/j.jmpt.2005.07.020. [DOI] [PubMed]
- 99.Vernon HT, Aker P, Burns S, Viljakaanen S, Short L. Pressure pain threshold evaluation of the effect of spinal manipulation in the treatment of chronic neck pain: a pilot study. J Manipulative Physiol Ther. 1990;13:13–16. [PubMed] [Google Scholar]
- 100.Vicenzino B, Collins D, Benson H, Wright A. An investigation of the interrelationship between manipulative therapy-induced hypoalgesia and sympathoexcitation. J Manipulative Physiol Ther. 1998;21:448–453. [PubMed] [Google Scholar]
- 101.Vicenzino B, Collins D, Wright A. The initial effects of a cervical spine manipulative physiotherapy treatment on the pain and dysfunction of lateral epicondylalgia. Pain. 1996;68:69–74. doi: 10.1016/S0304-3959(96)03221-6. [DOI] [PubMed] [Google Scholar]
- 102.Vicenzino B, Paungmali A, Buratowski S, Wright A. Specific manipulative therapy treatment for chronic lateral epicondylalgia produces uniquely characteristic hypoalgesia. Man Ther. 2001;6:205–212. doi: 10.1054/math.2001.0411. [DOI] [PubMed] [Google Scholar]
- 103.Wright TW, Glowczewskie F, Wheeler D, Miller G, Cowin D. Excursion and strain of the median nerve. J Bone Joint Surg Am. 1996;78:1897–1903. doi: 10.2106/00004623-199612000-00013. [DOI] [PubMed] [Google Scholar]
- 104.Zanette G, Marani S, Tamburin S. Extra-median spread of sensory symptoms in carpal tunnel syndrome suggests the presence of pain-related mechanisms. Pain. 2006;122:264–270. doi: 10.1016/j.pain.2006.01.034. http://dx.doi.org/10.1016/j.pain.2006.01.034. [DOI] [PubMed]