Abstract
Alzheimer disease (AD) is the most common form of dementia. The amyloid-β (Aβ) peptide has become a major therapeutic target in AD on the basis of pathological, biochemical and genetic evidence that supports a role for this molecule in the disease process. Active and passive Aβ immunotherapies have been shown to lower cerebral Aβ levels and improve cognition in animal models of AD. In humans, dosing in the phase II clinical trial of the AN1792 Aβ vaccine was stopped when ~6% of the immunized patients developed meningoencephalitis. However, some plaque clearance and modest clinical improvements were observed in patients following immunization. As a result of this study, at least seven passive Aβ immunotherapies are now in clinical trials in patients with mild to moderate AD. Several second-generation active Aβ vaccines are also in early clinical trials. On the basis of preclinical studies and the limited data from clinical trials, Aβ immunotherapy might be most effective in preventing or slowing the progression of AD when patients are immunized before or in the very earliest stages of disease onset. Biomarkers for AD and imaging technology have improved greatly over the past 10 years and, in the future, might be used to identify presymptomatic, at-risk individuals who might benefit from Aβ immunization.
Introduction
Alzheimer disease (AD) is the leading cause of dementia, affecting more than 26 million people worldwide.1 Clinically, the disease is characterized by progressive memory loss and a decline in cognitive abilities. Several symptomatic treatments are in use for AD; however, no disease-modifying therapies are currently available. The two major pathological hallmarks of AD are extra cellular amyloid plaques, which are formed mainly from the amyloid-β (Aβ) peptide, and intracellular neurofibrillary tangles (NFTs), which contain hyperphosphorylated tau. Other pathological changes in the brain include gliosis, inflammation, neuritic dystrophy, neuron loss, and changes in neurotransmitter levels.2,3 In AD, the development of pathology in the brain is thought to precede cognitive symptoms and, hence, diagnosis of the disease, by many years.
The Aβ peptide, which comprises 40–42 amino acids, is generated following proteolytic cleavage of the amyloid precursor protein (APP).4 Several findings suggest that Aβ, particularly the 42 amino acid form (Aβ1–42), is a major factor in the pathogenesis of AD. Mutations in APP and in the genes that encode presenilins 1 and 2, (proteins involved in cleavage of APP) are associated with AD in a small number of families. Furthermore, Aβ is deposited in plaques and blood vessels in the brain early in the disease process. Finally, Aβ oligomers and fibrillar aggregates are toxic to neurons.5–7
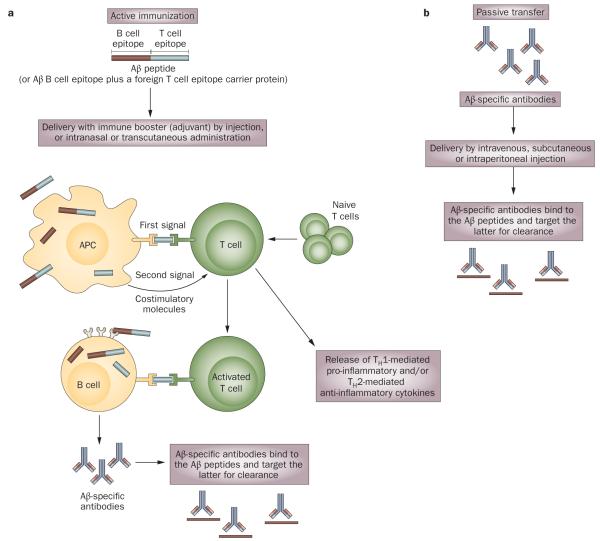
The ‘amyloid cascade hypothesis’ (Box 1) emphasizes a central role for Aβ in the pathogenesis of AD. Thus, Aβ has become a major therapeutic target, with various anti-Aβ strategies being pursued. These strategies include lowering the production of the peptide by inhibiting the enzymes responsible for Aβ generation, preventing the formation of Aβ aggregates, and increasing the rate of Aβ clearance from the brain. Aβ immunotherapy uses anti-Aβ antibodies, generated following vaccination or introduced passively, to increase the rate of clearance and prevent aggregation of this peptide (Figure 1).
Box 1. The amyloid cascade hypothesis.
The ‘amyloid cascade’ hypothesis places the formation of early, toxic amyloid-β(Aβ) oligomers and the accumulation of Aβ aggregates at the center of Alzheimer disease pathogenesis. This hypothesis states that over time, an imbalance in Aβ production and/or clearance leads to gradual accumulation and aggregation of the peptide in the brain, initiating a neurodegenerative cascade that involves amyloid deposition, inflammation, oxidative stress, and neuronal injury and loss.3,102,103 Supporting this hypothesis, in vitro and in vivo studies in animal models have shown that oligomeric and fibrillar forms of Aβ cause long-term potentiation impairment104,105 and synaptic dysfunction,106–108 and accelerate the formation of neurofibrillary tangles that eventually cause synaptic failure and neuronal death.109
Figure 1.
Active and passive immunization approaches. a | Vaccination (active immunization) activates the body’s immune system to produce antigen-specific antibodies. In AD, full-length Aβ or a fragment of Aβ conjugated to a foreign T cell epitope carrier protein can be used as an antigen, which is delivered into the body alongside an immune system booster (adjuvant). The humoral immune response is generated when APCs, which take up and process the antigen, present T cell epitopes to naive TH lymphocytes, activating the latter (first signal). Binding of co-stimulatory molecules on the surfaces of APCs and T cells provides a secondary signal that enhances T cell activation. Meanwhile, the soluble antigen binds to B cell receptors, via the B cell epitope, and this antigen is presented to activated T cells to help the B cell make antibodies against the antigen. Activated T cells also produce cellular immune responses. A TH1 cellular immune response leads to the release of pro-inflammatory cytokines, whereas a TH2 response causes release of anti-inflammatory cytokines. b | Passive immunization bypasses the need for the body to mount an immune response to produce antigen-specific antibodies. In both active and passive Aβ immunization, anti-Aβ antibodies bind Aβ, targeting the peptide for clearance. Abbreviations: Aβ, amyloid-β; APC, antigen presenting cell; TH, T helper.
Over the past 10 years, Aβ immunotherapy has emerged from preclinical studies in transgenic mouse models of AD to enter clinical trials in humans. Presently, at least 13 different Aβ immunotherapies are in clinical trials worldwide.8 Adverse events, including meningo encephalitis and vasogenic edema, have been noted in some of these clinical trials. Nevertheless, studies of active and passive Aβ immunotherapies are continuing to move forward, with an estimated total enrollment of >9,000 patients. On the basis of results from preclinical and clinical studies, we believe that Aβ immunotherapy has strong potential for preventing AD if patients are immunized before disease onset or in the earliest stages of the disorder. In this Review, we will provide an overview of the preclinical studies in animal models that supported a role for Aβ immunotherapy in the prevention of AD pathogenesis and cognitive deficits. We also will summarize the details of the AN1792 vaccine trial and deliver an update on the active and passive Aβ immunotherapies currently in clinical trials. Lastly, we will outline what we perceive to be important considerations in the development of Aβ immunotherapy for preventing AD.
Preclinical evidence
In the mid-1990s, Solomon and colleagues demonstrated that anti-Aβ monoclonal antibodies dissolved Aβ aggregates and prevented Aβ monomers from aggregating in vitro.9,10 Subsequently, anti-Aβ antibody-based therapies for AD have emerged.
Active immunization
In 1999, Schenk et al. reported the first evidence that active Aβ immunotherapy could reduce Aβ pathology in vivo. The researchers vaccinated PDAPP mice–transgenic animals that exhibit amyloid plaque pathology–with pre-aggregated, synthetic Aβ1–42. The anti-Aβ antibodies generated following immunization both prevented plaque deposition and led to a reduction of the number of preestablished plaques in the brains of these animals.11 In addition, Vaccination attenuated gliosis and neuritic dystrophy. Numerous studies have confirmed and extended these findings in several transgenic AD mouse models, using various immunogens, adjuvants and routes of administration. In such models, active Aβ immunization initiated before or at the onset of AD-like pathogenesis lowered brain Aβ levels effectively12–16 and attenuated behavioral deficits.17,18 These findings demonstrated the potential of active Aβ immuniza tion for preventing cerebral Aβ ac cumulation and cognitive impairment.
The effects of active Aβ immunotherapy on transgenic animals exhibiting well-established AD-like pathology have been variable. In plaque-bearing PDAPP mice (aged 11–15 or 18 months), vaccination with Aβ1–42 prevented the formation of new plaques and, presumably, led to a reduction in established plaques.11 In Tg2576 mice (a plaque-bearing transgenic mouse model of AD), however, although Aβ1–42 vaccination in young, pre-plaque animals prevented plaque deposition, the same vaccine was much less effective in clearing pre-established plaques in aged mice.14 In 18 month-old 3xTg-AD mice–transgenic animals that exhibit both amyloid plaques and NFTs– active vaccination with fibrillar Aβ1–42 did not reduce plaque number, or the levels of insoluble Aβ or insoluble tau. Immunization did, however, lead to an improvement in behavioral performance and a reduction in soluble Aβ levels in these animals, suggesting that soluble Aβ species (for example, oligomers) might be directly linked to the behavioral impairment observed in the 3xTg-AD model.19 By contrast, in aged beagles that exhibited diffuse plaques and cognitive deficits, Aβ vaccination led to a reduction in plaque burden but did not alter cerebral Aβ oligomer levels or improve cognition.20
In general, the anti-Aβ antibodies described in the studies above recognized various B cell epitopes within the first 15 amino acids of Aβ,12,21–25 whereas the major T cell epitopes were mapped mainly to the middle region and carboxyl terminus of the peptide.24,26 An Aβ-specific T cell epitope has, however, been reported within the first 16 amino acids of the peptide in Tg2576 mice on a C57BL/6 background and in some human leukocyte antigen transgenic mice.27
Key points.
Preclinical studies support the idea that Alzheimer disease (AD) can be prevented by amyloid-β (Aβ) immunotherapies
Adverse events, but some efficacy, were observed in clinical trials of the AN1792 Aβ vaccine and the passive Aβ immunotherapy bapineuzumab
At least 13 Aβ immunotherapies are in clinical trials in patients with mild to moderate AD
Second-generation Aβ vaccines that avoid the adverse events observed with AN1792 and improve antibody generation might improve Aβ immunotherapy efficacy
Aβ immunization might be considerably more efficacious in AD if administered before Aβ aggregation, thereby protecting the brain from downstream neurodegenerative effects
Passive immunization
In young PDAPP mice, passive immunization with an anti-Aβ monoclonal antibody (m266), which recognized an epitope in the middle region of the peptide (Aβ13–28), prevented plaque deposition, and led to a reduction in the levels of soluble and insoluble Aβ, as well as an increase in the levels of Aβ–anti-Aβ antibody complexes in the blood. These findings supported a role for passive Aβ immunotherapy in the prevention of AD.28 In older plaque-bearing PDAPP mice, only passive immunization with monoclonal antibodies that recognized the amino-terminal epitopes of Aβ (that is, not the mid-region or carboxy-terminal epitopes) led to a reduction in pre-established plaques.29 Generally, studies in aged transgenic mice have reported that passive Aβ immuno therapy, when initiated after the onset of pathology, has lowered the plaque burden and cerebral Aβ1–42 levels,30 reduced neuritic dystrophy,29 and reversed behavioral deficits (in some studies behavioral improvements occurred in the absence of changes in brain Aβ levels).31,32 Systemic injections of some anti-Aβ anti bodies, however, while lowering Aβ-plaque burden, led to an increase in the occurrence of microhemorrhages in areas of cerebral amyloid angiopathy (CAA).33,34 In one study, passive immunization with anti-Aβ mono clonal antibodies directed against the carboxyl terminus of the peptide (Aβ28–40) led to a reduction in plaque burden and an improvement in behavioral deficits, despite an increase in microhemorrhage.35 Direct intra hippocampal injection of anti-Aβ monoclonal antibodies lowered the plaque burden and reduced the rate of early aggregation of phosphorylated tau in 12 month-old 3xTg-AD mice.36 This treatment did not, however, have any effect on more advanced tau pathology, such as NFTs, in older 3xTg-AD mice,36 suggesting that early lowering of Aβ might have downstream effects on tau pathology.
Passive Aβ immunization has been shown to have beneficial effects on synaptic plasticity and neuronal function. Intracerebroventricular infusion of anti-Aβ antibodies protected against synaptic loss and gliosis in Tg2576 transgenic mice.37,38 Furthermore, mono clonal anti-Aβ antibodies against the amino terminus and the middle region of Aβ prevented synaptic plasticity disruption induced by intracerebroventricular infusion of naturally occurring, cell-derived Aβ oligomers,39 or of Aβ dimers from human AD cerebrospinal fluid (CSF).40 An anti-Aβ monoclonal antibody (3D6) that recognizes the free amino terminus was shown to protect dendritic spines and improve structural plasticity in the brains of PDAPP mice.41
Taken together, preclinical studies of Aβ immunotherapy suggest that the overall efficacy of this treatment approach might be higher when immunization occurs before the aggregation of Aβ and tau, and before amyloid accumulates in cerebral blood vessels. Several of the first-generation Aβ immunotherapies that have been tested in animal models have already moved into the later phases of clinical trials. Furthermore, numerous second-generation treatments are under investigation in animal models and early clinical trials (Box 2). The mechanisms of Aβ clearance following immunization might vary depending on the stage of disease (Box 3).
Box 2. Second-generation amyloid-β immunotherapies.
Efforts are underway to develop novel active and passive amyloid-β (Aβ) immunotherapies that overcome the safety concerns associated with the AN1792 vaccine and exhibit an increase in efficacy. Most of the treatments described below are currently in preclinical stages of investigation, although some have reached human clinical trials.
An ideal Aβ vaccine would stimulate a T-helper (TH) 2 immune response (rather than a TH1-mediated cellular immune response) to generate robust anti-Aβ antibody production that prevents or slows cognitive decline. Examples of second-generation active Aβ vaccines include: nontoxic, soluble Aβ derivative immunogens;15 phage display of Aβ3–6;110 short amino-terminal Aβ fragments that target the B cell epitope while avoiding T cell activation;16,111–113 herpes simplex virus amplicons coding for Aβ;114 the TH2-biased amino-terminal UBITh® Aβ vaccine (United Biomedical, Hauppauge, NY, USA);115 nonviral DNA Aβ vaccines;116 DNA vaccines encoding Aβ amino-terminal fragments, a promiscuous T cell epitope and molecular adjuvants;117,118 and Aβ ‘retroparticles’ (Aβ1–15 displayed on retrovirus-like particles fused to the transmembrane domain of platelet-derived growth factor).119 In addition to the different types of vaccines, various adjuvants and routes of vaccine delivery (such as oral, intranasal and transcutaneous delivery) are under investigation to improve the safety, efficacy and ease of use of these immunotherapies.
Passive Aβ immunotherapies are also evolving to include not only site-directed humanized monoclonal antibodies (directed to the amino terminus, carboxyl terminus or middle region of Aβ), but also conformation-specific antibodies. The latter group includes antibodies that recognize toxic, soluble Aβ oligomers120 and Aβ-derived diffusible ligands.121 This group also comprises short-chain variable fragment anti-Aβ antibodies,122–124 anti-Aβ intrabodies, which target intraneuronal Aβ,125 and antibodies directed against toxic, modified amino-terminal species of Aβ.126
Box 3. Mechanisms of amyloid-β clearance and immunotherapy efficacy.
Several mechanisms could explain how antibodies directed at amyloid-β (Aβ) promote the clearance of this peptide in vivo. When anti-Aβ antibodies form complexes with Aβ peptides, the Fc portion of the antibodies might bind the Fc receptor on microglia, inducing phagocytosis of these complexes.30 This mechanism would require that anti-Aβ antibodies cross the blood–brain barrier (BBB) and bind Aβ within the CNS. Some evidence has been reported to support this mechanism; however, two studies have demonstrated that Fc-mediated phagocytosis is not required for immunotherapy-induced Aβ clearance.127,128 In an alternative mechanism, anti-Aβ antibodies in the blood might cause a shift in the concentration gradient of Aβ across the BBB, thus resulting in an increase in Aβ efflux from the brain to the periphery.28,129 Aβ antibodies might bind and remove small Aβ aggregates, thereby neutralizing the effects of toxic Aβ species on synapses.39 One study suggests that certain antibodies (for example, a midregion Aβ antibody, m266) sequester monomeric Aβ inside the brain, facilitating clearance of the peptide and preventing build up of toxic, aggregated forms of Aβ.130
These proposed mechanisms of Aβ clearance by immunotherapy are not mutually exclusive and might be disease stage-dependent. For example, a preventive vaccine administered before cerebral amyloid accumulation might not require that the antibodies cross the BBB to enhance Aβ clearance and maintain Aβ in its monomeric state. By contrast, a therapeutic vaccine (delivered once plaque deposition is well underway) would probably benefit from the transport of Aβ antibodies into the CNS to induce Aβ phagocytosis and neutralize local Aβ toxicity. Circulating anti-Aβ antibodies in the periphery might pull Aβ from brain to blood for clearance, thereby preventing further deposition.
Clinical evidence
The An1792 trials
Design
In the late 1990s, elan and Wyeth developed an Aβ vaccine for use in humans. This vaccine, AN1792, consisted of a synthetic form of the Aβ1–42 peptide and the surface-active saponin adjuvant QS-21. To date, the data from the two AN1792 trials are the only results of major active Aβ immunization clinical trials that have been made widely available. The first AN1792 trial, initiated in December 1999, was a phase I safety study conducted in 80 patients with mild to moderate AD. These individuals were randomly assigned to one of four treatment groups: AN1792, AN1792 without QS-21, QS-21 only, or placebo. Over the subsequent 6 month period, patients in each group received four intramuscular vaccinations. This phase I trial did not report any notable adverse events.42
Following the results of the phase I study, a phase IIa 15 month trial was initiated in October 2001 to evaluate the efficacy of AN1792 plus QS-21 in AD. A total of 372 patients with mild to moderate AD were enrolled in this double-blind, placebo-controlled, multicenter study. Of the 300 patients who received AN1792 (plus QS-21), 223 completed the study. In the other arm of the trial, 53 of the 72 patients who received placebo completed the study. This trial was stopped in January 2002 after ~6% of patients developed aseptic meningoencephalitis and leukoencephalopathy.43,44 The trial design was sub sequently revised to determine the safety and tolerability of AN1792 at 9 months after the last dose of vaccine. The results of the AN1792 trials are summarized below.
Immune response
QS-21 strongly induces T-helper (TH) 1 lymphocytes. Thus, the AN1792 vaccine was designed to induce a strong cell-mediated immune response, which is needed in the elderly to generate a robust antibody response.45 In the phase I trial, however, overall serum anti-Aβ antibody titers were low, reaching above 1:1,000 in only ~23% of the patients.42 During the later stages of the phase I trial, the addition of polysorbate 80 to enhance solubility of the peptide increased the number of antibody responders to almost 60% and modified the immune response from a predominantly TH2 response to a proinflammatory TH1 response.46
In the phase IIa study, a slight improvement in the antibody titers following AN1792 immunization was obtained, with ~20% of the patients showing serum antibody titers above 1:2,200 after between one and three vaccinations. In these patients, immunization resulted in generation of anti-Aβ antibodies targeting the amino terminus of Aβ.47 This trial, as stated earlier, was discontinued after 18 of the 300 patients developed meningoencephalitis following between one and three immunizations. This finding led to the conclusion that AN1792 promoted the production of Aβ-reactive autoimmune T cells, which was probably related to activation of TH1 lymphocytes. The TH1 response might have been related to the adjuvant (QS-21) or to the presence of T cell and B cell recognition epitopes in the Aβ peptide. The B cell epitope (within the first 11–15 amino acids of Aβ) is considered to be important for generation of anti-Aβ antibodies, whereas the most common T cell epitope (within amino acids 15–42 of Aβ)48 has been proposed to have initiated the T responses that triggered the me ningoencephalitis in some AN1792-vaccinated patients.
Cognitive, MRI and biochemical outcomes
The phase I study was designed to test the safety of the AN1792 vaccine; however, during the trial, immunized patients were noted to display a tendency towards slower cognitive decline than controls.42 Thus, in the phase trial, the primary end points included safety, tolerability, and pilot efficacy measures–multiple cognitive measures, volumeric MRI, and CSF levels of Aβ1–42 and phosphorylated tau (phospho-tau). In a single-center analysis of a subgroup of 30 patients involved in the phase IIa trial, improvements were reported in some measures of cognitive performance in six patients with high antibody titers.49 Nevertheless, in the phase IIa trial as a whole, no significant effects on cognitive performance–as measured by several neuropsychological tests–were reported in the antibodyresponder group.44 A few of the tests related to memory (for example, the z-score across the neuro psychological test battery) showed that the antibody responders exhibited a slower rate of memory deterio ration than did the placebo-treated patients (P = 0.02). A follow-up study in a subset of patients 4.6 years after AN1792 dosing was halted demon strated that most of the antibody responders still had detectable anti-Aβ titers (17 of 19 individuals tested). Moreover, these indivi duals had a markedly reduced rate of functional decline on the Disability Assessment for Dementia Scale and the Dependence Scale compared with placebo-treated patients.50
Volumetric MRI studies revealed that antibody responders had increased brain volume loss compared with controls, and that a dissociation existed between brain volume loss and cognitive function in these indivi duals.51 One possible explanation for this dissociation could be that the removal of amyloid by immuno therapy might have occurred late in the progression of the disease at a point when the neuro degenerative pathology and atrophy in the hippocampus was very advanced. Alternatively, the MRI results might have indicated that amyloid removal was accompanied by dynamic changes in fluids, which led to an extensive reduction in hippocampal volume. The dissociation between brain volume loss and cog nitive performance might also reflect some negative effects of the vaccination on fiber or white matter volume. Of note, although the hippocampal volume was small in the ANe observed difference in p-tau levels in this subset of patients might not be representative of the entire study. CSF Aβ1–42 levels in the immunized patient subset were not markedly altered from baseline by AN1792. This lack of difference might be explained by inter-patient variability and the small number of indivi duals assessed. In conclusion, although the AN1792 trial was halted, this study provided the first indication that Aβ immunization might affect the pathology of AD, as was predicted by studies in animal models.
Pathological outcomes
Experimental studies in APP transgenic mice showed clear evidence that Aβ immunotherapy led to a reduction in Aβ pathology; thus, most case reports of AN1792-vaccinated patients have focused on investigating the effects of the treatment on plaques. In the considera tion of these reports, and in the future assessment of Aβ immunotherapy-treated patients, other aspects of AD neuro pathology, as well as indicators of neuro degeneration, should be con sidered. AD is a complex neuro degenerative disorder that specifically damages limbic structures, the association neo cortical pathways52–54 and the cholinergic system.55,56 Amyloid plaques2 and NFTs57 are key neuropathological diagnostic features of AD; however, the neuro degenerative process in AD might be initiated by damage to the synap tic terminals.58,59 Indeed, early synaptic pathology has been postulated to lead to axonal abnormalities,60 dendritic spine61 and dendrite atrophy62 and, eventually, neuronal loss.58
Of the 80 patients enrolled in the phase I trial, 42 died before or during follow-up. eight of these AN1792vaccinated individuals were analyzed neuro pathologically.63 This study showed that the Aβ load in the AN1792 group (2.1% area of neuropil covered by amyloid) was consistently reduced relative to the age-matched, unvaccinated AD group (5.1%). Furthermore, the extent of plaque removal was significantly associated with the mean anti-Aβ antibody titer for up to 84 weeks after the first dose of vaccine (Kruskal–Wallis test, P = 0.02). Despite plaque removal, the eight vaccinated indivi duals exhibited severe dementia at the time of death. A number of factors might account for this discrepancy between amyloid load and extent of dementia. Most of these patients only received one or two vaccinations and did not complete the trial. Moreover, the removal of pre-existing amyloid deposits might not be sufficient to ameliorate established memory deficits. In addition, by the time the amyloid was removed, the ongoing neurodegenerative pathological processes might have been at an advanced stage and, hence, irreversible.
Neuropathological studies were initially conducted on the brains from 3 of the 18 individuals who developed meningoencephalitis during the AN1792 trial. These studies reported the presence of an unusual form of meningo encephalitis and leukoencephalopathy, with numerous T cells and macrophages infiltrating the white matter and perivascular spaces in these brains.64,65 Amyloid plaques were sparse or absent throughout areas of the neocortex in these patients (suggesting a favorable clearance of Aβ), while other hallmarks of an AD brain, including CAA and NFTs, were identified in the CNS. Similarly, in a single case report of a patient with dementia with Lewy bodies, who exhibited globally stable functional and cognitive features, Aβ immunization resulted in a marked clearance of amyloid deposits, with tau and synuclein pathology remaining unchanged.66
A neuropathological case study was performed on the brain of a 71 year-old patient with AD who was immunized with AN1792 but did not develop encepha litis (Figure 2).67 No amyloid plaques were noted in the frontal cortex in this case, although abundant Aβ-immunoreactive macrophages were observed in this region. The presence of NFTs and CAA was an indication that the pathology was ongoing. In this patient, the white matter seemed normal, and minimal lymphocytic infiltration of the leptomeninges was observed. Consistent with the findings from this case, a subsequent study of three immunized patients revealed extensive clearance of amyloid and tau-containing neurites. However, other features of tau pathology (NFTs and neuropil threads) as well as CAA were still present.65 In fact, a previous study had showed that although parenchymal amyloid was focally disaggregated, vascular deposits were relatively preserved or even increased in two patients with AD approximately 1 year after immunization with AN1792.68 Immunoassays revealed that the total soluble Aβ levels in the gray and white matter were sharply increased in these vaccinated patients compared with unimmunized patients with AD and healthy controls.68 In another study, brains were examined from nine AD patients 4 months to 5 years after AN1792 vaccination. The brains from these individuals had markedly increased cerebrovascular Aβ1–42 and Aβ1–40 deposition compared with brains from unimmunized patients with AD.69 Remarkably, a complete absence of both plaques and CAA was noted in the brains of two patients who died 4 and 5 years after the first immunization,69 raising the possibility that, given time, Aβ is eventually cleared from the cerebral vasculature.
Figure 2.
Neuropathological findings in an AN1792-immunized patient with Alzheimer disease. A 71 year-old male patient with a 10 year history of dementia was administered three doses of AN1792. He died a year later due to failure to thrive, but did not develop meningoencephalitis. a | Gross morphology of the brain showed preservation of the limbic structures and cortical ribbon. b | In the frontal cortex, although amyloid-β immunoreactive macrophages were abundant, no amyloid plaques were detected. c | Cortical vessels exhibited persistent amyloidosis despite removal of the surrounding amyloid plaques. d | CD4-positive T cells (indicated by brown staining) were frequently located around blood vessels. e | In areas where amyloid was removed, the neuritic network (labeled with an anti-neurofilament antibody) appeared preserved.
Current trials
Passive immunization
At least seven passive AD immunotherapies are in clinical trials in patients with mild to moderate AD (Table 1).8 In 2008, elan and Wyeth reported that a phase IIa trial of bapineuzimab (AAB-001)–a humanized monoclonal antibody that recognizes the amino terminus of Aβ–in patients with mild to moderate AD did not meet the study’s end points for cognitive efficacy, although a trend for cognitive stabilization was observed.70 Post hoc analysis, however, demonstrated significant cognitive benefits from this treatment in multiple tests for patients who did not carry the apolipoprotein E (APOE) ε4 allele– a major genetic risk factor for AD. By contrast, only a trend towards benefit was observed in APOE ε4 ca rriers, possibly because of accelerated pathogenesis in these individuals. The outcomes from the post hoc analysis are encouraging; however, these results should be interpreted with caution, as the use of multiple comparisons in post hoc analyses is controversial.
Table 1.
Ongoing clinical trials of passive Aβ immunotherapies
| Therapy | Sponsors | Antibody | Phase and number of trials |
Estimated patient enrollment |
treatment duration |
Primary outcome measures |
Estimated completion date of final trial |
|---|---|---|---|---|---|---|---|
| Bapineuzumab (AAB-001)* |
Elan; Wyeth; JANSSEN Alzheimer Immunotherapy |
Anti-Aβ amino terminal MAb |
III; one trial | 1,350 | 2.5 years | Safety and efficacy‡ | July 2012 |
| Bapineuzumab (AAB-001)* |
Elan; Wyeth; JANSSEN Alzheimer Immunotherapy |
Anti-Aβ amino terminal MAb |
III; five trials | 4,650§ | 18 months | Cognition and global function |
April 2011 |
| Solanezumab (LY2062430) |
Eli Lilly | Anti-Aβ mid-region MAb |
III; two trials | 2,000 | 19 months | Cognition and global function |
July 2012 |
| Gammagard™ IVIg (10%)∥ |
Baxter Healthcare; Alzheimer’s Disease Consortium Study |
Pooled human antibodies |
III; one trial | 360 | 18 months | Cognition and global function |
July 2011 |
| Bapineuzumab (AAB-001)¶ |
Wyeth | Anti-Aβ mid-region MAb |
II; one trial | 120 | 6 months | Treatment-related adverse effects |
March 2010 |
| PF-04360365 | Pfizer | Anti-Aβ MAb | II; two trials | 211 | 12–18 months |
Safety, tolerability and pharmacokinetics |
November 2011 |
| R1450 | Hoffman-LaRoche | Fully human MAb | II; one trial | 60 | 3–12 months | Safety and tolerability | May 2009 |
| IVIg (10%) | Octapharma | Pooled human antibodies |
II; one trial | 56 | 6 months | Changes in plasma Aβ levels |
September 2009 |
| GSK933766A | GlaxoSmithKline | Anti-Aβ antibody | I: one trial | 122 | 12 months | Safety, tolerability and treatment-related adverse effects |
August 2010 |
Delivered by intravenous injection.
Long-term extension of earlier AAB-001 phase II trial.
Participants stratified according to apolipoprotein E genotype.
Gammagard produced by Baxter Healthcare, Deerfield, IL, USA.
Delivered by subcutaneous injection. Abbreviations: Aβ, amyloid-β; IVIg, intravenous immunoglobulin; MAb, monoclonal antibody.
Ongoing clinical trials that include the separation of APOE ε4 carriers into different treatment groups, as well as additional cognitive measures, should help to resolve whether bapineuzimab demonstrates efficacy in AD. Several large phase III bapineuzumab trials are currently underway. In five of these studies, more than 4,000 patients have been stratified according to APOE ε4 status to see whether this genetic difference affects the efficacy of the drug. In these trials, carriers of APOE ε4 are only receiving the lowest dose (0.5 mg/kg) of the treatment as a result of incidences of transient vasogenic edema observed in the phase II study, particularly in this subgroup. An extension study involving 1,350 patients with AD who participated in early bapineuzumab trials is also ongoing. Participants in this study will receive bapi neuzumab by intravenous injection for 2.5 years. In April 2009, elan and Wyeth dropped the highest of the three bapi neuzumab doses (2 mg/kg) in APOE ε4 non-carriers because of the risk of vasogenic edema; these patients are now being dosed with 1 mg/kg.71 In addition, sub cutaneous injection of bapineuzumab is being in vestigated in 120 patients with AD in a phase II clinical trial.
In 2008, eli Lilly reported that a 12 week phase II trial of solanezumab (LY2062430)–a humanized mono clonal antibody that recognizes the middle region of Aβ and binds soluble forms of the peptide–successfully met the safety and tolerability end points. In this study, solane zumab was administered to 52 patients with mild to moderate AD in doses of up to 400 mg per week. The Alzheimer Disease Assessment Scale-Cognition scores and CSF tau levels were unchanged in these patients.72,73 Interestingly, amino-terminal truncated Aβ species (including pyroglutamate-modified Aβ) were detected in patients’ blood following, but not before, passive immuniza tion, suggesting a sequestration of Aβ from the brain to the periphery.74 Two large phase III studies of solanezu mab in a total of 2,000 patients with mild to moderate AD are underway.
Another strategy for passive immunization is the injection of intravenous immunoglobulin (IvIg), a pooled mixture of natural human immunoglobulins that include Aβ antibodies (those recognizing Aβ oligomers and fibrils, among others).75 IvIg antibodies have been shown to interfere with the oligomerization and fibrillization of Aβ,76,77 protect neurons against Aβ-mediated toxicity,76 and promote Aβ clearance from the brain.78 In a pilot study, IvIg treatment led to a reduction in total Aβ levels in CSF, an increase in serum Aβ levels, and stabilization of cognition in five patients with AD.79 In one 18 month study in eight patients with mild AD, IvIg therapy was administered for 6 months, stopped for 3 months, and then resumed for 9 months. IvIg-treated patients showed signs of cognitive improvement after 6 months. However, cognitive function declined to baseline during the washout period, and then stayed at baseline during the subsequent 9 months.80 Aβ levels in CSF were reduced only during the periods of IvIg infusion. In addition, anti-Aβ antibodies were detected in the CSF of the patients following IvIg treatment, indicating that IvIg antibodies might cross the blood–brain barrier and lower Aβ levels in the brain. Baxter Healthcare and the Alzheimer’s Disease Consortium Study have initiated a phase III IvIg study. One other IvIg study, sponsored by Octapharma, is in progress. In addition, several phase I trials of other passive Aβ immuno therapies are underway (Table 1).
Active immunization
Following halting of the dosing in the phase II AN1792 trial for safety reasons, the second-generation Aβ vaccines currently in clinical trials have been designed to avoid stimulating adverse immune responses (Table 2).8 elan and Wyeth’s Aβ vaccine ACC-001, an Aβ aminoterminal immunoconjugate, was shown to be safe in a phase I study and is currently in phase II clinical trials in ~360 patients with mild to moderate AD. This phase II trial was put on hold briefly because of a skin lesion in one patient; however, none of the adverse effects observed in the AN7192 trial have been observed in this new study.81
Table 2.
Ongoing clinical trials of active amyloid-β immunotherapies
| Therapy | Sponsors | Vaccine | Phase and number of trials |
Estimated patient enrollment |
treatment duration |
Primary outcome measures |
Estimated completion date of final trial |
|---|---|---|---|---|---|---|---|
| ACC-001 | Elan; Wyeth | Aβ amino-terminal conjugate |
II; three trials | 360 | 24 months | Safety, tolerability and treatment-related adverse effects |
May 2012 |
| CAD-105 | Novartis | Aβ1–5coupled to Qb virus-like particles |
II; three trials | 84 | 12–24 months |
Safety and tolerability | June 2011 |
| Affitope AD01; Affitope AD02 |
Affiris AG; GlaxoSmithKline |
Aβ amino-terminal mimotope ± adjuvant |
Ib; two trials | 48 | 12 months | Safety and tolerability* |
December 2009 |
| V950 | Merck | Aβ amino-terminal peptides conjugated to ISCO-MATRIX®‡ |
I; one trial | 124 | 48 months | Safety and tolerability | April 2014 |
| UB311 | United Biochemical | Aβ1–14 using UBITh®§ | I; one trial | 18 | 7 months | Safety and tolerability | December 2010 |
Long-term extension of previous phase I trial.
ISCO-MATRIX® produced by CSL Behring, King of Prussia, PA, USA.
UBITh® produced by United Biomedical, Hauppauge, NY, USA. Abbreviation: Aβ, amyloid-β.
Novartis also has an Aβ vaccine (CAD-106) in clinical trials. CAD-106 comprises multiple copies of Aβ1–6 coupled to Qb virus-like particles. A first-in-man trial of CAD-106 was conducted in Sweden in 58 patients with mild to moderate AD. Individuals in this trial received three 50 μg or 150 μg doses of the vaccine.82 Low antibody titers were observed with the 50 μg dose of CAD-106; however, at the 150 μg dose, antibody titers were twofold higher. No differences were observed in the exploratory outcome measures–clinical assessment, Aβ CSF levels or whole brain volume by MRI–between CAD-106- treated and placebo-treated patients. A phase II trial of this vaccine is underway in ~84 patients with mild AD.
Affiris AG have reported that in separate phase I clinical trials, each conducted in 24 patients with mild to moderate AD, two peptide vaccines mimicking parts of the aminoterminus of the Aβ sequence, AD01 and AD02, were found to be safe after four single-dose vaccinations were given 4 weeks apart.83 Long-term tolerability phase 1b studies are underway for these vaccines. Merck and united Biochemical both also have active Aβ vaccines in phase I trials (Table 2).
Improving amyloid-β immunotherapy
Predicting Alzheimer disease
Aβ immunotherapy has a high potential for lowering cerebral Aβ levels and protecting cognition; however, as indicated in animal models, this approach to therapy seems to be more effective when administered early in the disease course. Thus, one of the biggest hurdles to preventing AD by immunization is our ability to identify people at risk of developing the disease beyond those individuals bearing genetic mutations in APP or the presenilin genes. Currently, a definitive diagnosis of AD is made at autopsy, on the basis of neuropathological presence of amyloid plaques and NFTs—lesions that accumulate over a number of years before neuronal loss and clinical dementia. Progress in biomarkers and imaging technology, combined with more-sophisticated neuro psychological testing has increased the likelihood that cohorts of healthy, middle-aged and elderly indivi duals at risk of developing AD can be identified. The presence of an APOE ε4 allele increases the risk of AD but does not determine exactly when, or indeed if, an individual will develop the disease. Fagan and colleagues have shown that in individuals with very mild or mild AD (Clinical Dementia Ratings 0.5 and 1, respectively), CSF levels of Aβ1–42 are reduced compared with healthy aged-matched controls, while levels of CSF tau and p-tau181 are both increased.84 A similar profile of CSF changes was reported in patients with mild cognitive impairment (MCI) who later converted to AD.85 In another study, low CSF Aβ1–42 levels were associated with a reduction in whole-brain volume, as measured by structural MRI. This finding suggested that as Aβ aggregates and forms cerebral plaques, brain volume undergoes atrophy.73 In this study, increases in the levels of CSF tau and p-tau181 occurred after CSF Aβ levels decreased. Moreover, these increases in tau levels were more strongly associated with neuronal damage and cognitive decline than were CSF Aβ levels. One study has reported that 20–40% of elderly individuals without dementia had at least some, if not complete, neuropathological findings of AD at autopsy. More over, this study revealed that the presence of AD lesions was associated with cognitive dysfunction in specific tests of episodic memory, semantic knowledge, visual spatial ability, and/or executive function.87
Brain imaging, when combined with the afore mentioned biomarkers, greatly increases our ability to predict who is at risk of developing AD several years before disease onset. Brain volume measurements by structural MRI can discriminate between MCI and AD and predict conversion of MCI to AD with high accuracy.88 ventricular volume, as measured by MRI, was shown to predict MCI in healthy elderly individuals followed up for 15 years.89 Another advance in AD brain imaging is PeT with 11C-labeled Pittsburgh compound B (PIB), which labels amyloid in the brains of living individuals.90 In AD, PIB binding has been associated with brain atrophy,91 a reduction in CSF Aβ1–42 levels,84 and a decrease in glucose metabolism.92 Somewhat surprisingly, high PIB binding has been reported in healthy elderly individuals, suggesting that cerebral amyloid deposition precedes cognitive impairment in AD.93–96 Thus, in the future, a combination of imaging (for example, MRI and/or PIB-PeT) and biomarkers (such as low CSF Aβ1–42 levels and high CSF tau and phospho-tau181 levels) might be used to identify and define groups of individuals for AD prevention trials.
Immunotherapy considerations
Aβ immunization looks like a promising approach for combating AD, yet a number of issues pertaining to the treatments themselves need to be considered for this strategy to succeed, including the type of immunotherapy (Box 4). Indeed, long-term prevention might be more feasible with active than with passive immuniza tion, especially when one considers vaccinating very large populations over long periods of time. Strategies targeting specific Aβ conformations that are known to be toxic to neurons might benefit from antibodies directed against the amino terminus (immunodominant epitope of Aβ), given the poor accessibility of other portions of Aβ in aggregates.25 Whether dissolving existing plaques will result in the release of soluble toxic Aβ species (for example, oligomers) and, if so, for how long, remains unclear. Immunization before plaque deposition might avoid this problem. As a result of Aβ immunotherapy-induced microhemorrhage in transgenic mouse models of AD with CAA and vasogenic edema in humans (especially in APOE ε4 carriers), immunization might need to be administered before the build up of amyloid in blood vessel walls to avoid these adverse effects. Intraneuronal Aβ might act as a nidus for Aβ deposition (intra neuronally and extracellularly) in the soma and along processes and terminals of affected neurons. Thus, limiting intra neuronal Aβ formation at an early stage of AD development might be protective.97,98 An immunological approach for the reduction of Aβ formation by targeting the β-secretase cleavage site of APP has been proposed.99
Box 4. Active versus passive amyloid-β immunization.
Both active and passive amyloid-β (Aβ) immunotherapies have their advantages and disadvantages. Active Aβ immunotherapy has the potential to be more cost-effective and long-lasting, with fewer visits to the doctor, than passive immunization, which requires monthly infusions of costly humanized monoclonal antibodies. Active therapies might have additional benefits. One study has reported that Aβ vaccination in nonhuman primates led to an increase in the production of crossreactive, potentially protective Aβ autoantibodies.131 Such antibodies are typically lower in patients with AD than in age-matched healthy individuals;132 therefore, boosting the levels of these proteins might be beneficial.
Vaccination usually involves delivery of a strong adjuvant to boost antibody production. Such adjuvants could potentially induce an undesirable immune response, especially in elderly individuals in whom proinflammatory cytokines are already above normal levels.100 In these cases, a passive Aβ immunotherapy might be beneficial. One way to circumvent this problem for Aβ vaccines could be to use anti-inflammatory T helper 2-biased adjuvants, which might help avoid unwanted adverse effects while still increasing antibody production. Another problem for active therapies is that if an adverse event does occur, the immune response to the active vaccine can be difficult to stop quickly. Passive Aβ immunotherapies, on the other hand, can be stopped at any time. Another advantage to the passive therapeutic approach is the use of antibodies that are specific for particular Aβ conformations or species thought to be most toxic, thereby avoiding removaof all Aβ from the brain. One disadvantage of the passive approach is the potential for patients to eventually develop neutralizing antibodies against the passive therapy.
The ability of the human body to generate a strong humoral response or fight infection declines with aging, a condition known as immunosenescence.100,101 In addition, the immune system might participate in AD pathogenesis. Chronic exposure of humans and transgenic mice to Aβ aggregates seems to lead to cellular and humoral hyporesponsiveness to the peptide, which could, in turn, contribute to the disease process.26 Thus, a preventive AD vaccine started before the build up of aggregated Aβ and/or immunosenescence (for example, at 50–60 years of age) might lead to a more robust and long-lasting humoral immune response and, hence, high levels of production of Aβ-lowering antibodies. A prevention trial, however, could take many years and incur high costs. Identifying individuals at risk of developing AD might improve the feasibility of such trials.
Many healthy middle-aged individuals with a family history of AD might be willing to participate in a clinical AD prevention trial; however, before any such trial could take place, safety and efficacy of the immunotherapy in patients with AD will probably be required. unfortunately, little evidence exists that Aβ immunotherapy can reverse cognitive impairment in patients with mild to moderate AD. Indeed, stopping the progression of neuro degeneration and cognitive decline once it is well underway might be diffi cult, even in the context of plaque clearing,63 suggest ing again that early immunization might improve the efficacy of Aβ immunotherapy. Ongoing clinical trials should provide some further clarification about whether Aβ immunotherapy is effective in patients who already have AD. These trials, if negative, will still not determine whether AD can be prevented if Aβ is lowered presymptomatically, before the onset of substantial neurodegeneration.
Conclusions
Aβ immunotherapy continues to show promise as a strategy for preventing AD or treating the disease in its early stages. Preclinical studies in animal models have shown clear evidence that Aβ immunization can prevent cerebral amyloid pathology, synaptic degeneration and cognitive deficits. early human clinical trials of the AN1792 Aβ vaccine were associated with adverse effects but also showed some signs of efficacy. Indeed, on the basis of these studies, at least 13 Aβ immunotherapies are currently in human clinical trials, mostly in patients with mild to moderate AD. If successful, these trials could lead to the first evidence of a disease-modifying treatment for AD. Moreover, such an outcome would indicate successful translation of preclinical studies in animal models to clinical efficacy in humans.
Aβ immunotherapy might, in the future, be an effective treatment for preventing AD. Imaging and bio markers have improved dramatically over the past 10 years, increasing the probability of identifying at-risk indivi duals before clinical symptom onset and allowing the Aβ immunotherapy response to be monitored. If given early, before AD pathogenesis is well underway, Aβ immunization might be able to prevent aggregation of neurotoxic forms of Aβ, thereby preventing downstream effects, such as synaptic dy sfunction, neuronal damage and cognitive impairment.
Review criteria.
Articles for review were identified from PubMed and Google searches using the following terms: “Aβ immunotherapy”, “immunotherapy and Alzheimer’s disease”, “Alzheimer’s vaccine”, “Aβ vaccine”, “Aβ immunization”, “AN1792”, “CAD106”, “bapineuzumab”, “solaneuzumab”, “Alzheimer’s disease clinical trials”, “affitope”, and “Alzheimer’s disease treatments”. Only articles published in English and from 1995 were retrieved and considered for review. Information regarding Aβ immunotherapy clinical trials was found on clinicaltrials.gov and company websites. Abstracts and reports from meetings were used to find the most recent results of clinical trials.
Acknowledgments
The authors would like to thank Douglas Galasko (UCSD, La Jolla, CA, USA) for his helpful comments and Anahit Ghochikyan (Institute for Molecular Medicine, Huntington Beach, CA, USA) for her help in the design of Figure 1. This work was supported by NIH grants to C. A. Lemere (RO1AG20159) and E. Masliah (AG5131, AG18440, AG02074 and AG10435).
Footnotes
Competing interests C. A. Lemere declares an association with the following companies: Elan, Wyeth. See the article online for full details of the relationships. E. Masliah declares no competing interests.
References
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi H. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2.Dickson DW. The pathogenesis of senile plaques. J. Neuropathol. Exp. Neurol. 1997;56:321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe MS. Shutting down Alzheimer’s. Sci. Am. 2006;294:72–79. doi: 10.1038/scientificamerican0506-72. [DOI] [PubMed] [Google Scholar]
- 5.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 6.Klein W. Aβ toxicity in Alzheimer’s disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem. Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 7.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 8.ClinicalTrials.gov 2009 [online], http://www.clinicaltrials.gov.
- 9.Solomon B, Koppel R, Hanan E, Katzav T. Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer β-amyloid peptide. Proc. Natl Acad. Sci. USA. 1996;93:452–455. doi: 10.1073/pnas.93.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon B, Koppel R, Frenkel D, Hanan-Aharon E. Disaggregation of Alzheimer β-amyloid by site-directed mAb. Proc. Natl Acad. Sci. USA. 1997;94:4109–4112. doi: 10.1073/pnas.94.8.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schenk D, et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 12.Lemere CA, et al. Nasal Aβ treatment induces anti-Aβ antibody production and decreases cerebral amyloid burden in PD-APP mice. Ann. NY Acad. Sci. 2000;920:328–331. doi: 10.1111/j.1749-6632.2000.tb06943.x. [DOI] [PubMed] [Google Scholar]
- 13.Weiner HL, et al. Nasal administration of amyloid-β peptide decreases cerebral amyloid burden in a mouse model of Alzheimer’s disease. Ann. Neurol. 2000;48:567–579. [PubMed] [Google Scholar]
- 14.Das P, Murphy M, Younkin L, Younkin S, Golde T. Reduced effectiveness of Aβ1–42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiol. Aging. 2001;22:721–727. doi: 10.1016/s0197-4580(01)00245-7. [DOI] [PubMed] [Google Scholar]
- 15.Sigurdsson EM, Scholtzova H, Mehta PD, Frangione B, Wisniewski T. Immunization with a nontoxic/nonfibrillar amyloid-β homologous peptide reduces Alzheimer’s disease-associated pathology in transgenic mice. Am. J. Pathol. 2001;159:439–447. doi: 10.1016/s0002-9440(10)61715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maier M, et al. Short amyloid-β (Aβ) immunogens reduce cerebral Aβ load and learning deficits in an Alzheimer’s disease mouse model in the absence of an Aβ-specific cellular immune response. J. Neurosci. 2006;26:4717–4728. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janus C, et al. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 18.Morgan D, et al. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 19.Oddo S, et al. Reduction of soluble Aβ and tau, but not soluble Aβ alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J. Biol. Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- 20.Head E, et al. A two-year study with fibrillar β-amyloid (Aβ) immunization in aged canines: effects on cognitive function and brain Aβ. J. Neurosci. 2008;28:3555–3566. doi: 10.1523/JNEUROSCI.0208-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemere CA, Maron R, Selkoe DJ, Weiner HL. Nasal vaccination with β-amyloid peptide for the treatment of Alzheimer’s disease. DNA Cell Biol. 2001;20:705–711. doi: 10.1089/10445490152717569. [DOI] [PubMed] [Google Scholar]
- 22.Town T, et al. Characterization of murine immunoglobulin G antibodies against human amyloid-β1–42. Neurosci. Lett. 2001;307:101–104. doi: 10.1016/s0304-3940(01)01951-6. [DOI] [PubMed] [Google Scholar]
- 23.McLaurin J, et al. Therapeutically effective antibodies against amyloid-β peptide target amyloid-β residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat. Med. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- 24.Cribbs DH, et al. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with β-amyloid. Int. Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardberg AS, et al. Molecular basis for passive immunotherapy of Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 2007;104:15659–15664. doi: 10.1073/pnas.0705888104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monsonego A, Maron R, Zota V, Selkoe D, Weiner H. Immune hyporesponsiveness to amyloid-β peptide in amyloid precursor protein transgenic mice: implications for the pathogenesis and treatment of Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 2001;98:10273–10278. doi: 10.1073/pnas.191118298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das P, Chapoval S, Howard V, David CS, Golde TE. Immune responses against Aβ1–42 in HLA class II transgenic mice: implications for Aβ1–42 immune-mediated therapies. Neurobiol. Aging. 2003;24:969–976. doi: 10.1016/s0197-4580(03)00036-8. [DOI] [PubMed] [Google Scholar]
- 28.DeMattos R, et al. Peripheral anti-Aβ antibody alters CNS and plasma clearance and decreases brain Aβ burden in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bard F, et al. Epitope and isotype specificities of antibodies to β-amyloid for protection against Alzheimer’s disease-like neuropathology. Proc. Natl Acad. Sci. USA. 2003;100:2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bard F, et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 31.Kotilinek LA, et al. Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J. Neurosci. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dodart JC, et al. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer’s disease model. Nat.Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 33.Pfeifer M, et al. Cerebral hemorrhage after passive anti-Aβ immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- 34.Racke MM, et al. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid β. J. Neurosci. 2005;25:629–636. doi: 10.1523/JNEUROSCI.4337-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilcock DM, et al. Passive immunotherapy against Aβ in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J. Neuroinflammation. 2004;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Aβ immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Chauhan NB, Siegel GJ. Reversal of amyloid β toxicity in Alzheimer’s disease model Tg2576 by intraventricular antiamyloid β antibody. J. Neurosci. Res. 2002;69:10–23. doi: 10.1002/jnr.10286. [DOI] [PubMed] [Google Scholar]
- 38.Chauhan NB, Siegel GJ. Intracerebroventricular passive immunization with anti-Aβ antibody in Tg2576. J. Neurosci. Res. 2003;74:142–147. doi: 10.1002/jnr.10721. [DOI] [PubMed] [Google Scholar]
- 39.Klyubin I, et al. Amyloid β protein immunotherapy neutralizes Aβ oligomers that disrupt synaptic plasticity in vivo. Nat. Med. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- 40.Klyubin I, et al. Amyloid β protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J. Neurosci. 2008;28:4231–4237. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spires-Jones TL, et al. Passive immunotherapy rapidly increases structural plasticity in a mouse model of Alzheimer disease. Neurobiol. Dis. 2009;33:213–220. doi: 10.1016/j.nbd.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayer AJ, et al. Evaluation of the safety and immunogenicity of synthetic Aβ42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- 43.Orgogozo JM, et al. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 44.Gilman S, et al. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 45.Wisniewski T, Frangione B. Immunological and anti-chaperone therapeutic approaches for Alzheimer disease. Brain Pathol. 2005;15:72–77. doi: 10.1111/j.1750-3639.2005.tb00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pride M, et al. Progress in the active immunotherapeutic approach to Alzheimer’s disease: clinical investigations into AN1792-associated meningoencephalitis. Neurodegener. Dis. 2008;5:194–196. doi: 10.1159/000113700. [DOI] [PubMed] [Google Scholar]
- 47.Lee M, et al. Aβ42 immunization in Alzheimer’s disease generates Aβ N-terminal antibodies. Ann. Neurol. 2005;58:430–435. doi: 10.1002/ana.20592. [DOI] [PubMed] [Google Scholar]
- 48.Monsonego A, et al. Increased T cell reactivity to amyloid β protein in older humans and patients with Alzheimer disease. J. Clin. Invest. 2003;112:415–422. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hock C, et al. Antibodies against β-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 50.Vellas B. Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr. Alzheimer Res. 2009;6:144–151. doi: 10.2174/156720509787602852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fox NC, et al. Effects of Aβ immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- 52.Masliah E, et al. Re-evaluation of the structural organization of neuritic plaques in Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 1993;52:619–632. doi: 10.1097/00005072-199311000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Braak E, Braak H, Mandelkow EM. A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol. 1994;87:554–567. doi: 10.1007/BF00293315. [DOI] [PubMed] [Google Scholar]
- 54.Hof P, Morrison J. In: Alzheimer Disease. Terry R, Katzman R, Bick K, editors. Raven Press; New York: 1994. pp. 197–230. [Google Scholar]
- 55.Perry EK, et al. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br. Med. J. 1978;2:1457–1459. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perry E. Cholinergic signaling in Alzheimer disease: therapeutic strategies. Alzheimer Dis. Assoc. Disord. 1995;9(Suppl. 2):1–2. [PubMed] [Google Scholar]
- 57.Trojanowski JQ, et al. Altered tau and neurofilament proteins in neuro-degenerative diseases: diagnostic implications for Alzheimer’s disease and Lewy body dementias. Brain Pathol. 1993;3:45–54. doi: 10.1111/j.1750-3639.1993.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 58.Terry RD, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 59.Masliah E, et al. Synaptic and neuritic alterations during the progression of Alzheimer’s disease. Neurosci. Lett. 1994;74:67–72. doi: 10.1016/0304-3940(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 60.Stokin GB, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 61.Spires TL, et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J. Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moolman DL, Vitolo OV, Vonsattel JP, Shelanski ML. Dendrite and dendritic spine alterations in Alzheimer models. J. Neurocytol. 2004;33:377–387. doi: 10.1023/B:NEUR.0000044197.83514.64. [DOI] [PubMed] [Google Scholar]
- 63.Holmes C, et al. Long-term effects of Aβ42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 64.Ferrer I, Boada Rovira M, Sánchez Guerra ML, Rey MJ, Costa-Jussá F. Neuropathology and pathogenesis of encephalitis following amyloid-β immunization in Alzheimer’s disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicoll JA, et al. Aβ species removal after Aβ42 immunization. J. Neuropathol. Exp. Neurol. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- 66.Bombois S, et al. Absence of β-amyloid deposits after immunization in Alzheimer disease with Lewy body dementia. Arch. Neurol. 2007;64:583–587. doi: 10.1001/archneur.64.4.583. [DOI] [PubMed] [Google Scholar]
- 67.Masliah E, et al. Aβ vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- 68.Patton RL, et al. Amyloid-β peptide remnants in AN-1792-immunized Alzheimer’s disease patients: a biochemical analysis. Am. J. Pathol. 2006;169:1048–1063. doi: 10.2353/ajpath.2006.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boche D, et al. Consequence of Aβ immunization on the vasculature of human Alzheimer’s disease brain. Brain. 2008;131:3299–3310. doi: 10.1093/brain/awn261. [DOI] [PubMed] [Google Scholar]
- 70.Grundman M, Black R. Clinical trials of bapineuzumab, a β-amyloid-targeted immunotherapy in patients with mild to moderate Alzheimer’s disease [abstract O3-04-05] Alzheimers Dementia. 2008;4:T166. [Google Scholar]
- 71.Elan and Wyeth plan to amend bapineuzumab phase 3 protocols. 2009 Press release [online], http://newsroom.elan.com/phoenix.zhtml?c=88326&p=irolnewsArticle&ID=1272546&highlight.
- 72.Siemers ER, et al. Safety, tolerability and biomarker effects of an Aβ monoclonal antibody administered to patients with Alzheimer’s disease [abstract P4–346] Alzheimers Dementia. 2008;4:T774. [Google Scholar]
- 73.Siemers ER, et al. Measurement of cerebrospinal fluid total tau and phospho-tau in phase 2 trials of therapies targeting Aβ [abstract] Alzheimers Dementia. 2009;5:P258. [Google Scholar]
- 74.De Mattos RB, et al. Identification, characterization, and comparison of aminoterminally truncated Aβ42 peptides in Alzheimer’s disease brain tissue and in plasma from Alzheimer’s patients receiving solanezumab immunotherapy treatment [abstract] Alzheimers Dementia. 2009;5:P156–P157. [Google Scholar]
- 75.Relkin NR. Natural human antibodies targeting amyloid aggregates in intravenous immunoglobulin [abstract S1-02-02] Alzheimers Dementia. 2008;4:T101. [Google Scholar]
- 76.Du Y, et al. Human anti-β-amyloid antibodies block β-amyloid fibril formation and prevent β-amyloid-induced neurotoxicity. Brain. 2003;126:1935–1939. doi: 10.1093/brain/awg191. [DOI] [PubMed] [Google Scholar]
- 77.Ma QL, et al. Antibodies against β-amyloid reduce Aβ oligomers, glycogen synthase kinase-3β activation and τ phosphorylation in vivo and in vitro. J. Neurosci. Res. 2006;83:374–384. doi: 10.1002/jnr.20734. [DOI] [PubMed] [Google Scholar]
- 78.Istrin G, Bosis E, Solomon B. Intravenous immunoglobulin enhances the clearance of fibrillar amyloid-β peptide. J. Neurosci. Res. 2006;84:434–443. doi: 10.1002/jnr.20886. [DOI] [PubMed] [Google Scholar]
- 79.Dodel RC, et al. Intravenous immunoglobulins containing antibodies against β-amyloid for the treatment of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2004;75:1472–1474. doi: 10.1136/jnnp.2003.033399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Relkin NR, et al. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol. Aging. 2008;30:1728–1736. doi: 10.1016/j.neurobiolaging.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 81.Strobel G. An eFAD prevention trial—one man’s view. Alzheimer Research Forum. 2009 [online], http://www.alzforum.org/new/detail.asp?id=2273.
- 82.Bengt G. Results of the first-in-man study with the active Aβ immunotherapy CAD106 in Alzheimer patients [abstract] Alzheimers Dementia. 2009;5:P113–P114. [Google Scholar]
- 83.Schneeberger A, Mandler M, Zauner W, Mattner F, Schmidt W. Development of Alzheimer AFFITOPE vaccines—from concept to clinical testing [abstract] Alzheimers Dementia. 2009;5:P257. doi: 10.1007/s12603-009-0070-5. [DOI] [PubMed] [Google Scholar]
- 84.Fagan AM, et al. Cerebrospinal fluid tau/ β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch. Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 85.Mattsson N, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 86.Fagan AM, et al. Decreased cerebrospinal fluid Aβ42 correlates with brain atrophy in cognitively normal elderly. Ann. Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Price JL, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol. Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Craig-Schapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer’s disease. Neurobiol. Dis. 2009;35:128–140. doi: 10.1016/j.nbd.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carlson NE, et al. Trajectories of brain loss in aging and the development of cognitive impairment. Neurology. 2008;70:828–833. doi: 10.1212/01.wnl.0000280577.43413.d9. [DOI] [PubMed] [Google Scholar]
- 90.Mathis CA, et al. Synthesis and evaluation of11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J. Med. Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 91.Archer HA, et al. Amyloid load and cerebral atrophy in Alzheimer’s disease: an 11C-PIB positron emission tomography study. Ann. Neurol. 2006;60:145–147. doi: 10.1002/ana.20889. [DOI] [PubMed] [Google Scholar]
- 92.Klunk W, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburg Compound-B. Ann. Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 93.Fagan A, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann. Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 94.Pike KE, et al. β-Amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 95.Rowe CC, et al. Imaging β-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 96.Mormino EC, et al. Episodic memory loss is related to hippocampal-mediated β-amyloid deposition in elderly subjects. Brain. 2008;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gouras GK, et al. Intraneuronal Aβ42 accumulation in human brain. Am. J. Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tampellini D, et al. Internalized antibodies to the Aβ domain of APP reduce neuronal Aβ and protect against synaptic alterations. J. Biol. Chem. 2007;282:18895–18906. doi: 10.1074/jbc.M700373200. [DOI] [PubMed] [Google Scholar]
- 99.Arbel M, Solomon B. A novel immunotherapy for Alzheimer’s disease: antibodies against the β-secretase cleavage site of APP. Curr. Alzheimer Res. 2007;4:437–445. doi: 10.2174/156720507781788792. [DOI] [PubMed] [Google Scholar]
- 100.Effros RB, et al. Workshop on HIV infection and aging: what is known and future research directions. Clin. Infect. Dis. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ghochikyan A. Rationale for peptide and DNA based epitope vaccines for Alzheimer’s disease immunotherapy. CNS Neurol. Disord. Drug Targets. 2009;8:128–143. doi: 10.2174/187152709787847298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 103.Hardy J, Higgins G. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 104.Winklhofer KF, Tatzelt J, Haass C. The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. EMBO J. 2008;27:336–349. doi: 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Knobloch M, Farinelli M, Konietzko U, Nitsch RM, Mansuy IM. Aβ oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms. J. Neurosci. 2007;27:7648–7653. doi: 10.1523/JNEUROSCI.0395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shankar GM, et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 108.Chiba T, et al. Amyloid-β causes memory impairment by disturbing the JAK2/STAT3 axis in hippocampal neurons. Mol. Psychiatry. 2009;14:206–222. doi: 10.1038/mp.2008.105. [DOI] [PubMed] [Google Scholar]
- 109.Selkoe DJ. Toward a comprehensive theory for Alzheimer’s disease. Hypothesis: Alzheimer’s disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann. NY Acad. Sci. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- 110.Frenkel D, Dewachter I, Van Leuven F, Solomon B. Reduction of β-amyloid plaques in brain of transgenic mouse model of Alzheimer’s disease by EFRH-phage immunization. Vaccine. 2003;21:1060–1065. doi: 10.1016/s0264-410x(02)00609-6. [DOI] [PubMed] [Google Scholar]
- 111.Agadjanyan MG, et al. Prototype Alzheimer’s disease vaccine using the immunodominant B cell epitope from β-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J. Immunol. 2005;174:1580–1586. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- 112.Petrushina I, et al. Alzheimer’s disease peptide epitope vaccine reduces insoluble but not soluble/oligomeric Aβ species in amyloid precursor protein transgenic mice. J. Neurosci. 2007;27:12721–12731. doi: 10.1523/JNEUROSCI.3201-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seabrook TJ, et al. Dendrimeric Aβ1–15 is an effective immunogen in wildtype and APP tg mice. Neurobiol. Aging. 2006;28:813–823. doi: 10.1016/j.neurobiolaging.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 114.Bowers WJ, et al. HSV amplicon-mediated Aβ vaccination in Tg2576 mice: differential antigenspecific immune responses. Neurobiol. Aging. 2005;26:393–407. doi: 10.1016/j.neurobiolaging.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 115.Wang CY, et al. Site-specific UBITh amyloid-β vaccine for immunotherapy of Alzheimer’s disease. Vaccine. 2007;25:3041–3052. doi: 10.1016/j.vaccine.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 116.Okura Y, et al. Nonviral Abeta DNA vaccine therapy against Alzheimer’s disease: long-term effects and safety. Proc. Natl Acad. Sci. USA. 2006;103:9619–9624. doi: 10.1073/pnas.0600966103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Movsesyan N, et al. Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine—a novel immunotherapeutic strategy. PLoS ONE. 2008;3:e2124. doi: 10.1371/journal.pone.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Movsesyan N, et al. DNA epitope vaccine containing complement component C3d enhances anti-amyloid-β antibody production and polarizes the immune response towards a Th2 phenotype. J. Neuroimmunol. 2008;205:57–63. doi: 10.1016/j.jneuroim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bach P, et al. Vaccination with Aβ-displaying virus-like particles reduces soluble and insoluble cerebral Aβ and lowers plaque burden in APP transgenic mice. J. Immunol. 2009;182:7613–7624. doi: 10.4049/jimmunol.0803366. [DOI] [PubMed] [Google Scholar]
- 120.Lee EB, et al. Targeting amyloid-β peptide (Aβ) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Aβ precursor protein (APP) transgenic mice. J. Biol. Chem. 2006;281:4292–4299. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- 121.Lambert MP, et al. Monoclonal antibodies that target pathological assemblies of Aβ. J. Neurochem. 2007;100:23–35. doi: 10.1111/j.1471-4159.2006.04157.x. [DOI] [PubMed] [Google Scholar]
- 122.Levites Y, et al. Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid beta, amyloid β40, and amyloid β42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice. J. Neurosci. 2006;26:11923–11928. doi: 10.1523/JNEUROSCI.2795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang YJ, et al. Intramuscular delivery of a single chain antibody gene reduces brain Aβ burden in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 2009;30:364–376. doi: 10.1016/j.neurobiolaging.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 124.Meli G, Visintin M, Cannistraci I, Cattaneo A. Direct in vivo intracellular selection of conformation-sensitive antibody domains targeting Alzheimer’s amyloid-β oligomers. J. Mol. Biol. 2009;387:584–606. doi: 10.1016/j.jmb.2009.01.061. [DOI] [PubMed] [Google Scholar]
- 125.Sudol K, et al. Generating differentially targeted amyloid-β specific intrabodies as a passive vaccination strategy for Alzheimer’s disease. Mol. Ther. 2009;17:2031–2040. doi: 10.1038/mt.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Acero G, et al. Immunodominant epitope and properties of pyroglutamate-modified Aβ-specific antibodies produced in rabbits. J. Neuroimmunol. 2009;213:39–46. doi: 10.1016/j.jneuroim.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Das P, et al. Amyloid-β immunization effectively reduces amyloid deposition in FcRγ-/- knock-out mice. J. Neurosci. 2003;23:8532–8538. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bacskai BJ, et al. Non-Fc-mediated mechanisms are involved in clearance of amyloid-β in vivo by immunotherapy. J. Neurosci. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lemere CA, et al. Evidence for peripheral clearance of cerebral Aβ protein following chronic, active Aβ immunization in PSAPP mice. Neurobiol. Dis. 2003;14:10–18. doi: 10.1016/s0969-9961(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 130.Yamada K, et al. Aβ immunotherapy: intracerebral sequestration of Aβ by an anti-Aβ monoclonal antibody 266 with high affinity to soluble Aβ. J. Neurosci. 2009;29:11393–11398. doi: 10.1523/JNEUROSCI.2021-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Britschgi M, et al. Neuroprotective natural antibodies to assemblies of amyloidogenic peptides decrease with normal aging and advancing Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 2009;106:12145–12150. doi: 10.1073/pnas.0904866106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weksler ME, et al. Patients with Alzheimer’s disease have lower levels of serum anti-amyloid peptide antibodies than healthy elderly individuals. Exp. Gerontol. 2002;37:943–948. doi: 10.1016/s0531-5565(02)00029-3. [DOI] [PubMed] [Google Scholar]