Abstract
The present study compared the performance of individuals with Huntington's disease (HD) and Alzheimer's disease (AD) on three types of California Verbal Learning Test–Second Edition (CVLT-II) recognition discriminability indices (RDI): Source, Novel, and Total. The HD and AD groups did not differ significantly on Source RDI (all 16 targets versus the 16 previously presented, List B, distractors). However, HD patients performed significantly better than AD patients on Total RDI (all 16 targets versus all 32 distractors) and Novel RDI (all 16 targets versus 16 new distractors). Implications of these findings on the differentiation of the memory disorders associated with HD and AD are discussed.
Introduction
Huntington's disease (HD) is marked by early damage to the basal ganglia, particularly the caudate nucleus (Vonsattel, 2000; Vonsattel et al., 1985), which has extensive projections to frontal-lobe regions (Alexander, Crutcher, & DeLong, 1990; Crosson et al., 2003; Cummings, 1993). In contrast, the early stages of Alzheimer's disease (AD) are characterized by pathological changes in mesial-temporal/hippocampal areas that over time encroach into cortical association areas, with relative sparing of most subcortical structures (Braak & Braak, 1991; Hyman, Van Hoesen, Damasio, & Barnes, 1984). The different patterns of neurodegeneration associated with these two disorders yield distinct memory profiles (Butters, Wolfe, Granholm, & Martone, 1986; Delis et al., 1991; Salmon & Filoteo, 2007). Specifically, patients with HD present with significant deficits in recall of information, but they exhibit disproportionate improvement on recognition memory testing (Delis et al., 1991; Tröster et al., 1993). In contrast, patients with AD exhibit poor learning, abnormally rapid forgetting, and poor recognition memory (Delis et al., 1991; Tröster et al., 1993). These profiles have been interpreted as indicating that the locus of the memory impairment may be at the retrieval level in HD and at the encoding/storage level in AD (Butters et al., 1986; Butters, Wolfe, Martone, Granholm, & Cermak, 1985; Delis et al., 1991; Martone, Butters, Payne, Becker, & Sax, 1984; Moss, Albert, Butters, & Payne, 1986). However, a recent meta-analysis (Montoya et al., 2006) of studies examining episodic memory performance in HD indicates that both recognition and recall abilities are impaired in HD, suggesting that HD patients have at least some degree of encoding impairment.
In addition to the retrieval/encoding distinction, HD and AD patients have been found to differ in terms of their source memory abilities. A loss of contextual or source memory is thought to occur when remembrance of recently acquired items of information is relatively intact, in the context of more pronounced difficulties remembering when or where the information was acquired (F. J. Evans & Thorn, 1966; Shimamura & Squire, 1987, 1991). Furthermore, there is evidence that source memory problems are related to frontal-lobe impairment (Baldo, Delis, Kramer, & Shimamura, 2002; Craik, Morris, Morris, & Loewen, 1990; Duarte, Ranganath, & Knight, 2005; Janowsky, Shimamura, & Squire, 1989; Schacter, Harbluk, & McLachlin, 1984; Simons et al., 2002). For example, Baldo et al. (2002) found that on delayed recognition testing, patients with focal frontal lesions had difficulties determining which of the two word lists from the California Verbal Learning Test–Second Edition (CVLT-II; Delis, Kramer, Kaplan, & Ober, 2000) a particular item was derived from. Specifically, after being presented with Lists A and B, subjects were later asked on a recognition task if an item had been on List A. The patients with frontal lesions were able to differentiate between List A items and novel distractors, but they exhibited deficits in distinguishing List A items from List B distractor items, thereby implicating a source memory impairment. Furthermore, Brandt, Bylsma, Aylward, Rothlind, and Gow (1995) found that HD patients and frontal patients could not remember whether they had learned target information during the experiment or prior to the study, suggesting that HD patients may be similar to patients with frontal-lobe dysfunction in their tendency to confuse the source of newly learned information. From an anatomical perspective, this prediction is quite plausible given HD-associated pathology in the striatum (Aylward et al., 1996; de la Monte, Vonsattel, & Richardson, 1988; Harris et al., 1992), a consequence being disruption to frontal-striatal circuits (Alexander et al., 1990).
Although most memory measures, including the original CVLT (Delis, Kramer, Kaplan, & Ober, 1987), provide only a single measure of recognition discriminability, the CVLT-II (Delis et al., 2000) includes measures that parse yes/no recognition memory performance into several component functions. That is, the CVLT-II provides not only a Total Recognition Discriminability score, but also additional subtypes of Source and Novel Recognition Discriminability. Source Recognition Discriminability reflects the ability to discriminate between the 16 target items and the 16 List B items that are included as distractors on the yes/no recognition trial. Novel Recognition Discriminability measures the ability to discriminate between the 16 target items and 16 distractor items that were not previously presented on List A or List B. Total Recognition Discriminability reflects the ability to discriminate between the 16 target items and all 32 distractor items. The present study investigated the utility of these indices for characterizing the neurocognitive mechanism of deficient recognition memory performance in HD and AD. It was hypothesized that, first, HD patients would perform as poorly as AD patients on the Source Recognition Discriminability index. Second, we predicted that HD patients would perform significantly better than AD patients on the Novel Recognition Discriminability index. Lastly, we predicted that HD patients would perform significantly better than AD patients on the Total Recognition Discriminability index. The last two predictions were premised on the evidence that AD patients typically show a more profound encoding/storage deficit than HD patients and thus would be expected to show greater difficulties on those recognition indices less dependent on source memory abilities.
Method
Participants
A total of 16 patients diagnosed with probable Alzheimer's disease (AD) and 17 patients diagnosed with Huntington's disease (HD) participated in the study. The 17 HD patients received neuropsychological testing as part of their participation in the Huntington's Disease Clinical Research Program at the University of California, San Diego, School of Medicine. All HD patients were diagnosed with HD by a senior staff neurologist on the basis of unequivocal motor signs (i.e., chorea) and a positive family history for HD. In some cases, genetic confirmation of expanded CAG repeats on chromosome 4 was also available. All participants gave informed written consent for participation in the study.
The AD patients were selected from a larger pool of patients followed by the Alzheimer's Disease Research Center at the University of California, San Diego, School of Medicine in order to provide the closest match to the HD subjects on the Mattis Dementia Rating Scale (DRS; Mattis, 1973) and demographic variables. The diagnosis of AD was made by two senior staff neurologists according to the criteria for “probable AD” developed by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (McKhann et al., 1984). None of the patients from either group had a reported history of stroke, brain tumor, brain surgery, head injury with loss of consciousness for more than five minutes, or substance abuse/dependence within the past year.
Measures and analyses
The CVLT-II was administered to all participants by trained psychometrists using standardized procedures (Delis et al., 2000). Individuals are read a list of 16 target words (List A) taken from four categories, with 4 words per category. List A is learned across five trials, after which an interference list is provided. The interference list (List B) is also made up of 16 words (4 words from each of four semantic categories). Immediately following the presentation of List B, free and cued recall of List A is elicited. After a delay period of 20 minutes during which time nonverbal tests are administered, free and cued recall of the List A words is assessed. After the delayed recall trials, yes/no recognition testing is conducted. The recognition portion of the test consists of all 16 List A target words as well as 32 distractors, of which 16 are the List B words, and 16 are novel distractors. Individuals are instructed to say “yes” to identify the 16 target words from List A and to say “no” if the recognition item was not from List A.
On the first edition of the CVLT (Delis et al., 1987), indices of recognition discriminability and response bias were computed based on nonparametric formulas provided by Underwood (1974). However, these formulas are not well suited for recognition tasks that have uneven numbers of targets and distractors, such as those found on the CVLT and CVLT-II (Corwin, 1994; Delis et al., 2000). Thus, the CVLT-II employs a parametric “d prime” recognition measure (d′) that is well suited for recognition tests that have unequal target items and distractors, because the calculation of d′ is based on hit and false positive rates, rather than absolute numbers. The raw d′ score is analogous to a contrast z score in that it reflects the absolute difference in standard deviation units between the examinee's hit rate (signal) and false-positive rate (noise). If an examinee's hit rate and false-positive rate are both at 50% accuracy, then d′ is zero. The d′ raw score on the CVLT-II can range from a high of +4.02 (16 hits, 0 false positives) to a low of −4.02 (0 hits, 32 false positives).
Total Recognition Discriminability is defined on the CVLT-II as the ability to endorse the 16 target items and reject all 32 distractors. Source Recognition Discriminability is defined as the ability to endorse the 16 List A target items and reject the 16 distractors that comprise List B and thus assesses the ability to recognize the source of a word (originating from List A or List B). Novel Recognition Discriminability is defined as the ability to endorse the 16 target items and reject those 16 distractors that are not found on List B. Patients with the most severe types of memory deficits often obtain low scores on this measure (Delis et al., 2000).
A split-plot analysis of variance (ANOVA) was utilized, with group (HD and AD) as the between-subjects factor and recognition discriminability subtype (Total, Source, and Novel) as the within-subjects factor. In order to investigate the presence of differences in the groups on recall measures, an additional split-plot ANOVA was utilized, with group (HD and AD) as the between-subjects factor and recall subtype (short delay free, short cued, long free, and long cued) as the within-subjects factor. Data were analyzed using age-, education-, and gender-corrected z-scores based on the CVLT-II national normative database.
Participants were also administered the DRS, which provides a screening test of global cognitive functioning. The DRS assesses several cognitive domains, including attention, memory, conceptual reasoning, and visuospatial abilities, and was used to match the HD and AD groups on dementia severity. In addition, each AD patient received a Clinical Dementia Rating score (CDR; Hughes, Berg, Danziger, Coben, & Martin, 1982; Morris, 1997). The CDR is a rating scale for clinical staging of dementia of Alzheimer's type that integrates direct assessment by a physician with an interview with a knowledgeable informant to ascertain overall function in six domains (memory, orientation, problem solving, community affairs, home and hobbies, and self care). Scores on the CDR are commonly evaluated in terms of overall stage (0 = normal, 0.5 = questionable/very mild dementia, 1 = mild dementia, 2 = moderate dementia, 3 = severe dementia). As part of the evaluation of the HD patients, a clinician completed the Shoulson– Fahn Total Functional Capacity scale (TFC) from the Unified Huntington's Disease Rating Scale (UHDRS; Huntington Study Group, 1996). The TFC is a 14-unit scale (range 0 to 13), with higher scores indicating higher levels of functioning by occupation, major activities of daily living (ADLs; finances, domestic chores), and level and type of care required. Scores on TFC can be segregated into five stages (Marder et al., 2000): Stage I (scores from 11–13); Stage II (scores from 7–10); Stage III (scores from 3–6); Stage IV (scores 1–2); and Stage V (score of 0).
Results
Demographics, CDR, DRS, TFC, and recall performance
Table 1 summarizes demographic variables and DRS, CDR, and TFC scores for the two patient groups. No significant differences were found between groups for education level or DRS scores (ps > .60 and .80, respectively). The AD group was significantly older than the HD group (p < .001), an expected finding given that AD typically affects individuals later in life than HD. In addition, the HD group had significantly more females than males (p < .05). For these reasons, the CVLT-II results from the two patient groups were analyzed using demographically corrected standardized score. CDR scores in the AD group ranged from questionable/very mild dementia to moderate dementia, which accords with the observed range of scores on the DRS. TFC scores in the HD group ranged from Stage II to Stage IV.
Table 1.
Demographics and DRS scores for HD and AD patients
| HD | AD | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | N | M | SD | % | N | M | SD | % |
| N | 17 | 16 | ||||||
| Agea | 47.1 | 15.4 | 73.6 | 12.6 | ||||
| Educationa | 13.7 | 1.9 | 14.5 | 2.8 | ||||
| % Female | 77 | 38 | ||||||
| DRS | 120.4 | 10.5 | 120.8 | 10.0 | ||||
| CDR (IQR) | — | 1b | 0.75 | |||||
| TFC (IQR) | 4b | 2 | — | |||||
Note. HD = Huntington's disease. AD = Alzheimer's disease. DRS = Mattis Dementia Rating Scale. CDR = Clinical Dementia Rating scale. TFC = Shoulson–Fahn Total Functional Capacity scale. IQR = interquartile range.
In years.
Median value.
Recall Measures
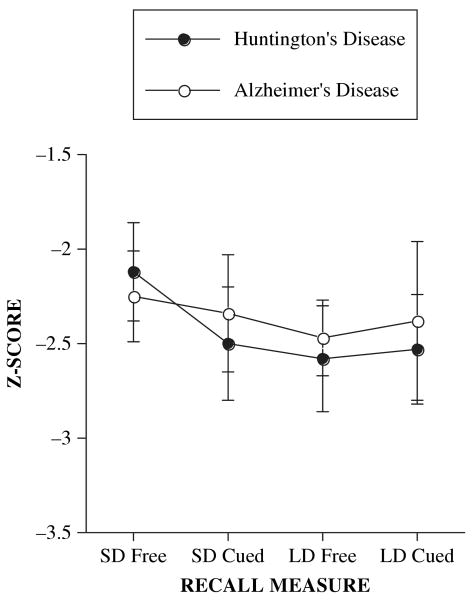
Both the AD and HD groups exhibited comparable levels of impairment across the delayed recall trials. Analysis revealed no significant effects of group, F(1, 31) = 0.04, p > .80, or type, F(3, 93) = 1.62, p > .15, on any of the delayed recall trials, nor a significant interaction effect, F(3, 93) = 0.35, p > .75; see Figure 1.
Figure 1.
Mean (±1 SE) recall measures by subtype and diagnosis.
Recognition discriminability measures
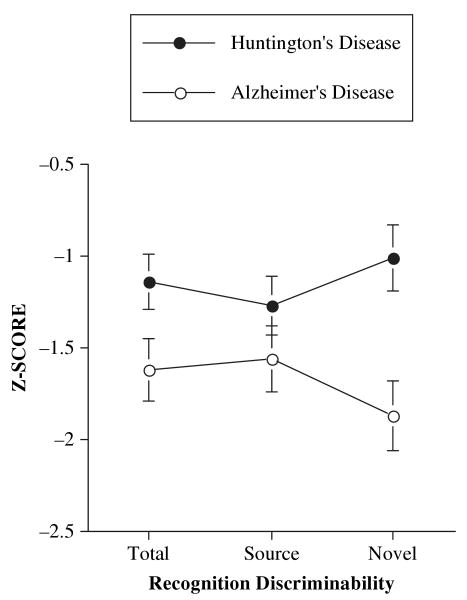
Analyses demonstrated a significant interaction between group and subtype of recognition discriminability, F(2, 62) = 12.8, p < .001. Follow-up analyses revealed that the HD patients' performance on Source Recognition Discriminability failed to differ significantly from that of the AD patients (p > .20; see Table 2 for effect sizes and confidence intervals). However, HD patients performed significantly better than AD patients on Novel Recognition Discriminability (p < .01) and on Total Recognition Discriminability (p < .05; see Figure 2).
Table 2.
Effect sizes and confidence intervals for comparisons between the HD and AD groups on the recognition discriminability indices
| Variable | Mean diff. | 95% CI for diff. | Cohen's d ES |
|---|---|---|---|
| NRD (AD vs. HD) | −0.860 | −1.40, −0.325 | 1.14 |
| TRD (AD vs. HD) | −0.481 | −0.950, −0.012 | 0.727 |
| SRD (AD vs. HD) | −0.283 | −0.774, 0.206 | 0.405 |
Note. HD = Huntington's disease. AD = Alzheimer's disease. CI = confidence interval. ES = effect size. SRD = Source Recognition Discriminability. NRD = Novel Recognition Discriminability. TRD = Total Recognition Discriminability.
Figure 2.
Mean (±1 SE) recognition discriminability scores by subtype and diagnosis.
Recall versus recognition
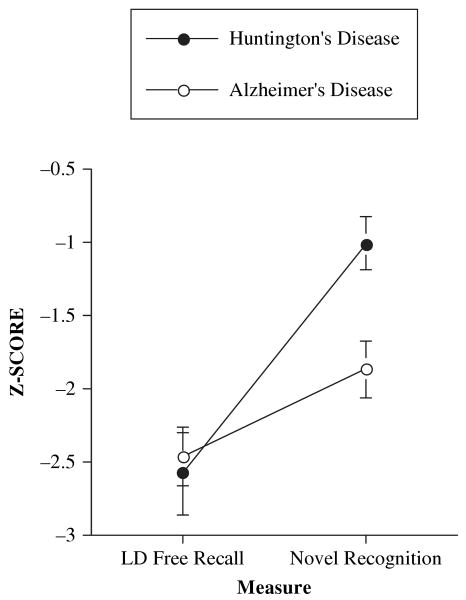
The prototypical memory profiles associated with AD and HD patients predict an interaction between diagnosis and type of memory measure (recall and recognition). The findings of the present study suggest that the best measure of recognition on the CVLT-II for distinguishing between the memory profiles of AD and HD patients may be Novel Recognition Discriminability, since it parses out the contributions of source memory. From a retrieval perspective, Long Delay Free Recall is likely the most difficult measure on the CVLT-II (Delis et al., 2000). Thus, an additional between-subjects by within-subjects ANOVA was conducted, with diagnosis as the between-subjects factor and type of measure (Long Delay Free Recall and Novel Recognition Discriminability) as the within-subjects factor. Analysis revealed a significant interaction between diagnosis and measure, F(1, 31) = 7.9, p < .01, indicating no difference between groups on Long Delay Free Recall, but significantly better Novel Recognition Discriminability for the HD group than for the AD group. These findings are illustrated in Figure 3.
Figure 3.
Mean (±1 SE) Long Delay Free Recall and Novel Recognition Discriminability scores by diagnosis, indicating a significant interaction between measure and diagnosis.
Discussion
The present study attempts to further characterize the differential memory profiles associated with Huntington's disease and Alzheimer's disease. Many of the past studies in this area have treated recognition discriminability as a unitary construct and have found that HD patients tend to perform better than AD patients on yes/no recognition testing (Butters et al., 1986; Butters et al., 1985; Delis et al., 1991). These findings have, in part, contributed to theories about the neurocognitive mechanisms underlying the distinct memory profiles in HD and AD patients—namely, that AD patients' memory difficulties are primarily the outcome of an encoding/storage deficit, whereas HD patients exhibit predominantly a retrieval deficit. The present findings continue to provide support for this dichotomy. Specifically, HD patients performed significantly better than AD patients on Total Recognition Discriminability, but not on Long Delay Free Recall, indicating that HD patients benefited from the reduced retrieval demands associated with the recognition testing format. Moreover, the evidence that the AD patients did not benefit to the same degree as HD patients from the retrieval cues afforded by the yes– no recognition paradigm likely reflects their more extensive encoding/storage level deficits in AD.
The present study adds to this literature by examining the utility of the CVLT-II recognition discriminability subtypes for distinguishing between patients with HD and AD. Specifically, the CVLT-II provides measures of Source and Novel Recognition Discriminability, in addition to the traditional Total Recognition Discriminability measure. The analyses of these various recognition discriminability indices revealed that the HD patients' performance on Source Recognition Discriminability did not differ significantly from that of the AD patients. This result is generally consistent with past findings indicating disproportionate difficulties with source memory in individuals with frontal dysfunction in general and HD patients in particular (Baldo et al., 2002; Brandt et al., 1995; Duarte et al., 2005; Janowsky et al., 1989). For example, Brandt et al. (1995) reported that the size of the basal ganglia (particularly the left caudate nucleus) in HD patients is negatively correlated with the accuracy of their source memory.
Novel Recognition Discriminability requires individuals to differentiate target words from new distractor items that were never presented in the prior recall trials. Thus, this index assesses recognition memory performance under conditions in which the burden on source memory is substantially minimized. Deficits on this measure are usually associated with damage to medial temporal structures, such as what occurs in AD (Delis et al., 1991). As predicted, HD patients performed significantly better than AD patients on this measure. The AD patients were significantly impaired on all of the subtypes of yes/no recognition discriminability, including the Source Recognition Discriminability index. These results suggest that AD patients do not have a specific source memory impairment, rather a generalized encoding/storage deficit that affects all aspects of yes/no recognition memory.
The findings of the present study suggest that the classic retrieval versus encoding/storage distinction that has been used to characterize the distinct memory disorders of HD and AD may be more complex than traditionally thought. Specifically, HD patients manifested a relatively circumscribed impairment remembering the context or source of the target information, a finding consistent with the nature of their frontal-system pathology (Baldo et al., 2002; Brandt et al., 1995; Craik et al., 1990; Duarte et al., 2005; Janowsky et al., 1989; Schacter et al., 1984; Simons et al., 2002). In contrast, AD patients exhibited a generalized yes/no recognition memory impairment, likely owing to extensive mesial-temporal pathology. Therefore, the current study indicates that analysis of recognition discriminability subtypes may provide useful information for parsing the frontal versus mesial-temporal contributions to recognition memory performance.
A relative strength of the CVLT-II is its ability to evaluate learning and memory across a range of clinical and research conditions. But like most neuropsychological tests with broad clinical applications, the CVLT-II cannot assess a specific component memory process, such as source memory, as precisely as do experimental paradigms that are designed exclusively for that purpose. For example, a commonly employed experimental method for evaluating source memory involves presenting two word lists an equivalent number of times during the study phase, the result being that the lists are of equal familiarity. This, however, is not the measurement approach taken in the CVLT-II (List A items are presented on five occasions; List B items are presented only once).
Another potential limitation of the current study includes the relatively small sample size. While the magnitude of the group differences was clearly largest on the Novel Recognition Discriminability index, the HD group did indeed perform somewhat stronger than the AD group on the Source Recognition Discriminability index, suggesting that with a larger sample significant group differences in source memory might manifest. Lastly, although matched on the DRS, it is conceivable that there are group differences in the functional implications of dementia severity, given the potential impact of motor impairment on the DRS total score. Thus, some of the observed difference on, for example, the Novel Discriminability Index could in part reflect greater overall cognitive impairment in the AD group. Future studies including larger samples of AD and HD patients would allow for a more precise clarification of the relationship between dementia severity and performance on the various recognition discriminability indices. Nevertheless, the current findings suggest that the traditional dissociations that are used to characterize cortical and subcortical dementia, such as encoding versus retrieval, may be more nuanced, particularly when considering component memory processes like source memory.
Acknowledgments
Preparation of this article was supported in part by a Veterans Administration Merit Review Grant (DD), a VA Career Development Award (MJ), and National Institute on Aging Grants RO1 AG12674 (MB), P50 AG05131 (UCSD ADRC), and R01 AG12963 (DS).
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article maybe used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progress in Brain Research. 1990;85:119–146. [PubMed] [Google Scholar]
- Aylward EH, Codori AM, Barta PE, Pearlson GD, Harris GJ, Brandt J. Basal ganglia volume and proximity to onset in presymptomatic Huntington disease. Archives of Neurology. 1996;53:1293–1296. doi: 10.1001/archneur.1996.00550120105023. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Delis D, Kramer J, Shimamura AP. Memory performance on the California Verbal Learning Test-II: Findings from patients with focal frontal lesions. Journal of the International Neuropsychological Society. 2002;8:539–546. doi: 10.1017/s135561770281428x. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brandt J, Bylsma FW, Aylward EH, Rothlind J, Gow CA. Impaired source memory in Huntington's disease and its relation to basal ganglia atrophy. Journal of Clinical and Experimental Neuropsychology. 1995;17:868–877. doi: 10.1080/01688639508402436. [DOI] [PubMed] [Google Scholar]
- Butters N, Wolfe J, Granholm E, Martone M. An assessment of verbal recall, recognition and fluency abilities in patients with Huntington's disease. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior. 1986;22:11–32. doi: 10.1016/s0010-9452(86)80030-2. [DOI] [PubMed] [Google Scholar]
- Butters N, Wolfe J, Martone M, Granholm E, Cermak LS. Memory disorders associated with Huntington's disease: Verbal recall, verbal recognition and procedural memory. Neuropsychologia. 1985;23:729–743. doi: 10.1016/0028-3932(85)90080-6. [DOI] [PubMed] [Google Scholar]
- Corwin J. On measuring discrimination and response bias: Unequal numbers of targets and distractors and two classes of distractors. Neuropsychology. 1994;8:110–117. [Google Scholar]
- Craik FI, Morris LW, Morris RG, Loewen ER. Relations between source amnesia and frontal lobe functioning in older adults. Psychology and Aging. 1990;5:148–151. doi: 10.1037/0882-7974.5.1.148. [DOI] [PubMed] [Google Scholar]
- Crosson B, Benefield H, Cato MA, Sadek JR, Moore AB, Wierenga CE, et al. Left and right basal ganglia and frontal activity during language generation: Contributions to lexical, semantic, and phonological processes. Journal of the International Neuropsychological Society. 2003;9:1061–1077. doi: 10.1017/S135561770397010X. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Vonsattel JP, Richardson EP., Jr Morphometric demonstration of atrophic changes in the cerebral cortex, white matter, and neostriatum in Huntington's disease. Journal of Neuropathology and Experimental Neurology. 1988;47:516–525. doi: 10.1097/00005072-198809000-00003. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test–II, Second Edition. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Delis DC, Massman PJ, Butters N, Salmon DP, Cermak LS, Kramer JH. Profiles of demented and amnesic patients on the California Verbal Learning Test: Implications for the assessment of memory disorders. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1991;3:19–26. [Google Scholar]
- Duarte A, Ranganath C, Knight RT. Effects of unilateral prefrontal lesions on familiarity, recollection, and source memory. The Journal of Neuroscience. 2005;25:8333–8337. doi: 10.1523/JNEUROSCI.1392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans FJ, Thorn WA. Two types of post-hypnotic amnesia: Recall amnesia and source amnesia. The International Journal of Clinical and Experimental Hypnosis. 1966;14:162–179. doi: 10.1080/00207146608412959. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Pearlson GD, Peyser CE, Aylward EH, Roberts J, Barta PE, et al. Putamen volume reduction on magnetic resonance imaging exceeds caudate changes in mild Huntington's disease. Annals of Neurology. 1992;31:69–75. doi: 10.1002/ana.410310113. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Huntington Study Group. Unified Huntington's disease rating scale: Reliability and consistency. Movement Disorders. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: Cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989;27:1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- Marder K, Zhao H, Myers RH, Cudkowicz M, Kayson E, Kieburtz K, Orme C, Paulsen J, Penney JB, Jr, Siemers E, Shoulson I, Huntington Study Group Rate of functional decline in Huntington's disease. Neurology. 2000;54:452–458. doi: 10.1212/wnl.54.2.452. [DOI] [PubMed] [Google Scholar]
- Martone M, Butters N, Payne M, Becker JT, Sax DS. Dissociations between skill learning and verbal recognition in amnesia and dementia. Archives of Neurology. 1984;41:965–970. doi: 10.1001/archneur.1984.04050200071020. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale. Odessa, FL: Psychological Assessment Resources; 1973. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Montoya A, Pelletier M, Menear M, Duplessis E, Richer F, Lepage M. Episodic memory impairment in Huntington's disease: A meta-analysis. Neuropsychologia. 2006;44:1984–1994. doi: 10.1016/j.neuropsychologia.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Morris JC. Clinical Dementia Rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. International Psychogeriatrics. 1997;9(Suppl. 1):173–176. doi: 10.1017/s1041610297004870. [DOI] [PubMed] [Google Scholar]
- Moss MB, Albert MS, Butters N, Payne M. Differential patterns of memory loss among patients with Alzheimer's disease, Huntington's disease, and alcoholic Korsakoff's syndrome. Archives of Neurology. 1986;43:239–246. doi: 10.1001/archneur.1986.00520030031008. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Filoteo JV. Neuropsychology of cortical versus subcortical dementia syndromes. Seminars in Neurology. 2007;27:7–21. doi: 10.1055/s-2006-956751. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Harbluk JL, McLachlin DR. Retrieval without recollection: An experimental analysis of source amnesia. Journal of Verbal Learning and Verbal Behavior. 1984;23:593–611. [Google Scholar]
- Shimamura AP, Squire LR. A neuropsychological study of fact memory and source amnesia. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13:464–473. doi: 10.1037//0278-7393.13.3.464. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Squire LR. The relationship between fact and source memory: Findings from amnesic patients and normal subjects. Psychobiology. 1991;19:1–10. [Google Scholar]
- Simons JS, Verfaellie M, Galton CJ, Miller BL, Hodges JR, Graham KS. Recollection-based memory in frontotemporal dementia: Implications for theories of long-term memory. Brain: A Journal of Neurology. 2002;125:2523–2536. doi: 10.1093/brain/awf247. [DOI] [PubMed] [Google Scholar]
- Troster AI, Butters N, Salmon DP, Cullum CM, Jacobs D, Brandt J, et al. The diagnostic utility of savings scores: Differentiating Alzheimer's and Huntington's diseases with the logical memory and visual reproduction tests. Journal of Clinical and Experimental Neuropsychology. 1993;15:773–788. doi: 10.1080/01688639308402595. [DOI] [PubMed] [Google Scholar]
- Underwood BJ. The role of the association in recognition memory. Journal of Experimental Psychology Monographs. 1974;102:917–939. [Google Scholar]
- Vonsattel JP. Neuropathology of Huntington's disease. NeuroScience News. 2000;3:45–53. [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington's disease. Journal of Neuropathology and Experimental Neurology. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]