Abstract
Neuropsychological studies show that cognitive deficits associated with Alzheimer's disease (AD) are distinct from age-associated cognitive decline. Quantitative and qualitative differences are apparent across many cognitive domains, but are especially obvious in episodic memory (particularly delayed recall), semantic knowledge, and some aspects of executive functions. The qualitatively distinct pattern of deficits is less salient in very old AD patients than in younger AD patients. Although decline in episodic memory is usually the earliest cognitive change that occurs prior to the development of the AD dementia syndrome, asymmetry in cognitive abilities may also occur in this “preclinical” phase of the disease and predict imminent dementia. Discrete patterns of cognitive deficits occur in AD and several neuropathologically distinct age-associated neurodegenerative disorders. Knowledge of these differences helps to clinically distinguish among various causes of dementia and provides useful models for understanding brain-behavior relationships that mediate cognitive abilities affected in various neurodegenerative diseases.
Keywords: cognition, memory, Alzheimer's disease
Introduction
The detection and characterization of cognitive deficits associated with age-related neurodegenerative diseases such as Alzheimer's disease (AD) is the focus of growing clinical research interest as increasing numbers of people survive into older age. This interest is fueled by the need to accurately detect the onset of cognitive changes that signal the beginning of a progressive dementia syndrome and to differentiate among disorders with distinct etiologies and sites of pathology. This can be a particularly difficult task given the insidious onset and slow progression of most neurodegenerative diseases, but it is critically important given the lack of a reliable biological marker that can distinguish AD from normal aging or other neurodegenerative disorders that lead to dementia. Accurate clinical diagnosis of dementia and its underlying cause is crucial for prognosis and the early and appropriate application of disease-specific treatments that are currently available or in development.
Neuropsychological research on dementia has focused on AD because it is the most common cause of dementia and is primarily defined by its impact on cognition. This research has led to increased knowledge about the particular cognitive deficits that occur in the earliest stages of AD, and this has enhanced the ability to clinically diagnosis the disease early in its course. The impact of aging on the ability to detect AD has been described, and subtle cognitive changes that might foreshadow the development of dementia in those with “preclinical” AD have been identified. The cognitive manifestations of AD have been compared and contrasted to those of other age-related neurodegenerative disorders in order to improve differential diagnosis and provide information about the neurological basis of various cognitive abilities that are affected. The contributions of this research to the neuropsychological assessment of dementia are reviewed below.
Neuropsychological Detection of Alzheimer's Disease
Alzheimer's disease is an age-related degenerative brain disorder characterized by neuronal atrophy, synapse loss, and the abnormal accumulation of amyloidogenic plaques and neurofibrillary tangles in medial temporal lobe limbic structures (e.g., entorhinal cortex, hippocampus) and the association cortices of the frontal, temporal, and parietal lobes (Braak & Braak 1991). Consistent with these widespread neuropathological changes, the primary clinical manifestation of AD is a progressive global dementia syndrome that usually begins in later life (i.e., ages 60–70). In the usual case, the dementia syndrome is characterized by prominent amnesia with additional deficits in language and semantic knowledge, abstract reasoning, executive functions, attention, and visuospatial abilities (Salmon & Bondi 1999). These cognitive deficits and the decline in everyday function they produce are the core features of the AD dementia syndrome and are the focus of clinical assessment of the disease.
Although the pattern of progression of AD pathology is not fully known, evidence suggests that the earliest changes occur in medial temporal lobe structures (e.g., hippocampus, entorhinal cortex) that are critical for episodic memory (Braak & Braak 1991). This is consistent with a wealth of neuropsychological evidence showing that episodic memory impairment (i.e., amnesia) is usually the earliest and most salient aspect of the AD dementia syndrome (for review, see Salmon 2000). Studies of the clinical utility of episodic memory measures for the early detection of AD have identified a number of characteristics that are quite effective in differentiating between mildly demented AD patients and normal older adults. First, patients with very early AD are particularly impaired on measures of delayed recall (i.e., have abnormally rapid forgetting), with several studies showing that absolute delayed recall scores or “savings” scores (i.e., amount recalled after the delay divided by the amount recalled on the immediate learning trial) can differentiate mildly demented AD patients from healthy elderly controls with approximately 85% to 90% accuracy (for review, see Salmon 2000). Second, to-be-remembered information is not accessible after a delay even if retrieval demands are reduced by the use of recognition testing (e.g., Delis et al. 1991). Third, AD patients exhibit an abnormal serial position effect characterized by an attenuation of the primacy effect (i.e., recall of words from the beginning of a list), suggesting that they cannot effectively transfer information from primary memory to secondary memory (e.g., Bayley et al. 2000). Fourth, semantic encoding is less effective in improving the episodic memory performance of patients with AD than normal elderly individuals (for review, see Bäckman & Small 1998). Fifth, patients with AD have an enhanced tendency to produce intrusion errors (i.e., when previously learned information is produced during the attempt to recall new material) on both verbal and nonverbal memory tests, presumably due to increased sensitivity to interference and/or decreased inhibitory processes (Butters et al. 1987, Jacobs et al. 1990). Evaluation of these characteristics of the memory deficit associated with AD is incorporated into several memory tests that are effective for early detection of the disease (e.g., Buschke 1973, Buschke et al. 1997, Knopman & Ryberg 1989) and in clinical algorithms developed to differentiate AD from other types of dementia (e.g., Delis et al. 1991).
As the neuropathology of AD spreads beyond medial temporal lobe structures to the association cortices of the temporal, frontal, and parietal lobes (Braak & Braak 1991), a number of higher-order cognitive abilities are affected. Patients with AD develop a semantic memory deficit that manifests itself as a loss of general knowledge and impairment of language abilities (i.e., aphasia). Patients with AD are often impaired on tests of confrontation naming, verbal fluency, and semantic categorization, and have a reduced ability to recall overlearned facts (e.g., the number of days in a year) (for reviews, see Chan et al. 1998, Hodges & Patterson 1995, Nebes 1989). Interestingly, patients are highly consistent in the individual items they miss across different semantic memory tests that employ unique modes of access and output (e.g., fluency versus confrontation naming; Chertkow & Bub 1990, Hodges et al. 1992) or within the same test across unique evaluations (Norton et al. 1997). This suggests that AD results in a true loss of semantic knowledge rather than only an impaired ability to retrieve information from intact semantic memory stores (also see Salmon et al. 1999). A similar loss of knowledge is thought to contribute to the severe deficit that patients with AD exhibit in the ability to remember past events that were successfully remembered prior to the onset of the disease (i.e., retrograde amnesia) (for review, see Salmon 2000).
Deficits in executive functions responsible for concurrent mental manipulation of information, concept formation, problem solving, and cue-directed behavior occur early in the course of AD (Perry & Hodges 1999). The ability to perform concurrent manipulation of information appears to be particularly vulnerable. Lefleche & Albert (1995) demonstrated that very mildly demented patients with AD were significantly impaired relative to elderly normal control subjects on tests that required set shifting, self-monitoring, or sequencing, but not on tests that required cue-directed attention or verbal problem solving. Patients with AD have also been shown to be impaired on (a) difficult problem-solving tests such as the Tower of London puzzle (Lange et al. 1995) and the modified Wisconsin Card Sorting Task (Bondi et al. 1993), (b) tests of relational integration (Waltz et al. 2004), and (c) various other clinical neuropsychological tests that assess executive functions such as the Porteus Maze Task, Part B of the Trail-Making Test, and the Raven Progressive Matrices Task (e.g., Grady et al. 1988).
Deficits in attention and visuospatial abilities develop during the course of AD, but are usually less salient than other cognitive deficits in the early stages of disease (Butters et al. 1988, Storandt et al. 1984). When attention deficits do occur, they are usually evident on dual-processing tasks, tasks that require the disengagement and shifting of attention, and working memory tasks that are dependent upon the control of attentional resources (for reviews, see Parasuraman & Haxby 1993, Perry & Hodges 1999). Visuospatial deficits associated with AD usually affect visuoconstructional abilities assessed by the Block Design Test, the Clock Drawing Test, and complex figure copying (i.e., apraxia), and visuoperceptual abilities tapped by tests such as Judgment of Line Orientation or the Money Road Map Test (for reviews, see Cronin-Golomb & Amick 2001, Freedman et al. 1994).
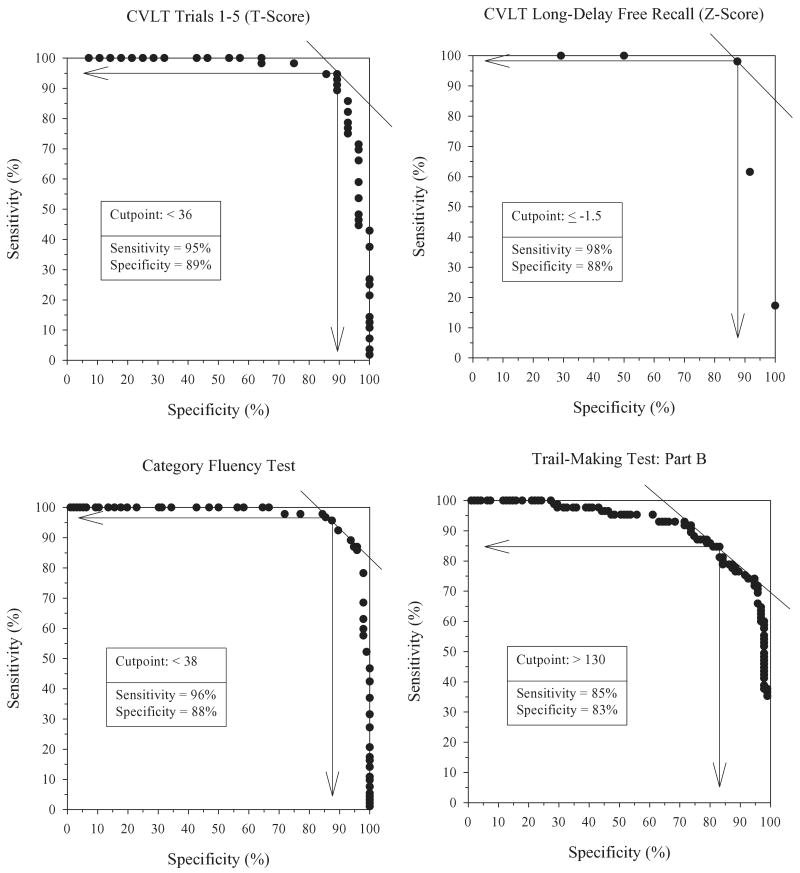
The neuropsychological research reviewed above suggests that in the usual case, AD is associated with a specific pattern of cognitive deficits that can effectively differentiate the disease from normal aging. This was confirmed in a study by Salmon and colleagues (2002) that compared the performances of 98 patients with early AD (i.e., scored ≥24 on the Mini-Mental State Exam) and 98 gender-, age-, and education-matched normal control subjects on sensitive measures of learning and memory, executive abilities, language, and visuospatial abilities. The diagnosis of AD was verified in each of the AD patients by subsequent autopsy or longitudinal clinical evaluations that showed a typical course for the disease. Receiver Operating Characteristic curve analyses showed excellent sensitivity and specificity for the detection of very mild AD for learning and delayed recall measures from the California Verbal Learning Test (sensitivity: 95%–98%, specificity: 88%– 89%), the category fluency test (sensitivity: 96%, specificity: 88%), and Part B of the Trail-Making Test (sensitivity: 85%, specificity: 83%) (see Figure 1). A diagnostic model obtained using a nonparametric recursive partitioning procedure (classification tree analysis) showed that a combination of performance on the category fluency test (a measure of semantic memory and executive function) and the delayed recall measure of the Visual Reproduction Test accurately classified 96% of the patients with AD and 93% of the elderly normal control subjects, a level of accuracy higher than achieved with any individual cognitive measure. These results support the view that deficits in episodic memory (e.g., rapid forgetting), certain executive functions (e.g., cognitive set shifting), and semantic knowledge are particularly characteristic of early AD.
Figure 1.
Receiver Operating Characteristic curves comparing sensitivity and specificity for the accurate diagnosis of early Alzheimer's disease (AD) achieved with the Trial 1–5 Learning measure from the California Verbal Learning Test (CVLT), the Long-Delay Free Recall measure from the CVLT, the Category Fluency Test (a semantic memory and executive function measure), and Part B of the Trail-Making Test (an executive function measure). The maximally effective cut-point for memory and executive function measures showed excellent sensitivity and specificity in distinguishing between very mild AD and normal aging. (Adapted from Salmon et al. 2002.)
The Impact of Aging on the Neuropsychological Detection of Alzheimer's Disease
Although much progress has been made in identifying the typical pattern of cognitive deficits associated with early AD, the boundaries between normal age-related cognitive change and early signs of AD remain especially difficult to delineate in very elderly individuals (i.e., over the age of 80). This is because many of the early structural and functional brain changes of AD overlap with changes observed in normal aging. Normal aging is associated with mild brain atrophy and increased white matter abnormality seen on magnetic resonance imaging (MRI) scans (e.g., Jack et al. 1998, Jernigan et al. 2001, Pfefferbaum et al. 1994), decreased hemodynamic response seen on functional MRI scans (D'Esposito et al. 1999), and reduced synaptic density evident upon histopathological examination of brain tissue (Masliah et al. 1993). These brain changes are thought to mediate age-related decline in information processing speed, executive function, learning efficiency, and effortful retrieval (for review, see Hedden & Gabrieli 2004). Because normal aging can detrimentally affect many of the same cognitive abilities affected by AD, the prominence of specific deficits related to AD may be much less evident in the Very-Old (over the age of 80) than in the Young-Old (below the age of 70), especially after performance is standardized to that of the age-appropriate normal cohort. As a result, a less distinct and somewhat atypical cognitive deficit profile is associated with AD in the Very-Old compared to the Young-Old.
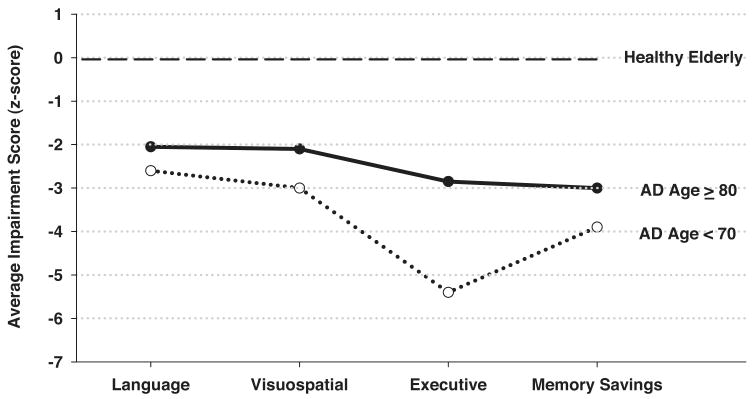
This difference in profiles was illustrated in a study that directly compared the neuropsychological test performance of AD patients who were Very-Old or Young-Old (Bondi et al. 2003). Despite achieving similar raw scores on all neuropsychological measures, the Young-Old and Very-Old AD patients differed in the severity and pattern of the cognitive deficits they exhibited in relation to their age-appropriate controls (see Figure 2). The Young-Old AD patients were generally more impaired than the Very-Old patients and showed a typical AD profile. That is, they exhibited worse deficits in episodic memory (i.e., savings scores) and executive functions than in other cognitive domains. The Very-Old AD patients, in contrast, exhibited a similar level of impairment across all cognitive domains so that their deficit profile lacked the disproportionate saliency of memory and executive function deficits typical of the disease. Because the raw scores of the Young-Old and Very-Old AD patients were similar, the driving force behind their unique deficit profiles was the age-related differences in the performance of the normal control cohorts. Thus, normal aging can significantly affect the severity and pattern of neuropsychological deficits associated with early AD and reduce the saliency of the deficit profile as a diagnostic marker of the disease. This finding has important clinical implications because it identifies the significant risk of false negative diagnostic errors in very elderly AD patients if the clinician expects to see the typical deficit pattern characteristic of younger AD patients. Accurate detection of AD in the very elderly patient may require a multifaceted approach to diagnosis that integrates neuropsychological assessment, neuroimaging, and genetic factors.
Figure 2.
The average composite impairment score achieved by Alzheimer's disease (AD) patients older than age 80 or younger than age 70 in the cognitive domains of language, visuospatial abilities, executive functions, and memory (savings scores). The presented scores are z-scores referenced to the patient groups' respective age-appropriate healthy elderly control cohort. (Adapted from Bondi et al. 2003.)
Neuropsychological Detection of “Preclinical” Alzheimer's Disease
It is commonly accepted that the neurodegenerative changes of AD begin well before clinical manifestations of the disease become apparent (e.g., Katzman 1994). As the pathologic changes of AD gradually accumulate, a threshold for the initiation of the clinical symptoms of the disease is eventually reached. Once this threshold is crossed, cognitive deficits become evident and gradually worsen in parallel with continued neurodegeneration. When the cognitive deficits become global and severe enough to interfere with normal social and occupational functioning, established criteria for dementia and a clinical diagnosis of AD are met. It is clear from this sequence of events that subtle cognitive decline is likely to occur in a patient with AD well before the clinical diagnosis can be made with any certainty. Identification of the cognitive changes that occur during this “preclinical” phase of the disease might provide a reliable way to detect AD in its earliest stages, when potential disease-modifying treatments might be most effective (Thal 1999). Because of the importance of this goal, the attempt to identify preclinical cognitive changes of AD is one of the most active areas of neuropsychological research.
In light of neuropathological evidence that the earliest changes of AD usually occur in the medial temporal lobe structures that are known to be critical for episodic memory (Braak & Braak 1991), it is not surprising that the search for preclinical cognitive markers of the disease has focused largely on this aspect of cognition. Indeed, a number of prospective longitudinal studies of cognitive function in nondemented older adults have shown that a subtle decline in episodic memory often occurs prior to the emergence of the obvious cognitive and behavioral changes required for a clinical diagnosis of AD (for review, see Twamley et al. 2006). These findings led to the development of formal criteria for mild cognitive impairment (MCI), a pre-dementia condition in elderly individuals that is characterized by both subjective and objective memory impairment that occurs in the face of relatively preserved general cognition and functional abilities (for reviews, see Albert & Blacker 2006, Collie & Maruff 2000, Petersen et al. 2001).
The course of episodic memory change during the preclinical phase of AD has been the focus of a number of studies (Bäckman et al. 2001, Chen et al. 2001, Rubin et al. 1998, Small et al. 2000, Storandt et al. 2002). These studies suggest that memory performance may be poor but stable a number of years prior to the development of the dementia syndrome in those with AD, and then decline rapidly in the period immediately preceding the dementia diagnosis. Small et al. (2000) and Bäckman et al. (2001), for example, found that episodic memory was mildly impaired six years prior to dementia onset, but changed little over the next three years. In contrast, Chen et al. (2001) and Lange et al. (2002) showed a significant and steady decline in episodic memory beginning about three years prior to the dementia diagnosis in individuals with preclinical AD. These results indicate that an abrupt decline in memory in an elderly individual might better predict the imminent onset of dementia than poor but stable memory ability.
Although the search for cognitive changes in preclinical AD has largely focused on episodic memory, several recent reviews and meta-analyses suggest that largely nonspecific cognitive decline occurs in the two to three years preceding a dementia diagnosis (Bäckman et al. 2004, 2005; Twamley et al. 2006). Although these studies consistently find a decline in episodic memory, they also often reveal additional deficits in executive functions, perceptual speed, verbal ability, visuospatial skill, and attention during the preclinical phase of AD. This widespread decline in cognitive abilities mirrors evidence that multiple brain regions (e.g., medial temporal lobes, frontal lobes, anterior cingulate cortex) are impaired in preclinical AD (Albert et al. 2001, Small et al. 2003).
Consistent with this broader view, Jacobson and colleagues (2002) found that asymmetry in cognitive performance can be a marker of preclinical AD. Based upon prior research documenting lateralized cognitive deficits (e.g., greater verbal than visuospatial deficits, or vice versa) in subgroups of mildly demented AD patients, these investigators compared cognitively normal elderly adults with preclinical AD (i.e., they were diagnosed with AD approximately one year later) and age- and education-matched normal control subjects on a derived neuropsychological test measure that reflected the absolute difference between verbal and visuospatial ability (i.e., a measure of cognitive asymmetry). Although the groups performed similarly on individual cognitive tests of memory, language, and visuospatial ability, a greater proportion of the preclinical AD patients than the controls had asymmetric cognitive changes in either the verbal or visuospatial direction that were obscured when cognitive scores are averaged over the entire group. Thus, the consideration of both cognitive asymmetry and subtle declines in memory may improve the ability to detect AD in its earliest, preclinical stages.
Alzheimer's Disease as a Disconnection Syndrome
A growing body of evidence indicates that an important early consequence of AD is the loss of effective interaction between various regions of the cortex (e.g., De Lacoste & White 1993). From an anatomical perspective, neurofibrillary tangles have been shown to have a strong predilection for cortical layers (e.g., layer-III and layer-V) and cell types (e.g., midsize pyramidal neurons) that support connections between functionally related cortical association areas. This is most clearly seen in the limbic system, where neurofibrillary tangle pathology in midsize pyramidal neurons of the entorhinal cortex disconnects the hippocampus from neocortex (e.g., Hyman et al. 1984). Although less obvious, this disconnection also occurs in the neocortex, where AD pathology in layer-III and layer-V pyramidal neurons selectively disrupts corticocortical pathways that connect functionally related cortical association areas (for review of the corticocortical disconnection, see Hof & Morrison 1999).
Neurophysiologically, cortical disconnection appears to lead to marked abnormalities in the interregional pattern of blood-flow activation elicited during the performance of cognitive tasks (for review, see Delbeuck et al. 2003). It also appears to underlie reduced coherence (i.e., synchronization) between electroencephalography signals measured at different scalp surface electrode sites that correspond to neocortical association areas that must work in concert during integrative cognitive tasks (e.g., cross-modal stimulus processing) (e.g., Dunkin et al. 1995, Hogan et al. 2003, Jelic et al. 1996, Knott et al. 2000, Stevens et al. 2001). Evoked potential refractory effects related to presentation of intermodal stimuli (i.e., auditory and visual) are also abnormally reduced in patients with AD, consistent with impaired interaction between visual and auditory cortical systems (Golob et al. 2001).
Few studies have directly examined the behavioral consequences of functional disconnectivity in patients with AD, but those that have tend to find a selective impairment in information integration (Della Sala et al. 2000, Freedman & Oscar-Berman 1997, Kurylo et al. 1996, Lakmache et al. 1998, Tippett et al. 2003). This was illustrated in a study by Foster and colleagues (1999) that examined the impact of AD on “feature binding” (Treisman 1996), the moment-by-moment ability to combine discrete sensory inputs analyzed in distinct cortical regions (e.g., color, shape, location) into a coherent representation of a single object. Feature binding was hypothesized to be particularly sensitive to cortical disconnection in AD because defective interaction among neocortical areas should produce a specific deficit in effectively integrating distinct stimulus features despite an intact ability to process each feature separately. Consistent with this notion, Foster and colleagues (1999) found that patients with AD exhibited disproportionately greater response times (compared to normal controls) when required to identify targets on the basis of a conjunction of two or more features (i.e., a conjunction search) than when required to identify targets solely on the basis of a single feature (i.e., a feature search). Tales et al. (2002) recently extended this finding by demonstrating that the selective impairment of patients with AD on conjunction search tasks could not be attributed to different attentional demands inherent in conjunction versus single-feature tasks.
Building upon these previous findings, Festa and colleagues (2005) examined the impact of corticocortical disconnectivity in AD on the ability to integrate motion and color information that is processed in distinct visual processing “streams.” These streams are functionally segregated parallel cortical circuits that analyze different aspects of the visual scene (e.g., Ungerleider & Mishkin 1982). The dorsal stream projecting from striate cortex to parietal cortex selectively analyzes motion and luminance contrast information, while the ventral stream projecting from striate cortex to temporal cortex selectively analyzes form and color information. Previous research has shown that neurologically intact individuals can integrate (i.e., bind) either type of surface feature (color or luminance) with motion information in order to substantially reduce thresholds for motion detection (e.g., Croner & Albright 1997). Color or luminance information is equally effective in this regard even though enhancement of motion detection from color cues places relatively greater demand on cross-cortical interaction since it requires the integration of information across ventral (motion) and dorsal (color) cortical streams (Dobkins & Albright 1998). Enhancement of motion detection from luminance cues only requires the integration of information within the ventral stream.
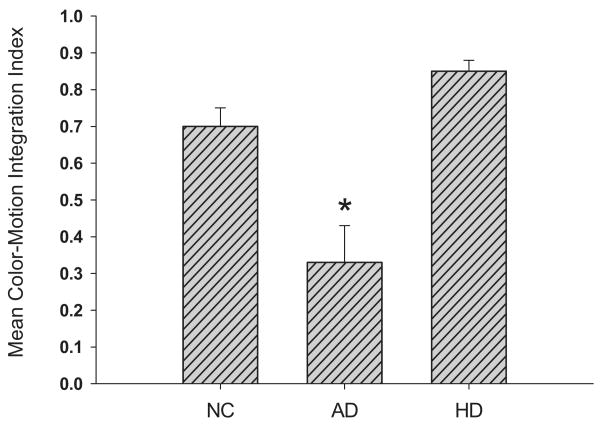
Festa and colleagues (2005) showed that AD patients had normal enhancement of motion detection with luminance cues, but enhancement was significantly less than normal with color cues (see Figure 3). That is, patients could effectively bind information processed within one visual stream, but could not cross-cortically bind information processed in separate cortical streams. This deficit could not be easily attributed to general cognitive dysfunction because both luminance-motion and color-motion integration were normal in demented patients with Huntington's disease who do not have prominent cortical dysfunction. Rather, these results provide psychophysical evidence for cortical disconnectivity in AD and suggest that AD might serve as a model system for investigating the neurocognitive substrates of sensory integration. The specificity of cortical disconnectivity in AD suggests that it may have potential as a cognitive marker for detecting and tracking progression of the disease.
Figure 3.
The mean color-motion integration index scores achieved by normal control (NC) subjects, Alzheimer's disease (AD) patients, and Huntington's disease (HD) patients on a visual sensory integration task. The color-motion integration index reflects the gain in motion direction detection derived from using color information that segments coherently moving targets from distracters. Patients with AD, but not those with HD, were significantly (*) impaired in integrating motion and color information. (Adapted from Festa et al. 2005.)
Distinguishing Alzheimer's Disease from Other Age-Related Causes of Dementia
Although AD is the leading cause of dementia in the elderly, it has been known for some time that dementia can arise from a wide variety of etiologically and neuropathologically distinct disorders that give rise to different patterns of relatively spared and impaired cognitive abilities. Knowledge of these differences may lead to better understanding of the neurobiological basis of specific cognitive deficits (and normal cognition) and improve differential diagnosis of various neurodegenerative disorders. The remaining sections review similarities and differences in the cognitive deficits of AD and those of other age-related causes of dementia including Huntington's disease (HD), dementia with Lewy bodies (DLB), frontotemporal dementia (FTD), and vascular dementia.
Alzheimer's Disease versus Huntington's Disease
HD is an inherited, autosomal dominant disease that results in the midlife (i.e., ages 30– 40) development of movement disorder (e.g., chorea, dysarthria, gait disturbance, oculomotor dysfunction), behavioral changes (e.g., depression, irritability, anxiety) and dementia. These deficits arise primarily from a progressive deterioration of the neostriatum (caudate nucleus and putamen) (Vonsattel & Di Figlia 1998) that disrupts frontostriatal loops that consist of projections from the frontal neocortex to the striatum, striatum to the globus pallidus, globus pallidus to thalamus, and thalamus back to specific regions of frontal cortex (e.g., dorsolateral prefrontal, orbitofrontal, and anterior cingulate cortex) (Alexander et al. 1986). These circuits are believed to provide a subcortical influence on both motor control and higher cognitive functions (Alexander et al. 1986). The cognitive and behavioral deficits associated with HD have been described as a “subcortical dementia” syndrome that is broadly characterized by slowness of thought, impaired attention, executive dysfunction, poor learning, visuoperceptual and constructional deficits, and personality changes such as apathy and depression (McHugh & Folstein 1975). This syndrome differs from the “cortical dementia” syndrome of AD (described above), and the two disorders are often used as a model to study the cortical-subcortical dementia distinction.
Qualitative differences between AD and HD exist in many aspects of cognition, and they may aid in differentiating between subcortical and cortical dementia syndromes. As mentioned above, a severe deficit in episodic memory is characteristic of AD and has been attributed to ineffective consolidation (i.e., storage) of new information (Salmon 2000). Patients with HD, in contrast, exhibit a mild-to-moderate memory impairment that appears to result from a general deficit in the ability to initiate and carry out systematic retrieval of successfully stored information (Butters et al. 1985, 1986). This distinction was illustrated in a study by Delis and colleagues (1991) that directly compared AD and HD patients on a rigorous test of verbal learning and memory, the California Verbal Learning Test. Although the HD and AD patients had comparable immediate and delayed free-recall deficits (based on age-corrected normative data), they differed in several important ways. First, patients with AD exhibited equivalent deficits when memory was assessed using free recall or recognition procedures, whereas patients with HD were less impaired with recognition testing than free recall testing. The significant improvement with recognition testing suggests that HD patients' memory impairment is attenuated when the need for effortful, strategic retrieval is reduced (Butters et al. 1985, 1986). A similar improvement with recognition testing is observed in the remote memory test performance of patients with HD (but not AD patients), presumably a reflection of ineffective retrieval during free recall (Sadek et al. 2004). Second, patients with AD exhibited significantly faster forgetting over a delay interval than did patients with HD. Whereas HD patients retained approximately 70% of the initially acquired information over a 20-minute delay, AD patients retained less than 20%. The qualitative difference in the performances of AD and HD patients is consistent with the notion that information is not effectively consolidated and rapidly dissipates in patients with AD, whereas information can be successfully stored but not effectively retrieved by patients with HD. This is not to say, however, that impaired retrieval is the only cause of the episodic memory deficit in HD. Some residual memory deficit is apparent even when retrieval demands are reduced (Brandt et al. 1992; for review, see Montoya et al. 2006).
Qualitative differences in the language and semantic knowledge deficits exhibited by patients with AD and HD are evident on tests of naming, verbal fluency, and semantic categorization. Patients with AD exhibit a significant confrontation naming deficit (e.g., Bayles & Tomoeda 1983) that is not shared by patients with HD (Hodges et al. 1991), and the two groups produce distinct patterns of naming errors wherein a greater proportion of AD errors are semantically based (e.g., superordinate errors such as calling a “camel” an “animal”) and a greater proportion of HD errors are perceptually based (e.g., calling a “pretzel” a “snake”) (Hodges et al. 1991). On tests of verbal fluency, patients with HD are severely and equivalently impaired on both letter-fluency (i.e., generate words that begin with the letters F, A, or S) and category-fluency (i.e., generate exemplars of animals, fruits, or vegetables) tasks, whereas patients with AD are more impaired on category-fluency than on letter-fluency tasks (for reviews, see Henry et al. 2004, 2005). In addition, the temporal dynamics of retrieval from semantic memory during the letter- and category-fluency tasks indicate that patients with AD have a lower-than-normal mean latency consistent with the notion that they effectively draw exemplars from a semantic set that is abnormally small due to a loss of semantic knowledge, whereas patients with HD have a higher-than-normal mean response latency, consistent with the view that they have a normal-size semantic set but draw exemplars abnormally slowly due to a disruption of retrieval processes (Rohrer et al. 1999). Studies using multidimensional modeling techniques indicate that the network of semantic associations for patients with HD is virtually identical to that of control subjects, whereas that of patients with AD is characterized by weaker and more conceptually concrete associations (for review, see Chan et al. 1998). Thus, AD appears to be characterized by a decline in the structure and organization of semantic knowledge that does not occur in HD.
Deficits in attention, working memory, and executive functions occur in both AD and HD, but specific aspects of these cognitive processes are differentially affected in the two disorders. A general deficit in attention is usually more salient in patients with HD than in those with AD (e.g., Butters et al. 1988). A deficit in shifting or allocating attention is often quite apparent in HD (Hanes et al. 1995, Lange et al. 1995, Lawrence et al. 1996) and appears to be particularly evident when attentional shifts must be internally regulated (Sprengelmeyer et al. 1995). The ability to effectively shift attention between stimulus dimensions in a visual discrimination task in which first one stimulus dimension (e.g., color) and then another (e.g., shape) was reinforced as correct was impaired in moderately to severely demented patients with HD, but not in patients with AD or in mildly demented patients with HD (Lange et al. 1995, Lawrence et al. 1996). All aspects of working memory are affected relatively early in HD, including the maintenance of information in the temporary memory buffers (e.g., as evidenced by poor digit-span performance), inhibition of irrelevant information, and the use of strategic aspects of memory (e.g., planning, organization) to enhance free recall (for review, see Salmon et al. 2001). In contrast, AD is initially characterized by relatively mild working-memory deficits that primarily involve disruption of the central executive with sparing of the phonological loop and visuospatial scratchpad (Baddeley et al. 1991, Collette et al. 1999). It is not until later stages of AD that all aspects of the working memory system become compromised (Baddeley et al. 1991, Collette et al. 1999).
The prominent deficits in attention and working memory that occur in HD are accompanied by impairment of various executive functions involved in planning and problem solving such as goal-directed behavior, the ability to generate multiple response alternatives, the capacity to resist distraction and maintain response set, and the cognitive flexibility to evaluate and modify behavior (for review, see Brandt & Bylsma 1993). Deficits in these abilities are apparent on a variety of tests that require executive functions such as the Wisconsin Card Sorting Test (Paulsen et al. 1995, Peinemann et al. 2005, Pillon et al. 1991, Ward et al. 2006), the Stroop Test (Peinemann et al. 2005, Ward et al. 2006), the Tower of London Test (Lange et al. 1995), the Gambling Decision Making task (Stout et al. 2001), and tests of verbal concept formation (Hanes et al. 1995). These deficits progress throughout the course of disease (Ho et al. 2003, Ward et al. 2006) but are not unique to HD. A number of studies have shown that extensive executive dysfunction also occurs in AD (for review, see Perry & Hodges 1999). Specific aspects of executive dysfunction may be more common in one dementia syndrome than in another, but few studies have directly compared this aspect of cognition in the two disorders.
Although visuospatial deficits are characteristic of both AD (for review, see Cronin-Golomb & Amick 2001) and HD (Ward et al. 2006; for review, see Brandt & Butters 1986), relatively little is known about the specific components of visuospatial processing that might be differentially affected in the two disorders. In one of the few studies to directly address this issue, Brouwers and colleagues (1984) found that patients with AD, but not those with HD, were impaired on tests of visuoconstructional ability that required extrapersonal orientation (e.g., copying a complex figure), whereas patients with HD, but not those with AD, were impaired on visuospatial tasks that required personal orientation (e.g., the Money Road Map Test). This dissociation was supported by the results of another study that examined the ability to mentally rotate representations of objects (Lineweaver et al. 2005). Patients with HD were significantly slower than normal control subjects in performing mental rotation (perhaps due to general bradyphrenia) but were as accurate as controls in making the rotation and reporting the correct side of the target. Patients with AD, in contrast, performed the mental rotation as quickly as controls but were significantly impaired in making an accurate rotation and reporting the correct side of the target. This may reflect a deficit in extrapersonal visual orientation in AD secondary to neocortical damage in brain regions thought to be involved in processing visual motion (e.g., the middle temporal gyrus).
Alzheimer's Disease versus Dementia with Lewy Bodies
DLB is a clinico-pathologic condition characterized by a dementia syndrome that occurs in the presence of cell loss and the deposition of Lewy bodies (abnormal intracytoplasmic eosinophilic neuronal inclusion bodies) in a subcortical pattern similar to that of Parkinson's disease (e.g., in brain stem nuclei including the substantia nigra, locus ceruleus, dorsal motor nucleus of the vagus, and substantia innominata), the presence of Lewy bodies diffusely distributed throughout the limbic system (e.g., cingulate, insula, amygdala, hippocampus, entorhinal cortex, and transentorhinal cortex) and neocortex (e.g., temporal, parietal, and frontal lobes), and in many cases AD pathology (i.e., neuritic plaques, neurofibrillary tangles) that occurs in the same general distribution throughout the brain as in “pure” AD (for review, see Ince & Perry 2005). There is widespread depletion of cortical choline acetyltransferase in the neocortex and striatum in DLB (e.g., Tiraboschi et al. 2002) and a disruption of dopaminergic input to the striatum due to the loss of pigmented substantia nigra neurons (Ince & Perry 2005). DLB is not rare and may occur in approximately 20% of all elderly demented patients (McKeith et al. 1996).
The distribution of neuropathologic changes in DLB and AD is quite similar, so it is not surprising that the two disorders result in similar dementia syndromes. Both disorders are initially characterized by the insidious onset of cognitive decline with no other prominent neurological abnormalities (Hansen et al. 1990, McKeith et al. 1996). Memory impairment is often the earliest feature of both disorders, but with time, cognitive deficits become widespread and inexorably progress to severe dementia. Because of these similarities, patients with DLB are often clinically diagnosed as having probable or possible AD during life (e.g., Merdes et al. 2003). However, several clinical features occur with a higher prevalence in patients with DLB than in those with pure AD. These features include mild spontaneous motor features of Parkinsonism (e.g., bradykinesia, rigidity, and masked facies, but without a resting tremor), recurrent and well-formed visual hallucinations, and fluctuating cognition with pronounced variations in attention or alertness (for review, see McKeith et al. 2005). These clinical distinctions form the basis for consensus criteria adopted by the International Consortium on DLB to clinically diagnose DLB and distinguish it from AD (McKeith et al. 1996, 2005).
Given the difficulty in clinically differentiating DLB from AD, a number of studies of autopsy-confirmed or clinically diagnosed patients have attempted to delineate the two disorders further based on patterns of neuropsychological deficits. These studies have consistently shown that the most salient neuropsychological difference between the two disorders is a disproportionately severe visuospatial and visuo-constructive deficit in patients with DLB. This has been shown using tests of visual perception (e.g., segregation of overlapping figures), tests of visual search (e.g., parallel search tasks that usually elicit the pop-out phenomenon), and tests that require drawing simple and complex two-dimensional figures or the construction of three-dimensional objects (for review, see Salmon & Hamilton 2006). These particularly severe deficits in visuospatial and visuoperceptual abilities are often apparent even when DLB patients perform better than do AD patients on tests of verbal memory (e.g., Lambon Ralph et al. 2001).
The prominence of visuoperceptual, visuospatial, and visuoconstructional deficits in patients with DLB may be related to occipital cortex dysfunction that does not usually occur in patients with AD. Studies using positron emission tomography (PET) or single-photon emission computerized tomography (SPECT) neuroimaging have shown that relatively early DLB is characterized by hypometabolism and decreased blood flow in primary visual and visual-association cortex that is not evident in AD (e.g., Minoshima et al. 2001). These metabolic changes are paralleled by pathologic changes in occipital cortex of patients with DLB that include white matter spongiform change with coexisting gliosis (Higuchi et al. 2000) and, in some cases, deposition of Lewy bodies (e.g., Gomez-Tortosa et al. 1999). Because occipital cortex pathology is rare in pure AD, it is not surprising that the visuoperceptual and visuospatial abilities that may be dependent upon these cortices are disproportionately impaired in patients with DLB.
Patients with DLB often also have disproportionately severe deficits in executive functions and attention in comparison to equally demented patients with pure AD. This difference is evident on tests of attention such as the Wechsler Adult Intelligence Scale-Revised Digit Span subtest or the Cancellation Test, tests of initiation and systematic retrieval from semantic memory such as the Initiation/Perseveration subscale of the Mattis Dementia Rating Scale or the phonemic verbal fluency test, and tests of abstract reasoning such as the Raven Colored Progressive Matrices or the Wechsler Adult Intelligence Scale-Revised Similarities subtest (for review, see Salmon & Hamilton 2006). A series of studies using a computer-based testing paradigm (i.e., the Cambridge Neuropsychological Test Automated Battery) demonstrated that patients with DLB were more impaired than patients with AD on a conditional pattern-location paired-associates learning task (Galloway et al. 1992), a delayed matching-to-sample task (Sahgal et al. 1992a), a visual search task that assessed the ability to focus attention (Sahgal et al. 1992b), and a spatial working-memory task that assessed both spatial memory and the ability to use an efficient search strategy (Sahgal et al. 1995). These prominent attention and executive function deficits are similar to those that occur in patients with basal ganglia dysfunction that interrupts frontostriatal circuits (e.g., HD). These circuits may be affected in two ways in patients with DLB: by direct neocortical Lewy body pathology in the association areas of the frontal lobes and by substantia nigra pathology that interrupts dopaminergic projections to the striatum. When superimposed upon the AD pathology that is also often present in the frontal cortex of patients with DLB, these pathological changes may result in disproportionately severe deficits in executive function and attention.
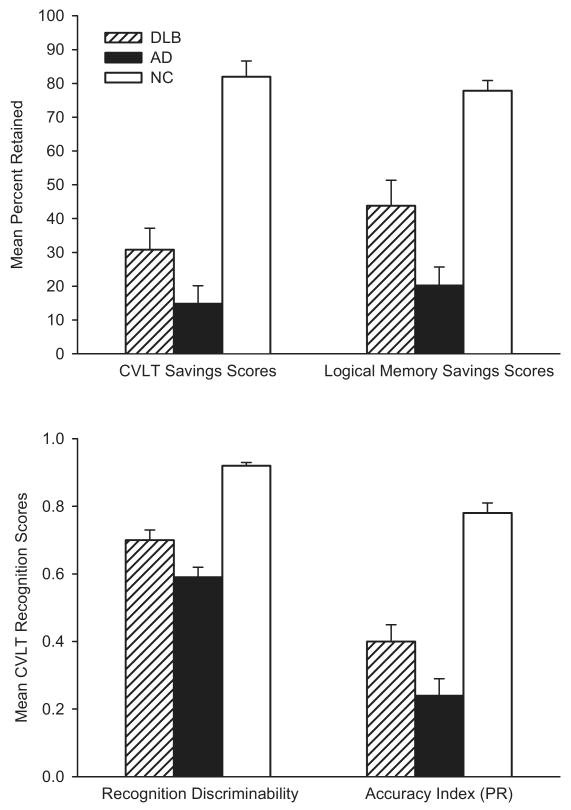
In contrast to DLB patients' disproportionately severe deficits in visuospatial abilities, executive functions, and attention, their memory deficit is generally less severe than that of AD patients and may reflect a qualitative difference in the processes affected. This was illustrated in a study that directly compared the performances of patients with autopsy-confirmed DLB (all with concomitant AD pathology) or pure AD on the California Verbal Learning Test and the Wechsler Memory Scale-Revised Logical Memory Test (Hamilton et al. 2004). Although the two groups were equally impaired in their ability to learn new verbal information on these tests, DLB patients exhibited better retention and better recognition memory than did patients with pure AD. These results suggest that a deficit in retrieval plays a greater role in the memory impairment of patients with DLB than in that of patients with AD. Although the pattern of deficits does not rule out the possibility that poor encoding contributes to memory impairment in both disorders, it appears that DLB patients have better retention than do patients with AD when retrieval demands are reduced through the use of the recognition format (see Figure 4). The observed differences are consistent with neuropathologic (Lippa et al. 1998) and MRI (Barber et al. 2001, Hashimoto et al. 1998) evidence that medial temporal lobe structures important for memory (e.g., hippocampus, entorhinal cortex, parahip-pocampal gyrus) are less severely affected in DLB than in AD. A combination of only moderate medial temporal lobe damage and frontostriatal dysfunction might explain the less severe retention deficit and greater impact of deficient retrieval processes in DLB than in AD.
Figure 4.
The average scores achieved by normal control (NC) subjects, patients with Alzheimer's disease (AD), and patients with dementia with Lewy bodies (DLB) on various learning and memory measures from the California Verbal Learning Test (CVLT) and the Wechsler Adult Intelligence Scale-Revised Logical Memory Test. Despite similar levels of global cognitive impairment, the DLB patients were less impaired than the AD patients on measures of retention (memory savings score) and recognition memory (recognition discriminability and recognition accuracy index). PR, percent retained. (Adapted from Hamilton et al. 2004.)
The general pattern of greater visuospatial, attention, and executive function impairment in DLB than AD, and greater memory impairment in AD than DLB, has been confirmed in a number of recent studies that compared clinically diagnosed or autopsy-diagnosed patient groups on batteries of neuropsychological tests (Ferman et al. 2006, Guidi et al. 2006, Johnson et al. 2005, Kraybill et al. 2005, Stavitsky et al. 2006). Consideration of these patterns of deficits (particularly those of visuospatial abilities) may have important clinical utility in distinguishing between AD and DLB in mildly demented patients (Tiraboschi et al. 2006).
Alzheimer's Disease versus Frontotemporal Dementia
Frontotemporal dementia (FTD) is a clinico-pathologic condition characterized by deterioration of personality and cognition associated with prominent frontal and temporal lobar atrophy. A number of conditions fall under the rubric of FTD including Pick's disease, familial chromosome 17-linked frontal lobe dementia, dementia lacking distinctive histopathology, semantic dementia, and primary progressive aphasia (for review, see Kertesz 2006). Although each of these variants has a unique clinical presentation, the most common variant of FTD typically begins with the insidious onset of personality and behavioral changes (e.g., inappropriate social conduct, apathy, disinhibition, perseverative behavior, loss of insight, hyperorality, decreased speech output) that are accompanied or soon followed by cognitive deficits that include alterations in executive functions, attention, and/or language, often with relative sparing of visuospatial abilities and memory (for reviews, see Boxer & Miller 2006, Grossman 2002, Neary 2005). FTD accounts for approximately 6%–12% of all cases of dementia (Kertesz 2006).
Recent attempts to differentiate FTD and AD based on the nature and severity of behavioral symptoms have met with some success (see Kertesz 2006). However, the disorders are clinically similar and remain difficult to distinguish during life (Mendez et al. 1993, Varma et al. 1999). This has led some investigators to propose that consideration of the patterns of cognitive deficits associated with FTD and AD might aid in clinically distinguishing between the two disorders. A number of studies suggest that patients with FTD are more impaired than those with AD on tests of verbal fluency (Frisoni et al. 1995, Lindau et al. 1998, Mathuranath et al. 2000) or less impaired on tests of memory (Binetti et al. 2000, Frisoni et al. 1995, Lindau et al. 1998, Pachana et al. 1996, Thomas-Anterion et al. 2000) and visuospatial abilities (Elfgren et al. 1994, Mendez et al. 1996). Unfortunately, these findings are often based on relatively small, clinically defined (not autopsy-confirmed) patient samples that are susceptible to cross-contamination, and on studies that compared FTD and AD patients who were at different stages of illness. In addition, the ability to detect differences was attenuated by the choice of neuropsychological test in some studies, such as those that may have failed to find a significant difference in the visuospatial-constructional abilities of FTD and AD patients because they used a Rey-Osterrieth Complex Figure task that is known to require attention and organizational abilities dependent on the frontal lobes (Frisoni et al. 1995, Lindau et al. 1998, Pachana et al. 1996, Varma et al. 1999).
Several studies that examined profiles of cognitive deficits associated with FTD and AD suggest that FTD patients have a greater deficit in executive functions than in other cognitive abilities, whereas AD patients have executive dysfunction that is proportional to their deficits in language and visuospatial abilities and less prominent than their episodic memory deficit (Forstl et al. 1996, Rascovsky et al. 2002, Starkstein et al. 1994). In a study that retrospectively compared the cognitive profiles of patients with autopsy-confirmed FTD or AD who were matched for education and level of dementia at the time of testing, Rascovsky and colleagues (2002) found that FTD patients performed significantly worse than AD patients on word-generation tasks that are sensitive to frontal lobe dysfunction (i.e., letter and category fluency tests), but significantly better on tests of memory (i.e., Mattis Dementia Rating Scale Memory subscale) and visuospatial abilities (i.e., Block Design and Clock Drawing tests) that are sensitive to dysfunction of medial temporal and parietal association cortices. A logistic regression model using letter fluency, memory subscale, and Block Design test scores provided good discriminability between the groups, correctly classifying 91% of AD patients and 77% of FTD patients. Similar levels of diagnostic accuracy were observed in studies comparing clinically diagnosed patients on executive function, visuospatial, and memory tests (Elfgren et al. 1994, Gregory et al. 1997, Libon et al. 2007, Lipton et al. 2005).
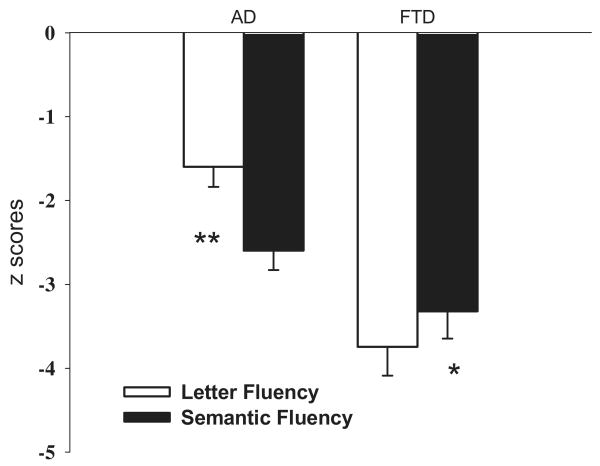
In a related study, Rascovsky and colleagues (2007) compared the performances of autopsy-confirmed FTD and AD patients on letter and semantic category fluency tests to determine if distinct patterns of deficits might be evident on these relatively simple tasks. Although both verbal fluency tasks utilize frontal lobe–mediated executive processes, distinct patterns were hypothesized because semantic category fluency requires a search through semantic or conceptual memory and is critically dependent upon knowledge of the physical and/or functional attributes that define a particular semantic category, whereas letter fluency requires the use of phonemic cues to guide retrieval and may thus require greater effort and more active strategic search than semantic category fluency. Results showed that despite similar age, education, and dementia severity, FTD patients performed worse than AD did patients overall, and letter fluency was worse than semantic fluency for the FTD patients, whereas semantic fluency was worse than letter fluency for the AD patients (see Figure 5). A derived measure of the disparity between letter and semantic fluency (the Semantic Index) correctly classified 92% of AD patients and 85% of FTD patients for an overall correct classification of nearly 90%. The unique patterns of fluency deficits in FTD and AD may be indicative of differences in the relative contribution of frontal lobe–mediated retrieval deficits (most prominent in FTD) and temporal lobe–mediated semantic deficits (most prominent in AD) in the two disorders.
Figure 5.
Mean z-scores achieved by patients with Alzheimer's disease (AD) and patients with frontotemporal dementia (FTD; excluding semantic dementia) on the letter fluency and semantic category fluency tests. FTD patients were more impaired on the letter fluency than semantic fluency task, whereas AD patients were more impaired on the semantic fluency than letter fluency task. *p < 0.05, **p < 0.01. (Adapted from Rascovsky et al. 2007.)
Alzheimer's Disease versus Vascular Dementia
Vascular dementia (VaD) refers to a cumulative decline in cognitive functioning secondary to multiple or strategically placed infarctions, ischemic injury, or hemorrhagic lesions. The clinical and neuropathologic presentation of VaD is quite heterogeneous, and a variety of conditions fall under the general rubric of VaD. As Hodges &Graham (2001) pointed out, these conditions generally fall into three large categories: multi-infarct dementia associated with multiple large cortical infarctions (usually affecting 10cc or more of brain tissue), dementia due to strategically placed infarction (e.g., left angular gyrus damage related to infarction of the posterior branch of the medial cerebral artery), and subcortical ischemic vascular dementia due to subcortical small vessel disease that results in multiple lacunar strokes, leukoaraiosis (Binswanger's disease), or diffuse white matter pathology.
Specific research criteria for the broadly defined diagnosis of VaD have been proposed (e.g., Chui et al. 1992, Roman et al. 1993). In general, these guidelines require that multiple cognitive deficits (i.e., dementia) occur in the presence of focal neurological signs and symptoms and/or laboratory (e.g., computerized tomography or MRI scan) evidence of cerebrovascular disease that is thought to be etiologically related to the cognitive impairment. A relationship between dementia and cerebrovascular disease is often indicated if the onset of dementia occurs within several months of a recognized stroke, cognitive functioning abruptly deteriorates, or the course of cognitive deterioration is fluctuating or stepwise. In one set of diagnostic criteria (Roman et al. 1993), VaD can be subcategorized on the basis of the suspected type of vascular pathology (as determined by clinical, radiologic, and neuropathologic features), and possible or probable VaD may be assigned depending on the certainty of the contribution of cerebrovascular disease to the dementia syndrome. Definite VaD is diagnosed only on the basis of histopathologic evidence of cerebrovascular disease that occurs in the absence of neurofibrillary tangles and neuritic plaques exceeding those expected for age (i.e., AD) and without clinical evidence of any other disorder capable of producing dementia (e.g., Pick's disease, diffuse Lewy body disease).
Recent studies of the neuropsychological deficits associated with VaD have primarily focused on differentiating between subcortical VaD and AD. These studies largely show that patients with subcortical VaD are more impaired than those with AD on tests of executive functions, whereas patients with AD are more impaired than those with subcortical VaD on tests of episodic memory (particularly delayed recall) (Desmond 2004, Graham et al. 2004, Kertesz & Clydesdale 1994, Lafosse et al. 1997, Lamar et al. 1997). In addition, these studies suggest that the executive dysfunction associated with subcortical VaD is its most prominent deficit, perhaps because subcortical pathology interrupts frontosubcortical circuits that mediate this aspect of cognition. Indeed, a study by Price and colleagues (2005) showed that VaD patients with a significant volume of white matter abnormality on imaging exhibited a profile of greater executive/visuoconstructional impairment than impairment of memory and language abilities.
Although neuropsychological studies provide consistent evidence for distinct cognitive profiles in subcortical VaD and AD, most of these studies employed clinically diagnosed patients without autopsy confirmation of diagnosis. This may have led to some degree of misclassification of patients across groups because AD and VaD are quite heterogeneous and can overlap in their clinical presentations. To avoid this potential confound, Reed and colleagues (2007) recently compared the profiles of neuropsychological deficits exhibited by patients with autopsy-confirmed subcortical VaD or AD. Consistent with previous studies of clinically diagnosed patients, patients with AD had a deficit in episodic memory (both verbal and nonverbal) that was significantly greater than their executive function deficit. In contrast, patients with subcortical VaD had a deficit in executive functions that was greater than their deficit in verbal (but not nonverbal) episodic memory, but this difference was not significant. An analysis of individual patient profiles was carried out to explore these differences further. This analysis showed that 71% of AD patients exhibited a profile with memory impairment more prominent than executive dysfunction, whereas only 45% of patients with subcortical VaD exhibited a profile with more prominent executive dysfunction than memory impairment. Interestingly, relatively severe cerebrovascular disease at autopsy was often not associated with clinically significant cognitive decline. When the profile analysis was restricted to those patients who exhibited significant cognitive impairment at their clinical assessment, the distinction between subcortical VaD and AD patients was more pronounced, with 79% of AD patients exhibiting a low memory profile (5% with a low executive profile) and 67% of subcortical VaD patients exhibiting a low executive profile (0% with a low memory profile). The results of this study suggest that relatively distinct cognitive deficit profiles might be clinically useful in differentiating between subcortical VaD and AD, but additional research with autopsy-diagnosed patients is needed to further define the deficit profile that will be most useful in this regard.
Conclusions
Considerable progress has been made in differentiating between the cognitive changes that occur as a normal consequence of aging and those that signal the onset of a dementia syndrome caused by AD or another neurodegenerative disease. Clinical and experimental neuropsychological research has identified many of the basic cognitive processes that are adversely affected by AD and is beginning to uncover the earliest preclinical cognitive changes that might predict the subsequent development of dementia and AD in nondemented individuals. Neuropsychological research has also made considerable progress in delineating different patterns of relatively preserved and impaired cognitive abilities that distinguish between AD and other age-associated neurodegenerative disorders. Greater understanding of the cognitive distinctions between these disorders can aid in the development of better differential diagnosis and has important implications for the nature of brain-behavior relationships underlying memory, language, executive functions, and other cognitive abilities.
Summary Points.
Cognitive deficits associated with AD can be differentiated from age-associated cognitive decline by quantitative and qualitative differences in episodic memory, semantic knowledge, and some aspects of executive functions. However, the qualitatively distinct pattern of deficits is less salient in very old AD patients than in younger AD patients.
Decline in episodic memory (particularly delayed recall) is usually the earliest cognitive change that occurs prior to the development of the AD dementia syndrome and may predict imminent dementia. Recent evidence suggests that asymmetry in cognitive abilities may also occur in this preclinical phase of AD.
The cortical neuropathology of AD appears to result in a loss of functional connectivity that allows effective interaction between distinct and relatively intact cortical information-processing systems. This loss has been demonstrated in AD patients' impaired ability to bind distinct visual stimulus features that are effectively processed in different cortical streams (i.e., motion and color). This behavioral manifestation of cortical disconnectivity has potential as a cognitive marker for detecting and tracking progression of AD.
Distinct patterns of cognitive deficits occur in AD and other age-associated neurodegenerative disorders such as Huntington's disease, dementia with Lewy bodies, frontotemporal dementia, and vascular dementia. Differences in the cognitive profiles associated with these various disorders can aid in differential diagnosis and provide a useful model for understanding brain-behavior relationships that mediate the affected cognitive abilities.
Future Issues.
The early diagnosis of AD in a preclinical stage that might be most amenable to treatments that halt or slow disease progression remains an extremely important goal. It is essential to recognize and verify the accuracy of subtle cognitive abnormalities (e.g., poor delayed recall performance, cognitive asymmetry) that might identify those nondemented elderly individuals who are destined to develop dementia.
The role of cortical disconnectivity in producing the specific pattern of cognitive deficits that occurs in early AD needs to be determined. Furthermore, the identification of cognitive processes that are particularly vulnerable to the effects of cortical disconnectivity in early AD might provide a cognitive marker that could be used to assess the effects of medications that specifically target cortical function (e.g., the N-methyl-D-aspartate receptor antagonist memantine).
It remains difficult to estimate rate of cognitive decline in AD and other age-related neurodegenerative diseases, but emerging evidence suggests that certain aspects of current cognitive performance can predict subsequent rate of global cognitive decline in patients with AD (e.g., Chan et al. 1995). Further research is needed to confirm this possibility and to generalize it to other neurodegenerative disorders such as DLB and FTD.
There is a continuing need to identify differences in the profiles of cognitive deficits associated with AD and other age-related neurodegenerative diseases (e.g., DLB, FTD) and to determine how these profiles can be incorporated with other clinical features to improve the accuracy of differential diagnosis in very mildly demented individuals. Accurate early diagnosis is a particularly important goal since the various neurodegenerative disorders are likely to respond differently to the potential treatments for dementia that are in development.
Acknowledgments
The preparation of this review was supported by funds from NIA grants AG-05131, AG-12963, and AG-12674 to the University of California, San Diego.
- Dementia
syndrome of acquired intellectual impairment of sufficient severity to interfere with social or occupational functioning caused by brain dysfunction
- Executive functions
higher-order cognitive processes involved in planning, concept formation, problem solving, cue-directed behavior, and the concurrent manipulation and retention of information
- Episodic memory
memory for autobiographical events and episodes that depend upon temporal and/or spatial contextual cues for their retrieval
- Semantic memory
general fund of knowledge that consists of overlearned facts and concepts that are not dependent upon contextual cues for retrieval (e.g., meanings of words and well-known geographical, historical, and arithmetical facts)
- MRI
magnetic resonance imaging
- Corticocortical disconnection
the loss of effective interaction between functionally related cortical association areas
- HD
Huntington's disease
- DLB
dementia with Lewy bodies
- FTD
frontotemporal dementia
- PET
positron emission tomography
- VaD
vascular dementia
Footnotes
The U.S. Government has the right to retain a nonexclusive, royalty-free license in and to any copyright covering this paper.
Disclosure Statement: The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
Literature Cited
- Albert MS, Blacker D. Mild cognitive impairment and dementia. Annu Rev Clin Psychol. 2006;2:379–88. doi: 10.1146/annurev.clinpsy.1.102803.144039. [DOI] [PubMed] [Google Scholar]
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7:631–39. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]; Measures of episodic memory and executive function predicted the development of dementia within three years in patients with mild memory difficulty.
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Jones S, Berger AK, Laukka EJ, Small BJ. Multiple cognitive deficits during the transition to Alzheimer's disease. J Intern Med. 2004;256:195–204. doi: 10.1111/j.1365-2796.2004.01386.x. [DOI] [PubMed] [Google Scholar]; A review showing that multiple cognitive domains (i.e., episodic memory, executive function, and perceptual speed) are adversely affected several years before a clinical diagnosis of AD.
- Bäckman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer's disease: a meta-analysis. Neuropsychology. 2005;19:520–31. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Small BJ. Influences of cognitive support on episodic remembering: tracing the process of loss from normal aging to Alzheimer's disease. Psychol Aging. 1998;13:267–76. doi: 10.1037//0882-7974.13.2.267. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer's disease. Brain. 2001;124:96–102. doi: 10.1093/brain/124.1.96. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Bressi S, Della Sala S, Logie R, Spinnler H. The decline of working memory in Alzheimer's disease: a longitudinal study. Brain. 1991;114:2521–42. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- Barber R, McKeith IG, Ballard C, Gholkar A, O'Brien JT. A comparison of medial and lateral temporal lobe atrophy in dementia with Lewy bodies and Alzheimer's disease: magnetic resonance imaging volumetric study. Dement Geriatr Cogn Disord. 2001;12:198–205. doi: 10.1159/000051258. [DOI] [PubMed] [Google Scholar]
- Bayles KA, Tomoeda CK. Confrontation naming impairment in dementia. Brain Lang. 1983;19:98–114. doi: 10.1016/0093-934x(83)90057-3. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Salmon DP, Bondi MW, Bui BK, Olichney J, et al. Comparison of the serial position effect in very mild Alzheimer's disease, mild Alzheimer's disease, and amnesia associated with electroconvulsive therapy. J Int Neuropsychol Soc. 2000;6:290–98. doi: 10.1017/s1355617700633040. [DOI] [PubMed] [Google Scholar]
- Binetti G, Locascio JJ, Corkin S, Vonsattel JP, Growdon JH. Differences between Pick disease and Alzheimer disease in the clinical appearance and rate of cognitive decline. Arch Neurol. 2000;57:225–32. doi: 10.1001/archneur.57.2.225. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Salmon DP, Corey-Bloom J, Katzman R, et al. Neuropsychological deficits associated with Alzheimer's disease in the very old: discrepancies in raw vs standardized scores. J Int Neuropsychol Soc. 2003;9:783–95. doi: 10.1017/S1355617703950119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Monsch AU, Butters N, Salmon DP, Paulsen JS. Utility of a modified version of the Wisconsin Card Sorting Test in the detection of dementia of the Alzheimer type. Clin Neuropsychol. 1993;7:161–70. doi: 10.1080/13854049308401518. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Miller BL. Clinical features of frontotemporal dementia. Alzheimer Dis Assoc Disord. 2006;19:S3–6. doi: 10.1097/01.wad.0000183086.99691.91. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brandt J, Butters N. The neuropsychology of Huntington's disease. Trends Neurosci. 1986;9:118–20. [Google Scholar]
- Brandt J, Bylsma FW. The dementia of Huntington's disease. In: Parks RW, Zec RF, Wilson RS, editors. Neuropsychology of Alzheimer's Disease and Other Dementias. New York: Oxford Univ. Press; 1993. pp. 265–82. [Google Scholar]
- Brandt J, Corwin J, Krafft L. Is verbal recognition memory really different in Huntington's and Alzheimer's disease? J Clin Exp Neuropsychol. 1992;14:773–84. doi: 10.1080/01688639208402862. [DOI] [PubMed] [Google Scholar]
- Brouwers P, Cox C, Martin A, Chase T, Fedio P. Differential perceptual-spatial impairment in Huntington's and Alzheimer's dementias. Arch Neurol. 1984;41:1073–76. doi: 10.1001/archneur.1984.04050210071017. [DOI] [PubMed] [Google Scholar]
- Buschke H. Selective reminding for analysis of memory and learning. J Verb Learn Verb Behav. 1973;12:543–50. [Google Scholar]
- Buschke H, Sliwinski MJ, Kuslansky G, Lipton RB. Diagnosis of early dementia by the double memory test. Neurology. 1997;48:989–97. doi: 10.1212/wnl.48.4.989. [DOI] [PubMed] [Google Scholar]
- Butters N, Granholm E, Salmon DP, Grant I, Wolfe J. Episodic and semantic memory: a comparison of amnesic and demented patients. J Clin Exp Neuropsychol. 1987;9:479–97. doi: 10.1080/01688638708410764. [DOI] [PubMed] [Google Scholar]
- Butters N, Salmon DP, Cullum CM, Cairns P, Troster AI, et al. Differentiation of amnesic and demented patients with the Wechsler Memory Scale-Revised. Clin Neuropsychol. 1988;2:133–48. [Google Scholar]
- Butters N, Wolfe J, Granholm E, Martone M. An assessment of verbal recall, recognition and fluency abilities in patients with Huntington's disease. Cortex. 1986;22:11–32. doi: 10.1016/s0010-9452(86)80030-2. [DOI] [PubMed] [Google Scholar]
- Butters N, Wolfe J, Martone M, Granholm E, Cermak LS. Memory disorders associated with Huntington's disease: verbal recall, verbal recognition and procedural memory. Neuropsychologia. 1985;23:729–43. doi: 10.1016/0028-3932(85)90080-6. [DOI] [PubMed] [Google Scholar]
- Chan AS, Salmon DP, Butters N. Semantic network abnormalities in patients with Alzheimer's disease. In: Parks RW, Levine DS, Long DL, editors. Fundamentals of Neural Network Modeling. Cambridge, MA: MIT Press; 1998. pp. 381–93. [Google Scholar]
- Chan AS, Salmon DP, Butters N, Johnson S. Semantic network abnormality predicts rate of cognitive decline in patients with Alzheimer's disease. J Int Neuropsychol Soc. 1995;1:297–303. doi: 10.1017/s1355617700000291. [DOI] [PubMed] [Google Scholar]
- Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Arch Gen Psychiatry. 2001;58:853–58. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Bub D. Semantic memory loss in dementia of Alzheimer's type. Brain. 1990;113:397–417. doi: 10.1093/brain/113.2.397. [DOI] [PubMed] [Google Scholar]
- Chui H, Victoroff J, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic Treatment Centers. Neurology. 1992;42:473–80. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- Collette F, Van Der Linden M, Bechet S, Salmon E. Phonological loop and central executive functioning in Alzheimer's disease. Neuropsychologia. 1999;37:905–18. doi: 10.1016/s0028-3932(98)00148-1. [DOI] [PubMed] [Google Scholar]
- Collie A, Maruff P. The neuropsychology of preclinical Alzheimer's disease and mild cognitive impairment. Neurosci Biobehav Rev. 2000;24:365–74. doi: 10.1016/s0149-7634(00)00012-9. [DOI] [PubMed] [Google Scholar]
- Croner LJ, Albright TD. Image segmentation enhances discrimination of motion in visual noise. Vision Res. 1997;37:1415–27. doi: 10.1016/s0042-6989(96)00299-4. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A, Amick M. Spatial abilities in aging, Alzheimer's disease, and Parkinson's disease. In: Boller F, Cappa SF, editors. Handbook of Neuropsychology, Vol 6: Aging and Dementia. 2nd Amsterdam: Elsevier; 2001. pp. 119–43. [Google Scholar]
- De Lacoste M, White CL. The role of cortical connectivity in Alzheimer's disease pathogenesis: a review and model system. Neurobiol Aging. 1993;14:1–16. doi: 10.1016/0197-4580(93)90015-4. [DOI] [PubMed] [Google Scholar]
- Delbeuck X, Van Der Linden M, Collette F. Alzheimer's disease as a disconnection syndrome? Neuropsychol Rev. 2003;13:79–92. doi: 10.1023/a:1023832305702. [DOI] [PubMed] [Google Scholar]
- Delis DC, Massman PJ, Butters N, Salmon DP, Cermak LS, Kramer JH. Profiles of demented and amnesic patients on the California Verbal Learning Test: implications for the assessment of memory disorders. Psychol Assess. 1991;3:19–26. [Google Scholar]
- Della Sala S, Kinnear P, Spinnler H, Stangalino C. Color-to-figure matching in Alzheimer's disease. Arch Clin Neuropsychol. 2000;15:571–85. doi: 10.1016/s0887-6177(99)00047-5. [DOI] [PubMed] [Google Scholar]
- Desmond DW. The neuropsychology of vascular cognitive impairment: Is there a specific cognitive impairment? J Neurol Sci. 2004;226:3–7. doi: 10.1016/j.jns.2004.09.002. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the BOLD hemodynamic response. NeuroImage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Albright TD. The influence of chromatic information on visual motion processing in the primate visual system. In: Watanabe T, editor. In High-Level Motion Processing—Computational, Neurobiological and Psychophysical Perspectives. Cambridge, MA: MIT Press; 1998. pp. 53–94. [Google Scholar]
- Dunkin JJ, Osato S, Leuchter AF. Relationships between EEG coherence and neuropsychological tests in dementia. Clin Electroencephal. 1995;26:47–59. doi: 10.1177/155005949502600107. [DOI] [PubMed] [Google Scholar]
- Elfgren C, Brun A, Gustafson L, Johanson A, Minton L, et al. Neuropsychological tests as discriminators between dementia of Alzheimer's type and frontotemporal dementia. Int Geriatr Psychiatry. 1994;9:635–42. [Google Scholar]
- Ferman TJ, Smith GE, Boeve BF, Graff-Radford NR, Lucas JA, et al. Neuropsychological differentiation of dementia with Lewy bodies from normal aging and Alzheimer's disease. Clin Neuropsychol. 2006;20:623–36. doi: 10.1080/13854040500376831. [DOI] [PubMed] [Google Scholar]
- Festa E, Insler RZ, Salmon DP, Paxton J, Hamilton JM, Heindel WC. Neocortical disconnectivity disrupts sensory integration in Alzheimer's disease. Neuropsychology. 2005;19:728–38. doi: 10.1037/0894-4105.19.6.728. [DOI] [PubMed] [Google Scholar]; AD patients exhibited specific deficits in binding visual features when cross-cortical integration was required (e.g., motion-color versus motion-luminance).
- Förstl H, Besthorn C, Geiger-Kabisch C, Sattel H, Schreitter-Gasser U. Frontal lobe degeneration and Alzheimer's disease: a controlled study on clinical findings, volumetric brain changes and quantitative electroencephalography data. Dementia. 1996;7:27–34. doi: 10.1159/000106849. [DOI] [PubMed] [Google Scholar]
- Foster JK, Behrmann M, Stuss DT. Visual attention deficits in Alzheimer's disease: simple versus conjoined feature search. Neuropsychology. 1999;13:223–45. doi: 10.1037//0894-4105.13.2.223. [DOI] [PubMed] [Google Scholar]; AD patients had abnormal increase in reaction time with increasing array size during conjoined feature search suggesting impaired perceptual integration.
- Freedman M, Leach L, Kaplan E, Winocur G, Shulman KI, Delis DC. Clock Drawing: A Neuropsychological Analysis. New York: Oxford Univ. Press; 1994. [Google Scholar]
- Freedman M, Oscar-Berman M. Breakdown of cross-modal function in dementia. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:102–6. [PubMed] [Google Scholar]
- Frisoni GB, Pizzolato G, Geroldi C, Rossato A, Bianchetti A, Trabucchi M. Dementia of the frontal type: neuropsychological and 99[TC-HMPAO] SPECT features. J Geriatr Psychiatry Neurol. 1995;8:42–48. [PubMed] [Google Scholar]
- Galloway PH, Sahgal A, McKeith IG, Lloyd S, Cook JH, et al. Visual pattern recognition memory and learning deficits in senile dementias of Alzheimer and Lewy body types. Dementia. 1992;3:101–7. [Google Scholar]
- Golob EJ, Miranda GG, Johnson JK, Starr A. Sensory cortical interactions in aging, mild cognitive impairment, and Alzheimer's disease. Neurobiol Aging. 2001;22:755–63. doi: 10.1016/s0197-4580(01)00244-5. [DOI] [PubMed] [Google Scholar]
- Gómez-Tortosa E, Newell K, Irizarry MC, Albert M, Growdon JH, Hyman BT. Clinical and quantitative pathologic correlates of dementia with Lewy bodies. Neurology. 1999;53:1284–91. doi: 10.1212/wnl.53.6.1284. [DOI] [PubMed] [Google Scholar]
- Grady CL, Haxby JV, Horwitz B, Sundaram M, Berg G, et al. Longitudinal study of the early neuropsychological and cerebral metabolic changes in dementia of the Alzheimer type. J Clin Exp Neuropsychol. 1988;10:576–96. doi: 10.1080/01688638808402796. [DOI] [PubMed] [Google Scholar]
- Graham NL, Emery T, Hodges JR. Distinct cognitive profiles in Alzheimer's disease and subcortical vascular dementia. J Neurol Neurosurg Psychiatry. 2004;75:61–71. [PMC free article] [PubMed] [Google Scholar]
- Gregory CA, Orrell M, Sahkian B, Hodges J. Can frontotemporal dementia and Alzheimer's disease be differentiated using a brief battery of tests? Int Geriatr Psychiatry. 1997;12:357–83. doi: 10.1002/(sici)1099-1166(199703)12:3<375::aid-gps518>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Grossman M. Frontotemporal dementia: a review. J Int Neuropsychol Soc. 2002;8:566–83. doi: 10.1017/s1355617702814357. [DOI] [PubMed] [Google Scholar]
- Guidi M, Paciaroni L, Paolini S, DePadova S, Scarpino O. Differences and similarities in the neuropsychological profile of dementia with Lewy bodies and Alzheimer's disease in the early stage. J Neurol Sci. 2006;248:120–23. doi: 10.1016/j.jns.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Hamilton JM, Salmon DP, Galasko D, Delis DC, Hansen LA, et al. A comparison of episodic memory deficits in neuropathologically-confirmed dementia with Lewy bodies and Alzheimer's disease. J Int Neuropsychol Soc. 2004;10:689–97. doi: 10.1017/S1355617704105043. [DOI] [PubMed] [Google Scholar]
- Hanes KR, Andrewes DG, Pantelis C. Cognitive flexibility and complex integration in Parkinson's disease, Huntington's disease, and schizophrenia. J Int Neuropsychol Soc. 1995;1:545–53. doi: 10.1017/s1355617700000679. [DOI] [PubMed] [Google Scholar]
- Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, et al. The Lewy body variant of Alzheimer's disease: a clinical and pathologic entity. Neurology. 1990;40:1–8. doi: 10.1212/wnl.40.1.1. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Kitagaki H, Imamura T, Hirono N, Shimomura T, et al. Medial temporal and whole-brain atrophy in dementia with Lewy bodies. Neurology. 1998;51:357–62. doi: 10.1212/wnl.51.2.357. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev. 2004;5:87–97. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer's type: a meta-analysis. Neuropsychologia. 2004;42:1212–22. doi: 10.1016/j.neuropsychologia.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR, Phillips LH. A meta-analytic review of verbal fluency deficits in Huntington's disease. Neuropsychology. 2005;19:243–52. doi: 10.1037/0894-4105.19.2.243. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Tashiro M, Arai H, Okamura N, Hara S, et al. Glucose hypometabolism and neuropathological correlates in brains of dementia with Lewy bodies. Exp Neurol. 2000;162:247–56. doi: 10.1006/exnr.2000.7342. [DOI] [PubMed] [Google Scholar]
- Ho AK, Sahakian BJ, Brown RG, Barker RA, Hodges JR, et al. Profile of cognitive progression in early Huntington's disease. Neurology. 2003;61:1702–6. doi: 10.1212/01.wnl.0000098878.47789.bd. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Graham NL. Vascular dementias. In: Hodges JR, editor. Early Onset Dementia: A Multidisciplinary Approach. Oxford: Oxford Univ. Press; 2001. pp. 319–37. [Google Scholar]
- Hodges JR, Patterson K. Is semantic memory consistently impaired early in the course of Alzheimer's disease? Neuroanatomical and diagnostic implications Neuropsychologia. 1995;33:441–59. doi: 10.1016/0028-3932(94)00127-b. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. The nature of the naming deficit in Alzheimer's and Huntington's disease. Brain. 1991;114:1547–58. doi: 10.1093/brain/114.4.1547. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Semantic memory impairment in Alzheimer's disease: failure of access or degraded knowledge? Neuropsychologia. 1992;30:301–14. doi: 10.1016/0028-3932(92)90104-t. [DOI] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. The cellular basis of cortical disconnection in Alzheimer's disease and related dementing conditions. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer Disease. New York: Raven; 1999. pp. 207–32. [Google Scholar]
- Hogan MJ, Swanwick GRJ, Kaiser J, Rowan M, Lawlor S. Memory-related EEG power and coherence reductions in mild Alzheimer's disease. Int J Psychophysiol. 2003;49:147–63. doi: 10.1016/s0167-8760(03)00118-1. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Damasio AR, Van Hoesen GW, Barnes CL. Alzheimer's disease: cell specific pathology isolates the hippocampal formation. Science. 1984;225:1168–70. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Ince PG, Perry EK. Pathology of dementia with Lewy bodies. In: Burns A, O'Brien J, Ames D, editors. Dementia. 3rd London: Hodder Arnold; 2005. pp. 615–33. [Google Scholar]
- Jacobs D, Salmon DP, Tröster AI, Butters N. Intrusion errors in the figural memory of patients with Alzheimer's and Huntington's disease. Arch Clin Neuropsychol. 1990;5:49–57. [PubMed] [Google Scholar]
- Jacobson MW, Delis DC, Bondi MW, Salmon DP. Do neuropsychological tests detect preclinical Alzheimer's disease? Individual-test versus cognitive discrepancy analyses Neuropsychology. 2002;16:132–39. doi: 10.1037//0894-4105.16.2.132. [DOI] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, Waring SC, O'Brien PC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1998;49:786–94. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelic V, Shigeta M, Julin P, Almkvist O, Winblad B, Wahlund LO. Quantitative electroencephalography power and coherence in Alzheimer's disease and mild cognitive impairment. Dementia. 1996;7:314–23. doi: 10.1159/000106897. [DOI] [PubMed] [Google Scholar]
- Jernigan TJ, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–94. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Johnson DK, Morris JC, Galvin JE. Verbal and visuospatial deficits in dementia with Lewy bodies. Neurology. 2005;65:1232–38. doi: 10.1212/01.wnl.0000180964.60708.c2. [DOI] [PubMed] [Google Scholar]
- Katzman R. Apolipoprotein E and Alzheimer's disease. Curr Opin Neurobiol. 1994;4:703–7. doi: 10.1016/0959-4388(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Progress in clinical neurosciences: frontotemporal dementia–Pick's disease. Can J Neurol Sci. 2006;33:141–48. doi: 10.1017/s0317167100004893. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Clydesdale S. Neuropsychological deficits in vascular dementia vs Alzheimer's disease: frontal lobe deficits prominent in vascular dementia. Arch Neurol. 1994;51:1226–31. doi: 10.1001/archneur.1994.00540240070018. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46:141–45. doi: 10.1001/archneur.1989.00520380041011. [DOI] [PubMed] [Google Scholar]
- Knott V, Mohr E, Mahoney C, Ilivitsky V. Electroencephalographic coherence in Alzheimer's disease: comparisons with a control group and population norms. J Geriatr Psychiatry Neurol. 2000;13:1–8. doi: 10.1177/089198870001300101. [DOI] [PubMed] [Google Scholar]
- Kraybill ML, Larson EB, Tsuang DW, Teri L, McCormick WC, et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology. 2005;64:2069–73. doi: 10.1212/01.WNL.0000165987.89198.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurylo DD, Corkin S, Rizzo JF, Growdon JH. Greater relative impairment of object recognition than of visuospatial abilities in Alzheimer's disease. Neuropsychology. 1996;10:74–81. [Google Scholar]
- Lafosse JM, Reed BR, Mungas D, Sterling SB, Wahbeh H, Jagust WJ. Fluency and memory differences between ischemic vascular dementia and Alzheimer's disease. Neuropsychology. 1997;11:514–22. doi: 10.1037//0894-4105.11.4.514. [DOI] [PubMed] [Google Scholar]
- Lakmache Y, Lassonde M, Gauthier S, Frigon JY, Lepore F. Interhemispheric disconnection syndrome in Alzheimer's disease. Proc Natl Acad Sci USA. 1998;95:9042–46. doi: 10.1073/pnas.95.15.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar M, Podell K, Carew TG, Cloud BS, Resh R, et al. Perseverative behavior in Alzheimer's disease and subcortical ischemic vascular dementia. Neuropsychology. 1997;11:523–34. doi: 10.1037//0894-4105.11.4.523. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Powell J, Howard D, Whitworth AB, Garrard P, Hodges JR. Semantic memory is impaired in both dementia with Lewy bodies and dementia of Alzheimer's type: a comparative neuropsychological study and literature review. J Neurol Neurosurg Psychiatry. 2001;70:149–56. doi: 10.1136/jnnp.70.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange KL, Bondi MW, Salmon DP, Galasko D, Delis DC, et al. Decline in verbal memory during preclinical Alzheimer's disease: examination of the effect of APOE genotype. J Int Neuropsychol Soc. 2002;8:943–55. doi: 10.1017/s1355617702870096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange KW, Sahakian BJ, Quinn NP, Marsden CD, Robbins TW. Comparison of executive and visuospatial memory function in Huntington's disease and dementia of Alzheimer type matched for degree of dementia. J Neurol Neurosurg Psychiatry. 1995;58:598–606. doi: 10.1136/jnnp.58.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AD, Sahakian BJ, Hodges JR, Rosser AE, Lange KW, Robbins TW. Executive and mnemonic functions in early Huntington's disease. Brain. 1996;119:1633–45. doi: 10.1093/brain/119.5.1633. [DOI] [PubMed] [Google Scholar]
- Lefleche G, Albert MS. Executive function deficits in mild Alzheimer's disease. Neuropsychology. 1995;9:313–20. [Google Scholar]; Executive function deficits in early AD involve concurrent manipulation of information (e.g., set-shifting, sequencing) rather than cued-directed attention or problem solving.
- Libon DJ, Xie SX, Moore P, Farmer J, Antani S, et al. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007;68:369–75. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- Lindau M, Almkvist O, Johansson SE, Wahlund LO. Cognitive and behavioral differentiation of frontal lobe degeneration of the non-Alzheimer's type and Alzheimer's disease. Dementia Geriatr Cogn Disord. 1998;9:205–13. doi: 10.1159/000017048. [DOI] [PubMed] [Google Scholar]
- Lineweaver TT, Salmon DP, Bondi MW, Corey-Bloom J. Distinct effects of Alzheimer's disease and Huntington's disease on performance of mental rotation. J Int Neuropsychol Soc. 2005;11:30–39. doi: 10.1017/S1355617705050034. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Johnson R, Smith TW. The medial temporal lobe in dementia with Lewy bodies: a comparative study with Alzheimer's disease. Ann Neurol. 1998;43:102–6. doi: 10.1002/ana.410430117. [DOI] [PubMed] [Google Scholar]
- Lipton AM, Ohman KA, Womack KB, Hynan LS, Ninman ET, Lacritz LH. Subscores of the FAB differentiate frontotemporal lobar degeneration from AD. Neurology. 2005;65:726–31. doi: 10.1212/01.wnl.0000174437.73416.7b. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen LA, DeTeresa R, Terry RD. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993;43:192–97. doi: 10.1212/wnl.43.1_part_1.192. [DOI] [PubMed] [Google Scholar]
- Mathuranath PS, Nestor PJ, Berrios GE, Rakowicz W, Hodges JR. A brief cognitive test battery to differentiate Alzheimer's disease and frontotemporal dementia. Neurology. 2000;55:1613–20. doi: 10.1212/01.wnl.0000434309.85312.19. [DOI] [PubMed] [Google Scholar]
- McHugh PR, Folstein MF. Psychiatric symptoms of Huntington's chorea: a clinical and phenomenologic study. In: Benson DF, Blumer D, editors. Psychiatric Aspects of Neurological Disease. New York: Raven; 1975. pp. 267–85. [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry E, Dickson D, et al. Clinical and pathological diagnosis of dementia with Lewy bodies (DLB): Report of the Consortium on Dementia with Lewy Bodies (CDLB) International Workgroup. Neurology. 1996;47:1113–24. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Cherrier M, Perryman K, Pachana N, Miller B, Cummings JL. Frontotemporal dementias versus Alzheimer's disease: differential cognitive features. Neurology. 1996;47:1189–94. doi: 10.1212/wnl.47.5.1189. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Selwood A, Mastri AR, Frey WH. Pick's disease versus Alzheimer's disease: a comparison of clinical characteristics. Neurology. 1993;43:289–92. doi: 10.1212/wnl.43.2.289. [DOI] [PubMed] [Google Scholar]
- Merdes AR, Hansen LA, Jeste DV, Galasko D, Hofstetter CR, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003;60:1586–90. doi: 10.1212/01.wnl.0000065889.42856.f2. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Foster NL, Sima A, Frey KA, Albin RL, Kuhl DE. Alzheimer's disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol. 2001;50:358–65. doi: 10.1002/ana.1133. [DOI] [PubMed] [Google Scholar]; Neuropsychological deficit profiles distinguished between AD (memory worse than executive dysfunction) and small vessel cerebrovascular disease (memory equal to executive dysfunction).
- Montoya A, Pelletier M, Menear M, Duplessis, Richer F, Lepage M. Episodic memory impairment in Huntington's disease: a meta-analysis. Neuropsychologia. 2006;44:1984–94. doi: 10.1016/j.neuropsychologia.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Neary D. Frontotemporal dementia. In: Burns A, O'Brien J, Ames D, editors. Dementia. 3rd London: Hodder Arnold; 2005. pp. 667–77. [Google Scholar]
- Nebes R. Semantic memory in Alzheimer's disease. Psychol Bull. 1989;106:377–94. doi: 10.1037/0033-2909.106.3.377. [DOI] [PubMed] [Google Scholar]
- Norton LE, Bondi MW, Salmon DP, Goodglass H. Deterioration of generic knowledge in patients with Alzheimer's disease: evidence from the Number Information Test. J Clin Exp Neuropsychol. 1997;19:857–66. doi: 10.1080/01688639708403766. [DOI] [PubMed] [Google Scholar]
- Pachana NA, Boone KB, Miller BL, Cummings JL, Berman N. Comparison of neuropsychological functioning in Alzheimer's disease and frontotemporal dementia. J Int Neuropsychol Soc. 1996;2:505–10. doi: 10.1017/s1355617700001673. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Haxby JV. Attention and brain function in Alzheimer's disease. Neuropsychology. 1993;7:242–72. [Google Scholar]
- Paulsen JS, Salmon DP, Monsch AU, Butters N, Swenson M, Bondi MW. Discrimination of cortical from subcortical dementias on the basis of memory and problem-solving tests. J Clin Psychol. 1995;51:48–58. doi: 10.1002/1097-4679(199501)51:1<48::aid-jclp2270510109>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Schuller S, Pohl C, Jahn T, Weindl A, Kassubek J. Executive dysfunction in early stages of Huntington's disease is associated with striatal and insular atrophy: a neuropsychological and voxel-based morphometric study. J Neurol Sci. 2005;239:11–19. doi: 10.1016/j.jns.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer's disease: a critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–87. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pillon B, Dubois B, Ploska A, Agid Y. Severity and specificity of cognitive impairment in Alzheimer's, Huntington's, and Parkinson's diseases and progressive supranuclear palsy. Neurology. 1991;41:634–43. doi: 10.1212/wnl.41.5.634. [DOI] [PubMed] [Google Scholar]
- Price CC, Jefferson AL, Merino JG, Heilman KM, Libon DJ. Subcortical vascular dementia: integrating neuropsychological and neuroradiologic data. Neurology. 2005;65:376–82. doi: 10.1212/01.wnl.0000168877.06011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Hansen LA, Thal LJ, Galasko D. Disparate phonemic and semantic fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer's disease. Neuropsychology. 2007;21:20–30. doi: 10.1037/0894-4105.21.1.20. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Ho GJ, Galasko D, Peavy GM, et al. Cognitive profiles differ in autopsy-confirmed fronto-temporal dementia and Alzheimer's disease. Neurology. 2002;58:1801–8. doi: 10.1212/wnl.58.12.1801. [DOI] [PubMed] [Google Scholar]; Neuropsychological deficit profiles distinguished between AD (memory worse than executive dysfunction) and small vessel cerebrovascular disease (memory equal to executive dysfunction).
- Reed BR, Mungas DM, Kramer JH, Ellis W, Vinters HV, et al. Profiles of neuropsychological impairment in autopsy-defined Alzheimer's disease and cerebrovascular disease. Brain. 2007;130:731–39. doi: 10.1093/brain/awl385. [DOI] [PubMed] [Google Scholar]; Neuropsychological deficit profiles distinguished between AD (memory worse than executive dysfunction) and small vessel cerebrovascular disease (memory equal to executive dysfunction).
- Rohrer D, Salmon DP, Wixted JT, Paulsen JS. The disparate effects of Alzheimer's disease and Huntington's disease on semantic memory. Neuropsychology. 1999;13:381–88. doi: 10.1037//0894-4105.13.3.381. [DOI] [PubMed] [Google Scholar]
- Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, et al. Vascular dementia: diagnostic criteria for research studies. Neurology. 1993;43:250–60. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- Rubin EH, Storandt M, Miller JP, Kinscherf DA, Grant EA, et al. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Arch Neurol. 1998;55:395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- Sadek JR, Johnson SA, White DA, Salmon DP, Taylor KI, et al. Retrograde amnesia in dementia: comparison of HIV-associated dementia, Alzheimer's disease and Huntington's disease. Neuropsychology. 2004;18:692–99. doi: 10.1037/0894-4105.18.4.692. [DOI] [PubMed] [Google Scholar]
- Sahgal A, Galloway PH, McKeith IG, Edwardson JA, Lloyd S. A comparative study of attentional deficits in senile dementias of Alzheimer and Lewy body types. Dementia. 1992b;3:350–54. doi: 10.1001/archneur.1992.00530340059019. [DOI] [PubMed] [Google Scholar]
- Sahgal A, Galloway PH, McKeith IG, Lloyd S, Cook JH, et al. Matching-to-sample deficits in patients with senile dementias of the Alzheimer and Lewy body types. Arch Neurol. 1992a;49:1043–46. doi: 10.1001/archneur.1992.00530340059019. [DOI] [PubMed] [Google Scholar]
- Sahgal A, McKeith IG, Galloway PH, Tasker N, Steckler T. Do differences in visuospatial ability between senile dementias of the Alzheimer and Lewy body types reflect differences solely in mnemonic function? J Clin Exp Neuropsychol. 1995;17:35–43. doi: 10.1080/13803399508406579. [DOI] [PubMed] [Google Scholar]
- Salmon DP. Disorders of memory in Alzheimer's disease. In: Cermak LS, editor. Handbook of Neuropsychology, Vol 2: Memory and Its Disorders. 2nd Amsterdam: Elsevier; 2000. pp. 155–95. [Google Scholar]
- Salmon DP, Bondi MW. Neuropsychology of Alzheimer's disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer Disease. 2nd Philadelphia, PA: Lippincott: Williams & Wilkens; 1999. pp. 39–56. [Google Scholar]
- Salmon DP, Hamilton JM. Neuropsychological features of dementia with Lewy bodies. In: O'Brien J, McKeith I, Ames D, Chiu E, editors. Dementia with Lewy Bodies. London: Taylor & Francis; 2006. pp. 49–72. [Google Scholar]
- Salmon DP, Hamilton JM, Peavy GM. Neuropsychological deficits in Huntington's disease: implications for striatal function in cognition. In: Boller F, Cappa SF, editors. Handbook of Neuropsychology, Vol 6: Aging and Dementia. 2nd Amsterdam: Elsevier; 2001. pp. 373–402. [Google Scholar]
- Salmon DP, Heindel WC, Lange KL. Differential decline in word generation from phonemic and semantic categories during the course of Alzheimer's disease: implications for the integrity of semantic memory. J Int Neuropsychol Soc. 1999;5:692–703. doi: 10.1017/s1355617799577126. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Thomas RG, Pay MM, Booth A, Hofstetter CR, et al. Alzheimer's disease can be accurately diagnosed in very mildly impaired individuals. Neurology. 2002;59:1022–28. doi: 10.1212/wnl.59.7.1022. [DOI] [PubMed] [Google Scholar]; Neuropsychological deficit profiles distinguished between AD (memory worse than executive dysfunction) and small vessel cerebrovascular disease (memory equal to executive dysfunction).
- Small BJ, Fratiglioni L, Viitanen M, Winblad B, Bäckman L. The course of cognitive impairment in preclinical Alzheimer disease: three- and six-year follow-up of a population-based sample. Arch Neurol. 2000;57:839–44. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- Small BJ, Mobly JL, Laukka EJ, Jones S, Bäckman L. Cognitive deficits inpreclinical Alzheimer's disease. Acta Neurol Scand Suppl. 2003;179:29–33. doi: 10.1034/j.1600-0404.107.s179.6.x. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Lange H, Homberg V. The pattern of attentional deficits in Huntington's disease. Brain. 1995;118:145–52. doi: 10.1093/brain/118.1.145. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Migliorelli R, Teson A, Sabe L, Vazquez S, et al. Specificity of changes in cerebral blood flow in patients with frontal lobe dementia. J Neurol Neurosurg Psychiatry. 1994;57:790–96. doi: 10.1136/jnnp.57.7.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavitsky K, Brickman AM, Scarmeas N, Torgan RL, Tang MX, et al. The progression of cognition, psychiatric symptoms, and functional abilities in dementia with Lewy bodies and Alzheimer's disease. Arch Neurol. 2006;63:1450–56. doi: 10.1001/archneur.63.10.1450. [DOI] [PubMed] [Google Scholar]
- Stevens A, Kircher T, Nickola M, Bartels M, Rosellen N, Wormstall H. Dynamic regulation of EEG power and coherence is lost early and globally in probable DAT. Eur Arch Psychiatry Clin Neurosci. 2001;251:199–204. doi: 10.1007/s004060170027. [DOI] [PubMed] [Google Scholar]
- Storandt M, Botwinick J, Danziger WL, Berg L, Hughes CP. Psychometric differentiation of mild senile dementia of the Alzheimer type. Arch Neurol. 1984;41:497–99. doi: 10.1001/archneur.1984.04050170043013. [DOI] [PubMed] [Google Scholar]
- Storandt M, Grant EA, Miller JP, Morris JC. Rates of progression in mild cognitive impairment and early Alzheimer's disease. Neurology. 2002;59:1034–41. doi: 10.1212/wnl.59.7.1034. [DOI] [PubMed] [Google Scholar]
- Stout JC, Rodawalt WC, Siemers ER. Risky decision making in Huntington's disease. J Int Neuropsychol Soc. 2001;7:92–101. doi: 10.1017/s1355617701711095. [DOI] [PubMed] [Google Scholar]
- Tales A, Butler SR, Fossey J, Gilchrist ID, Jones RW, Troscianko T. Visual search in Alzheimer's disease: a deficiency in processing conjunctions of features. Neuropsychologia. 2002;40:1849–57. doi: 10.1016/s0028-3932(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Thal LJ. Clinical trials in Alzheimer disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer Disease. 2nd Philadelphia, PA: William & Wilkens; 1999. pp. 423–39. [Google Scholar]
- Thomas-Anterion C, Jacquin K, Laurent B. Differential mechanisms of impairment of remote memory in Alzheimer's and frontotemporal dementia. Dement Geriatr Cogn Disord. 2000;11:100–6. doi: 10.1159/000017221. [DOI] [PubMed] [Google Scholar]
- Tippett LJ, Blackwood K, Farah MJ. Visual object and face processing in mild-to-moderate Alzheimer's disease: from segmentation to imagination. Neuropsychologia. 2003;41:453–68. doi: 10.1016/s0028-3932(02)00140-9. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P, Hansen LA, Alford M, Merdes A, Masliah E, et al. Early and widespread cholinergic losses differentiate dementia with Lewy bodies from Alzheimer disease. Arch Gen Psychiatry. 2002;59:946–51. doi: 10.1001/archpsyc.59.10.946. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P, Salmon DP, Hansen LA, Hofstetter CR, Thal LJ, Corey-Bloom J. What best differentiates Lewy body from Alzheimer's disease in early-stage dementia? Brain. 2006;129:729–35. doi: 10.1093/brain/awh725. [DOI] [PubMed] [Google Scholar]
- Treisman A. The binding problem. Curr Opin Neurobiol. 1996;6:171–78. doi: 10.1016/s0959-4388(96)80070-5. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Ropacki SAL, Bondi MW. Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease. J Int Neuropsychol Soc. 2006;12:707–35. doi: 10.1017/S1355617706060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Mansfield RJW, Goodale MS, editors. The Analysis of Visual Behavior. Cambridge, MA: MIT Press; 1982. pp. 549–86. [Google Scholar]
- Varma AR, Snowden JS, Lloyd JJ, Talbot PR, Mann DMA, Neary D. Evaluation of the NINCDS-ADRDA criteria in the differentiation of Alzheimer's disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1999;66:184–88. doi: 10.1136/jnnp.66.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel JP, Di Figlia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–84. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Knowlton BJ, Holyoak KJ, Boone KB, Back-Madruga C, et al. Relational integration and executive function in Alzheimer's disease. Neuropsychology. 2004;18:296–305. doi: 10.1037/0894-4105.18.2.296. [DOI] [PubMed] [Google Scholar]
- Ward J, Sheppard JM, Shpritz B, Margolis RL, Rosenblatt A, Brandt J. A four-year study of cognitive functioning in Huntington's disease. J Int Neuropsychol Soc. 2006;12:445–54. [PubMed] [Google Scholar]