Abstract
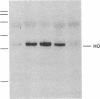
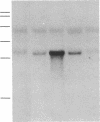
We have shown that UVA (320-380 nm) radiation, hydrogen peroxide, and sodium arsenite induce a stress protein of approximately 32 kDa in human skin fibroblasts. The synthesis and cloning of cDNA from arsenite-induced mRNA populations have now allowed us to unequivocally identify the 32-kDa protein as heme oxygenase. By mRNA analysis we have shown that the heme oxygenase gene is also induced in cultured human skin fibroblasts by UVA radiation, hydrogen peroxide, cadmium chloride, iodoacetamide, and menadione. The known antioxidant properties of heme catabolites taken together with the observation of a high level of induction of the enzyme in cells from an organ not involved in hemoglobin breakdown strongly supports the proposal that the induction of heme oxygenase may be a general response to oxidant stress and constitutes an important cellular defense mechanism against oxidative damage.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. R., Marotti K. R., Whitaker-Dowling P. A. A candidate rat-specific gene product of the Kirsten murine sarcoma virus. Virology. 1979 Nov;99(1):31–48. doi: 10.1016/0042-6822(79)90034-5. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Caltabiano M. M., Koestler T. P., Poste G., Greig R. G. Induction of 32- and 34-kDa stress proteins by sodium arsenite, heavy metals, and thiol-reactive agents. J Biol Chem. 1986 Oct 5;261(28):13381–13386. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
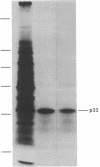
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Danpure H. J., Tyrrell R. M. Oxygen-dependence of near UV (365 NM) lethality and the interaction of near UV and X-rays in two mammalian cell lines. Photochem Photobiol. 1976 Mar;23(3):171–177. doi: 10.1111/j.1751-1097.1976.tb07238.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hiwasa T., Sakiyama S. Increase in the synthesis of a Mr 32,000 protein in BALB/c 3T3 cells after treatment with tumor promoters, chemical carcinogens, metal salts, and heat shock. Cancer Res. 1986 May;46(5):2474–2481. [PubMed] [Google Scholar]
- Johnston D., Oppermann H., Jackson J., Levinson W. Induction of four proteins in chick embryo cells by sodium arsenite. J Biol Chem. 1980 Jul 25;255(14):6975–6980. [PubMed] [Google Scholar]
- Kageyama H., Hiwasa T., Tokunaga K., Sakiyama S. Isolation and characterization of a complementary DNA clone for a Mr 32,000 protein which is induced with tumor promoters in BALB/c 3T3 cells. Cancer Res. 1988 Sep 1;48(17):4795–4798. [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Both near ultraviolet radiation and the oxidizing agent hydrogen peroxide induce a 32-kDa stress protein in normal human skin fibroblasts. J Biol Chem. 1987 Oct 25;262(30):14821–14825. [PubMed] [Google Scholar]
- Kikuchi G., Yoshida T. Function and induction of the microsomal heme oxygenase. Mol Cell Biochem. 1983;53-54(1-2):163–183. doi: 10.1007/BF00225252. [DOI] [PubMed] [Google Scholar]
- Lanks K. W. Modulators of the eukaryotic heat shock response. Exp Cell Res. 1986 Jul;165(1):1–10. doi: 10.1016/0014-4827(86)90528-8. [DOI] [PubMed] [Google Scholar]
- Maines M. D. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988 Jul;2(10):2557–2568. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Studies on the mechanism of induction of haem oxygenase by cobalt and other metal ions. Biochem J. 1976 Jan 15;154(1):125–131. doi: 10.1042/bj1540125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R. M., Taguchi H., Shibahara S. Nucleotide sequence and organization of the rat heme oxygenase gene. J Biol Chem. 1987 May 15;262(14):6795–6802. [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Marty L., Dani C., Panabieres F., El Sabouty S., Fort P., Jeanteur P. Post-transcriptional regulation of glyceraldehyde-3-phosphate-dehydrogenase gene expression in rat tissues. Nucleic Acids Res. 1984 Sep 25;12(18):6951–6963. doi: 10.1093/nar/12.18.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammartano L. J., Tuveson R. W. Escherichia coli strains carrying the cloned cytochrome d terminal oxidase complex are sensitive to near-UV inactivation. J Bacteriol. 1987 Nov;169(11):5304–5307. doi: 10.1128/jb.169.11.5304-5307.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton K. R., Todd J. M., Egle P. M. The induction of stress-related proteins by lead. J Biol Chem. 1986 Feb 5;261(4):1935–1940. [PubMed] [Google Scholar]
- Shiu R. P., Pouyssegur J., Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B., Small R. D., Jr The photoperoxidation of unsaturated organic molecules--XV. O21Delta g quenching by bilirubin and biliverdin. Photochem Photobiol. 1976 Jan;23(1):33–36. doi: 10.1111/j.1751-1097.1976.tb06767.x. [DOI] [PubMed] [Google Scholar]
- Stocker R., Ames B. N. Potential role of conjugated bilirubin and copper in the metabolism of lipid peroxides in bile. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8130–8134. doi: 10.1073/pnas.84.22.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Glazer A. N., Ames B. N. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Tyrrell R. M., Pidoux M. Correlation between endogenous glutathione content and sensitivity of cultured human skin cells to radiation at defined wavelengths in the solar ultraviolet range. Photochem Photobiol. 1988 Mar;47(3):405–412. doi: 10.1111/j.1751-1097.1988.tb02744.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M., Pidoux M. Endogenous glutathione protects human skin fibroblasts against the cytotoxic action of UVB, UVA and near-visible radiations. Photochem Photobiol. 1986 Nov;44(5):561–564. doi: 10.1111/j.1751-1097.1986.tb04709.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M., Pidoux M. Quantitative differences in host cell reactivation of ultraviolet-damaged virus in human skin fibroblasts and epidermal keratinocytes cultured from the same foreskin biopsy. Cancer Res. 1986 Jun;46(6):2665–2669. [PubMed] [Google Scholar]
- Yoshida T., Biro P., Cohen T., Müller R. M., Shibahara S. Human heme oxygenase cDNA and induction of its mRNA by hemin. Eur J Biochem. 1988 Feb 1;171(3):457–461. doi: 10.1111/j.1432-1033.1988.tb13811.x. [DOI] [PubMed] [Google Scholar]
- Zhang H., Scholl R., Browse J., Somerville C. Double stranded DNA sequencing as a choice for DNA sequencing. Nucleic Acids Res. 1988 Feb 11;16(3):1220–1220. doi: 10.1093/nar/16.3.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]