Abstract
In immune-induced inflammation, leukocytes are key mediators of tissue damage. Since A2A adenosine receptors (A2AR) are endogenous suppressors of inflammation, we examined cellular and molecular mechanisms of kidney damage to determine whether selective activation of A2AR will suppress inflammation in a rat model of glomerulonephritis. Activation of A2AR reduced the degree of kidney injury in both the acute inflammatory phase and the progressive phase of glomerulonephritis. This protection against acute and chronic inflammation was associated with suppression of the glomerular expression of the MDC/CCL22 chemokine and down-regulation of MIP-1α/CCL3, RANTES/CCL5, MIP-1β/CCL4, and MCP-1/CCL2 chemokines. The expression of anti-inflammatory cytokines, IL-4 and IL-10, also increased. The mechanism for these anti-inflammatory responses to the A2AR agonist was suppression of macrophages function. A2AR expression was increased in macrophages, macrophage-derived chemokines were reduced in response to the A2AR agonist, and chemokines not expressed in macrophages did not respond to A2AR activation. Thus, activation of the A2AR on macrophages inhibits immune-associated inflammation. In glomerulonephritis, A2AR activation modulates inflammation and tissue damage even in the progressive phase of glomerulonephritis. Accordingly, pharmacological activation of A2AR could be developed into a novel treatment for glomerulonephritis and other macrophage-related inflammatory diseases.
Keywords: chemokines, anti-inflammatory cytokines, tissue injury protection
INTRODUCTION
We still treat immunologically induced inflammatory diseases with nonspecific immunosuppressive drugs that can cause significant morbidity (1). For this reason defining the pathways of organ damage could lead to design of more specific therapies.
Crescentic glomerulonephritis is a rapidly progressive glomerular disease with a poor prognosis. Macrophages can play an important role in the induction and development of the disease and both the magnitude of proteinuria and the percentage of crescentic glomeruli are correlated with the number of macrophages that infiltrate the glomerulus; this is not the case with the number of CD8+ cells (2, 3). Macrophages are constituents of glomerular crescents in progressive crescentic glomerulonephritis and they probably play a major role in the irreversible scarring that leads to kidney failure (4–8). In Wistar-Kyoto rats (WKY), small doses of anti-glomerular basement membrane (GBM) antibody induce proliferative and necrotizing glomerulonephritis with crescent formation plus infiltration of CD8+ cells and macrophages into the glomeruli (9–13). In this model macrophages accumulate in glomeruli during later phases and contribute to the progressive decline in kidney function (3, 14).
Chemokines mediate selective attraction and activation of various subsets of leukocytes (6, 15–17). In the induction of anti-GBM glomerulonephritis in WKY rats CC chemokines play an important role. We found that CC chemokines, MCP-1/CCL2, MIP-1β/CCL4, RANTES/CCL5, and MDC/CCL22, and the CX3C chemokine, fractalkines/CX3CL1, are induced during the disease and that the expression of multiple chemokines coincides with the influx of CD8+ cell and ED1+ macrophages into the glomeruli. In in vitro studies these chemokines induced strong migratory response in inflammatory cells prepared from the nephritic glomeruli (9–11).
The degree of inflammation can be tightly regulated by endogenous anti-inflammation pathways, including, PPARγ, adiponectin, NO, and adenosine receptors (18–24). Genetic and pharmacological evidence support a non-redundant role for both endogenous adenosine and A2A receptors in protecting against acute inflammatory damage in models of inflammatory injury and systemic inflammation (25). The anti-inflammatory mechanism involves downregulation of activated immune cells in vivo (25). Thus, the A2AR could modulate inflammatory processes because it is expressed on most cells involved in inflammation, including a variety of hematopoietic cells, endothelial cells, and smooth muscle cells (19, 20, 25, 26). We have found that quiescent peritoneal macrophages do not express A2AR but following addition of LPS, these cells express abundant A2AR. In vitro studies indicate that A2AR are present on polymorphonuclear leukocytes (PMN) and can inhibit the expression of β2-integrins and adhesion, can suppress oxygen radical production, degranulation, and production of TNF-α (27–31). In addition, expression and activation of A2A receptor on macrophages can inhibit the production of IL-12, TNF-α, and nitric oxide, and enhance the secretion of IL-10 in response to lipopolysaccharide (LPS) (32–35). Adenosine can inhibit proliferation, activation, and production of inflammatory cytokines in peripheral T-cells it also can enhance the production of anti-inflammatory cytokines (36, 37).
Because of the potential for beneficial effects of pharmacological activation of A2AR as a therapeutic target, we tested the hypothesis that A2AR activation would protect against immune-mediated inflammation in experimental glomerulonephritis. Activation of A2AR significantly reduced leukocyte infiltration into the kidney and prevented kidney injury during the acute inflammatory phase and the progressive phase of glomerulonpehritis. Our results indicate that activation of A2AR represents a potential therapeutic strategy for glomerulonephritis and possibly other macrophage-mediated diseases.
MATERIAL AND METHODS
Induction, treatment, and analysis of anti-GBM glomerulonephritis
Animal studies were approved by the IACUC at Baylor College of Medicine. Male WKY rats (Harlan Sprague Dawley Inc., Indianapolis, IN), weighing 180–200 grams received an intravenous injection of 25µl/100g body weight of anti-GBM Ab as described (9, 10, 12, 38). We activated A2AR with a selective A2A receptor agonist, CGS 21680, (Tocris Cookson Inc., Ellisville, MO, 1.5mg/Kg i.p. twice a day, n = 6) and inactivated A2AR using a selective receptor antagonist, ZM241385 (Tocris, 2mg/Kg i.p. twice daily, n = 6) for a period of 5 days, as described (25). In initial experiments, we pharmacologically activated or inhibited A2AR during the acute inflammatory phase of the disease by starting treatment 8 hours after the injection of anti-GBM antibody (maximum glomerular deposition of IgG occurs one hour after injection of anti-GBM antibody (39)). In the second set of experiments we began treatment with A2AR agonist at day six after the injecting the anti-GBM antibody (the progressive phase of crescentic glomerulonephritis). Urine protein was assayed using sulfosalicylic acid and rats were euthanized at day six, or day 12 to collect blood and kidney tissues. During the first hour after injecting the A2AR agonist or the A2AR antagonist, tail-cuff blood pressure was measured in conscious rats (Visitech Systems, Apex NC) as described (40).
mRNA expression of A2A R, chemokines, cytokines, and adhesion molecules
Rat A2AR nucleotides 459 to 801 (GenBank sequence accession number NM_053294) were used to generate A2AR probe from brain tissue by RT-PCR. MDC/CCL22 (400 bp), RANTES/CCL5 (246 bp), MIP-1a/CCL3 (284 bp), factalkine/CX3CL1 (420 bp), MIP-3β/CCL19 (380 bp), MIP-1β/CCL4 (210 bp), MCP-1/CCL2 (239 bp), VCAM-1 (371 bp), ICAM-1 (292 bp), and L-32 (92 bp) riboprobes were generated by PCR reaction using cDNA templates. rCK1 (BD Pharmingen, San Diego, CA) was used to investigate cytokine expression. Glomeruli were prepared by sequential sieving and total RNA was isolated from glomeruli (11, 41). Three µg of total RNA from each sample was used in an RNase protection assay using the Torrey Pines Biolabs kit (Houston, TX) as described (11, 16, 42, 43). Phosphoimage quantitiation was performed using the PhosphorImager SI scanning instrument and ImageQuaNT software (Molecular Dynamics, Sunnyvale, CA) (16, 44, 45).
Morphological analysis, immunohistochemical phenotyping, and quantitation of leukocytes
Kidney samples fixed in formalin or methanol-Carnoy fixative solution were embedded in paraffin. Two to three-µm sections were stained with periodic acid-Schiff reagent to assess glomerular hypercellularity, necrotizing lesions, and formation of glomerular crescents (crescentic glomeruli per 100 glomeruli was calculated and expressed as a percentage). Infiltrating leukocytes were immunohistochemically stained for CD8+ and ED1+, as described (9, 11, 12). Positively stained cells per 100 glomeruli were counted and expressed per glomerular section. All quantitative morphological analyses were performed in a blinded fashion.
Immunohistochemistry of A2AR, ED1+ cells, CD8+ cells, mesangial cells, and podocytes
Paraffin sections of methanol-Carnoy fixed tissue were stained with goat polyclonal anti-A2AR antibody (1:100, Santa Cruz Biotechnology, Santa Cruz, CA) and monoclonal antibody ED-1 against rat macrophages (Chemicon, Temecula, CA), or monoclonal OX-8 against rat CD8 (BD Biosciences Pharmingen). Two-color dual antigen immunostaining was obtained by serial avidin-biotin peroxidase and alkaline phosphatase staining reactions with final chromogenic substrates of diamibenzidine (brown color) and Fast Red (red color), respectively as described (12, 46, 47).
Kidney sections were also stained with α-SMA (Dako, Carpinteria, CA), a marker of activated mesangial cells or α-Actinin-4 (AXXORA, LLC, San Diego, CA), a marker for podocytes (48, 49).
RESULTS
A2A R expression in nephritic glomeruli
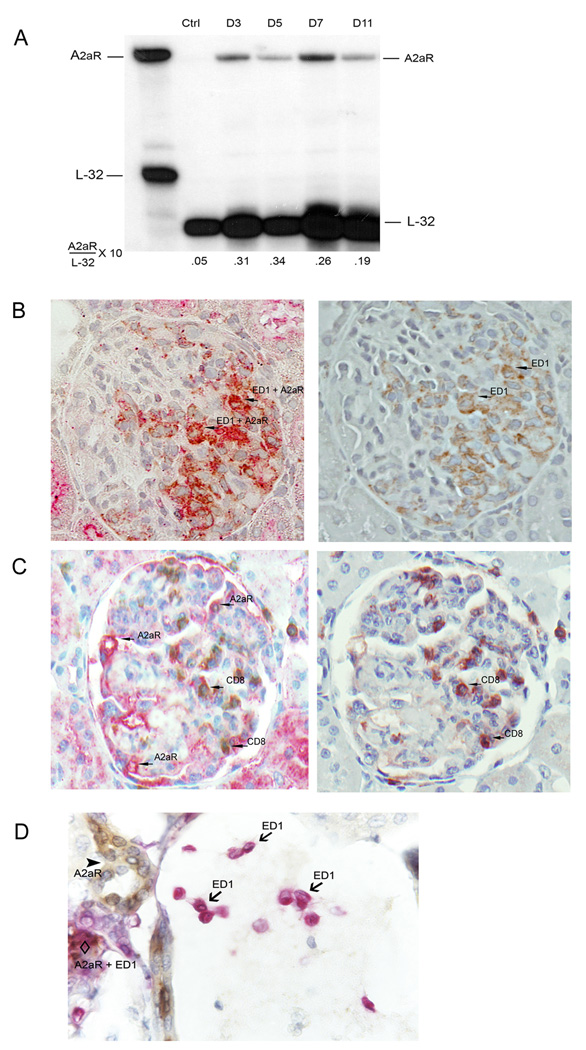
Normal glomeruli do not express A2A R. In response to the anti-GBM antibody, however, there was strong expression of A2AR from day 3 onwards (Figure 1 A). The glomerular level of A2AR mRNA was 6.2 fold higher in anti-GBM Ab-treated rats than in normal glomeruli on day 3. A2AR expression peaked at day 5 (6.8-fold increase) and started to decrease by day 7 (5.2-fold increase). Double immunohistochemical staining of ED1+ cells and A2AR demonstrated that A2AR was virtually confined to macrophages. There was some staining in the location of glomerular endothelial cells (Figure 1 B) but A2AR was not expressed in either CD8+ cells (Figure 1 C) or in monocytes located in extraglomerular vessels (Figure 1 D). These results suggest that increased expression of A2AR in macrophages might yield an anti-inflammatory defense against kidney damage.
1.
Induction of A2AR in nephritic glomeruli. (A) RNase protection analysis was performed to determine A2AR mRNA expression in the glomeruli of anti-GBM glomerulonephritis in WKY rats. A2AR was induced from day 3 onwards during anti-GBM glomerulonephritis. The expression of A2AR peaked at day 5 and started to decrease by day 7. Rat ribosomal L-32 gene was used as a housekeeping gene. Probe contains polylinker regions and is longer than the protected bands. The expression levels of A2AR mRNA are expressed relative to the mRNA levels of the housekeeping gene L-32. Data shown is a representative of three separate experiments. (B) Double immunohistochemical staining of ED1+ cells (brown color) overlap with A2AR (red color), thus A2AR was mainly in Mϕ. (C) Interestingly, A2AR (red color) was not expressed in CD8+ cells (brown color) or (D) monocytes (red color) located in extraglomerular vessels. (B) and (C), right panel, show ED1+ or CD8+ cells staining after removal of red color (A2AR staining) from (B) and (C) slides at left panel to demonstrate the overlap of A2AR expression in ED1+ but not in CD8+ cells. Sections for staining were sampled on day 7 after anti-GBM antibody injection.
A2AR activation prevents glomerulonephritis
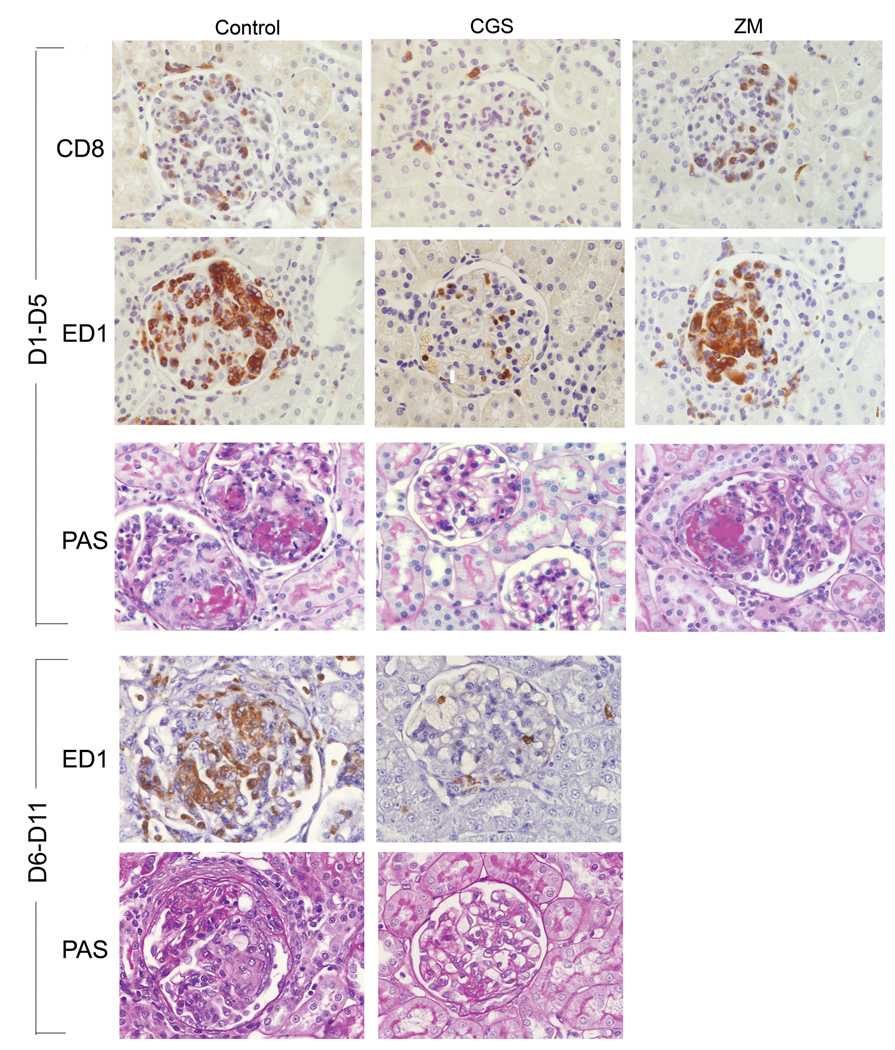
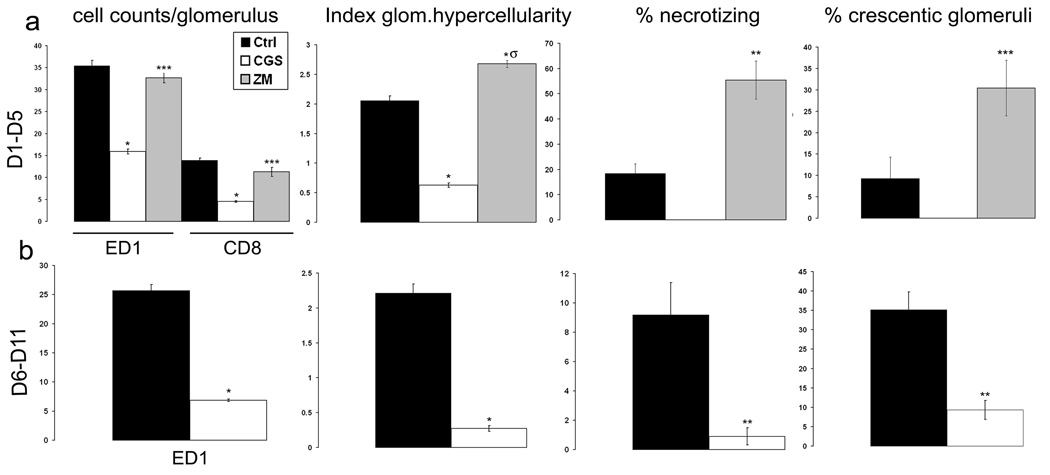
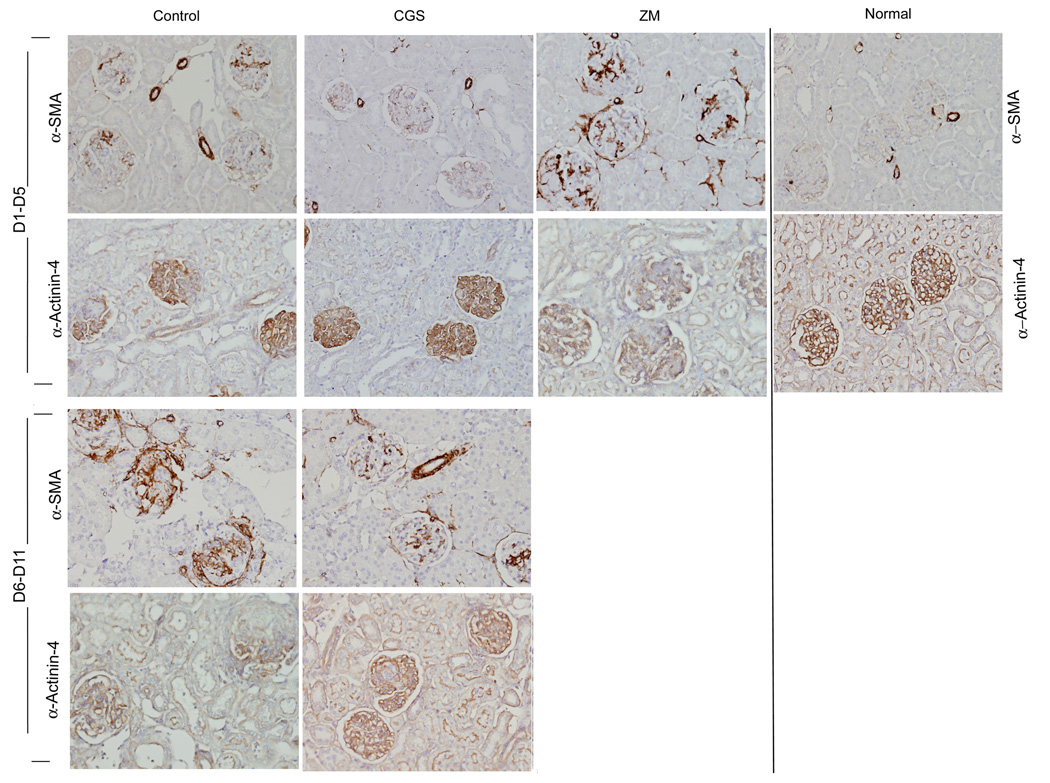
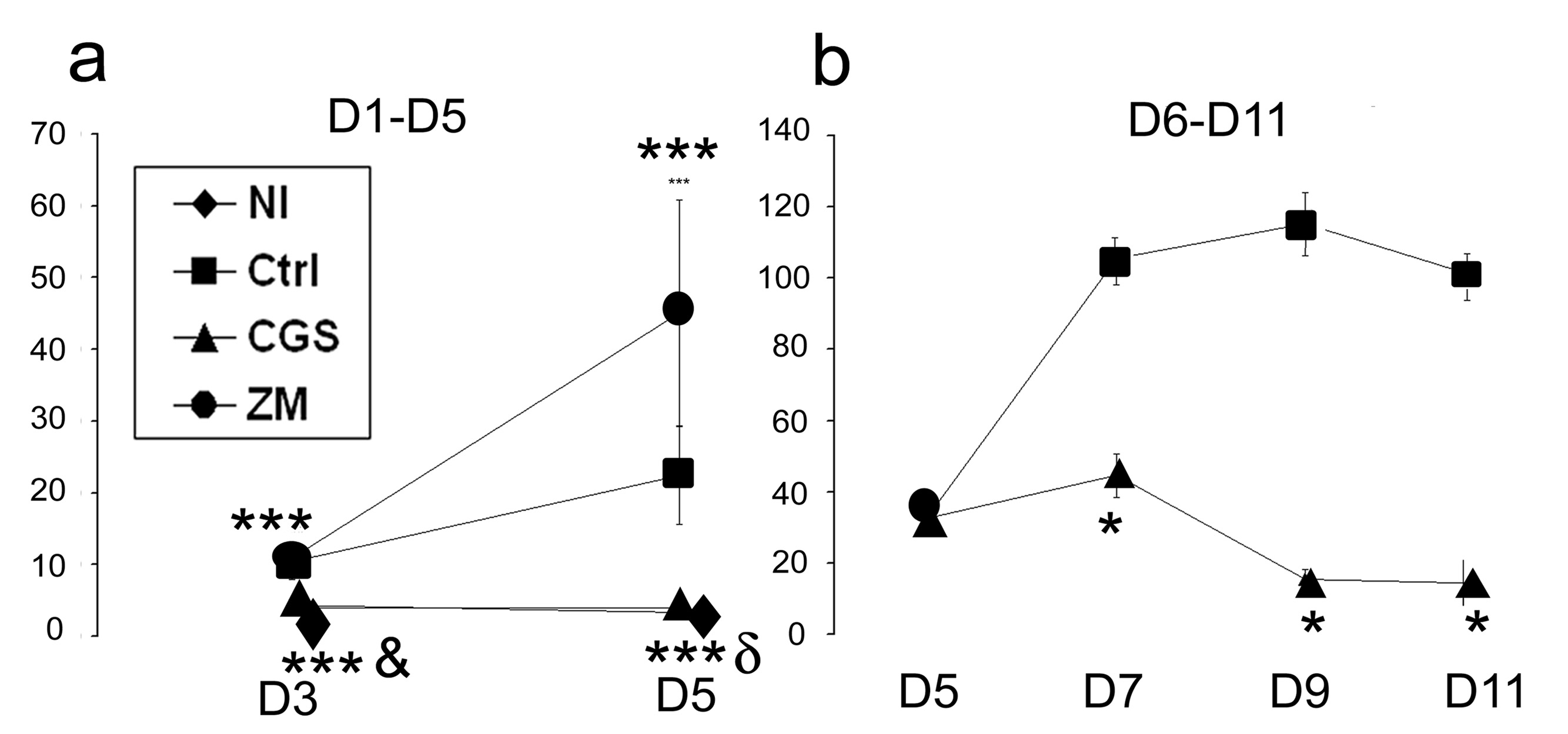
In the acute (day 1 to day 5) phase of crescentic glomerulonephritis control rats exhibited severe glomerular hypercellularity, necrotizing lesions, and crescentic formation (Figure 2 and Figure 3). Increased expression of α-SMA, a marker for mesangial cells injury, was inversely correlated with reduced α-Actinin-4, a marker for podocytes, indicating the degree of glomerular injury (Figure 4). In rats treated with CGS21680, a specific A2AR agonist, however, there was only minimal damage to the kidneys. Notably, crescent formation, a major characteristic of this model, was abolished by CGS21680 treatment and necrotizing lesions were not observed while glomerular hypercellularity was markedly attenuated by ~ 70% (Figure 2 and Figure 3 a). Treatment with the A2AR agonist also decreased α-SMA expression and restored the expression of α-Actinin- 4 (Figure 4). There also was no proteinuria (Figure 5 a). In contrast, in rats with anti-GBM glomerulonephritis treated with a specific antagonist for A2AR, ZM241385, there was more severe glomerular hypercellularity, necrotizing lesions, and crescentic formation compared to control rats (Figure 2 and Figure 3 a). A2AR antagonist also enhanced the expression of α-SMA and increased the loss of α-Actinin- 4 (Figure 4). Finally, proteinuria was also significantly higher in ZM241385-treated vs. control rats with glomerulonephritis (Figure 5 a). Thus, selective activation of A2AR at acute phase (day 1 to day 5) confers kidney protection from damage.
2.
A2AR attenuates inflammatory infiltrates and renal injury during the acute inflammatory phase and progressive phase in anti-GBM glomerulonephritis. Immunohistochemistry stained for CD8+ cells or ED1+ monocytes/macrophages, and periodic acid-Schiff staining of kidney sections of control, CGS216809 (CGS), and ZM241385 (ZM)-treated rats with anti-GBM glomerulonephritis. In the acute phase of the disease (day 1 to day 5), the control group of glomerulonephritis rats display severe glomerular hypercellularity, necrotizing lesions, crescentic formation, and prominent accumulation of CD8+ and ED1+ macrophages infiltration. In CGS-treated rats, crescentic formation and necrotizing lesions were prevented, and CD8+ and ED1+ macrophages infiltrates attenuated. Worsening of glomerular injury was observed in rats treated with ZM. In the progressive phase of the disease (day 6 to day 11) CGS markedly reduced glomerular lesion and ED1+ cell infiltrate.
3.
Quantitation of CD8+ and ED1+ cell infiltration, glomerular hypercellularity, necrotizing lesions, and crescent formation in the glomeruli from WKY rats with anti-GBM glomerulonephritis that were treated with vehicle, CGS21680 (CGS) or ZM241385 (ZM) during day 1 to day 5 and day 6 to day 11. One hundred glomeruli per section were counted. Each data point represents sections sampled from six rats and is expressed as mean ± SEM. *p<0.0001 vs. control, **p<0.005 vs. control, ***p<0.05 vs. control, σp<0.0001 vs. CGS
4.
A2AR activation decreases α-SMA expression and restores α-Actinin-4 expression in anti-GBM GN. Immunohistochemistry stained for α-SMA and α-Actinin-4 of kidney sections of normal, control, CGS216809 (CGS), and ZM241385 (ZM)-treated rats with anti-GBM glomerulonephritis. In the acute phase of the disease (day 1 to day 5), the control group of glomerulonephritis rats display increased expression of α-SMA and lost of α-Actinin-4. In CGS-treated rats, the expression of α-SMA was reduced and the expression of α-Actinin-4 restored. Increased expression of α-SMA and enhanced loss of α-Actinin-4 was observed in rats treated with ZM. In the progressive phase of the disease (day 6 to day 11) CGS markedly reduced α-SMA and restored α-Actinin-4.
5.
Determination of proteinuria (milligrams of urine protein per 24 h) in Wistar-Kyoto rats with anti-GBM glomerulonephritis that were treated with vehicle, CGS21680 (CGS), and ZM241385 (ZM). In the acute phase of the disease, CGS completely blocked proteinuria in anti-GBM antibody-injected rats. Proteinuria was significantly higher in ZM-treated rats than the control group. In the progressive phase of glomerulonephritis, CGS markedly reduced proteinuria. Treatment Samples were sampled from six rats per group and expressed as mean ± SEM. *p<0.0001 vs. control, **p<0.005 vs. control, ***p<0.05 vs. control, δp<0.005 vs. ZM, &p<0.0001 vs. ZM
Increased or normal blood pressure has been reported in mice lacking A2AR. We determined if A2AR antagonism could increase blood pressure and contribute to kidney damage (46, 50). There was no significant difference in systolic blood pressure among the groups at day 4 or day 6 after induction of anti-GBM GN (D4: 147.75 ± 18.7, 141.0 ± 14.2, 149.7 ± 14.1; D6: 134.6 ± 8.01, 131.0 ± 9.43, 137.0 ± 5.6 in control, A2AR agonist, andA2AR antagonist treated rats respectively).
Next, we treated rats in the progressive phase of glomerulonephritis (day 6 to day 11) using the A2AR agonist, CGS21680. As shown in Figure 2 and Figure 3b, glomerular hypercellularity, necrotizing lesions, and crescentic formation were markedly reduced at day 12 compared to the results in the control group (88%, 90%, and 74%, respectively). A2AR activation also decreased α-SMA expression and restored the expression of α-Actinin-4 vs. results in the control group (Figure 4). Finally, CGS21680 reduced proteinuria within 24 hours of administration; the inhibitory effect was sustained during the treatment period (Figure 5 b). Thus, selective activation of A2AR also reduces kidney injury and improves proteinuria during the progressive phase of glomerulonephritis.
Prevention of glomerulonephritis by A2AR activation reduces lymphocyte/macrophage infiltration
To understand the mechanism for the prevention of kidney-injury, we examined the infiltration of leukocytes into the kidney. As shown in Figure 2 and Figure 3a there was a prominent accumulation of CD8+cells and ED1+ macrophages in glomeruli of control rats during the acute (day 1 to 5) inflammatory phase of glomerulonephritis. Treatment with CGS21680 dramatically attenuated infiltration of CD8+ cells and ED1+ macrophages into glomeruli. Pharmacological activation of A2AR in the progressive phase (from day 6 to day 11) blocked macrophage infiltration to levels only 26% of the level in glomeruli of control rats (Figure 2 and Figure 3 b). This model of glomerulonephritis is characterized by an early infiltration of CD8+ cells with a maximum increase on day 3 after the injection of anti-GBM Ab (13). Consequently, CD8+ cells were not observed in either control or CGS21680 treated rats.
A2A R activation modulates the expression of chemokines and cytokines in anti-GBM glomerulonephritis
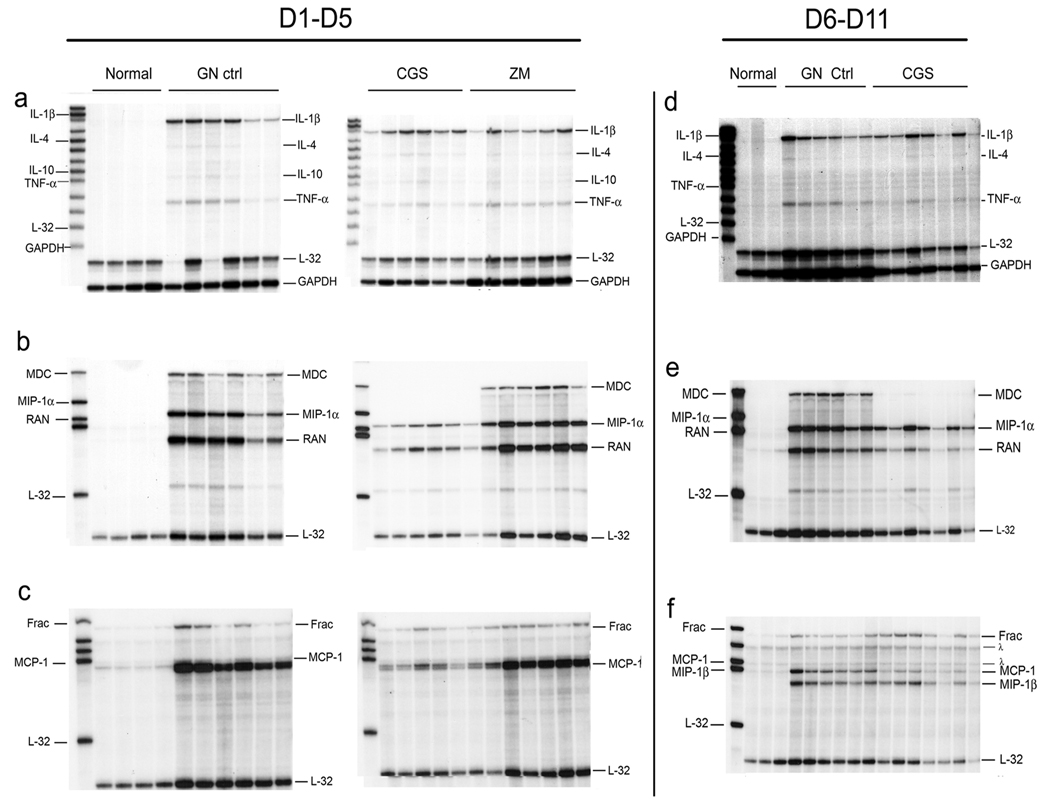
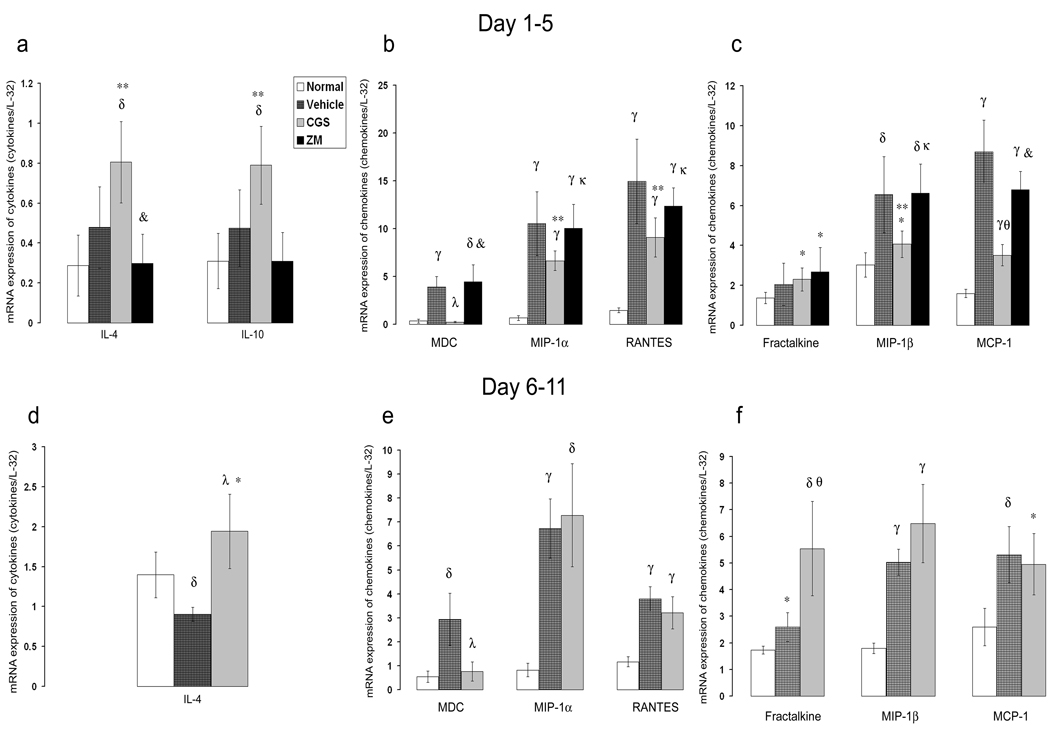
A potential mechanism by which activation of A2AR protects from inflammatory damage and leukocyte infiltration could be via suppression of cytokines/chemokines. We found that pharmacological A2AR activation in the acute inflammatory phase (day 1 to day 5) did not reduce glomerular expression of pro-inflammatory cytokines IL-1β or TNF-α mRNA. In contrast, it significantly increased the expression of anti-inflammatory cytokines, IL-4 and IL-10 (69% and 66% respectively), compared to levels in glomeruli of control rats (Figure 6 a and Figure 7a). Activation of A2AR abolished the induction of MDC/CCL22 and significantly attenuated the expression of MIP-1a/CCL3, MIP-1β/CCL4, RANTES/CCL5, and MCP-1/CCL2 (Figure 6 b, c and 7 b, c) but did not affect expression of fractalkine/CX3CL1 (Figure 6 c and Figure 7 c). Interestingly, inhibition of A2AR by ZM241385 did not significantly change the expression of these chemokines. Expression of anti-inflammatory cytokines seemed to be attenuated by ZM241385 but the changes were not statistically significant compared to the control results. When compared with levels in CGS21680-treated rats, IL-10 and IL-4 were significantly reduced in rats treated with ZM241385 (64.2% and 61% respectively) (Figure 6 a and Figure 7 a).
6.
Selective A2AR activation alters anti-GBM glomerulonephritis-induced cytokine expression. RNase protection assay of cytokines and chemokines expressed in the glomeruli of anti-GBM glomerulonephritis in WKY rats. CGS in the acute inflammatory phase of the disease (day 1 to day 5) increased the expression of IL-4 and IL-10 (a), abolished the increase of the expression of MDC/CCL22, and attenuated the expression of MIP-1α/CCL3, RANTES/CCL5, MIP-1β/CCL4, and MCP-1/CCL2 (b,c). ZM reduced IL-4 and IL-10 expression compared with CGS group (a). In the progressive phase of the disease (day 6 to day 11), CGS significantly enhanced the expression of IL-4 (d) and suppressed the increase of the expression of MDC/CCL22 (e). λ Refers to undigested probe. Each lane represents a single rat.
7.
Selective A2AR activation alters anti-GBM glomerulonephritis-induced cytokine expression. Densitometric analysis of blots from RNase protection assay of cytokines and chemokines expressed in the glomeruli of anti-GBM glomerulonephritis in WKY rats. The data are presented as a ratio of the cpm for the specific mRNA/L-32 mRNA to ensure a constant quantity of RNA in each sample. Results were sampled from six rats per group and expressed as mean ± SD. *p<0.05 vs. nl, δp<0.005 vs. nl, γp<0.0005 vs. nl, **p<0.05 vs. ctrl, θp<0.005 vs. ctrl, λp<0.0005 vs. ctrl, κp<0.05 vs. CGS, &p<0.005 vs. CGS. nl refers to normal rat.
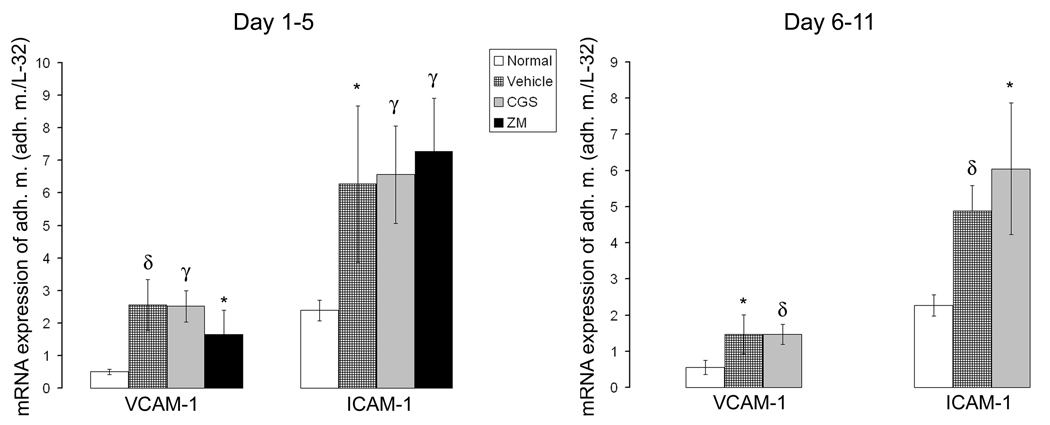
We also investigated the effects of the A2AR agonist and antagonist on the expression of VCAM-I and ICAM-1 in anti-GBM glomerulonephritis. Increased expression of VCAM-1 and ICAM-1 was observed in anti-GBM glomerulonephritis and A2AR activation or inactivation did not significantly modify their expression in the acute inflammatory phase (day 1 to day 5) compared to control rats (Figure 8).
8.
Expression of adhesion molecules is not modified by pharmacological activation of A2AR. Densitometric analysis of blots from RNase protection assay of VCAM-1 and ICAM-1 expressed in the glomeruli in anti-GBM glomerulonephritis. The data are presented as a ratio of the cpm for the specific mRNA/L-32 mRNA to ensure a constant quantity of RNA in each sample. Results were sampled from six rats per group and expressed as mean ± SD. *p<0.05 vs. nl, δp<0.005 vs. nl, γp<0.0005 vs. nl,
Pharmacological activation of A2AR in rats with established glomerulonephritis (day 6 to day 11), significantly enhanced the expression of the anti-inflammatory cytokine IL-4 (104%) but not of IL-10 (Figure 6 d and Figure 7 d). The induction of MDC/CCL22 chemokine was suppressed, but the expression of other chemokines and VCAM-1 or ICAM-1 did not change. Fractalkine/CX3CL1 expression significantly increased (Figure 6 e, f;Figure 7 e, f, and Figure 8).
These results suggest that A2AR pharmacological activation modulates renal injury by altering expression of inflammatory and anti-inflammatory cytokines in the kidney.
DISCUSSION
Our results indicate that activation of adenosine A2A receptors prevents infiltration of leukocytes into the kidney, suppresses glomerular inflammation, and protects the kidney from inflammatory injuries induced by anti-GBM glomerulonephritis. These benefits were observed both in the acute inflammatory phase and during the progressive phase of glomerulonephritis. The protection can be linked to the ability of activated A2AR to suppress infiltration of leukocytes, the key mediators of kidney damage.
We found that A2AR activation alters the expression of chemokines. Specifically, pharmacological activation of A2AR suppresses MDC/CCL22 expression and markedly reduces the expression of other chemokines during the acute inflammatory phase of glomerulonephritis (Figure 6). These changes in chemokine expression can explain the decreased infiltration of CD8+ and macrophages (6, 9–11). During the progressive phase of glomerulonephritis (day 6 to day 11), we found suppressed MDC/CCL22 induction while the expression of other chemokines (except for fractalkine/CX3CL1) was not modified. These results are consistent with the report that MDC/CCL22 plays a critical role in stimulating the influx of macrophages into glomeruli in anti-GBM glomerulonephritis (11). Notably, MDC/CCL22 expression in anti-GBM glomerulonephritis is confined to macrophages (11). As shown in Figure 1, at day 7 after injecting the anti-GBM antibody, macrophages are the major cellular site of A2AR expression in nephritic glomeruli. When MDC/CCL22 expression is inhibited by the A2AR agonist, CGS21680, there also is a dramatic inhibition of ED1+ macrophage infiltration. These results raise the following possibilities: 1) Activation of macrophage-specific A2AR suppresses MDC/CCL22 expression resulting in decreased macrophage infiltration in the acute phase of glomerulonephritis; and 2) macrophage infiltration in later stages of glomerulonephritis depends on MDC/CCL22 because blocking expression of this chemokine ameliorates the degree of functional damage. Interestingly, A2AR activation in the progressive phase of glomerulonephritis did not alter the expression of other chemokines, except for fractalkine/CX3CL1. Possibly increased fractalkine/CX3CL1 expression occurs because of endothelial cell activation in the nephritic glomeruli. This interpretation is suggested because fractalkine/CX3CL1 is mainly expressed by endothelial cells and we have shown that fractalkine/CX3CL1 is induced in the endothelium of nephritic glomeruli (10). In contrast, we have not found A2AR in cultured glomerular endothelial cells (quiescent or activated) or HUVEC (quiescent). Thus, a plausible explanation for the finding that A2AR occupancy during the progressive phase of glomerulonephritis does not alter the expression of other chemokines, is that the chemokines at day 6 to day11 could be produced by A2AR-negative kidney cells.
Our finding that activation of A2AR did not suppress the expression of pro-inflammatory cytokines, IL-1β and TNF-α, (Figure 5 a and d) is consistent with the notion that macrophages are not the predominant source of IL-1β and TNF-α in this model of glomerulonephritis (51, 52). On the other hand, in vitro studies indicate that IL-1β and TNF-α can up-regulate A2AR in THP-1 cells, human dermal microvascular endothelial cells, and PC12 cells (35, 53, 54). Our results, therefore, suggest that IL-1β and TNF-α could induce A2AR expression in other cells and contribute to the beneficial effect of A2AR agonists in preventing glomerular damage.
In addition, we found that the expression of the anti-inflammatory cytokines, IL-10 and/or IL-4, was enhanced by the A2AR activation during anti-GBM glomerulonephritis. These cytokines might also contribute to the beneficial effects of A2AR activation on kidney injury (55, 56).
Increasing evidence suggest that A2AR tissue-protection requires activation of receptors expressed on bone marrow-derived cells (57, 58). Our data indicate that macrophages are the target cells mediating the benefits of A2AR activation in both the acute and progressive phases of glomerulonephritis. First, A2AR expression was increased in macrophages but not in CD8+ cells during anti-GBM GN (Figure 1B and 1C). Second, macrophage derived-chemokines such as MDC/CCL22, MCP-1/CCL2, RANTES/CCL5, and MIP-1α/CCL3 were decreased in response to A2AR agonist. Third, chemokines not expressed in macrophages (e.g., fractalkine/CX3CL1) were unaffected by the A2AR agonist. Finally, macrophages are present in the crescents and in progressive glomerulonephritis and are correlated with the degree of glomerular injury (5, 6, 8). It has been found that depletion of macrophages in this and other models of glomerulonephritis suppresses glomerular crescent formation and reduces proteinuria (3, 7, 59). Accordingly, suppression of the expression of MDC/CCL22 and reduced expression of other chemokines when macrophage-A2AR activation occurs could explain in part the beneficial effect of CGS21680 in both acute and progressive phases of anti-GBM glomerulonephritis.
Notably, we found that the A2AR agonist also was beneficial in the late phase of anti-GBM glomerulonephritis when macrophage influx and crescent formation were at their maximum (day 6) (13, 60). These responses were associated with a significant reduction in macrophage infiltration (74%) suggesting that activation of A2AR on macrophages was responsible for the suppression of glomerular injury.
To examine mechanisms underlying the beneficial effects of A2AR we determined the expression of α-SMA, a marker for mesangial cell injury and activation (49)and α-Actinin-4, a marker for podocytes, in glomeruli of rats with anti-GBM glomerulonephritis. Earlier reports have demonstrated that changes in podocytes contribute to the severity of anti-GBM glomerulonephritis (48, 61, 62). We found that the expression of α-SMA is increased and that α-Actinin- 4 expression is reduced in anti-GBM GN. Treatment with A2AR agonist significantly decreased α-SMA expression and restored the expression of α-Actinin- 4. In contrast, A2AR antagonist enhanced the expression of α-SMA and increased the loss of the podocyte marker, α-Actinin- 4, compared to the controls rats. These data suggest that A2AR agonist may attenuate kidney injury through effects on mesangial cells and podocytes. Future experiments will determine if A2AR activation prevents mesangial cells injury and restores α-Actinin-4 by reducing inflammation or through a direct effect on mesangial cells and podocytes.
A2AR activation or inactivation did not modify blood pressure in rats with anti-GBM glomerulonephritis. Earlier reposts in mice lacking A2AR provide inconsistent changes in blood pressure (46, 50). The inconsistent results could reflect differences in strains of mice, administration of A2AR agonists, conscious vs. anesthetized animals, etc. Regardless, the effects of the A2AR agonist and antagonist we found are not related to changes in blood pressure.
Prolonged exposure to adenosine agonists could produce desensitization of A2AR (63–65). In certain reports, desensitization was dose-dependent and reversible; suggested mechanisms for desensitization have included down-regulation of the expression of A2AR or of Gs proteins involved in the cellular signaling or phosphorylation of the A2A, G protein-coupled receptor (GPCR) by GPCR kinases. There are also reports, however, providing evidence that prolonged stimulation of A2AR does not lead to loss of functional response to adenosine agonists (66, 67). In our experiments, treatment with adenosine agonists increased expression of A2AR in glomeruli of rats with anti-GBM glomerulonephritis, providing a mechanism that would prevent desensitization (Supplementary results). Interestingly, in rats treated with ZM241385, we found decreased expression of A2AR. This finding could eliminate responses to endogenous adenosine-induced activation of A2AR and lead to additional kidney damage.
In summary, we have demonstrated that pharmacological activation of A2AR attenuates leukocyte infiltration into the kidney during both the acute and progressive phases of anti-GBM glomerulonephritis. Subsequent responses include decreased expression of chemokines and upregulation of anti-inflammatory cytokines with suppressed inflammation. These events are associated with marked protection against glomerular injury. Consequently, A2AR activation could be a novel therapeutic strategy for modifying macrophage-mediated glomerulonephritis or other diseases dependent on macrophages.
Supplementary Material
ACKNOWLEDGMENTS
The untimely death of our dear colleague Dr. Lili Feng deprived us of a superb scientist. She not only contributed substantially to our understanding of mechanisms of inflammation in the kidney but also influenced the training of young scientists. Her enthusiasm, knowledge, and beautiful spirit will be greatly missed.
This work was supported in part by National Institute of Health George O’Brien Center grant (P50 DK064233, G.E.G, L.D.T. and L.F.). We thank Dr. William Mitch for critical review of the manuscript.
REFERENCES
- 1.Javaid B, Quigg RJ. Treatment of glomerulonephritis: Will we ever have options other than steroids and cytotoxics? Kidney Int. 2005;67:1692–1703. doi: 10.1111/j.1523-1755.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 2.Anders HJ, Vielhauer V, Schlondorff D. Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int. 2003;63:401–415. doi: 10.1046/j.1523-1755.2003.00750.x. [DOI] [PubMed] [Google Scholar]
- 3.Isome M, Fujinaka H, Adhikary LP, Kovalenko P, El-Shemi AG, Yoshida Y, Yaoita E, Takeishi T, Takeya M, Naito M, Suzuki H, Yamamoto T. Important role for macrophages in induction of crescentic anti-GBM glomerulonephritis in WKY rats. Nephrol Dial Transplant. 2004;19:2997–3004. doi: 10.1093/ndt/gfh558. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulos E, Seron D, Hartley RB, Cameron JS. Lupus nephritis: correlation of interstitial cells with glomerular function. Kidney Int. 1990;37:100–109. doi: 10.1038/ki.1990.14. [DOI] [PubMed] [Google Scholar]
- 5.Ikezumi Y, Hurst LA, Masaki T, Atkins RC, Nikolic-Paterson DJ. Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int. 2003;63:83–95. doi: 10.1046/j.1523-1755.2003.00717.x. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, Gutierrez-Ramos JC. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997;185:1371–1380. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolic-Paterson DJ, Lan HY, Hill PA, Atkins RC. Macrophages in renal injury. Kidney Int Suppl. 1994;45:S79–S82. [PubMed] [Google Scholar]
- 8.Schreiner GF, Cotran RS, Pardo V, Unanue ER. A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med. 1978;147:369–384. doi: 10.1084/jem.147.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Bacon KB, Li L, Garcia GE, Xia Y, Lo D, Thompson DA, Siani MA, Yamamoto T, Harrison JK, Feng L. In vivo inhibition of CC and CX3C chemokine-induced leukocyte infiltration and attenuation of glomerulonephritis in Wistar-Kyoto (WKY) rats by vMIP-II. J Exp Med. 1998;188:193–198. doi: 10.1084/jem.188.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng L, Chen S, Garcia GE, Xia Y, Siani MA, Botti P, Wilson CB, Harrison JK, Bacon KB. Prevention of crescentic glomerulonephritis by immunoneutralization of the fractalkine receptor CX3CR1 rapid communication. Kidney Int. 1999;56:612–620. doi: 10.1046/j.1523-1755.1999.00604.x. [DOI] [PubMed] [Google Scholar]
- 11.Garcia GE, Xia Y, Harrison J, Wilson CB, Johnson RJ, Bacon KB, Feng L. Mononuclear cell-infiltrate inhibition by blocking macrophage-derived chemokine results in attenuation of developing crescentic glomerulonephritis. Am J Pathol. 2003;162:1061–1073. doi: 10.1016/S0002-9440(10)63903-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia GE, Xia Y, Ku G, Johnson RJ, Wilson CB, Feng L. IL-18 translational inhibition restricts IFN-gamma expression in crescentic glomerulonephritis. Kidney Int. 2003;64:160–169. doi: 10.1046/j.1523-1755.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 13.Kawasaki K, Yaoita E, Yamamoto T, Kihara I. Depletion of CD8 positive cells in nephrotoxic serum nephritis of WKY rats. Kidney Int. 1992;41:1517–1526. doi: 10.1038/ki.1992.221. [DOI] [PubMed] [Google Scholar]
- 14.Wada T, Yokoyama H, Furuichi K, Kobayashi KI, Harada K, Naruto M, Su SB, Akiyama M, Mukaida N, Matsushima K. Intervention of crescentic glomerulonephritis by antibodies to monocyte chemotactic and activating factor (MCAF/MCP-1) Faseb J. 1996;10:1418–1425. [PubMed] [Google Scholar]
- 15.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 16.Feng L, Xia Y, Yoshimura T, Wilson CB. Modulation of neutrophil influx in glomerulonephritis in the rat with anti-macrophage inflammatory protein-2 (MIP-2) antibody. J Clin Invest. 1995;95:1009–1017. doi: 10.1172/JCI117745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, Dolecki GJ, Sherry B, Zagorski J, Lefkowith JB. Chemokines are expressed in a myeloid cell-dependent fashion and mediate distinct functions in immune complex glomerulonephritis in rat. J Immunol. 1997;158:3917–3924. [PubMed] [Google Scholar]
- 18.Colasanti M, Persichini T. Nitric oxide: an inhibitor of NF-kappaB/Rel system in glial cells. Brain Res Bull. 2000;52:155–161. doi: 10.1016/s0361-9230(00)00262-8. [DOI] [PubMed] [Google Scholar]
- 19.Cronstein BN. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol. 1994;76:5–13. doi: 10.1152/jappl.1994.76.1.5. [DOI] [PubMed] [Google Scholar]
- 20.Fredholm BB, Zhang Y, van der Ploeg I. Adenosine A2A receptors mediate the inhibitory effect of adenosine on formyl-Met-Leu-Phe-stimulated respiratory burst in neutrophil leucocytes. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:262–267. doi: 10.1007/BF00171056. [DOI] [PubMed] [Google Scholar]
- 21.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, Kihara S, Funahashi T, Takata M, Temaru R, Sato A, Yamazaki K, Nakamura N, Kobayashi M. Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res. 2005;97:1245–1252. doi: 10.1161/01.RES.0000194328.57164.36. [DOI] [PubMed] [Google Scholar]
- 23.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 24.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 25.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 26.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 27.Bullough DA, Magill MJ, Firestein GS, Mullane KM. Adenosine activates A2 receptors to inhibit neutrophil adhesion and injury to isolated cardiac myocytes. J Immunol. 1995;155:2579–2586. [PubMed] [Google Scholar]
- 28.Cronstein BN, Rosenstein ED, Kramer SB, Weissmann G, Hirschhorn R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol. 1985;135:1366–1371. [PubMed] [Google Scholar]
- 29.Richter J. Effect of adenosine analogues and cAMP-raising agents on TNF-, GM-CSF-, and chemotactic peptide-induced degranulation in single adherent neutrophils. J Leukoc Biol. 1992;51:270–275. doi: 10.1002/jlb.51.3.270. [DOI] [PubMed] [Google Scholar]
- 30.Thiel M, Chambers JD, Chouker A, Fischer S, Zourelidis C, Bardenheuer HJ, Arfors KE, Peter K. Effect of adenosine on the expression of beta(2) integrins and L-selectin of human polymorphonuclear leukocytes in vitro. J Leukoc Biol. 1996;59:671–682. doi: 10.1002/jlb.59.5.671. [DOI] [PubMed] [Google Scholar]
- 31.Thiel M, Chouker A. Acting via A2 receptors, adenosine inhibits the production of tumor necrosis factor-alpha of endotoxin-stimulated human polymorphonuclear leukocytes. J Lab Clin Med. 1995;126:275–282. [PubMed] [Google Scholar]
- 32.Gessi S, Varani K, Merighi S, Ongini E, Borea PA. A(2A) adenosine receptors in human peripheral blood cells. Br J Pharmacol. 2000;129:2–11. doi: 10.1038/sj.bjp.0703045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. Faseb J. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 34.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157:4634–4640. [PubMed] [Google Scholar]
- 35.Khoa ND, Montesinos MC, Reiss AB, Delano D, Awadallah N, Cronstein BN. Inflammatory cytokines regulate function and expression of adenosine A(2A) receptors in human monocytic THP-1 cells. J Immunol. 2001;167:4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong JM, Chen JF, Schwarzschild MA, Apasov S, Smith PT, Caldwell C, Chen P, Figler H, Sullivan G, Fink S, Linden J, Sitkovsky M. Gene dose effect reveals no Gs-coupled A2A adenosine receptor reserve in murine T-lymphocytes: studies of cells from A2A-receptor-gene-deficient mice. Biochem J. 2001;354:123–130. doi: 10.1042/0264-6021:3540123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 38.Kanellis J, Garcia GE, Li P, Parra G, Wilson CB, Rao Y, Han S, Smith CW, Johnson RJ, Wu JY, Feng L. Modulation of inflammation by slit protein in vivo in experimental crescentic glomerulonephritis. Am J Pathol. 2004;165:341–352. doi: 10.1016/S0002-9440(10)63301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unanue ER, Dixon FJ. Experimental Glomerulonephritis. V. Studies on the Interaction of Nephrotoxic Antibodies with Tissue of the Rat. J Exp Med. 1965;121:697–714. doi: 10.1084/jem.121.5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, Jennette JC, O'Brien DA, Smithies O. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–148. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- 41.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 42.Garcia GE, Xia Y, Chen S, Wang Y, Ye RD, Harrison JK, Bacon KB, Zerwes HG, Feng L. NF-kappaB-dependent fractalkine induction in rat aortic endothelial cells stimulated by IL-1beta, TNF-alpha, and LPS. J Leukoc Biol. 2000;67:577–584. doi: 10.1002/jlb.67.4.577. [DOI] [PubMed] [Google Scholar]
- 43.Xia Y, Pauza ME, Feng L, Lo D. RelB regulation of chemokine expression modulates local inflammation. Am J Pathol. 1997;151:375–387. [PMC free article] [PubMed] [Google Scholar]
- 44.Feng L, Garcia GE, Yang Y, Xia Y, Gabbai FB, Peterson OW, Abraham JA, Blantz RC, Wilson CB. Heparin-binding EGF-like growth factor contributes to reduced glomerular filtration rate during glomerulonephritis in rats. J Clin Invest. 2000;105:341–350. doi: 10.1172/JCI2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia Y, Feng L, Yoshimura T, Wilson CB. LPS-induced MCP-1, IL-1 beta, and TNF-alpha mRNA expression in isolated erythrocyte-perfused rat kidney. Am J Physiol. 1993;264:F774–F780. doi: 10.1152/ajprenal.1993.264.5.F774. [DOI] [PubMed] [Google Scholar]
- 46.Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu L, Huang Z, Mariani J, Wang Y, Moskowitz M, Chen JF. Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med. 2004;10:1081–1087. doi: 10.1038/nm1103. [DOI] [PubMed] [Google Scholar]
- 48.Bariety J, Bruneval P, Meyrier A, Mandet C, Hill G, Jacquot C. Podocyte involvement in human immune crescentic glomerulonephritis. Kidney Int. 2005;68:1109–1119. doi: 10.1111/j.1523-1755.2005.00503.x. [DOI] [PubMed] [Google Scholar]
- 49.Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, Gordon K, Gown AM. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991;87:847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 51.Niemir ZI, Stein H, Dworacki G, Mundel P, Koehl N, Koch B, Autschbach F, Andrassy K, Ritz E, Waldherr R, Otto HF. Podocytes are the major source of IL-1 alpha and IL-1 beta in human glomerulonephritides. Kidney Int. 1997;52:393–403. doi: 10.1038/ki.1997.346. [DOI] [PubMed] [Google Scholar]
- 52.Timoshanko JR, Sedgwick JD, Holdsworth SR, Tipping PG. Intrinsic renal cells are the major source of tumor necrosis factor contributing to renal injury in murine crescentic glomerulonephritis. J Am Soc Nephrol. 2003;14:1785–1793. doi: 10.1097/01.asn.0000073902.38428.33. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen DK, Montesinos MC, Williams AJ, Kelly M, Cronstein BN. Th1 cytokines regulate adenosine receptors and their downstream signaling elements in human microvascular endothelial cells. J Immunol. 2003;171:3991–3998. doi: 10.4049/jimmunol.171.8.3991. [DOI] [PubMed] [Google Scholar]
- 54.Trincavelli ML, Costa B, Tuscano D, Lucacchini A, Martini C. Up-regulation of A(2A) adenosine receptors by proinflammatory cytokines in rat PC12 cells. Biochem Pharmacol. 2002;64:625–631. doi: 10.1016/s0006-2952(02)01222-4. [DOI] [PubMed] [Google Scholar]
- 55.Cook HT, Singh SJ, Wembridge DE, Smith J, Tam FW, Pusey CD. Interleukin-4 ameliorates crescentic glomerulonephritis in Wistar Kyoto rats. Kidney Int. 1999;55:1319–1326. doi: 10.1046/j.1523-1755.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- 56.El-Shemi AG, Fujinaka H, Matsuki A, Kamiie J, Kovalenko P, Qu Z, Bilim V, Nishimoto G, Yaoita E, Yoshida Y, Anegon I, Yamamoto T. Suppression of experimental crescentic glomerulonephritis by interleukin-10 gene transfer. Kidney Int. 2004;65:1280–1289. doi: 10.1111/j.1523-1755.2004.00536.x. [DOI] [PubMed] [Google Scholar]
- 57.Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Day YJ, Li Y, Rieger JM, Ramos SI, Okusa MD, Linden J. A2A Adenosine Receptors on Bone Marrow-Derived Cells Protect Liver from Ischemia-Reperfusion Injury. J Immunol. 2005;174:5040–5046. doi: 10.4049/jimmunol.174.8.5040. [DOI] [PubMed] [Google Scholar]
- 59.Holdsworth SR, Neale TJ, Wilson CB. Abrogation of macrophage-dependent injury in experimental glomerulonephritis in the rabbit. Use of an antimacrophage serum. J Clin Invest. 1981;68:686–698. doi: 10.1172/JCI110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawasaki K, Yaoita E, Yamamoto T, Tamatani T, Miyasaka M, Kihara I. Antibodies against intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1 prevent glomerular injury in rat experimental crescentic glomerulonephritis. J Immunol. 1993;150:1074–1083. [PubMed] [Google Scholar]
- 61.Le Hir M, Keller C, Eschmann V, Hahnel B, Hosser H, Kriz W. Podocyte bridges between the tuft and Bowman's capsule: an early event in experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2001;12:2060–2071. doi: 10.1681/ASN.V12102060. [DOI] [PubMed] [Google Scholar]
- 62.Moeller MJ, Soofi A, Hartmann I, Le Hir M, Wiggins R, Kriz W, Holzman LB. Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol. 2004;15:61–67. doi: 10.1097/01.asn.0000102468.37809.c6. [DOI] [PubMed] [Google Scholar]
- 63.Ramkumar V, Olah ME, Jacobson KA, Stiles GL. Distinct pathways of desensitization of A1- and A2-adenosine receptors in DDT1 MF-2 cells. Mol Pharmacol. 1991;40:639–647. [PMC free article] [PubMed] [Google Scholar]
- 64.Webb RL, Sills MA, Chovan JP, Peppard JV, Francis JE. Development of tolerance to the antihypertensive effects of highly selective adenosine A2a agonists upon chronic administration. J Pharmacol Exp Ther. 1993;267:287–295. [PubMed] [Google Scholar]
- 65.Willets JM, Parent JL, Benovic JL, Kelly E. Selective reduction in A2 adenosine receptor desensitization following antisense-induced suppression of G protein-coupled receptor kinase 2 expression. J Neurochem. 1999;73:1781–1789. doi: 10.1046/j.1471-4159.1999.0731781.x. [DOI] [PubMed] [Google Scholar]
- 66.Casati C, Monopoli A, Dionisotti S, Zocchi C, Bonizzoni E, Ongini E. Repeated administration of selective adenosine A1 and A2 receptor agonists in the spontaneously hypertensive rat: tolerance develops to A1-mediated hemodynamic effects. J Pharmacol Exp Ther. 1994;268:1506–1511. [PubMed] [Google Scholar]
- 67.Conti A, Lozza G, Monopoli A. Prolonged exposure to 5'-N-ethylcarboxamidoadenosine (NECA) does not affect the adenosine A2A-mediated vasodilation in porcine coronary arteries. Pharmacol Res. 1997;35:123–128. doi: 10.1006/phrs.1996.0125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.