Abstract
Human communication and survival depend on effective social information processing. Abundant behavioral evidence has shown that humans efficiently judge preferences for other individuals, a critical task in social interaction, yet the neural mechanism of this basic social evaluation, remains less than clear. Using a social-emotional preference task and connectivity analyses (psycho-physiological interaction) of fMRI data, we first demonstrated that cortical midline structures (medial prefrontal and posterior cingulate cortices) and the task-positive network typically implicated in carrying out goal-directed tasks (pre-supplementary motor area, dorsal anterior cingulate and bilateral frontoparietal cortices) were both recruited when subjects made a preference judgment, relative to gender identification, to human faces. Connectivity analyses further showed network interactions among these cortical midline structures, and with the task-positive network, both of which vary as a function of social preference. Overall, the data demonstrate the involvement of cortical midline structures in forming social preference, and provide evidence of network interactions which might reflect a mechanism by which an individual regularly forms and expresses this fundamental decision.
Keywords: fMRI BOLD, cortical midline structures, social cognition, functional connectivity, psycho-physiological interaction
Introduction
Social cognition is the set of functions allowing individuals to interact and navigate the day-to-day social world (Frith and Frith, 2007). It requires a well-developed set of social cognitive skills, such as perceiving emotion, discerning intentions, understanding dispositions and reflecting upon one's own subjective experience. Converging evidence suggests that regions in the midline of the human cerebral cortex (cortical midline structures, CMS), including the anterior medial frontal cortex (aMFC), ventral medial frontal cortex (vMFC), and posterior cingulate cortex (PCC), are important nodes that process social-cognitive information (e.g. Amodio and Frith, 2006; Northoff and Bermpohl, 2004; Uddin et al., 2007). Brain lesion studies have shown that patients with aMFC damage have difficulty with social reasoning (Adolphs, 1999; Apperly et al., 2004), in spite of otherwise intact intellectual ability. Following vMFC damage, adults with previously normal personalities develop abnormal social conduct, deficits in empathy, impaired emotional perspective-taking ability and difficulty assessing trustworthiness (Damasio et al., 1990; Hynes et al., 2006; Moretti et al., 2009). Additionally, neuroimaging research has established the role of these medial cortical structures in a range of social cognitive functions, such as emotion processing (Phan et al., 2002), person perception (e.g. Iacoboni et al., 2004; Mitchell et al., 2005b; Narumoto et al., 2001), attribution of mental states (e.g. Castelli et al., 2000; Frith and Frith, 1999; Mitchell et al., 2005a; Walter et al., 2004), and self-referential processing (e.g. Gusnard et al., 2001a; Johnson et al., 2005; Kelley et al., 2002; Northoff and Bermpohl, 2004).
One critical part of interpersonal behavior is social preference – namely, one's likes and dislikes toward others. As eminently social animals, humans have evolved efficient processes for judging other individuals. Empirical evidence suggests that first impressions form quickly (<100 msec), and may be evolutionarily important for rapid identification and coping with social encounters (Hassin and Trope, 2000; Willis and Todorov, 2006). This ability to rapidly form opinions of other people, however, does not necessarily preclude the involvement of higher-level cognitive mechanisms in extracting meanings from the deliberately shared social world (Frith and Frith, 2007). Appraising whether one likes a person or not also entails the attribution of personal relevance to the person, by weighing what matters to the individual (de Greck et al., 2008; Enzi et al., 2009); furthermore, data suggest that the importance and meaning of the stimulus for the individual is associated with self-relatedness (See: Northoff et al., 2006 for a critical meta-analysis). Although the neural correlates for forming first impressions about others has been recently identified (Schiller et al., 2009), the neurobiological underpinnings for elaborating social preference have received little attention.
Among the previous work investigating the neural correlates of social cognitive functions, those with explicit cognitive components such as elaborating introspective appraisals or evaluative decisions of stimuli often co-activate higher cortical regions that mediate cognitive control processing. For instance, when using paradigms that require the reflective appraisal of one's personal characteristics (e.g. Fossati et al., 2003, Kelley et al., 2002)(Moran et al., 2006), affective experiences (e.g. Gusnard et al., 2001a, Taylor et al., 2007), and the explicit evaluation of attitudes toward concepts or famous names (Cunningham et al., 2003, Cunningham et al., 2004), activity tends to increase in both medial and lateral frontoparietal cortices. The regions involved include aMFC, vMFC, and PCC, the core structures for social cognition, as well as the pre-supplementary motor area (pre-SMA), dorsal anterior cingulate (dACC), lateral frontal and lateral parietal cortices, a set of cortical structures commonly activated during the performance of cognitive tasks (termed the ‘task-positive’ network (Fox et al., 2005)). This latter set of regions has been associated with the continuous internal monitoring of actions and adjustment of goal-directed behaviors (Ridderinkhof et al., 2004), and is consistent with the notion that explicit appraisal of social cognitive information depends on controlled processing to guide contextually appropriate behavior. Nevertheless, co-activation does not necessarily reflect a functionally interacting network. Therefore, the network mechanism through which the core nodes for social cognition (aMFC, vMFC, PCC) integrate within themselves and with the structures that mediate controlled processing to enable goal-directed social-cognitive behaviors remains unclear.
Therefore, the primary goals in our study were to investigate the neural correlates of, and the network properties underlying, social preference.
In service of our first goal, we devised a ‘socio-emotional preference task’ (SePT), in which subjects viewed faces, with varying emotional expressions, and made appraisals of whether or not they liked the face (Preference). As a control condition, subjects also identified the gender of faces (Gender), which when contrasted with the social preference evaluations (Preference), permitted the isolation of the underlying process of evaluating subjective preference toward others while matching perception, decision-making and motoric responses. On the basis of the previous findings reviewed earlier, we first hypothesized that Preference (relative to Gender) would recruit more activity in cortical midline structures of interest: aMFC, vMFC and PCC. Additionally, increased activity in structures mediating cognitive control processes was also predicted.
A second contrast condition -- a passive baseline (a centered white fixation cross on a black screen) -- was also included in our paradigm to identify task-induced activity changes relative to a resting baseline. Recent neuroimaging research has identified a ‘default-mode network’ that includes these midline regions as well as the posterior lateral cortices (BA 19/22/39/40). A signature property of this network is that it maintains high metabolism during resting states and exhibits task-induced deactivation (TID) across a wide range of cognitive tasks (e.g. Gusnard et al., 2001b; Mazoyer et al., 2001; Shulman et al., 1997), accompanying increased activity in the task-positive network (Fox et al., 2005). The reciprocal, ‘see-saw’ activity between the two networks during cognitive task performance has been suggested to reflect reallocation of processing resources, such that TID reflects the suspension or interruption of processes that are carried out by the default-mode network when the mind is not engaged by external cognitive demands (McKiernan et al., 2006; McKiernan et al., 2003). If this is true, then one might expect that by engaging subjects in a social cognitive task (such as Preference) that demands activity from cortical midline structures, the default-mode network would be less susceptible to task-induced deactivation typically observed with cognitive/perceptual tasks (such as Gender). Hence, we further predicted that the cortical midline activity (aMFC, vMFC and PCC) observed in the Preference > Gender contrast would reflect decreased deactivation in the Preference condition (relative to passive baseline).
The second goal of this study was to analyze medial-cortical network properties that underlie social preference. A few reviews and meta-analysis have laid out a theoretical framework relevant for understanding the neural mechanisms underlying social cognitive processing, inclusive of making appraisals where one's self is the explicit referent (Amodio and Frith, 2006; Northoff and Bermpohl, 2004; Northoff et al., 2006). Within the CMS, it has been suggested that each node is associated with a distinct process important for the explicit appraisal and coding of everyday stimuli. Specifically, vMFC is primarily involved in the initial rapid appraisal and representation of the value of an environmental stimulus; whereas aMFC is characterized as a functional division for reappraisal, evaluation and explicit reasoning of the incoming stimulus. With its strong connection with the hippocampus implicated in autobiographic memory, PCC has a central role in integrating the temporal context of the stimuli. Additionally, strong reciprocal anatomical connections (Ongur and Price, 2000) and resting state intrinsic connectivity measured by low frequency BOLD fluctuations (Fox et al., 2005; Greicius et al., 2003) have been established among the cortical midline structures (aMFC, vMFC, and PCC), both providing grounds for a functionally interacting medial-cortical network for social cognition. Regarding the interaction between the CMS and higher-cognitive structures, a dorsal system including aMFC, pre-SMA/ dACC, lateral frontal cortex, among others, is proposed to integrate cognitive processes important for regulating behaviors (Ochsner and Gross, 2005; Phillips et al., 2003). Furthermore, widespread anatomical connections among these cortical structures bolster the possibility for a network-based functional interaction.
We employed a psycho-physiological interaction analysis (PPI) to identify functional networks that subserve neural processes underlying the SePT, as opposed to functionally-isolated structures that simply co-activate (Friston et al., 1997). PPI provides a within-subject measure of functional interactions (‘coupling’) between brain regions in relation to the experimental design. Based on the assumption that structures involved in the same functional network ‘co-modulate’ activity while carrying out specific tasks, connectivity is inferred by significant changes in correlation, as a function of task manipulation, between the time courses of regional neuronal activity at the within-subject level. On the basis of the neuroimaging literature reviewed above, we hypothesized that the cortical midline structures (aMFC, vMFC and PCC) co-activated during the SePT represent nodes of an interacting functional network, which may serve as a probable mechanism to integrate the distinct component processes relevant for social cognitive processing. We also hypothesized network interaction between the CMS and the task-positive network, potentially serving as a mechanism to integrate cognitive processes to guide contextually appropriate social-cognitive behavior.
Methods
Subjects
Twenty-one healthy individuals between the age of 23 and 51 years (15 males, mean age = 40 +/- 9.6 years, mean education = 16 +/- 3 years) were recruited from community advertisements. Subjects had normal or corrected-to-normal vision, reported no significant abnormal neurological or psychiatric history, and were not taking medication. Subjects gave written, informed consent for study participation after explanation of the purpose and risks of the study, in accordance with procedures approved by the University of Michigan institutional review board (IRBMED). After completion of the study, subjects were debriefed and reimbursed for their participation and time.
Stimuli and task
The SePT consisted of three sets of human facial emotions: positive (happy), negative (primarily fearful) and neutral expressions, all selected from a published dataset (Gur et al., 2002). Each facial emotion set contains an average of 22 faces, and the same actors portrayed different expressions for each set. Each stimulus was repeated for up to 7 times, and a total of 288 face instances were used throughout the study. For the Preference task, subjects were instructed to judge whether they liked the face, based on their immediate experience, without concerns for being right or wrong. As an experimental control condition for general face-processing, decision-making and motor response-related activities, a gender identification task was used (Gender). Subjects saw each face for 3 sec, with a word above indicating task condition (either Preference [“Like?”] or Gender [“Gender?”]). Subjects made their response with a button press of the index or middle finger of the right hand (yes/no for Preference, male/female for Gender). In neither task were subjects required to make their decisions based on valence of the stimuli.
Stimulus control and response recording occurred with E-prime (Psychology Software Tools, Inc.). Subjects viewed the stimuli via reflection using angled mirrors and a back-projection system. Each task block was twelve-second long, with 4 different face instances of the same valence. There were 18 pseudo-randomized task blocks per run, and each block was separated by a centered fixation cross (the ‘passive baseline’) which was presented in a jittered manner (range = 4 - 8 sec, mean = 6 sec).
Data acquisition
Magnetic resonance imaging (MRI) scanning occurred on a General Electric (Waukesha, WI) 3T Signa scanner (LX [8.3] release). The scanning began with structural acquisition of a standard T1 image (T1-overlay) for anatomic alignment. Functional images were acquired with a T2*-weighted (GRE; repetition time, 2000 msec; echo time, 30 msec; flip angle, 90°; field of view, 22 cm; 40 slices; 3.0 mm slice thickness/0 mm skip, equivalent to 64 × 64 matrix size), reverse spiral acquisition sequence, a method sensitive to signal in ventral medial frontal regions (Yang et al., 2002). T2* images were prescribed identical to the T1-overlay. The fMRI scans were made while subjects performing tasks: 166 volumes (including 4 initial, discarded volumes to allow for equilibration of scanner signal) were acquired each run, for a total of 664 volumes. After acquisition of functional volumes, a high-resolution T1 image (T1-spgr) was obtained for anatomic normalization.
Data analysis
Data processing began with the following preprocessing steps: fMRI data were first reconstructed off-line using custom code written in C (Noll et al., 1991). Subsequently, slice-timing and motion correction were done using the “slicetimer” and the “mcflirt” routines of the FSL fMRI analysis package (http://www.fmrib.ox.ac.uk/fsl/slicetimer/index.html) (Jenkinson et al., 2002). Realignment parameters were inspected as a proxy for subject movement, in order to ensure that movement did not exceed either 3 mm, or 1° rotation within a run. The remainder of preprocessing and image analysis was performed using Statistical Parametric Mapping SPM2 package (Wellcome Institute of Cognitive Neurology, London, United Kindom). The high-resolution T1 image (T1-spgr) was normalized to the Montreal Neurological Institute (MNI) 152 brain-template, yielding anatomical parameters that were applied to the co-registered time-series of functional volumes. An isotropic 5mm full-width half-maximum Gaussian kernel was then used to smooth the functional volumes. In the primary model to analyze effects of tasks, the design matrix consisted of 4 runs; each had six regressors of interest (two tasks crossed with the three face valences), and the passive baseline was modeled implicitly. All regressors were convolved with a canonical hemodynamic response function (HRF). The statistical model was estimated including a high pass filter (128 sec) and AR (1) temporal autocorrelation. Subsequently, the parameter estimates were derived from the magnitude (height) of the HRF. The focus of the present report is on the difference between Preference and Gender tasks, thus regressors for the different face valences were collapsed within each task. Contrast images, testing for difference relative to the implicit baseline and for task differences, were smoothed with a 5 mm Gaussian kernel to stabilize variance properties, and entered into a second-level random effect analysis. The group significance of the task effect was thresholded at p= 0.05 (FDR-corrected for whole brain multiple comparisons), and minimal cluster size of 15 contiguous voxels (equivalent to 405-mm3).
A psycho-physiological interaction analysis (PPI) allows one to test whether inter-regional correlation (‘functional coupling’) in neuronal activities (one from ‘Seed ROI’, one from ‘Coupled Region’) changes significantly as a function of task condition, while discounting mean activity due to task differences. Hence, this ‘functional connectivity’ analysis differs from the conventional activation mapping approaches (such as ‘cognitive subtraction’) in that PPI reveals differential interactions between brain regions on residual variances after removing task-related effects, and hence disambiguates inter-regional connectivity (‘truly covariant’) from differential task effects (Friston et al., 1997). Focusing on the medial cortical areas, we first identified regions of interest (seed ROIs) from the main effect of task (Preference > Gender) at the second-level random effect analysis. Each seed ROI was a sphere of 9mm radius (corresponding to the approximate average smoothness of the image) centered at the local maximum. For each seed ROI, the time-series of the first eigenvariate (from the primary model) was extracted for each subject, then deconvolved with a canonical hemodynamic response function (Gitelman et al., 2003), and multiplied by a binary vector coding for the task (‘psychological factor’: 1 for the Preference condition, -1 for the Gender condition), yielding an element-by-element product. This product was then convolved with the canonical hemodynamic response function and entered as the psycho-physiological interaction term (PPI.ppi) in the PPI model, with a high pass filter (128 sec) as well as AR1 temporal autocorrelation. Subsequently, positive and negative contrast weights were placed on the ‘PPI.ppi’ regressor to test for positive and negative psycho-physiological interactions, respectively. As a result, a significant positive PPI for a voxel implies that the correlation between this voxel (‘coupled region’) and the seed ROI is greater during Preference than that during Gender, and vice versa. To test for significance at the group level, the contrast images generated were smoothed with a 5mm Gaussian kernel first, and then entered into a second-level random effect analysis. The same statistical threshold was used (p<0.05 FDR-corrected; minimal cluster size >15 voxels).
Since PPI interactions reflect the change of regression slope between neuronal activities of two regions (a seed ROI and its coupled region) in two task conditions, we also repeated the analysis for Preference and Gender task separately, in order to determine the direction of the correlation (Etkin et al., 2006). Briefly, for each seed ROI at the fixed-effect level, the de-convolved time-series were multiplied by separate vectors for each task (‘psychological factor’: 1 for the Preference condition, 0 for the Gender condition; or, 0 for the Preference condition, 1 for the Gender condition), the products of which were then convolved with canonical hemodynamic response function to generate interaction terms for Preference and Gender task, respectively. Hence, the effects and interactions of the tasks were entered into the same model, but in separate columns. Contrast images for the interaction terms from each subject were similarly incorporated into a second-level random effect analysis, as previously described.
Results
Behavioral data
No significant effect of valence on gender identification accuracy was found [F (2,40) = 3.01, p=0.1]; In Preference task, the probability for a “Like” response varied as a function of valence: Subjects indicated that they liked positive faces more often than neutral faces, and neutral faces more often than negative ones [Ave. ± SE (%): 92.5 ± 2.4, 41.7 ± 5.8, 17.9 ± 4.5, respectively; F (2,40) = 99.38, p<0.001].
Mean reaction times for all our conditions of interest were calculated for each subject. There was a significant main effect of task, such that Preference took more time to perform than Gender [Ave. ± SE (msec): 1194.7 ± 35.6, 1106.2 ± 42.6, respectively; F (1,20) = 10.5, p=0.004].
Functional MRI data
Group Analysis of task effects
Relative to the resting baseline, both Preference and Gender tasks recruited: 1) Increased activity in a set of brain regions typically seen for perceptual processing and task execution, including: occipital lobe (fusiform face areas, FFA, included), precentral gyrus, dACC, pre-SMA, bilateral PFC and superior parietal lobule (SPL); and 2) decreased activity in several structures along the cortical midline, including medial superior/middle frontal gyri, PCC, precuneus, retrosplenial cortex and the inferior temporal/parietal cortices (Table 1 and Figure 1).
Table 1. Activation peaks -- Main effect of Tasks.
| Preference | Gender | |||||
|---|---|---|---|---|---|---|
| Region | (x, y, z)a | Clusterb | Z-scorec | (x, y, z)a | Clusterb | Z-scorec |
| Increases relative to baseline | ||||||
| Occipital lobe (BA 17/18/19/37) | 51, -51, -24 | 3816d | 6.27 | -39, -90, -9 | 3806d | 6.13 |
| pre-SMA/ dACC (BA 6/8/32) | 0, 12, 57 | 428 | 5.53 | 6, 21, 51 | 163 | 4.39 |
| SFG/MFG/ IFG (BA 6/8/9/45/46/47) | 48, 12, 30 | 908 | 5.42 | 48, 36, 33 | 378 | 4.72 |
| -45, 21, -3 | 114 | 4.36 | 39, 24, 3 | 81 | 3.83 | |
| SPL (BA 7/40) | 33, -57, 48 | 246 | 4.66 | 33, -57, 48 | 289 | 5.06 |
| -36, -51, 45 | 156 | 3.85 | -36, -48, 45 | 694 | 4.95 | |
| Precentral gyrus | -36, -3, 63 | 328 | 4.25 | -45, 3, 33 | 99 | 4.21 |
| Decreases relative to baseline | ||||||
| PCC/ Precuneus/ Retrosplenial cortex (BA 7/31) | 18, -54, 6 | 19714e | 6.96 | -18, -57, 3 | 18994e | 7.03 |
| Temporal gyri (BA 7/19/39/40/41/42) | 60, -60, 21 | 282 | 4.46 | |||
| 3, -21, 0 | 138 | 3.66 | ||||
Abbreviations – SFG, superior frontal gyrus; MFG, middle frontal gyrus; IFG, inferior frontal gyrus; SPL, superior parietal lobe.
Stereotactic coordinates from MNI152 reference, left/right, anterior/posterior and superior/inferior, respectively.
Cluster size in voxels.
All foci p < 0.05, FDR-corrected; Extend threshold: 15 contiguous voxels.
Also extended into cerebellum.
Also extended extensively into medial surface of SFG/ MFG, as well as temporal gyri.
Figure 1.
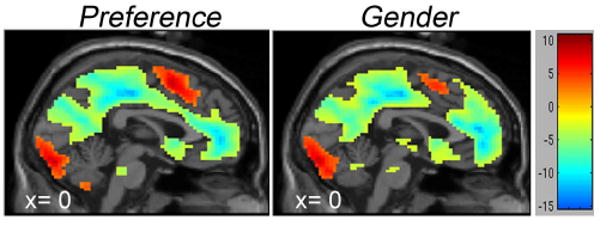
Main effect of tasks on cortical midline activity: Regions that showed significant activation (hot-colored) and deactivation (cool-colored) along the midline surface. Both Preference and Gender tasks showed a similar pattern of signal distribution. The coordinates and Z scores of all significant clusters are listed in Table 1.
Preference, when compared to Gender, recruited greater activity in several brain regions (Table 2), including the predicted foci on the medial cortical surface displayed in Figure 2a -- aMFC, vMFC and PCC, as well as the pre-SMA/ dACC. When examined relative to the passive baseline, this contrast (Preference > Gender) reflected two patterns of change, depending upon whether the region was deactivated relative to baseline (as seen in the default network activity) or active relative to baseline. Two conjunction analyses ([Preference – Gender] ∩ [(Baseline-Gender)-(Baseline-Preference)]; and [Preference – Gender] ∩ [(Preference – Baseline)-(Gender – Baseline)]) revealed that the cortical midline foci could be separated into two major categories along the y-axis (anterior-posterior). The anterior component, including our medial cortical structures of interest (aMFC, vMFC and PCC), was deactivated for both the Preference and Gender tasks, but less for Preference (termed ‘differential deactivation’; the yellow clusters in Figure 2b). In contrast, the posterior component, including pre-SMA/ dACC as well as bilateral frontoparietal cortices (not seen from the medial view), reflected ‘differential activation’: Greater activity (relative to passive baseline) in Preference than Gender (the red cluster in Figure 2b).
Table 2. Activation peaks -- Direct task comparisons.
| Region | (x, y, z)a | Clusterb | Z-scorec |
|---|---|---|---|
| Preference > Gender | |||
| pre-SMA/ dACC (BA 6/8/9/32) | -6, 33, 39 | 1690d | 6.13 |
| aMFCe | 0, 54, 30 | --e | 4.97 |
| vMFC | -3, 54, -18 | 34 | 3.37 |
| PCC | -6, -51, 33 | 31 | 3.30 |
| Lateral MFG/ IFG | -39, 21, -18 | 868 | 4.93 |
| 48, 30, -12 | 536 | 4.79 | |
| 45, 21, 45 | 236 | 4.73 | |
| SPL/IPS | -54, -60, 30 | 112 | 4.84 |
| 54, -57, 48 | 45 | 3.65 | |
| Cerebellum | -21, -84, -33 | 296 | 4.84 |
| 36, -81, -33 | 231 | 3.93 | |
| -6, -57, -42 | 88 | 3.66 | |
| Temporal pole | 48, 6, -42 | 56 | 4.06 |
| Caudate | -9, 9, 9 | 36 | 3.37 |
| Gender > Preference | |||
| Precuneus (BA 7/31) | 3, -48, 63 | 8121f | 5.45 |
| STG/ MTG | 60, -57, -6 | 43 | 4.30 |
| -54, -69, -3 | 97 | 4.10 |
Abbreviations – MFG, middle frontal gyrus; IFG, inferior frontal gyrus; SPL, superior parietal lobe; IPS, intraparietal sulcus; STG, superior temporal gyrus; MTG, middle temporal gyrus.
Stereotactic coordinates from MNI152 reference, left/right, anterior/posterior and superior/inferior, respectively.
Cluster size in voxels.
All foci p<0.05, FDR-corrected; Extend threshold: 15 contiguous voxels.
Also extended extensively into aMFC.
Identified as a local maxima within the pre-SMA/ dACC cluster.
Also extended into temporal-parietal junction.
Figure 2.
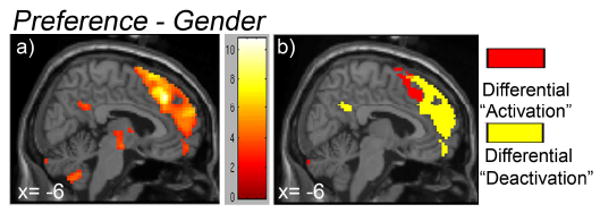
Direct comparison of tasks (Preference – Gender) on cortical midline activity.
2a) Regions that showed significant increases in activity during Preference than Gender were mapped onto the sagittal view of the cortical midline surface. The coordinates and Z scores of all significant clusters are listed in Table 2.
2b) Through conjunctional analyses, we further separated the ‘activation’ focus into: i) Difference in deactivation: Areas that include aMFC, vMFC and PCC; and ii) Difference in activation: Areas that include pre-SMA and dACC.
Gender, when compared to Preference, recruited greater activity in precuneus and posterior lateral cortices (temporal-parietal junction, TPJ; and superior/middle temporal gyri, STG/MTG), and these differences reflected greater deactivation from resting baseline during Preference than Gender tasks.
Functional connectivity analysis with PPI
For PPI analysis, we used the three cortical midline structures identified in the main subtraction analysis as seed ROIs to test for task-dependent ‘connectivity’: aMFC, peak activity (0, 54, 30); vMFC, peak activity (-3, 54, -18); PCC, peak activity (-6, -51, 33). As a function of social preference, each seed ROI identified overlapping, yet distinct, patterns of functional coupling with one another, as well as with the task-positive network.
Seed ROI – aMFC
When the seed ROI was placed at aMFC, PPI analysis showed that vMFC, PCC, pre-SMA/ dACC and bilateral frontoparietal cortices were among the very few regions that showed different functional coupling with aMFC between Preference and Gender task conditions (or, ‘task-dependent functional coupling’). More specifically, vMFC and PCC decreased correlation with aMFC as a function of social preference (Preference < Gender), whereas pre-SMA/dACC and bilateral frontoparietal cortices increased correlation (Preference > Gender; Table 3 and Figure 3a). When this interaction was examined further by testing for correlations between aMFC and the coupled regions for the two tasks separately, the analysis revealed that all these effects arose from differences in positive correlation (Figure 4). That is, for vMFC and PCC, the inter-regional correlation with aMFC was less positive during Preference than that during Gender; for pre-SMA/ dACC and bilateral frontoparietal cortices, the inter-regional correlation with aMFC was more positive during Preference than that during Gender (Figure 4b).
Table 3. PPI analyses -- Areas that showed task-dependent functional coupling with the medial cortical seed ROIs.
| Seed ROI | Coupled Region | (x, y, z)a | Clusterb | Z-scorec | Changes in Functional Couplingd |
|---|---|---|---|---|---|
| aMFC | PCC | -9, -57, 27 | 43 | 4.59 | Decreased |
| vMFC | 0, 51, -6 | 55 | 3.85 | Decreased | |
| STG | -60, -12, 0 | 72 | 4.11 | Decreased | |
| -63, -33, 15 | 42 | 4.09 | |||
| IPL | -45, -72, 33 | 46 | 3.87 | Decreased | |
| Precuneus | 45, 30, 27 | 25 | 3.67 | Decreased | |
| MFG/ IFG | 48, 9, 33 | 75 | 4.73 | Increased | |
| -54, 12, 33 | 58 | 4.36 | |||
| 45, 30, 27 | 25 | 3.67 | |||
| Pre-SMA/ dACC | 6, 24, 48 | 29 | 4.23 | Increased | |
| SPL/IPS | 36, -57, 48 | 110 | 4.43 | Increased | |
| -45, -48, 54 | 38 | 4.24 | |||
| Fusiform | 42, -60, -18 | 381 | 5.43 | Increased | |
| -36, -69, -18 | 157 | 5.07 | |||
| vMFC | aMFC | -3, 57, 9 | 138 | 4.39 | Decreased |
| PCC | -6, -66, 27 | 124 | 3.96 | Decreased | |
| Fusiform | -33, -69, -18 | 31 | 4.49 | Increased | |
| PCC | vMFC | 0, 42, -21 | 38 | 3.96 | Decreased |
| IPL | -42, -48, 33 | 15 | 3.73 | Decreased | |
| pre-SMA/ dACC | 0, 27, 51 | 97 | 4.37 | Increased | |
| SFG/MFG/ IFG | 42, 6, 30 | 15 | 3.44 | Increased | |
| SPL | 45, -48, 42 | 93 | 3.88 | Increased | |
| Fusiform | 39, -63, -21 | 39 | 4.07 | Increased | |
| Thalamus | 12, -3, 15 | 28 | 4.00 | Increased | |
| -15, -12,15 | 23 | 3.78 | |||
| Mid Cingulate | -3, -33, 27 | 111 | 3.95 | Increased |
Abbreviations – STG, superior temporal gyrus; IPL, inferior parietal lobe; MFG, middle frontal gyrus; IFG, inferior frontal gyrus; SPL, superior parietal lobe; IPS, intraparietal sulcus.
Stereotactic coordinates from MNI152 reference, left/right, anterior/posterior and superior/inferior, respectively.
Cluster size in voxels.
All foci p<0.05, FDR-corrected; Extend threshold: 15 contiguous voxels.
As a function of social preference (i.e. Preference, relative to Gender).
Figure 3.
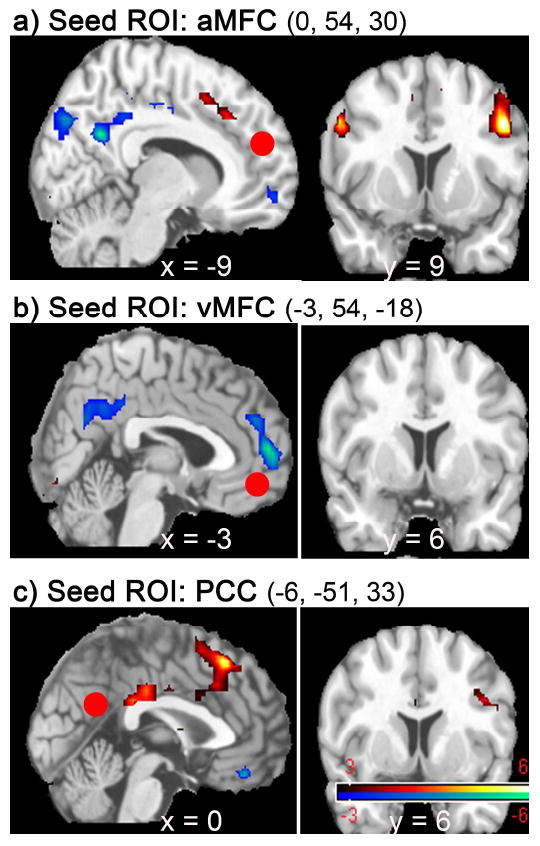
Sagittal/coronal views of regions that showed task-dependent functional coupling with the seed ROI (marked with red circles): a) aMFC; b) vMFC; c) PCC. The three cortical midline regions were identified by the main subtraction analysis (Preference – Gender), and used as seed ROIs in psycho-physiological interaction (PPI) analyses. Regions that decreased correlation with the seed ROIs, as a function of social preference, were predominantly components of the default-mode network (cool-colored); whereas regions that increased correlation with the seed ROIs belonged to the task-positive network (hot-colored). The coordinates and Z scores of all significant clusters are listed in Table 3. Displays are in neurological convention.
Figure 4.
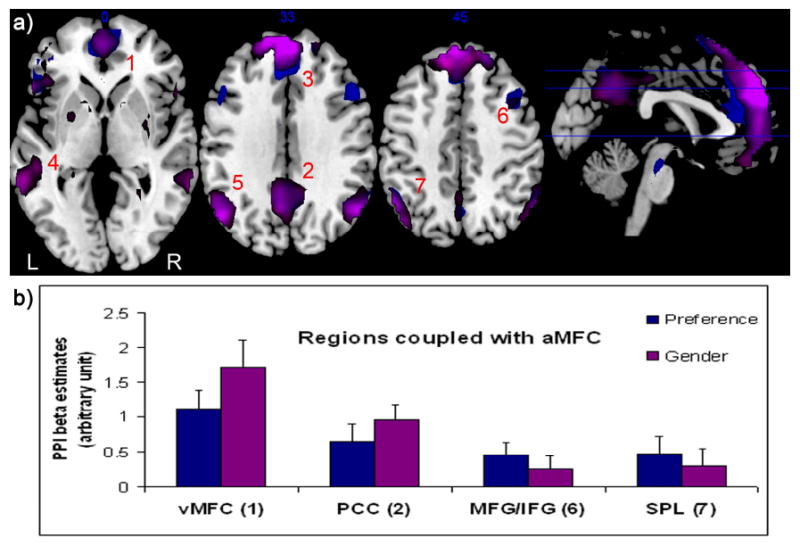
Representative results of a PPI analysis modeling separate task conditions.
4a) Regions that showed inter-regional correlations with the seed ROI aMFC during Preference (blue-colored) and Gender (purple-colored) tasks, respectively, were overlaid onto the single subject brain template. The cortical regions whose neuronal activities positively correlate with aMFC are as follows: 1. vMFC; 2. PCC; 3, aMFC; 4. superior temporal gyrus, STG; 5. inferior parietal lobule, IPL; 6. middle frontal gyrus/inferior frontal gyrus, MFG/IFG; 7. superior parietal lobule, SPL. No regions were found to negatively correlate with aMFC above the same statistical threshold (p=0.05 FDR-corrected for whole brain multiple comparison, minimal cluster size >15 voxels.) The number on the upper middle of each axial image indicates millimeters from the anterior commissure. R, right hemisphere; L, left hemisphere.
4b) Psychophysiological interaction beta estimates (± SE) for each task were separately extracted from 9mm-radius spheres centered on the regions previously identified to show task-dependent functional coupling with aMFC, such as vMFC, PCC, MFG/IFG, SPL (For coordinates details, see Table 3). In parenthesis are numbers corresponding to those in Fig 4a.
Seed ROI – vMFC
When the seed ROI was placed at vMFC, aMFC and PCC were the only regions that showed task-dependent functional coupling with vMFC. More specifically, both aMFC and PCC showed decreased correlation with vMFC as a function of social preference (Table 3 and Figure 3b); similarly, this occurred as a result of less positive inter-regional correlation during Preference than that during Gender.
Seed ROI – PCC
When the seed ROI was placed at PCC, PPI analysis revealed task-dependent functional coupling of the PCC with vMFC, with pre-SMA/ dACC, and with bilateral frontoparietal cortices, among few other regions. Specifically, vMFC showed decreased correlation with PCC as a function of social preference, whereas pre-SMA/ dACC and bilateral frontoparietal cortices showed increased correlation (Table 3 and Figure 3c); similarly, these were also found to be reflective of changes in positive correlation between the PCC and the coupled regions. That is, the inter-regional correlation between PCC and vMFC was less positive during Preference than that during Gender. For pre-SMA/ dACC and bilateral frontoparietal cortices, the inter-regional correlation with PCC was more positive during Preference than that during Gender.
In sum, the network interaction patterns revealed from PPI analyses showed that as a function of social preference (i.e. Preference, relative to Gender), there was a predominant pattern of decreases in positive coupling among all three cortical midline seed ROIs (aMFC, vMFC, PCC); on the other hand, only aMFC and PCC functionally interacted with the task-positive network mediating cognitive controlled processing, which was reflected as increases in positive coupling.
Discussion
As predicted, the SePT recruited cortical midline components of the default-mode network (aMFC, vMFC and PCC), reflecting reduced deactivation during Preference as compared to Gender. The SePT also activated brain areas involved in perceptual processing and task execution, reflecting increased activation during Preference relative to Gender. PPI connectivity analyses further provided evidence for network interactions of these three cortical midline structures that co-activated for the SePT. Specifically, our data showed that network interactions among the cortical midline structures, as well as that between the cortical midline structures and task-positive network vary as a function of social preference. Together, the results provide important insights into how social preference is carried out by large-scale networks.
Social functioning in the SePT and default-mode network activity
By employing an experimental design that allowed us to isolate the underlying process of evaluating subjective preference toward others (SePT), we first demonstrated the neural correlates of social preference and also tested the hypothesis that Preference modulates task-induced deactivations in the default-mode network. Our hypothesis was supported by direct comparison of the tasks, Preference and Gender, in combination with two conjunction analyses. As expected, several brain areas, including medial surface of SFG, vMFC, PCC and bilateral frontoparietal cortices, showed relatively greater activity in Preference than Gender. Moreover, the anterior portion of the frontal medial cluster (aMFC, along with vMFC) and PCC, resulted from less deactivation during Preference compared to Gender; the posterior portion of the same frontal medial cluster (pre-SMA, dACC), as well as bilateral frontoparietal cortices, arose from more activation during Preference.
The findings support and supplement the growing body of data for a CMS-based network invoked by social cognition. Current neuroimaging literature suggests that cortical midline structures support processes that integrate social information across time, allow representation and reflection of norms and intentionality, at a more abstract cognitive level (Uddin et al., 2007; Van Overwalle, 2008). Indeed, social preference requires an individual to attend to the social encounter beyond perceptual properties like gender. These preference evaluations may simply be based on emotion expression per se, or may involve more elaborative cognitive operations such as incorporating information from one's past experience, associations, or social stereotypes (e.g. squinty eyes tend to indicates untrustworthiness). Social preference lies in the eyes of the beholder – ultimately, it is up to the individual to sift through the available information, relate the information on a internal scale, and assess what is most personally relevant (de Greck et al., 2008; Enzi et al., 2009). Of note, a very recent study in impression formation also suggests that given the same person-descriptive information, the weights ascribed to each bit of information vary among individuals and can shape how first impressions are formed (Schiller et al., 2009). Further, Schiller and colleagues suggest PCC as part of a neural mechanism that codes for subjective valuation of social information. In line with the effect of expressing one's preferences for other individuals observed here in our study, a few other studies investigating the neural correlates of subjective preference for non-social objects (food or color) (Johnson et al., 2005; Paulus and Frank, 2003; Seger et al., 2004) have yielded similar results. Taken together, it is possible that the cortical midline signals in the present study may be attributed to the self-referential nature of the task (i.e. ‘Do I like the person?), in addition to the processing of socio-emotional information inherently conveyed by the facial stimuli; however, the experimental design of the SePT did not permit the separation of these processes. Nonetheless, robust signals from the cortical midline suggest it is an effective task for probing this basic aspect of social cognition.
Our results with regard to the task-induced deactivations (TID) were consistent with the current understanding of the default network. Both attention-demanding Preference and Gender tasks decreased activity in default-mode network areas -- Medial PFC (aMFC & vMFC), PCC/ precuneus/ retrosplenial cortex, inferior parietal lobule (IPL) and lateral temporal gyri. Further, our hypothesis that attending to the Preference task ‘counteracts’ TIDs in the default-mode network was also supported, as Preference had less deactivation in aMFC, vMFC and PCC than Gender.
Since its conceptualization over a decade ago (Shulman et al., 1997), the nature of TIDs in the default-mode network still needs to be clarified, although many believe they occur as a consequence of limited processing resources. In order to meet cognitive demands, resources are reallocated from the default-mode network to the task-positive network: The more demanding the task, the stronger the deactivations (e.g. Buckner et al., 2008; Mason et al., 2007; McKiernan et al., 2006; McKiernan et al., 2003). An equally probable, but not mutually exclusive, mechanism for TID is the switch from attending to processes that engage the default-mode network to attending to goal-directed processes as required by the cognitive demands. For instance, emotion processing and self-referential tasks that require activity from the cortical midline structures have been shown to have less task-induced deactivations (e.g. Gusnard et al., 2001a; Johnson et al., 2005; Pallesen et al., 2008; Simpson et al., 2001).
Our findings that the Preference task recruited cortical midline components of the default-mode network (aMFC, vMFC, PCC), as well as the task-positive network (pre-SMA/ dACC, bilateral frontoparietal cortices) to process social preference showed that a reciprocal, ‘see-saw’, relationship between networks described as ‘anti-correlated’ does not appear to be a necessary condition for network functioning. Furthermore, the data corroborated more with the notion that attending to social cognitive functions (such as Preference) demanded activities from the cortical midline structures, thereby modulating TIDs in the default-mode network. It is worth noting that while it is possible that decreased deactivation observed during Preference reflected a less demanding task and not the social cognitive nature of the Preference task per se, this possibility is not likely for the following reasons: First, reaction time, a useful behavioral index for task difficulty and attentional demand, was greater in Preference than Gender. A more cognitive-demanding task usually increases the magnitude of deactivation (McKiernan et al., 2003). Second, at the neuronal level, Preference recruited more activity in pre-SMA/ dACC and bilateral frontoparietal corticies, areas associated with action selection and performance monitoring (Ridderinkhof et al., 2004) and usually increase with greater cognitive demands (McKiernan et al., 2003).
Within the medial frontal cluster evoked by the task contrast ‘Preference > Gender’, there was a clear dissociation with regard to activation and deactivation signals. In the anterior portion of the cluster (aMFC, vMFC and PCC), the signals arose from less deactivation during Preference than during Gender; this ‘differential deactivation’ was suggestive of a modulation of the TIDs in the default-mode network by social cognition. In contrast, signals in the posterior portion of the cluster (pre-SMA/ dACC, and bilateral frontoparietal cortices) represented more activation during Preference. Across diverse cognitive demands, this set of regions has been implicated in action selection and performance monitoring (Duncan and Owen, 2000; Ridderinkhof et al., 2004). Hence, this ‘differential activation’ is reflective of a greater cognitive demand posed by the explicit appraisal of social preference. Altogether, this co-activation may reflect a functional interaction through which contextually appropriate social preference decisions are facilitated.
For the reverse contrast (Gender > Preference), brain regions that showed greater signals, including precuneus, TPJ and STG, reflected more deactivation of these regions in Preference condition. While these regions are also components of the default-mode network and have been implicated in the processing of social information (e.g. Britton et al., 2006; Frith and Frith, 1999), the predominance of deactivations in these regions is potentially interesting. We suggest it is possible that subjects attended to the perceptual properties of faces to identify gender, and hence engaged more lateral frontotemporoparietal network (Lieberman, 2007; Van Overwalle, 2008).
Task-dependent functional connectivity
We employed PPI analyses to investigate if the co-activated structures in the SePT functionally interacted, or ‘co-modulated’, with each other. As predicted, the three cortical midline ROIs identified overlapping, yet distinct, patterns of functional couplings as a function of social preference. In summary, of the three seed ROIs identified by the Preference > Gender contrast (aMFC, vMFC and PCC), all showed significant decreases in inter-correlations with one another as a function of social preference (i.e. Preference, relative to Gender). Moreover, such changes mainly reflected less positive coupling among the three regions when subjects performed the Preference task than Gender. For the task-positive network that also co-activated in the Preference > Gender contrast, on the other hand, significant increases in positive coupling during Preference occurred with aMFC and PCC.
Our current understanding of default-mode network connectivity is mainly based on seed voxel functional connectivity studies (Fox et al., 2005; Greicius et al., 2003) and independent component analysis of the resting state (Damoiseaux et al., 2006; Greicius and Menon, 2004). For example, Fox et al. analyzed bandpass filtered (0.009 < f <0.08 Hz) low frequency BOLD signal from subjects at rest, and found positive correlations within the default-mode network as well as negative correlations between the default-mode and task-positive networks (Fox et al., 2005). While intrinsic low frequency fluctuations in BOLD signals may inform us about brain organization in the absence of any task, they do not reveal connectivity dynamics of evoked BOLD responses during the performance of specific functions. Therefore, our PPI connectivity findings are complementary, and not necessarily in contradiction, to these resting state functional connectivity findings. Moreover, our data support and provide a network basis for social information processing.
Network interactions among co-activated cortical midline structures during social preference have implications for understanding the default-mode network. To date, only few studies have examined network properties of socio-emotional tasks (Das et al., 2005; Schmitz and Johnson, 2006). One particular intriguing observation from our data is how all three cortical midline default nodes (aMFC, vMFC & PCC) increase activity as a function of social preference, yet their residual variances showed decreased functional coupling. As each of the medial cortical default node has been associated with a distinct process in relation to social cognition (described above), it is tempting to speculate that this network interaction within the cortical midline default nodes may be a mechanism through which stimuli represented in vMFC are further modulated by an individual's cognitive evaluation (aMFC), as well as personal experience (PCC), in shaping social preference decisions (Uddin et al., 2007; Van Overwalle, 2008). However, a final interpretation of the PPI findings will require deeper investigation.
On the contrary, as the CMS and the task-positive network both increased activity in the Preference > Gender contrast, only aMFC and PCC showed increased functional couplings with the task-positive network as a function of social preference. These findings may be interpreted by two frameworks involving social cognitive functions laid out by Phillips (Phillips et al., 2003) and Northoff et al (Northoff et al., 2006). Reviewing findings from animal, human lesion and functional neuroimaging studies, Phillips and colleagues identified two neural systems critical for emotion processing: A ventral system that includes vMFC and subserves the identification of the emotional significance of environmental stimuli and the production of affective states; and a dorsal system (including aMFC, pre-SMA/ dACC and bilateral PFC) important for the performance of executive functions to regulate the initial appraisal and guide contextually appropriate goal-directed behavior (Phillips et al., 2003). Northoff and colleagues, via cluster and factor analyses, further suggested that PCC is also essential for temporal integration of self-referential stimuli (Northoff et al., 2006). Taken together, our findings provide connectivity-based evidence to support both frameworks: aMFC-/PCC-coupling with pre-SMA/ dACC and bilateral PFC suggest on-line support from cognitive operations to carry out evaluative social decisions, e. g. ‘Do I like this person?’ (Koechlin and Hyafil, 2007; Schmitz and Johnson, 2006) We further suggest that during social information processing (especially those with explicit requirements for evaluation or judgment, such as social preference considered herein), PCC may also be a part of the dorsal system as the autobiographical context of an individual could be critical in guiding the appropriate goal-directed behavior (Buckner et al., 2008).
The vMFC did not exhibit functional coupling with the task-positive network, possibly reflecting the fact that the initial stimulus appraisal is rapid and requires little cognitive effort, in line with the role for the vMFC suggested by others (Phillips et al., 2003; Schmitz and Johnson, 2006).
Neuroanatomically, this ‘socio-emotional neural circuitry’ is feasible as both direct and indirect projections between aMFC, vMFC and PCC have been characterized in human and monkey (Morris et al., 2000; Ongur and Price, 2000; Vogt et al., 2006). In addition, collaborative activities from structures associated with action monitoring/control and relating action to consequence are made possible by the reciprocal anatomical projections that connect aMFC and PCC with pre-SMA, dACC and bilateral PFC (Ongur and Price, 2000; Petrides and Pandya, 1999; Vogt et al., 2006). In contrast, the sparse neuroanatomical connection between vMFC and these cognitive function regions is consistent with the lack of connectivity observed during the SePT (Pandya and Yeterian, 1996; Petrides and Pandya, 2006). Other interpretations are certainly possible, and conclusions about signals not observed may reflect Type 2 errors, but the findings reported here do appear to converge with other work.
Limitations
The psycho-physiological interaction data have to be interpreted within the right framework. Importantly, since PPI takes data from the entire time series and assumes time-invariance in inter-regional correlations, the ‘positive’ and ‘negative’ coupling derived from linear regression have mathematical interpretations that should not be interpreted as ‘activation’ or ‘inhibition’ between spatially distinct brain regions. Rather, they only refer to the relative difference between the correlation slopes of the seed ROI with the coupled region for each condition. Therefore, no inference about any causal inter-regional relationships should be made for PPI (Friston et al., 2003). In addition, systematic analyses in future studies that compare correlations at different frequency ranges may provide additional insights about the relationship of PPI to other connectivity measures, including resting state spontaneous BOLD fluctuations.
Another issue concerns that fact that we did not collect post-scan ratings of the emotion dimensions such as ratings for valence or intensity. Although the same facial stimuli were used in both task conditions to exclude any potential pictorial confounds, our blocked-design paradigm was not suitable to address the question of whether the social preference signals observed may be confounded by emotion dimensions. Future studies employing an event-related design with post-scan ratings of personal association as well as emotion dimensions are necessary to better distinguish the influence of personal relevance and emotion dimensions of valence and intensity on this socio-emotional neural circuitry.
Conclusion
In this study, we employed a novel paradigm to study subjective preference evaluations toward social stimuli and identified a dynamic socio-emotional neural circuitry. Furthermore, to our knowledge, our connectivity analysis is the first study using PPI to show the functional dynamics of cortical midline structures with evoked fMRI BOLD responses. In summary, we present novel evidence to suggest that the functional interactions, both within- and between-network, depend on whether there is on-line task demand (resting state vs. non-resting state) and vary as a function of social preference. Our data also suggest that the functional interaction among the cortical midline structures may be an important mechanism in the formulation and expression of a fundamental social cognitive function – social preference. As resting state default-mode network connectivity have been documented to be altered in individuals with mental disorders characterized by abnormal social information processing, such as schizophrenia (Bluhm et al., 2007) and autism (Kennedy and Courchesne, 2008), we suggest that future research in brain networks during active social cognitive task performance may provide more understanding to the underlying mechanisms of these debilitating disorders.
Acknowledgments
The study was supported by the National Institute of Mental Health (R01 MH01258 to SFT). We thank Wendy Davis for subject recruitment and Keith Newnham for data acquisition.
Footnotes
This work has been presented in abstract form at the Society of Biological Psychiatry in Washington DC, May 2008; and the Social and Affective Neuroscience Conference in Boston, June 2008.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. Social cognition and the human brain. Trends Cogn Sci. 1999;3:469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Apperly IA, Samson D, Chiavarino C, Humphreys GW. Frontal and temporo-parietal lobe contributions to theory of mind: neuropsychological evidence from a false-belief task with reduced language and executive demands. J Cogn Neurosci. 2004;16:1773–1784. doi: 10.1162/0898929042947928. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: An fMRI study. Neuroimage. 2006;31:397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The Brain's Default Network: Anatomy, Function, and Relevance to Disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, Luxen A, Salmon E. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage. 2005;25:616–624. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Kemp AH, Liddell BJ, Brown KJ, Olivieri G, Peduto A, Gordon E, Williams LM. Pathways for fear perception: modulation of amygdala activity by thalamo-cortical systems. Neuroimage. 2005;26:141–148. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- de Greck M, Rotte M, Paus R, Moritz D, Thiemann R, Proesch U, Bruer U, Moerth S, Tempelmann C, Bogerts B, Northoff G. Is our self based on reward? Self-relatedness recruits neural activity in the reward system. Neuroimage. 2008;39:2066–2075. doi: 10.1016/j.neuroimage.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Enzi B, de Greck M, Prosch U, Tempelmann C, Northoff G. Is Our Self Nothing but Reward? Neuronal Overlap and Distinction between Reward and Personal Relevance and Its Relation to Human Personality. PLoS One. 2009;4:e8429. doi: 10.1371/journal.pone.0008429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD. Cerebral representation of one's own past: neural networks involved in autobiographical memory. J Neurosci. 1996;16:4275–4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds--a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Social cognition in humans. Curr Biol. 2007;17:R724–732. doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115:137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001a;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001b;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hassin R, Trope Y. Facing faces: studies on the cognitive aspects of physiognomy. J Pers Soc Psychol. 2000;78:837–852. doi: 10.1037//0022-3514.78.5.837. [DOI] [PubMed] [Google Scholar]
- Hynes CA, Baird AA, Grafton ST. Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia. 2006;44:374–383. doi: 10.1016/j.neuropsychologia.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Lieberman MD, Knowlton BJ, Molnar-Szakacs I, Moritz M, Throop CJ, Fiske AP. Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage. 2004;21:1167–1173. doi: 10.1016/j.neuroimage.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Kawahara-Baccus TN, Rowley HA, Alexander AL, Lee J, Davidson RJ. The cerebral response during subjective choice with and without self-reference. J Cogn Neurosci. 2005;17:1897–1906. doi: 10.1162/089892905775008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annu Rev Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, D'Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. Neuroimage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. General and specific contributions of the medial prefrontal cortex to knowledge about mental states. Neuroimage. 2005a;28:757–762. doi: 10.1016/j.neuroimage.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Neil Macrae C, Banaji MR. Forming impressions of people versus inanimate objects: social-cognitive processing in the medial prefrontal cortex. Neuroimage. 2005b;26:251–257. doi: 10.1016/j.neuroimage.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. J Cogn Neurosci. 2006;18:1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Moretti L, Dragone D, di Pellegrino G. Reward and social valuation deficits following ventromedial prefrontal damage. J Cogn Neurosci. 2009;21:128–140. doi: 10.1162/jocn.2009.21011. [DOI] [PubMed] [Google Scholar]
- Morris R, Paxinos G, Petrides M. Architectonic analysis of the human retrosplenial cortex. J Comp Neurol. 2000;421:14–28. doi: 10.1002/(sici)1096-9861(20000522)421:1<14::aid-cne2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Narumoto J, Okada T, Sadato N, Fukui K, Yonekura Y. Attention to emotion modulates fMRI activity in human right superior temporal sulcus. Brain Res Cogn Brain Res. 2001;12:225–231. doi: 10.1016/s0926-6410(01)00053-2. [DOI] [PubMed] [Google Scholar]
- Noll DC, Meyer CH, Pauly JM, Nishimura DG, Macovski A. A homogeneity correction method for magnetic resonance imaging with time-varying gradients. IEEE Trans Med Imaging. 1991;10:629–637. doi: 10.1109/42.108599. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Pallesen KJ, Brattico E, Bailey CJ, Korvenoja A, Gjedde A. Cognitive and Emotional Modulation of Brain Default Operation. J Cogn Neurosci. 2008 doi: 10.1162/jocn.2009.21086. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Comparison of prefrontal architecture and connections. Philos Trans R Soc Lond B Biol Sci. 1996;351:1423–1432. doi: 10.1098/rstb.1996.0127. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Frank LR. Ventromedial prefrontal cortex activation is critical for preference judgments. Neuroreport. 2003;14:1311–1315. doi: 10.1097/01.wnr.0000078543.07662.02. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways originating in the caudal prefrontal cortex in the macaque monkey. J Comp Neurol. 2006;498:227–251. doi: 10.1002/cne.21048. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn. 2008;17:457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA. A neural mechanism of first impressions. Nat Neurosci. 2009;12:508–514. doi: 10.1038/nn.2278. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Self-appraisal decisions evoke dissociated dorsal-ventral aMPFC networks. Neuroimage. 2006;30:1050–1058. doi: 10.1016/j.neuroimage.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Bermpohl F, Heinzel A, Rotte M, Walter M, Tempelmann C, Wiebking C, Dobrowolny H, Heinze HJ, Northoff G. The resting brain and our self: self-relatedness modulates resting state neural activity in cortical midline structures. Neuroscience. 2008;157:120–131. doi: 10.1016/j.neuroscience.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Seger CA, Stone M, Keenan JP. Cortical Activations during judgments about the self and an other person. Neuropsychologia. 2004;42:1168–1177. doi: 10.1016/j.neuropsychologia.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Jr, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: I. During cognitive task performance. Proc Natl Acad Sci U S A. 2001;98:683–687. doi: 10.1073/pnas.98.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: A meta-analysis. Hum Brain Mapp. 2008;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Adenzato M, Ciaramidaro A, Enrici I, Pia L, Bara BG. Understanding intentions in social interaction: the role of the anterior paracingulate cortex. J Cogn Neurosci. 2004;16:1854–1863. doi: 10.1162/0898929042947838. [DOI] [PubMed] [Google Scholar]
- Willis J, Todorov A. First impressions: making up your mind after a 100-ms exposure to a face. Psychol Sci. 2006;17:592–598. doi: 10.1111/j.1467-9280.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gu H, Zhan W, Xu S, Silbersweig DA, Stern E. Simultaneous perfusion and BOLD imaging using reverse spiral scanning at 3T: characterization of functional contrast and susceptibility artifacts. Magn Reson Med. 2002;48:278–289. doi: 10.1002/mrm.10196. [DOI] [PubMed] [Google Scholar]


