Abstract
Recent studies indicate that high-fat diets may attenuate cardiac hypertrophy and contractile dysfunction in chronic hypertension. However, it is unclear whether consuming a high-fat diet improves prognosis in aged individuals with advanced hypertensive heart disease or if differences in its fatty acid composition modulate its effects in this setting. In this study, aged spontaneously hypertensive heart failure rats were administered a standard high-carbohydrate diet or high-fat diets (42% of kcals) supplemented with Lard or high-linoleate safflower oil until death to examine dietary effects on disease progression and mortality. Both high-fat diets attenuated the cardiac hypertrophy, left ventricular chamber dilation and systolic dysfunction observed in rats consuming a high-carbohydrate diet. However, the lard diet significantly hastened heart failure mortality compared to the high-carbohydrate diet, whereas the linoleate diet significantly delayed mortality. Both high-fat diets elicited changes in the myocardial fatty acid profile, but had no effect on thromboxane excretion, or blood pressure. The pro-survival effect of the linoleate diet was associated with a greater myocardial content and linoleate-enrichment of cardiolipin, an essential mitochondrial phospholipid known to be deficient in the failing heart. This study demonstrates that despite having favorable effects on cardiac morphology and function in hypertension, a high-fat diet may accelerate or attenuate mortality in advanced hypertensive heart disease depending on its fatty acid composition. The precise mechanisms responsible for the divergent effects of the lard and linoleate-enriched diets merit further investigation, but may involve diet-induced changes in the content and/or composition of cardiolipin in the heart.
Keywords: diet, heart failure, hypertrophy, mortality, rats
Despite the success of neurohormonal inhibition and antihypertensive therapies used over the past decades, the prevalence of heart failure (HF) continues to increase with the aging population1,2 and 5-year mortality rates remain near ~50%.3 This has stimulated interest in identifying novel therapeutic approaches to the prevention and management of HF, including a particular focus on the roles of nutrition and dietary interventions.4,5 Specific dietary guidelines for the prevention and treatment of HF have not been established, but the American Heart Association currently recommends that total dietary fat consumption be limited to 30% of kcal intake for optimal cardiovascular health6,7. However, recent studies indicate that increasing dietary fat consumption (e.g., to 60% of kcal intake) attenuates the cardiac hypertrophy, contractile dysfunction, and mortality associated with hypertension induced by a high-salt diet in young Dahl salt-sensitive rats 8–10. Whether consumption of a high-fat diet attenuates the development of HF associated with advanced hypertensive heart disease in the senescent heart is not known. Also unclear is the extent to which differences in the fatty acid composition of a high-fat diet influence its effect on disease progression and mortality in this setting.
Linoleic acid (18:2n6; LA) is an essential polyunsaturated fatty acid (PUFA) that cannot be biosynthesized and therefore must be obtained in the diet. In addition to serving as the precursor to a variety of biologically important long-chain fatty acids and their derivatives11, LA is the primary fatty acid constituent of cardiac cardiolipin (CL), an abundant tetra-acyl mitochondrial phospholipid that is required for maintaining mitochondrial structure and function12,13. In the healthy mammalian heart, LA represents 80–90% of CL acyl chains, with ~77% of CL species containing four LA moieties (tetralinoleoyl CL; L4CL) 14,15. The critical importance of L4CL in human cardiac health has been recently underscored by the discovery that a genetic mutation leading to myocardial L4CL deficiency is the primary causative factor of severe cardiomyopathy in Barth Syndrome16. Decreases in the LA-enrichment of myocardial CL have also been reported in the more prevalent dilated and ischemic forms of HF in humans,17,18 and in animal models of pressure-overload hypertrophy.17,19 Recently, we reported that a progressive loss of L4CL correlates closely with the development of HF in aged spontaneously hypertensive HF (SHHF) rats.17,20 Collectively, these studies indicate that L4CL deficiency is common to many forms of HF, however no studies to date have examined its potential as a therapeutic target in the treatment of the disease. Dietary LA supplementation has been shown to effectively reverse decreases in L4CL induced by dietary LA restriction21, but its effects on L4CL deficiency and prognosis in hypertensive heart disease have not been previously investigated. In the present study, we hypothesized that a diet enriched in LA would improve cardiac L4CL levels and survival compared to a lard-supplemented diet and a standard high-carbohydrate diet in aged SHHF rats with advanced hypertensive heart disease.
Methods
Animal Model and Diets
Male lean SHHF rats (Mccfacp −/−) were maintained on a standard low-fat rat chow diet (Purina 5001) ad libitum until 18 months of age, when they routinely exhibit pronounced cardiac hypertrophy and marked reductions in cardiac L4CL17,20. Animals were then divided into three diet groups: a standard high-carbohydrate, low-fat (Purina 5001) chow diet (CON, n = 13), a high-fat diet consisting of 5001 chow with 20% (w/w) high-LA safflower oil (HLSO, n = 16), or a high-fat diet consisting of 20% (w/w) Lard (n = 13). The Purina 5001 chow was selected as the base diet because it is well tolerated by SHHF rats throughout their lifetime and derives only 13% of total carbohydrate kcals from simple sugars (7% of total kcal). This distinction has been noted due to recent evidence that a diet rich in simple sugars (e.g, 70% fructose) adversely influences mortality and cardiac function compared to complex carbohydrates in hypertensive rats.8 Animals were assigned to the three groups randomly, but care was taken to match the animals in each group on baseline body weight and echocardiography parameters. In addition, animals from the same litter were evenly distributed among the three groups to control for any inherited variations that might exist between litters.
Animals were maintained on the diets ad libitum until they died or were sacrificed due to overt terminal HF17,22 or severe age-related pathology22. Characterization of terminal HF was based on presentation of a combination of external clinical signs (e.g., orthopnea/dyspnea, cyanosis, piloerection and lethargy) and internal evidence of HF (e.g., ventricular dilatation and contractile dysfunction on echocardiography, atrial dilatation and thrombi, peritoneal fluid, pulmonary edema and pleural effusion) previously described in this model,17,22–25 which correlate with classic histological and biochemical markers of overt HF.23–26 An analysis of the precise nutrient and fatty acid compositions of the three diets and details regarding the determination of HF and non-HF mortality are available in the online supplement (please see http://hyper.ahajournals.org). All animals were treated according to the guidelines conforming to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and University of Colorado Animal Care and Use Committee (IACUC).
Myocardial CL, fatty acids and citrate synthase activity
The CL molecular species profile was determined in LV tissue homogenates by our previously published electrospray ionization mass spectrometry method.20 “Total CL” represents the m/z sum of the 8 most prevalent CL species detected 17. The global LV fatty acid profile of LV homogenates was determined by GC/MS methods previously described.20 Citrate synthase (CS) activity was determined in LV tissue27 to provide a comparative estimate of tissue mitochondrial density in the three experimental groups.
Blood pressure and thromboxane excretion
Tail cuff blood pressure measurements were obtained at baseline (18 months) a nd after 3 months on the diets (21 months) using the Gilson Duograph system in unheated isoflurane-anesthetized rats 28. Thromboxane A2 is a potent pro-hypertensive eicosanoid derived from endoperoxides synthesized by cyclooxgenase from arachidonic acid.11 Since arachidonic acid is primarily derived from linoleic acid consumed in the diet,11 renal thromboxane excretion (TXB2) was determined in duplicate by enzyme immunoassay (Cayman Chemical) in urine collected from rats placed in metabolic cages for 24 hr following 3 months on each of the experimental diets.
Echocardiography
Transthoracic echocardiography was performed on rats at baseline (18 months), after 3 months on the diets (21 months) and immediately prior to sacrifice (HF) under inhaled isoflurane anesthesia (5% initial, 2% maintenance) using a 12 MHz pediatric transducer connected to a Hewlett Packard Sonos 5500 Ultrasound as previously described.22
Statistical analyses
All data are presented as group means ± standard error. Cumulative survival probability was plotted on a Kaplan-Meier curve with pairwise comparisons of diets using the log-rank statistic. Group means for all data were compared using ANOVAs with Tukey tests post hoc when appropriate. Pearson correlation analyses were performed to determine the relationship between CL data and age of terminal HF. Statistical significance was established at P < 0.05 for all analyses.
Results
Animal characteristics and incidence of terminal HF
As recently reported in a survival study using aged SHHF rats22, some of the animals in this study died or developed age-related pathology (e.g., mammary and/or pituitary tumors) that necessitated euthanasia prior to manifestation of terminal HF (3 in CON and 5 in HLSO groups). These animals exhibited significantly lower heart and lung weights and lacked other classic internal features associated with terminal HF (e.g., pulmonary edema and pleural effusion, peritoneal ascites, ventricular dilatation and contractile dysfunction) and were therefore excluded from all subsequent analyses. Data from these animals excluded due to ‘non-HF mortality’ are available in the online data supplement (please see http://hyper.ahajournals.org). The mean age of animals sacrificed due to non-HF pathology was similar between the CON (22.8 ± 0.6 months) and HLSO (23.2 ± 1.2) groups. The absence of non-HF mortality in the Lard group may be explained by the relatively early onset of terminal HF in this group compared to CON and HLSO (Table 1, Figure 1), leading to death before the development of severe age-related pathology. Body and tissue weights of animals exhibiting terminal HF at the time of sacrifice are presented in Table 1. A significant loss of body weight occurred in all groups during the experimental period, with no significant differences among the three groups. Absolute and relative (normalized to brain weight) heart weight was significantly lower in animals fed the HLSO (P < 0.05) and Lard diets (P < 0.05), with no difference between the two high-fat diets. There were no significant differences among the groups on any other morphological parameters.
Table 1.
Mortality data and final animal characteristics.
| CON | Lard | HLSO | |
|---|---|---|---|
| Mortality data | |||
| Terminal HF, incidence | 10/13 (77%) | 13/13 (100%) | 11/16 (68%) |
| HF mortality, age | 23.5 ± 0.4 | 22.2 ± 1.5 | 25.0 ± 0.6*† |
| All cause mortality, age | 23.3 ± 0.3 | 22.2 ± 1.5 | 24.6 ± 0.5*† |
| Morphology | |||
| Initial BW, g | 426 ± 10 | 448 ± 6 | 457 ± 8 |
| Final BW, g | 369 ± 21 | 369 ± 6 | 395 ± 8 |
| Change in BW, % of initial | −13 ± 3 | −15 ± 1 | −13 ± 1 |
| Heart weight, g | 2.52 ± 0.15 | 2.05 ± 0.04* | 2.06 ± 0.02* |
| Heart/Brain weight, g/g | 1.24 ± 0.07 | 1.07 ± 0.02* | 1.02 ± 0.01* |
| Lung weight, g | 2.76 ± 0.21 | 2.45 ± 0.08 | 3.03 ± 0.07 |
Data are means ± standard error. Age data are in months. BW, body weight; HF, heart failure;
P < 0.05 vs. CON;
P < 0.05 vs. HLSO or Lard;
Figure 1.
Kaplan-Meier curves illustrating cumulative morality due to heart failure (A) and all causes (B) beginning at 18 months. The HLSO diet significantly improved survival resulting from heart failure (P < 0.01) and all causes (P < 0.01) compared to CON and Lard, while the Lard diet increased heart failure mortality.
Cumulative mortality
Mean age of mortality resulting from HF and all-causes was significantly greater in animals fed the HLSO diet compared to CON and Lard diets (Table 1). Figure 1 illustrates the effect of the diets on cumulative mortality resulting from HF (A) and all-causes (B). The HLSO diet significantly increased HF survival probability compared to the CON and Lard diets, while the Lard diet decreased HF survival probability (P < 0.05). Cumulative all-cause mortality rate was also significantly delayed in HLSO compared to Lard (P < 0.01) and CON (P < 0.05).
Myocardial Cardiolipin and Fatty Acid profile
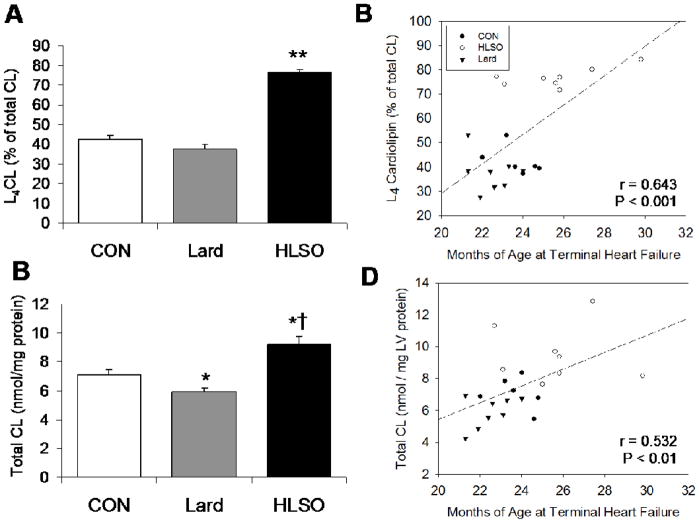
In the healthy mammalian heart, L4CL represents ~77% of total CL species15, which is similar to levels previously reported in 2 month old (non-failing) SHHF rats.17 In the present study, terminal HF (CON) was associated with a marked loss of L4CL (42% of total CL) as reported previously17,20 (Figure 2A). The HLSO diet markedly increased L4CL in the CL pool compared to CON and Lard (P < 0.001), restoring levels to 77% of total CL, while the Lard diet had slightly depressive, statistically insignificant effect (38% of total CL) (Figure 2A). HLSO also significantly increased total CL content compared to CON and Lard (P < 0.05), while the Lard diet elicited a slight decrease in CL content (P < 0.05 vs. CON) (Figure 2C). While a detailed assessment of tissue mitochondrial content was not performed, myocardial CS activity, an exclusive marker of the mitochondrial matrix and indirect index of mitochondrial density, was similar between CON (84 ± 15 nmol−1min−1mg protein−1), Lard (80 ± 10) and HLSO (78 ± 10), indicating that the observed changes in CL content were not likely due to changes in tissue mitochondrial content. Both L4CL and total CL levels correlated positively with age of mortality (Figure 2B,D), indicating that greater L4CL and total CL levels were directly associated with increased survival.
Figure 2.
Cardiolipin in left ventricular tissue from animals in terminal HF. L4CL expressed as a percent of total CL (A) and Total CL content (B); Significant positive correlations existed between age of death due to heart failure and L4CL (B) and total CL (D). Data are means ± SE. * P< 0.05 vs. CON. †P < 0.01 vs. Lard. ** P < 0.001 vs. CON.
The effects of the high-fat diets on the global myocardial fatty acid profile from rats in terminal HF are presented in Table 2. The HLSO diet resulted in significantly greater levels of LA and eicosapentaenoic acid (EPA, 20:5n3), while α-linolenic (α18:3n3), arachidonic (20:4n6) and docosahexaenoic acid (DHA, 22:6n3) levels were lower compared to CON and Lard groups. Palmitic acid (16:0) content was significantly greater in the Lard vs. CON and HLSO groups, while LA was significantly lower (P < 0.05).
Table 2.
Effect of diets on the global left ventricular fatty acid profile.
| Fatty Acid (% total) | CON | Lard | HLSO |
|---|---|---|---|
| Palmitic (16:0) | 9.8 ± 0.4 | 11.6 ± 0.2*† | 9.7 ± 0.3 |
| Stearic (18:0) | 17.6 ± 1.3 | 18.8 ± 0.7 | 19.5 ± 0.4 |
| Oleic (18:1n9) | 3.2 ± 0.3 | 3.7 ± 0.3 | 3.1 ± 0.1 |
| Linoleic (18:2n6) | 17.2 ± 0.9 | 12.5 ± 0.8* | 26.6 ± 1.2**†† |
| α-Linolenic (α18:3n3) | 0.13 ± 0.01 | 0.09 ± 0.01 | 0.06 ± 0.01*†† |
| Arachidonic (20:4n6) | 40.1 ± 1.9 | 43.4 ± 1.2 | 35.1 ± 1.3*† |
| EPA (20:5n3) | 0.37 ± 0.04 | 0.24 ± 0.01 | 0.66 ± 0.05**†† |
| DHA (22:6n3) | 11.6 ± 1.0 | 9.5 ± 1.0 | 5.3 ± 0.9*† |
DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid;
P < 0.05 vs. CON;
P < 0.05 vs. LA or Lard;
P < 0.01 vs. CON;
P < 0.01 vs. LA or Lard
Blood pressure and thromboxane excretion
There were no significant differences in systolic blood pressure between the CON (209 ± 5 mmHg), Lard (212 ± 3) or HLSO (210 ± 4) groups at baseline (prior to beginning the diets) or following 3 months on the diets (203 ± 4, 214 ± 3, and 209 ± 4, respectively). TXB2 excretion was not significantly different in the Lard (111 ± 50% of CON) or HLSO (104 ± 15% of CON) groups compared to CON (100 ± 21%) following 3 months on the diets.
Echocardiography
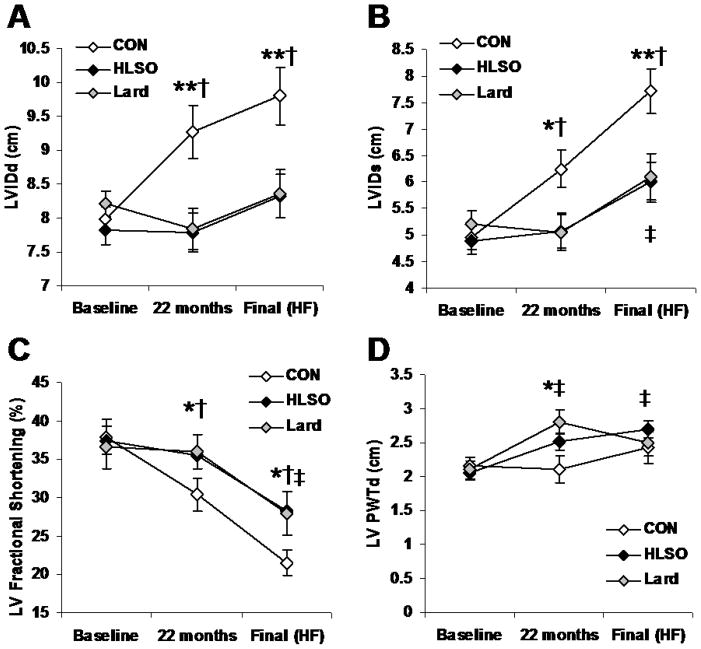
Echocardiography data obtained at baseline, following 3 months on the diets and in all surviving animals in terminal HF are presented in Figure 3. No significant differences on any measures existed between the groups at baseline. Significant increases in left ventricular internal diameter in diastole (LVIDd) and systole (LVIDs) were evident in the CON animals at 21 months and HF (P < 0.05), consistent with the development of dilated systolic HF. The Lard and HLSO diets prevented significant increases in LVIDd at both time points. No changes in LV posterior wall thickness in diastole (PWTd) were observed in CON animals throughout the course of the study, though the Lard and HLSO diets elicited sight increases relative to baseline values at both time points. Lard and HLSO diets attenuated the decline in fractional shortening (FS) seen in CON group at both time points. No significant differences were observed between Lard and HLSO on any echocardiography measures during the course of the study, indicating a similar effect of both high-fat diets on cardiac function and morphology during the pathogenesis of HF.
Figure 3.
Echocardiography data from animals at baseline (18 months of age), 22 months, and in final heart failure (HF). LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; LVPWTd, left ventricular posterior wall thickness in diastole. Data are means ± SE.* P < 0.10 for Lard and HLSO vs. CON; ** P < 0.05 for Lard and HLSO vs. CON; † P < 0.05 vs. Baseline within CON group; ‡ P<0.05 vs. Baseline within Lard and HLSO groups.
Discussion
The primary finding of this study is that two high-fat diets administered to animals with advanced hypertensive heart disease elicited opposite effects on HF mortality despite having similar effects on cardiac morphology and function. Our data corroborate previous evidence that high-fat diets attenuate hypertension-induced cardiac hypertrophy and systolic dysfunction,9,10 but demonstrate that the fatty acid composition of a high-fat is a critical determinant of its effect on HF mortality. The mechanisms by which the Lard and HLSO diets elicited such diametrically opposed opposite effects on survival are not entirely clear from this study, but our data demonstrate that they are independent of changes in systolic blood pressure and are closely associated with changes in the content and composition of myocardial cardiolipin.
Okere et al. 9,10 demonstrated that administration of high-fat diet (60% of kcal consisting primarily of stearate, 18:0) attenuates the cardiac hypertrophy, remodeling and contractile dysfunction associated with high-salt feeding in Dahl salt-sensitive rats without any reduction in systolic blood pressure. We observed nearly identical effects with the Lard and HLSO diets in aged SHHF rats in present study, indicating that high-fat diets of different fatty acid compositions appear have similar effects on cardiac morphology and function in the presence of chronic hypertension independent of any modulation of cardiac afterload. The mechanisms by which high-fat diets elicit these effects were not examined in the present study, but previous studies indicate that a suppression of insulin-mediated hypertrophic signaling and/or activation of peroxisome proliferator-activated receptor-α (PPARα) may be involved (recently reviewed by Sharma et al.4).
The primary aim of this study was to determine the effect of a LA-enriched diet on L4CL deficiency and mortality associated with terminal HF, based on evidence that cardiac L4CL deficiency is sufficient to cause lethal cardiomyopathy in humans16, and is associated with common forms of HF in humans17,18 and animal models.17,19,20 Dietary LA supplementation has been previously shown to reverse experimentally induced L4CL deficiency,21 but the present study is the first to demonstrate that a LA-enriched diet effectively preserves L4CL during the pathogenesis of hypertensive HF. The mechanism by which the HLSO and Lard diets altered L4CL levels was not directly examined in this study, but biosynthesis of L4CL is achieved by at least two CL remodeling pathways that require LA as a substrate (esterified to CoA29 or to glycerol in diacyl phospholipids such as phosphtidylcholine30). The extent to which dysfunction of CL remodeling pathways is responsible for L4CL deficiency in the failing heart is presently unknown, but the >50% increase in myocardial LA content induced by the HLSO diet suggests that L4CL levels may have been restored simply by mass action, overwhelming any deficiencies in CL remodeling capacity. However, at this point we cannot exclude the possibility that the HLSO and Lard diets directly modulated CL biosynthesis and/or degradation pathways. It has been previously demonstrated that palmitate (elevated in hearts from rats fed the Lard diet herein) decreases CL content in cardiomyocytes by interfering with biosynthesis of CL when doubly esterified to phosphatidylglyercol.31 In the present study, the Lard diet significantly decreased myocardial LA content compared to CON in the present study, despite having a greater LA content than the CON diet, suggesting that it may have altered CL synthesis, remodeling, and/or degradation processes. How LA and other dietary fatty acids directly modulate CL biosynthesis and remodeling requires further investigation.
The significant correlation of survival with myocardial L4CL and CL contents suggests that the diets may have affected mortality by modulating the content and/or composition of CL. Several proteins and processes involved in mitochondrial energy metabolism are known to require CL for optimal function,12 and impaired mitochondrial function likely contributes to the progression of HF.32–36 Furthermore, reductions in CL content can trigger apoptotic signaling in cardiomyocytes,37 which may also accelerate HF progression.38 Therefore, it is plausible that increasing myocardial levels of CL in its optimal L4 configuration may delay terminal HF by attenuating mitochondrial dysfunction and apoptosis during the advancing stages of the disease. Now that the survival benefit and CL restorative effects of the HLSO diet have been established, future investigations will focus on elucidating the cellular manifestations of this intervention during the various stages of hypertensive heart disease and HF.
While the pathologic consequences of myocardial CL deficiency have been well-established, the HLSO and Lard diets may have modulated HF mortality by other mechanisms. In addition to being associated with a pro-atherogenic serum lipid profile39,40, consumption of saturated and trans-fats present in the Lard diet may increase production of pro-inflammatory cytokines, such as tumor necrosis factor-α, in HF patients.41 Moreover, saturated fatty acids, particularly palmitate, are known to result in ceramide accumulation and apoptosis in cardiomyocytes42, however this has been recently reported following a saturated fat-rich diet independent of any adverse effects on cardiac function.43
Consumption of polyunsaturated fatty acids is generally associated with reduced cardiovascular risk44, but benefits have been ascribed primarily to the omega-3 PUFAs, principally, DHA (22:6n3) and EPA(20:5n3), due to their putative anti-arrhythmic, anti-hypertensive and anti-inflammatory effects45. In fact, some groups recommend that omega-6 PUFA intake be limited relative to n-3 PUFAs 46 given evidence that LA may limit DHA and EPA synthesis from α-linolenic acid (18:3n3, ALA)47, or promote increases in arachidonic acid (20:4n6) and its pro-inflammatory and pro-hypertensive metabolites. Interestingly, the HLSO diet in the present study improved survival despite decreasing myocardial ALA and DHA content, and had no effect on arachidonic acid content or excretion of its pro-hypertensive derivative TXA2. Elucidating the effects of chronic HLSO intake on PUFA metabolism is beyond the scope of this study, but our data clearly demonstrate that a LA-enriched diet is beneficial in the setting of advanced hypertensive heart disease despite its suppressive effect on some omega-3 PUFAs. Finally, consumption of LA may also benefit the hypertrophied heart by activating PPARα, which may be downregulated in the hypertrophied and failing heart.23,48 However, whether reactivation of PPARα in the hypertrophied heart is beneficial49,50 or harmful51 to long-term prognosis is not entirely clear.
Limitations of the study
As stated above, the primary aim of this study was to determine the effect of the selected diets on the cardiac CL profile and mortality in the SHHF rat model. While this investigation has provided novel insight into the effects of dietary fatty acid composition on long-term prognosis in hypertensive heart disease, there are limitations associated with the survival study design that warrant further comment. In particular, preparation of high-quality mitochondria for functional assessments was not feasible in this study given the inconsistent and unpredictable timing of animal death or sacrifice. However, it is also plausible that any subcellular effects elicited by the diets that could have influenced mortality might have occurred early during the course of disease progression and would no longer be evident when animals progressed to terminal HF. Therefore, the extent to which the selected diets elicit such changes during the early and late stages of disease progression requires further study and is currently under investigation in our laboratories. Finally, the decision to sacrifice animals and determination of HF vs. non-HF mortality was based on a well-documented series of clinical HF symptoms, tissue morphology and echocardiography indices that coincide with classic histological (e.g., fibrosis) and biochemical markers (myosin heavy chain isozymes and atrial natriuretic peptide) of HF previously established in this model.24–26 However, no additional analyses were performed to further characterize the precise cause of death or extent of disease progression at the time of sacrifice.
Perspectives
While consumption of a low-fat diet is currently recommended for optimal cardiovascular health,6 recent studies indicate that a high-fat diet can attenuate the progressive cardiac hypertrophy and contractile dysfunction associated with chronic hypertension without altering systolic blood pressure.4,9,10 The present study corroborates these findings in an established model of senescent hypertensive heart disease, but demonstrates that a high-fat diet may increase or decrease HF mortality depending on its fatty acid composition. The pro-survival effect of the HLSO diet may result in part from a preservation of a favorable CL profile in the heart, but further studies are needed to elucidate the physiological consequences of this effect. Moreover, it will be important to examine the many other effects that LA and/or HLSO may have on cardiovascular parameters in patients with advanced cardiac disease before considering the clinical feasibility of this intervention. It is worth noting, however, that serum and dietary intake of LA has been previously associated with reduced cardiovascular disease incidence and mortality in humans.44,52 Therefore, determining how LA and other dietary fatty acids modulate cardiac health and disease clearly merits further investigation.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported the American Heart Association Pacific Mountain Affiliate Grants 0265205Z (GCS) and 0755763Z (RLM) postdoctoral fellowship 0620009Z (AJC), and NIH grants R01 HL72790 (RLM), and U54 GM069338 (RCM).
Footnotes
Disclosures
None
References
- 1.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 2.McCullough PA, Philbin EF, Spertus JA, Kaatz S, Sandberg KR, Weaver WD. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39:60–69. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3:315–322. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 4.Sharma N, Okere IC, Duda MK, Chess DJ, O’Shea KM, Stanley WC. Potential impact of carbohydrate and fat intake on pathological left ventricular hypertrophy. Cardiovasc Res. 2007;73:257–268. doi: 10.1016/j.cardiores.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chess DJ, Stanley WC. Role of Diet and Fuel Overabundance in the Development and Progression of Heart Failure. Cardiovasc Res. 2008 doi: 10.1093/cvr/cvn074. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 7.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW, Jr, Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 8.Sharma N, Okere IC, Duda MK, Johnson J, Yuan CL, Chandler MP, Ernsberger P, Hoit BD, Stanley WC. High fructose diet increases mortality in hypertensive rats compared to a complex carbohydrate or high fat diet. Am J Hypertens. 2007;20:403–409. doi: 10.1016/j.amjhyper.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Okere IC, Chess DJ, McElfresh TA, Johnson J, Rennison J, Ernsberger P, Hoit BD, Chandler MP, Stanley WC. High-fat diet prevents cardiac hypertrophy and improves contractile function in the hypertensive dahl salt-sensitive rat. Clin Exp Pharmacol Physiol. 2005;32:825–831. doi: 10.1111/j.1440-1681.2005.04272.x. [DOI] [PubMed] [Google Scholar]
- 10.Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, Hoit BD, Ernsberger P, Chandler MP, Stanley WC. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006;48:1116–1123. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- 11.Das UN. Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J. 2006;1:420–439. doi: 10.1002/biot.200600012. [DOI] [PubMed] [Google Scholar]
- 12.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 13.Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- 14.Schlame M, Otten D. Analysis of cardiolipin molecular species by high-performance liquid chromatography of its derivative 1,3-bisphosphatidyl-2-benzoyl-sn-glycerol dimethyl ester. Anal Biochem. 1991;195:290–295. doi: 10.1016/0003-2697(91)90332-n. [DOI] [PubMed] [Google Scholar]
- 15.Schlame M, Ren M, Xu Y, Greenberg ML, Haller I. Molecular symmetry in mitochondrial cardiolipins. Chem Phys Lipids. 2005;138:38–49. doi: 10.1016/j.chemphyslip.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Schlame M, Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 2006;580:5450–5455. doi: 10.1016/j.febslet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, Maxey ML, McCune SA, Moore RL. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Heerdt PM, Schlame M, Jehle R, Barbone A, Burkhoff D, Blanck TJ. Disease-specific remodeling of cardiac mitochondria after a left ventricular assist device. Ann Thorac Surg. 2002;73:1216–1221. doi: 10.1016/s0003-4975(01)03621-9. [DOI] [PubMed] [Google Scholar]
- 19.Reibel DK, O’Rourke B, Foster KA, Hutchinson H, Uboh CE, Kent RL. Altered phospholipid metabolism in pressure-overload hypertrophied hearts. Am J Physiol. 1986;250:H1–6. doi: 10.1152/ajpheart.1986.250.1.H1. [DOI] [PubMed] [Google Scholar]
- 20.Sparagna GC, Johnson CA, McCune SA, Moore RL, Murphy RC. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J Lipid Res. 2005;46:1196–1204. doi: 10.1194/jlr.M500031-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Yamaoka S, Urade R, Kito M. Cardiolipin molecular species in rat heart mitochondria are sensitive to essential fatty acid-deficient dietary lipids. J Nutr. 1990;120:415–421. doi: 10.1093/jn/120.5.415. [DOI] [PubMed] [Google Scholar]
- 22.Chicco AJ, McCune SA, Emter CA, Sparagna GC, Rees ML, Bolden DA, Marshall KD, Murphy RC, Moore RL. Low-intensity exercise training delays heart failure and improves survival in female hypertensive heart failure rats. Hypertension. 2008;51:1096–1102. doi: 10.1161/HYPERTENSIONAHA.107.107078. [DOI] [PubMed] [Google Scholar]
- 23.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 24.Altschuld RA, Holycross BJ, Radin MJ, McCune SA. The failing SHHF rat heart. In: Singal PK, Dixon IMC, Kirshenbaum LA, Dhalla NS, editors. Cardiac Remodeling and Failure. Kluwer Academic Publishers; Boston, MA: 2003. pp. 67–74. [Google Scholar]
- 25.Emter CA, McCune SA, Sparagna GC, Radin MJ, Moore RL. Low-intensity exercise training delays onset of decompensated heart failure in spontaneously hypertensive heart failure rats. Am J Physiol Heart Circ Physiol. 2005;289:H2030–2038. doi: 10.1152/ajpheart.00526.2005. [DOI] [PubMed] [Google Scholar]
- 26.Heyen JR, Blasi ER, Nikula K, Rocha R, Daust HA, Frierdich G, Van Vleet JF, De Ciechi P, McMahon EG, Rudolph AE. Structural, functional, and molecular characterization of the SHHF model of heart failure. Am J Physiol Heart Circ Physiol. 2002;283:H1775–1784. doi: 10.1152/ajpheart.00305.2002. [DOI] [PubMed] [Google Scholar]
- 27.Srere P. Citrate synthase. Methods Enzymol. 1969;13:3–5. [Google Scholar]
- 28.Holycross BJ, Summers BM, Dunn RB, McCune SA. Plasma renin activity in heart failure-prone SHHF/Mcc-facp rats. Am J Physiol. 1997;273:H228–233. doi: 10.1152/ajpheart.1997.273.1.H228. [DOI] [PubMed] [Google Scholar]
- 29.Ma BJ, Taylor WA, Dolinsky VW, Hatch GM. Acylation of monolysocardiolipin in rat heart. J Lipid Res. 1999;40:1837–1845. [PubMed] [Google Scholar]
- 30.Xu Y, Malhotra A, Ren M, Schlame M. The enzymatic function of tafazzin. J Biol Chem. 2006;281:39217–39224. doi: 10.1074/jbc.M606100200. [DOI] [PubMed] [Google Scholar]
- 31.Ostrander DB, Sparagna GC, Amoscato AA, McMillin JB, Dowhan W. Decreased cardiolipin synthesis corresponds with cytochrome c release in palmitate-induced cardiomyocyte apoptosis. J Biol Chem. 2001;276:38061–38067. doi: 10.1074/jbc.M107067200. [DOI] [PubMed] [Google Scholar]
- 32.Quigley AF, Kapsa RM, Esmore D, Hale G, Byrne E. Mitochondrial respiratory chain activity in idiopathic dilated cardiomyopathy. J Card Fail. 2000;6:47–55. doi: 10.1016/s1071-9164(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 33.Buchwald A, Till H, Unterberg C, Oberschmidt R, Figulla HR, Wiegand V. Alterations of the mitochondrial respiratory chain in human dilated cardiomyopathy. Eur Heart J. 1990;11:509–516. doi: 10.1093/oxfordjournals.eurheartj.a059743. [DOI] [PubMed] [Google Scholar]
- 34.Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharov VG, Todor AV, Silverman N, Goldstein S, Sabbah HN. Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol. 2000;32:2361–2367. doi: 10.1006/jmcc.2000.1266. [DOI] [PubMed] [Google Scholar]
- 36.Hoppel CL, Tandler B, Parland W, Turkaly JS, Albers LD. Hamster cardiomyopathy. A defect in oxidative phosphorylation in the cardiac interfibrillar mitochondria. J Biol Chem. 1982;257:1540–1548. [PubMed] [Google Scholar]
- 37.McMillin JB, Dowhan W. Cardiolipin and apoptosis. Biochim Biophys Acta. 2002;1585:97–107. doi: 10.1016/s1388-1981(02)00329-3. [DOI] [PubMed] [Google Scholar]
- 38.Garg S, Narula J, Chandrashekhar Y. Apoptosis and heart failure: clinical relevance and therapeutic target. J Mol Cell Cardiol. 2005;38:73–79. doi: 10.1016/j.yjmcc.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 40.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 41.Lennie TA, Chung ML, Habash DL, Moser DK. Dietary fat intake and proinflammatory cytokine levels in patients with heart failure. J Card Fail. 2005;11:613–618. doi: 10.1016/j.cardfail.2005.06.434. [DOI] [PubMed] [Google Scholar]
- 42.Sparagna GC, Hickson-Bick DL, Buja LM, McMillin JB. Fatty acid-induced apoptosis in neonatal cardiomyocytes: redox signaling. Antioxid Redox Signal. 2001;3:71–79. doi: 10.1089/152308601750100524. [DOI] [PubMed] [Google Scholar]
- 43.Okere IC, Chandler MP, McElfresh TA, Rennison JH, Sharov V, Sabbah HN, Tserng KY, Hoit BD, Ernsberger P, Young ME, Stanley WC. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol. 2006;291:H38–44. doi: 10.1152/ajpheart.01295.2005. [DOI] [PubMed] [Google Scholar]
- 44.Erkkila A, de Mello VD, Riserus U, Laaksonen DE. Dietary fatty acids and cardiovascular disease: An epidemiological approach. Prog Lipid Res. 2008;47:172–187. doi: 10.1016/j.plipres.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Das UN. Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: but, why and how? Prostaglandins Leukot Essent Fatty Acids. 2000;63:351–362. doi: 10.1054/plef.2000.0226. [DOI] [PubMed] [Google Scholar]
- 46.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, Hargrove RL, Zhao G, Etherton TD. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179S–188S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 47.Emken EA, Adlof RO, Rohwedder WK, Gulley RM. Influence of linoleic acid on desaturation and uptake of deuterium-labeled palmitic and stearic acids in humans. Biochim Biophys Acta. 1993;1170:173–181. doi: 10.1016/0005-2760(93)90068-k. [DOI] [PubMed] [Google Scholar]
- 48.Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kelly DP. Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J Clin Invest. 2000;105:1723–1730. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diep QN, Benkirane K, Amiri F, Cohn JS, Endemann D, Schiffrin EL. PPAR alpha activator fenofibrate inhibits myocardial inflammation and fibrosis in angiotensin II-infused rats. J Mol Cell Cardiol. 2004;36:295–304. doi: 10.1016/j.yjmcc.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Goikoetxea MJ, Beaumont J, Diez J. Peroxisome proliferator-activated receptor alpha and hypertensive heart disease. Drugs. 2004;64 (Suppl 2):9–18. doi: 10.2165/00003495-200464002-00003. [DOI] [PubMed] [Google Scholar]
- 51.Young ME, Laws FA, Goodwin GW, Taegtmeyer H. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. J Biol Chem. 2001;276:44390–44395. doi: 10.1074/jbc.M103826200. [DOI] [PubMed] [Google Scholar]
- 52.Laaksonen DE, Nyyssonen K, Niskanen L, Rissanen TH, Salonen JT. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch Intern Med. 2005;165:193–199. doi: 10.1001/archinte.165.2.193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.