Abstract
Background
Repeated exposures to forced ethanol diets (EDs) or restraint stress sensitize anxiety-like behavior during a future ethanol withdrawal. The present investigation assessed whether pretreatment of rats with agents targeting receptor systems thought to be important in treating relapse in alcoholic patients would prevent sensitization of anxiety-like behavior.
Methods
Groups of rats were exposed to either (1) three 5-day cycles of ED with 2 days of withdrawal between cycles, (2) continuous ED, or (3) 5 days of ED in a single cycle preceded by 2 episodes of restraint stress 6 days apart. Drugs [baclofen, acamprosate, naloxone, lamotrigine, ifenprodil, dizocilpine (MK-801), CGS19755, diazepam, flumazenil, or 6-methyl-2-(phenylethynyl)pyridine] were given prophylactically during the first and second withdrawal periods only or, in separate baclofen experiments, acutely during the third withdrawal or during withdrawal from continuous ED. Baclofen administration preceded each stress session in the stress-withdrawal protocols. Anxiety-like behavior was assessed in the social interaction (SI) test 5 hours after the ethanol was removed or after 3 days of abstinence.
Results
Baclofen (1.25, 2.5, and 5 mg/kg), flumazenil (5 mg/kg), and diazepam (1 mg/kg) blocked the reduction in SI induced by ethanol withdrawal. Among the drugs that alter glutamate function, only acamprosate (300 mg/kg) was effective. In the stress protocols, baclofen (5 mg/kg) given before each of the 2 restraint stress sessions before ethanol exposure or before stress during abstinence also attenuated SI deficits.
Conclusions
These findings suggest that GABAB and GABAA, but not glutamate or opioid mechanisms, are involved in adaptive changes associated with anxiety-like behavior induced by these repeated ethanol-withdrawal and stress-withdrawal paradigms. The lack of action of agents attenuating different aspects of glutamate function suggests that acamprosate’s action is related to some other, as yet undetermined, mechanism.
Keywords: Repeated Ethanol Withdrawal, Anxiety-Like Behavior, Social Interaction, Acamprosate, Baclofen
RATS REPEATEDLY EXPOSED to 5-day cycles of ethanol in a liquid diet exhibit anxiety-like behavior following withdrawal from the third of 3 cycles (Overstreet et al., 2002). Furthermore, restraint stress substitutes for the first 2 cycles to sensitize anxiety during withdrawal from a future single cycle and elicits anxiety during abstinence (Breese et al., 2004, 2005a, 2005b). These protocols extend work with sensitized or “kindled” seizures in rats (e.g., McCown and Breese, 1990) and model sensitization of symptoms in human alcoholic individuals (Ballenger and Post, 1978; Malcolm et al., 2000; Sinha, 2001). This anxiety-like behavior (“anxiety”) in rats is manifest as a reduction in time spent in social interaction (SI), a deficit that can be prevented by pretreatment of the first 2 withdrawals by such drugs as the benzodiazepine (BZD) receptor antagonist flumazenil, the 5-HT1A receptor agonist buspirone, the 5-HT2C receptor antagonist SB242084, and the Type 1 corticotropin-releasing factor (CRF) antagonist CRA1000 (Knapp et al., 2005; Overstreet et al., 2003, 2004). These results suggest that such drugs may be useful for modifying emotional factors that may contribute to the development and/or maintenance of alcoholism.
In the present investigation, several agents from drug classes with demonstrated clinical utility in human alcoholism or potential anxiety-modulating effects in animal models were tested for effects in the repeated ethanol sensitization protocol. Acamprosate, baclofen, and naloxone were selected in part because they or closely related compounds have been used clinically in alcoholic patients. Acamprosate has been widely used in Europe (and more recently the United States) to reduce relapse in alcoholic subjects, and some studies show it to be modestly effective (e.g., Garbutt et al., 1999; Pelc et al., 1997; Verheul et al., 2005; Wilde and Wagstaff, 1997). Baclofen, a GABAB receptor agonist, has shown promise in early, limited clinical trials (Addolorato et al., 2002; Flannery et al., 2004) and appears to have anxiolytic properties (e.g., Drake et al., 2003; Flannery et al., 2004). Initial studies of withdrawal from relatively intense and/or extended ethanol treatment regimens suggest acute actions of baclofen against withdrawal anxiety and accompanying physical symptoms as well (Colombo et al., 2000; File et al., 1991, 1992). Naloxone, an opiate antagonist, suppresses ethanol intake in rats (e.g., Overstreet et al., 1999). The related compound naltrexone has been widely used in the United States and is also modestly successful in reducing relapse/drinking in many studies (reviewed in Anton and Swift, 2003) but not others (Krystal et al., 2001).
To explore possible mechanisms of an effect of acamprosate in this paradigm, several other compounds with actions against glutamate and/or N-methyl-d-aspartate (NMDA) receptors were investigated in the repeated ethanol sensitization protocol. While the mechanisms mediating acamprosate’s action in animal models and patients are not yet clear, it has been suggested that acamprosate may inhibit glutamatergic function (Harris et al., 2003; Littleton and Zieglgansberger, 2003; Mayer et al., 2002). Ifenprodil, lamotrigine, dizocilpine, CGS19755, and 6-methyl-2-(phenylethynyl)pyridine (MPEP) were selected for the current studies because each antagonizes glutamatergic activity in a unique manner. Ifenprodil has a novel indirect inhibitor mechanism at the NMDA receptor (Snell et al., 2000). Lamotrigine is a novel antiseizure drug that regulates the release of glutamate (Messenheimer, 1995), while dizocilpine and CGS19755 are noncompetitive and competitive blockers, respectively, of the NMDA receptor. 6-Methyl-2-(phenylethynyl)pyridine is an antagonist at the mGluR5 metabotropic glutamate receptor (Gasparini et al., 1999) and reportedly reduces ethanol drinking (Olive et al., 2005), cocaine reward (McGeehan and Olive, 2003) and anxiety in some models (Busse et al., 2004). If glutamatergic mechanisms participate in the adaptive changes associated with withdrawal from chronic ethanol and in the efficacy of acamprosate observed in the current experimental series and in clinical populations, then one would predict that 1 or more of these agents would counteract the reduced SI in ethanol-withdrawn rats. Diazepam and flumazenil were included in some experiments as positive controls based on previously reported results (Knapp et al., 2004, 2005).
METHODS
Animals
Sprague–Dawley rats (Charles-River, Raleigh, NC) were obtained at about 7 weeks of age (160–180 g) and housed in groups of 3 or 4 for several days with rat chow (LabDiet, Prolab 3000; Granville Milling Co., Creedmoor, NC) to adapt to the local conditions (a light:dark cycle of 12 hours:12 hours, with lights on between 09:00 and 21:00 hours). Thereafter, rats were housed individually with food and fluids presented as described below. All animal use was approved by the Institutional Animal Care and Use Committee at the University of North Carolina as per the Guide for the Care and Use of Laboratory Animals (National Research Council, Institute of Laboratory Animal Resources, Commission on Life Sciences, 1996).
Ethanol and Control Diets (CDs)
Rats were placed on nutritionally complete liquid diets similar to those used previously (e.g., Knapp et al., 2004; Moy et al., 1997, 2000; Overstreet et al., 2002, 2003). Briefly, the diet was lactalbumin/dextrose based and nutritionally complete (with concentrations of vitamins, minerals, and other nutrients derived from ICN Research Diets). Dextrose calories in the CD were equated with ethanol calories in the ethanol diet (ED). After 3 days on the CD, 4 subgroups (n = 8) of these rats were placed on a diet containing ethanol (4.5 or 7%), w/v either continuously for 23 days or for 1 or three 5-day cycles of ED. Cycles were separated by 2 days of CD.
A modified pair-feeding design was used, whereby rats maintained on CD were given a volume equivalent to the average volume consumed the previous day by the rats maintained on ED. The rats were weighed at weekly intervals, and volumes of diet were adjusted to ensure that the groups readily gained weight and had similar body weights. In general, behavioral assessments were conducted after 15 combined days of exposure to the ED between 5 and 6 hours after the removal of the ethanol. This time point was selected on the basis of previous observations of substantial anxiety-like behavior and zero blood ethanol levels in rats at this time (e.g., Breese et al., 2004; Knapp et al., 1998, Moy et al., 2000; Overstreet et al., 2002).
Restraint Stress
Groups of rats were exposed to two 60-minute restraint stress sessions (6 days apart) in plastic conical decapicones before ethanol exposure (Breese et al., 2004). Thus, these 2 stress sessions substituted for the first 2 cycles of ED exposure and withdrawal. Another group received 45 minutes of restraint stress 3 days into abstinence 30 minutes before SI testing (Breese et al., 2005b).
SI Test
The SI test, a validated index of anxiety-like behavior (File and Seth, 2003), involves placing a pair of animals in an arena and measuring the amount of time engaged in such behaviors as grooming, sniffing, and boxing. Locomotor activity is simultaneously recorded and provides a measure that is independent of SI (File and Seth, 2003; Overstreet et al., 2003, 2004). A modification of the standard SI test was used to reduce the number of animals needed for experiments. According to File and Seth (2003), the most sensitive procedure is to match up pairs of rats that have the same treatment on the basis of their body weights and then treat the total number of interactions by the pair as the unit of measure. In the present studies, pairs of rats with the same treatment were placed in the arena and the SI initiated by each member of the pair was recorded, thereby requiring fewer rats. Statistical analyses of several data sets revealed that this approach provided the same statistical outcome as treating the scores of the pair as a unit (Overstreet et al., 2003, 2004).
Experienced observers who were blind to the experimental condition carried out the SI test in a square open field (60 cm × 59 cm, with 16 squares marked out on the floor). The rats were unfamiliar with the open field, and the lighting conditions were low to generate an intermediate level of anxiety-related behavior. Rat pairs were matched on the basis of body weights and treatment conditions and placed simultaneously in the open field between hours 5 and 6 of ethanol withdrawal. During the 5-minute session, line crossings (by 2 forepaws) and time spent in SI (grooming, sniffing, following) were scored individually for each rat.
Drugs
Drugs were prepared in saline for soluble compounds or 0.5% carboxymethylcellulose (Sigma, St. Louis, MO) for suspensions (2 mL/kg). Drugs tested included acamprosate (Lipha Pharmaceuticals Inc., Lyon, France; 300 mg/kg), (+/−) baclofen (Sigma; 1.25, 2.5, or 5 mg/kg), naloxone HCl (RBI, Natick, MA; 5 or 20 mg/kg), ifenprodil tartrate (Sigma; 10 mg/kg), dizocilpine [(1)-MK-801; 0.3 mg/kg], CGS19755 (cis-4-phosphonomethyl-2-piperidine carboxylic acid; Ciba Geigy, Summit, NJ; 10 mg/kg), lamotrigine (GlaxoWellcome, Harlow, U.K.; 30 mg/kg), and MPEP (Sigma; 10 mg/kg). Diazepam (Research Biochemicals Inc., Natick, MA; 1 mg/kg) and flumazenil (Hoffman La Roche, Nutley, NJ; 5 mg/kg) were included in some experiments as positive controls based on previously reported results (Knapp et al., 2004, 2005). It is particularly important to note that, except for acute drug administration in Experiments 1, 6, and the abstinence portion of Experiment 10 below, drug injections were performed during the first and second withdrawals of the 3-withdrawal protocol, with animals thus being tested in a drug-free state. Similarly, drug injections in the stress studies were performed either prophylactically before each of the stress sessions preceding exposure to ED (Experiments 8 and 9) or acutely before the stress exposure on the SI testing day during abstinence (Experiment 10).
Experiment 1. Acute Baclofen Action Against Ethanol Withdrawal-Induced Anxiety
Baclofen was first examined in a dose range expected to be effective as acute treatment in counteracting the reduced SI during withdrawal from a single, long (23-day) exposure to 7% ED. It was expected that, should these doses of baclofen be effective acutely, they might lie within the effective range for subsequent prophylactic studies (Experiment 2). Diazepam and flumazenil were included as positive controls (Knapp et al., 2005). On the final day of ED exposure, approximately 4.5 hours after the ED was removed, the rats were injected with vehicle, 1 mg/kg diazepam, 5 mg/kg flumazenil, or 1.25 or 2.5 mg/kg baclofen and examined in the SI test. Rat pairs with similar body weights and the same treatment were placed in the SI arena 30 minutes (10 minutes for flumazenil) after the injections.
Experiments 2–4. Prophylactic Actions of Glutamate Inhibition on Multiple Withdrawal-Sensitized Anxiety
In these experiments, rats were exposed to CD throughout or to 15 days of 7% ED, in 3 cycles of 5 days, with a 2-day break between cycles 1 and 2 and cycles 2 and 3 (15 days of ED in total). This design also served to limit the magnitude of obvious physical withdrawal signs. Rats were injected i.p. 4 hours into the first and second withdrawals with saline vehicle, 300 mg/kg acamprosate, or 10 mg/kg ifenprodil (Experiment 2). Additional groups were exposed to three 5-day cycles of 7% ED and injected with vehicle, 30 mg/kg lamotrigine, 0.3 mg/kg dizocilpine, 10 mg/kg CGS19755 (Experiment 3), or MPEP (10 mg/kg, Experiment 4), at 4 hours into the first and second withdrawals.
Experiment 5. Prophylactic Naloxone and Flumazenil Actions Against Cycled 4.5% ED-Induced Anxiety
In this experiment, the focus was on the effects of naloxone against multiple withdrawal-sensitized anxiety. In addition, the intensity of the ethanol treatment was reduced by lowering the concentration of the ethanol in the diet from 7 to 4.5% to limit the locomotor effects of the cycling procedure (Overstreet et al., 2002). Flumazenil was included as a positive control. There were 4 treatment groups: 1 group on CD and 3 groups on 4.5% ED that received saline vehicle, 20 mg/kg naloxone (Overstreet et al., 1999), or 5 mg/kg flumazenil at 4 hours into the first and second withdrawals.
Experiment 6. Acute and Prophylactic Baclofen Actions Against Cycled 4.5% ED-Induced Anxiety
As cycled or continuous 7% EDs can have actions on SI as well as locomotor activity (e.g., Experiment 1), a follow-on experiment was conducted with acute or prophylactic baclofen treatments with ethanol treatments that elicited SI deficits during withdrawal in the absence of concomitant deficits in locomotor behavior. Rats were exposed to CD or to 15 days of 4.5% ED in three 5-day cycles because this protocol does not typically produce large changes in loco-motor activity (Overstreet et al., 2002). Thus, rats were exposed to the 4.5% ED and subjected to 1 of 3 treatments: prophylactic treatment with saline vehicle or 2.5 mg/kg baclofen during the first and second withdrawals, or acute treatment with 1.25 mg/kg baclofen during the third withdrawal, 30 minutes before being placed in the SI arena.
Experiment 7. Baclofen and Naloxone Actions Against Cycled 4.5% ED-Induced Anxiety: Additional Doses
Rats had 3 cycles of 4.5% alcohol with injections after the first and second cycles. The treatments were CD and 4 ED groups given injections of either vehicle, naloxone (5 mg/kg), or baclofen (1.25 or 5 mg/kg) 4 hours into the first and second withdrawals.
Experiment 8. Drug Actions Against Stress-Sensitized Ethanol Withdrawal Anxiety
While on CD, rats were exposed to restraint stress twice (6 days apart; 60 minutes each session) and then given 4.5% ED for 5 days before behavioral testing 5 hours into withdrawal. Ten (flumazenil) or 30 minutes (naloxone or baclofen) before each stress, rats were treated with vehicle, flumazenil (5 mg/kg), naloxone (20 mg/kg), or baclofen (2.5 mg/kg), respectively.
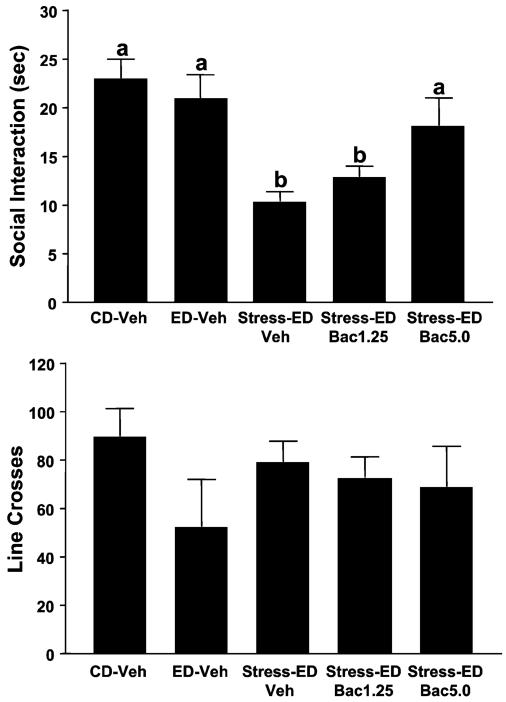
Experiment 9. Baclofen Actions Against Stress-Sensitized Ethanol Withdrawal Anxiety: Additional Doses
While on CD, rats were exposed to restraint stress and chronic ethanol as in Experiment 8 with treatments of vehicle or additional doses of baclofen (1.25 and 5.0 mg/kg) 30 minutes before stress. A fourth ED group was not subjected to stress.
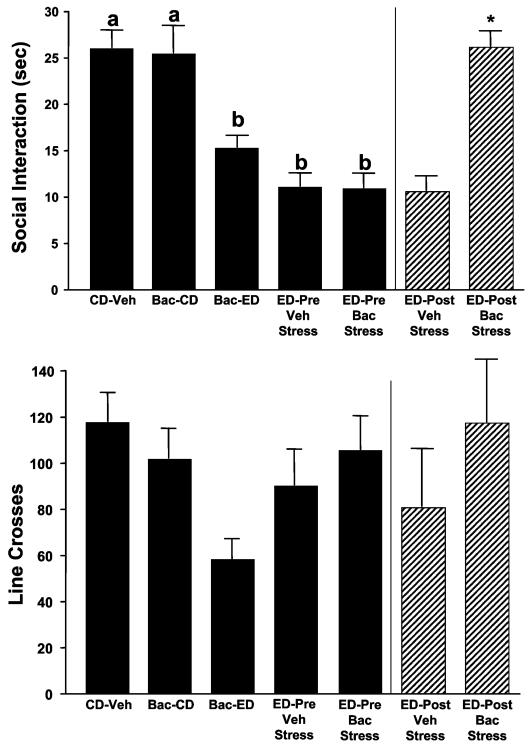
Experiment 10. Actions of Baclofen in Controls and in Abstinent-Stressed Rats
This experiment had 2 goals. First, the effects of 2 baclofen (5 mg/kg) injections (5 days apart) were assessed in CD-treated animals and in CD-treated animals that received 1 subsequent 5-day cycle of 4.5% ED withdrawal. Second, the prophylactic actions of baclofen were assessed in animals that were multiply withdrawn and stressed during abstinence. It was previously demonstrated that restraint stress for 45 minutes has an anxiogenic effect only in rats previously exposed to repeated cycles of ED (Breese et al., 2005b). Two groups of animals (n = 8 each) were given 3 repeated cycles of 5 days of 4.5% ED, while another group remained on CD throughout the study. Five hours after ethanol was removed, the rats were tested for SI, a time when blood ethanol levels have returned to zero in this model (Breese et al., 2004) and SI deficits are readily measured. Three days later while still consuming CD, these 2 repeatedly ethanol-cycled groups of rats were tested in the SI test again with different partners, but they were first subjected to 45 minutes of restraint stress 30 minutes before the test and were pretreated with vehicle or baclofen (5 mg/kg) 15 minutes before stress exposure.
Data Analysis
Rats were generally tested in batches of 40, with 5 treatment groups of 8 rats each. When more than 5 treatment groups were needed, data from 2 batches of rats were combined only if the control groups were not significantly different. Statistical analyses of SI, locomotor activity, alcohol intake, and body weights were carried out using the GBStat software package. The data were initially analyzed by 1-way ANOVAs. If the F values were statistically significant, post hoc analyses were performed using Tukey’s protected t-tests. A t-test was used to assess the effects of stress on multiply withdrawn rats in Experiment 10 and the ethanol intake in Experiment 4. Significance was set at p<0.05.
RESULTS
Experiment 1. Acute Baclofen Action Against Ethanol Withdrawal-Induced Anxiety
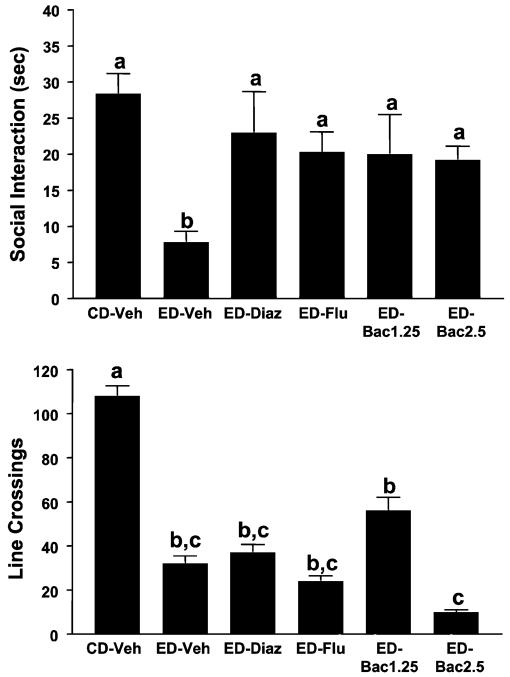
The acute effects of baclofen, diazepam, and flumazenil on withdrawal-induced anxiety are illustrated in Fig. 1. As expected, there were significant differences for time spent in SI [F(5, 34) = 4.51, p<0.01], with the ethanol-withdrawn rats spending less time in SI than the rats exposed to CD. There were also obvious signs of acute withdrawal syndrome including body/tail tremor. Nonetheless, acute treatment with either diazepam or flumazenil counteracted the deficit in SI, as expected, and so did both doses of baclofen. Ethanol-withdrawn rats also exhibited substantial deficits in line crossings [F(5, 34) = 11.71, p<0.0001], but none of the treatments counteracted this deficit in locomotor activity (Fig. 1). Indeed, there was a tendency for the higher dose of baclofen (2.5 mg/kg) to reduce activity even further. Thus, under the conditions used in this experiment, the drugs had selective anxiolytic-like effects.
Fig. 1.
Acute actions of baclofen, flumazenil, and diazepam on social interaction (SI) during withdrawal from prolonged 7% ethanol diet (ED). Rats were exposed to control diet (CD) throughout or 23 days of 7% ED before withdrawal. Rats exposed to ED were injected with 1 mg/kg diazepam (ED-Diaz), 5 mg/kg flumazenil (ED-Flu), or 1.25 or 2.5 mg/kg baclofen (ED-Bac1.25 or ED-Bac5.0) 30 min (10 for Flu) before the SI test. SI behavior and line crossings were recorded 5 h after withdrawal. Bars represent the means ± SEM. Groups with different letters are significantly different from each other (Tukey’s protected t-tests).
Experiments 2–4. Prophylactic Actions of Glutamate Inhibition on Multiple Withdrawal-Sensitized Anxiety
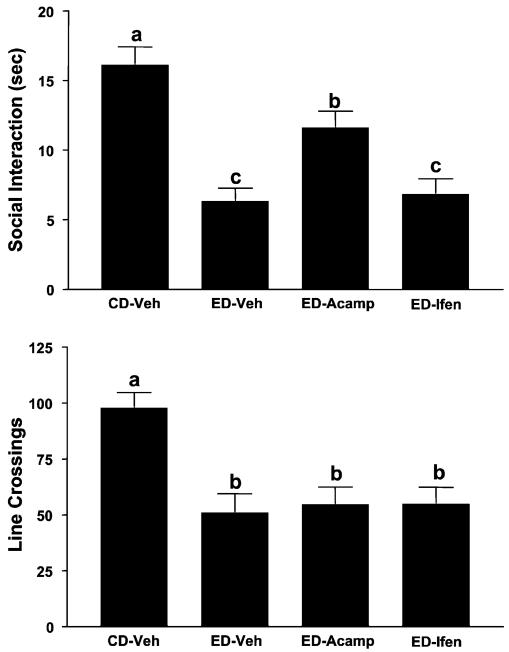
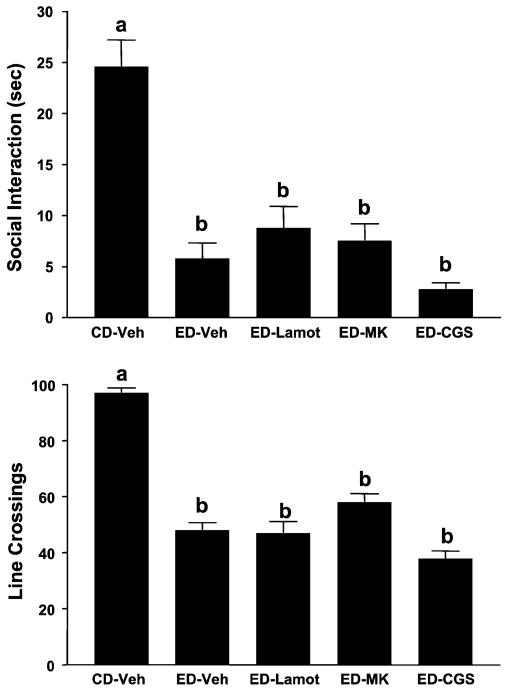
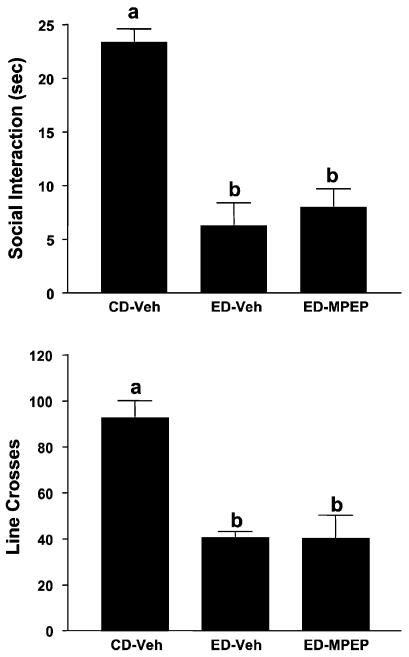
The effects of prophylactic treatment with acamprosate or ifenprodil on SI and locomotor activity are illustrated in Fig. 2. The differences in SI are quite substantial [F(3, 35) = 15.77, p<0.0001]; however, only acamprosate pretreatment (during first and second withdrawals) significantly counteracted the deficit in SI induced by repeated ethanol withdrawal. Repeated withdrawal also reduced line crossings [F(3, 35) = 6.78, p<0.001], but none of the treatments blocked this deficit. The effects of prophylactic treatment with lamotrigine, dizocilpine, CGS19755, or MPEP are illustrated in Figs. 3 and 4 for time spent in SI and locomotor activity. There were substantial differences in both time spent in SI [Fig. 3, F(4, 37) = 20.95, p<0.0001; Fig. 4, F(2, 21) = 20.00, p<0.0001] and line crossings [Fig. 3, F(4, 37) = 5.90, p<0.001; Fig. 4, F(2, 21) = 17.54, p<0.0001], but neither lamotrigine, dizocilpine, CGS19755, nor MPEP counteracted these effects (Figs. 3 and 4).
Fig. 2.
Prophylactic actions of acamprosate but not ifenprodil on the reduced social interaction (SI) time induced by repeated chronic exposures to 7% ethanol diet (ED). Rats were exposed to control diet (CD) throughout or 3 cycles of 5 days’ exposure to 7% ED. Rats exposed to ED were treated with vehicle (ED-Veh), 300 mg/kg acamprosate (ED-Acamp), or 10 mg/kg ifenprodil (ED-Ifen) 4 h into the first and second withdrawals. Social interaction behavior and line crossings were recorded 5 h after the last withdrawal. Data represent the means ± SEM. Groups with different letters are significantly different from each other (Tukey’s protected t-tests).
Fig. 3.
Failure of lamotrigine, dizocilpine (MK-801), or CGS19755 to prevent reduced social interaction (SI) time induced by repeated chronic exposures to 7% ethanol diet (ED). Rats were exposed to control diet (CD) throughout or 3 cycles of 5 days’ exposure to 7% ED. Rats exposed to ED were injected with vehicle (ED-V), 30 mg/kg lamotrigine (ED-Lamot), 0.3 mg/kg dizocilpine (ED-MK), or 10 mg/kg CGS19755 (ED-CGS) at 4 h into the first and second withdrawals. Social interaction behavior and line crossings were recorded 5 h into withdrawal after the third ethanol exposure was stopped. Bars represent the means ± SEM. Groups with different letters are significantly different from each other (Tukey’s protected t-tests).
Fig. 4.
Inactivity of 6-methyl-2-(phenylethynyl)pyridine (MPEP) against reduced social interaction (SI) time in rats repeatedly withdrawn from chronic 4.5% ethanol diet (ED). Rats were exposed to control diet (CD) throughout or 3 cycles of 5 days’ exposure to 4.5% ED. The rats exposed to ED were injected with vehicle (ED-Veh) or 10 mg/kg MPEP 4 h into the first and second withdrawal. Social interaction behavior and line crossings were recorded 5 h after the last ethanol exposure was stopped. Bars represent the means ± SEM. Groups with different letters are significantly different from each other (Tukey’s protected t-tests).
Experiment 5. Prophylactic Actions of Naloxone and Flumazenil Against Cycled 4.5% ED-Induced Anxiety
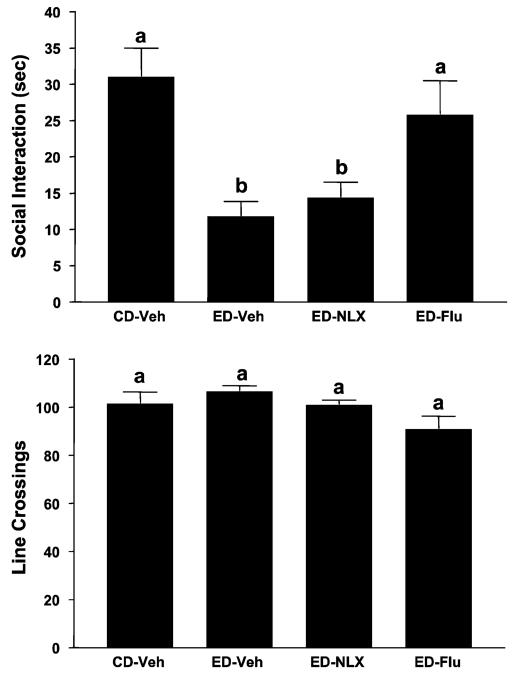
Figure 5 shows the effects of prophylactic treatment with naloxone or flumazenil on time spent in SI and locomotor activity. Once again, there were substantial differences in time spent in SI [F(3, 28) = 7.16, p<0.001], with the rats repeatedly withdrawn from ethanol (ED-Veh) spending significantly less time in SI than the rats exposed to CD. Pretreatment with naloxone during the first and second withdrawals did not counteract the deficit in SI whereas flumazenil, the positive control, did. In contrast to the large differences in time spent in SI, there were no differences in line crossings [F(3, 28) = 0.36, NS], probably because the rats were exposed to 4.5% ED (Overstreet et al., 2002).
Fig. 5.
Prophylactic effects of flumazenil but not naloxone on reduced social interaction (SI) behavior in rats repeatedly withdrawn from 4.5% ethanol diet (ED). Rats were exposed to control diet (CD) throughout or 3 cycles of 5 days’ exposure to 4.5% ED. Rats exposed to ED were injected with vehicle (ED-Veh), 20 mg/kg naloxone (ED-NLX), or 5 mg/kg flumazenil (ED-Flu) 4 h into the first and second withdrawals. Social interaction behavior and line crossings were recorded 5 h after the third ethanol exposure was stopped. Bars represent the means ± SEM. Groups with different letters are significantly different from each other (Tukey’s protected t-tests).
Experiment 6. Acute and Prophylactic Baclofen Actions Against Cycled 4.5% ED-Induced Anxiety
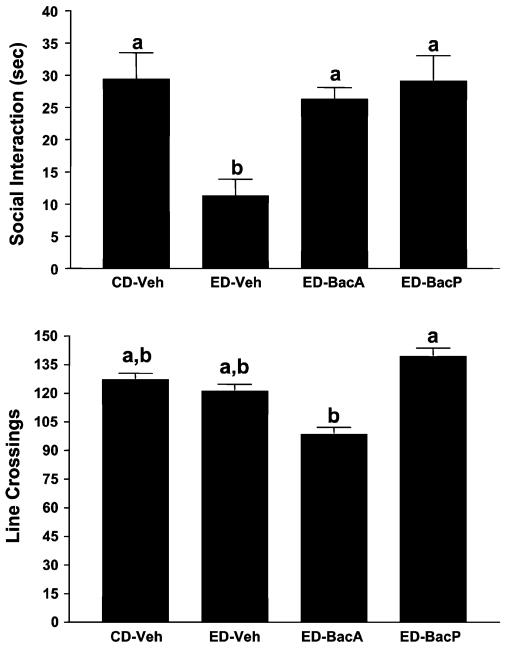
Initially, a dose of 2.5 mg/kg baclofen was given 4 hours into the first and second withdrawals from 4.5% ED. Because this dose had possible locomotor effects when given acutely, the 1.25 dose was selected for the acute-dose comparison. The remaining treatments were a control group exposed to CD and an experimental group exposed to 4.5% ED and given vehicle at 4 hours into the first and second withdrawals. As illustrated in Fig. 6, there were substantial differences for time spent in SI [F(3, 27) = 7.44, p<0.001], with the ethanol-withdrawn rats exhibiting the least amount. Baclofen was effective in counteracting this deficit in SI after either acute treatment (1.25 mg/kg) or prophylactic treatment (2.5 mg/kg). Differences in line crosses were slight [F(3, 27) = 2.65, p = 0.06], with only the 2 baclofen-treated groups being significantly different from each other (Fig. 5).
Fig. 6.
Acute and prophylactic effects of baclofen on reduced social interaction (SI) in rats repeatedly withdrawn from chronic 4.5% ethanol diet (ED). Rats were exposed to control diet (CD) throughout or 3 cycles of 5 days’ exposure to 4.5% ED. The rats exposed to ED were injected with vehicle (ED-Veh) or 2.5 mg/kg baclofen (ED-Bac2.5) 4 h into the first and second withdrawal or with 1.25 mg/kg baclofen (ED-Bac1.25) 4.5 h into the third withdrawal. Social interaction behavior and line crossings were recorded 5 h into withdrawal after the third ethanol exposure was stopped. Bars represent the means ± SEM. Groups with different letters are significantly different from each other (Tukey’s protected t-tests).
Experiment 7. Baclofen and Naloxone Actions Against Cycled 4.5% ED-Induced Anxiety: Additional Doses
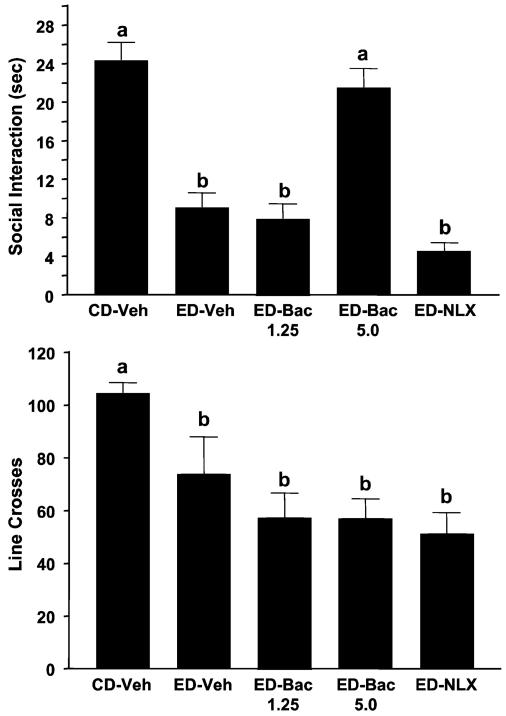
In this experiment, additional doses of baclofen and naloxone were assessed in multiply withdrawn rats. As shown in Fig. 7, there were significant differences for time spent in SI [F(4, 35) = 21.28, p<0.0001], with withdrawn groups showing the least SI. Whereas SI deficits were not blocked by prophylactic treatments with the lowest dose of baclofen (1.25 mg/kg), 5 mg/kg (much like 2.5 mg/kg in the previous study) was effective. The cycled ED also reduced locomotor behavior somewhat [F(4, 35) = 5.74, p<0.001], and neither drug reversed this effect. As with the 20 mg/kg naloxone dose in the previous study, the 5 mg/kg dose of naloxone was inactive in this experiment.
Fig. 7.
Prophylactic effects of baclofen against reduced social interaction (SI) in rats repeatedly withdrawn from 4.5% ethanol diet (ED). Rats were exposed to control diet (CD) throughout or 3 cycles of 5 days’ exposure to 4.5% ED. The rats exposed to ED were injected with vehicle (ED-Veh) or 1.25 or 5 mg/kg baclofen, or 5 mg/kg naloxone 4 h into the first and second withdrawal periods. Social interaction behavior and line crossings were recorded 5 h after the last ethanol exposure was stopped. Bars represent the means ± SEM. Groups with different letters are significantly different from each other (Tukey’s protected t-tests).
Experiment 8. Drug Actions Against Stress-Sensitized Ethanol Withdrawal Anxiety
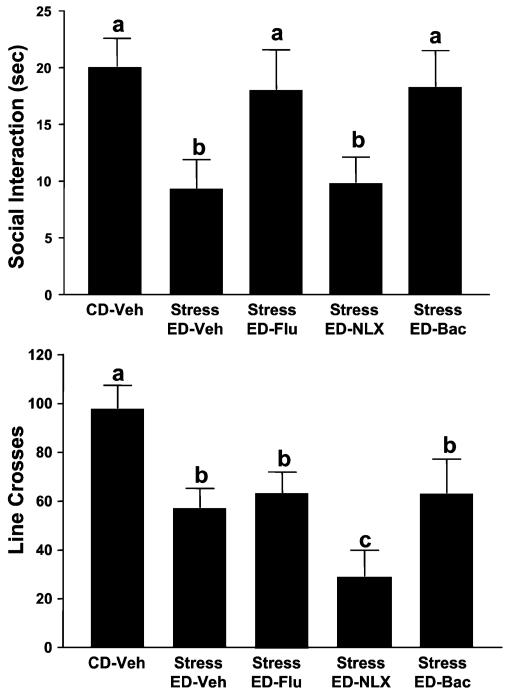
In the first stress experiment, rats received 2 stress episodes in lieu of the first 2 of the 3 withdrawal experiences. As shown in Fig. 8, there were significant differences for time spent in SI [F(4, 35) = 7.75, p<0.001], with withdrawn groups showing the least SI. While prophylactic naloxone (20 mg/kg) was inactive, flumazenil (5 mg/kg), and baclofen (2.5 mg/kg) both reversed the SI deficits of the stressed/withdrawn rats. A difference was found for locomotor activity [F(4, 35) = 5.52, p<0.01], whereby withdrawn rats tended to have somewhat lower locomotor behavior, an effect that was somewhat worsened by naloxone.
Fig. 8.
Actions of flumazenil and baclofen, but not naloxone, against stress-enhanced ethanol withdrawal anxiety. Rats were exposed to control diet (CD), restraint stressed twice (6 days apart; 45 min each session), and then given 4.5% ED for 5 days before social interaction (SI) testing. Thirty minutes before each stress (10 min for flumazenil), rats were treated with either vehicle, flumazenil (5 mg/kg), naloxone (20 mg/kg), or baclofen (2.5 mg/kg), respectively. Social interaction testing was carried out 5 h after ethanol exposure was stopped. Bars represent the means ± SEM. Groups with different letters are significantly different from each other (Tukey’s protected t-tests).
Experiment 9. Baclofen Actions Against Stress-Sensitized Ethanol Withdrawal Anxiety: Additional Doses
As with Experiment 8, rats in this experiment were exposed to 2 stresses in lieu of the first 2 of the 3 withdrawal experiences in the standard multiple withdrawal protocol. Before each stress, rats were administered vehicle or 1 of 2 additional doses of baclofen (1.25 or 5 mg/kg). As shown in Fig. 9, there were significant differences for time spent in SI [F(4, 35) = 6.76, p<0.001], with animals experiencing 1 withdrawal exhibiting no significant decline in SI as expected and rats experiencing stress and withdrawal appearing as if they had received multiple withdrawals. The high dose of prophylactically administered baclofen reversed this effect. Groups did not differ for locomotor behavior.
Fig. 9.
Baclofen (1.25 and 5 mg/kg) actions against stress-enhanced ethanol withdrawal anxiety: additional doses. Rats were treated as in Fig. 8, except that subgroups received either 1.25 or 5.0 mg/kg baclofen 30 min before the stress sessions. An additional group was not subjected to stress. Bars represent the means ± SEM. Groups with different letters are significantly different from each other (Tukey’s protected t-tests). CD, control diet.
Experiment 10. Actions of Baclofen in Controls and in Abstinent Stressed Rats
In this experiment, differences among the groups on the SI variable were significant [F(4, 35) = 11.75, p<0.0001]. Of these groups, rats maintained on CD showed no behavioral response to baclofen despite having been injected twice during the 2 weeks before testing (Fig. 10). However, other rats also treated with baclofen during the CD exposure period but then behaviorally tested following a subsequent 5-day cycle of 4.5% ED had significantly reduced SI scores. The greatest SI deficits appeared, as expected, in the 2 groups of rats given 3 cycles of chronic ethanol. When 1 of these groups was subsequently stressed 3 days into abstinence (a time frame during which SI behavior normally recovers; Overstreet et al., 2002) these animals maintained these deficits. In contrast, treatment of the second multiply withdrawn group acutely with baclofen before the stress completely reversed this SI deficit [t(14) = 6.94, p<0.0001]. Overall, locomotor activity among the groups was not different, although the baclofen-treated cycled animals tended to have reduced locomotor scores.
Fig. 10.
Acute actions of baclofen against stress-elicited social interaction (SI) deficits during abstinence from ethanol diet (ED) but no effects of prophylactic baclofen in animals not exposed to ED. Groups of rats were exposed to control diet (CD) and given either vehicle (CD-Veh) or baclofen (Bac-CD) twice or were similarly exposed to CD and baclofen, followed by one 5-day cycle of 4.5% ED (Bac-ED). Two additional groups were exposed to ED for 3 cycles, behaviorally tested to confirm the sensitization of anxiety (ED-Pre Veh stress and ED-Pre Bac stress) during withdrawal, and then retested immediately after administration of a single restraint stress (45 min) 3 days into abstinence. Baclofen had no effect on rats given only CD, tended to increase anxiety in animals subsequently exposed to 1 ED cycle (Bac-ED), and completely reversed stress-elicited anxiety in the multiply withdrawn abstinent rats (ED-Post Bac stress). Bars represent the means ± SEM. Groups with different letters are significantly different from each other (Tukey’s protected t-tests). *Significantly different from the stressed Veh-treated group (ED-Post Veh stress).
Ethanol Intakes and Body Weights
Good control over body weights was achieved, as illustrated in Table 1. There were no differences in the body weights of the various groups in all experiments. In addition, there were no significant differences in ethanol intakes among the groups receiving the ED. Therefore, the prophylactic drug treatments did not influence ethanol intake, and hence this variable could not account for their effects on SI behavior.
Table 1.
Body Weights and Ethanol Intakes in the Drug Treatment Groups Do Not Differa
| Treatment group | Ethanol intake (g/kg/d) | Body weight (g) |
|---|---|---|
| Experiment 1 | ||
| CD-Veh | NA | 271 ± 6 |
| ED-Veh | 13.0 ± 0.5 | 261 ± 8 |
| ED-Diaz | 13.2 ± 0.3 | 275 ± 10 |
| ED-Flu | 12.4 ± 0.7 | 241 ± 14 |
| ED-Bac1.25 | 13.3 ± 0.3 | 283 ± 9 |
| ED-Bac2.5 | 12.7 ± 0.3 | 274 ± 10 |
| F(4, 25) = 0.99; NS | F(5, 34) = 2.24; NS | |
| Experiment 2 | ||
| CD-Veh | NA | 302 ± 2 |
| ED-Veh | 11.2 ± 0.3 | 312 ± 10 |
| ED-Acamprosate | 11.1 ± 0.3 | 300 ± 6 |
| ED-Ifenprodil | 11.6 ± 0.4 | 308 ± 6 |
| F(2, 20) = 0.45; NS | F(3, 35) = 0.80; NS | |
| Experiment 3 | ||
| CD-Veh | NA | 302 ± 3 |
| ED-Veh | 11.3 ± 0.3 | 301 ± 6 |
| ED-Lamot | 11.3 ± 0.3 | 309 ± 5 |
| ED-Dizocilpine (MK-801) | 10.9 ± 0.3 | 294 ± 3 |
| ED-CGS | 10.8 ± 0.3 | 296 ± 2 |
| F(3, 30) = 0.95; NS | F(4, 37) = 2.03; NS | |
| Experiment 4 | ||
| CD-Veh | NA | 288 ± 5 |
| ED-Veh | 10.6 ± 0.5 | 299 ± 7 |
| ED-MPEP | 10.6 ± 0.3 | 298 ± 8 |
| t(14) = 0.02; NS | F[2, 21] = 0.83; NS | |
| Experiment 5 | ||
| CD-Veh | NA | 331 ± 2 |
| ED-Veh | 9.2 ± 0.1 | 328 ± 7 |
| ED-NLX | 8.9 ± 0.2 | 336 ± 5 |
| ED-Flu | 9.2 ± 0.9 | 333 ± 5 |
| F(2, 20) = 0.94; NS | F(3, 28) = 0.47; NS | |
| Experiment 6 | ||
| CD-Veh | NA | 325 ± 3 |
| ED-Vehicle | 8.6 ± 0.3 | 351 ± 8 |
| ED-BacA | 9.0 ± 0.3 | 350 ± 9 |
| ED-BacP | 9.2 ± 0.2 | 332 ± 12 |
| F(2, 20) = 0.64; NS | F(3, 27) 2.24; NS | |
| Experiment 7 | ||
| CD-Veh | NA | 312 ± 4.4 |
| ED-Veh | 8.8 ± 0.2 | 307 ± 2.8 |
| ED-Bac1.25 | 9.4 ± 0.3 | 313 ± 3.4 |
| ED-Bac5 | 8.6 ± 0.5 | 302 ± 6.5 |
| ED-NLX | 8.7 ± 0.4 | 309 ± 6.7 |
| F(3, 28) = 1.15; NS | F(4, 35) = 0.78; NS | |
| Experiment 8 | ||
| CD-Veh | NA | 301 ± 4.6 |
| ED-Veh | 10.1 ± 0.5 | 286 ± 5.3 |
| ED-Flum | 9.9 ± 0.4 | 289 ± 5.0 |
| ED-NLX | 9.7 ± 0.4 | 289 ± 4.5 |
| ED-Bac | 9.7 ± 0.3 | 290 ± 4.2 |
| F(3, 28) = 0.15; NS | F(4, 35) = 1.56; NS | |
| Experiment 9 | ||
| CD-Veh | NA | 305 ± 2.5 |
| ED-Veh | 9.2 ± 0.2 | 305 ± 6.1 |
| Stress-ED-Veh | 9.7 ± 0.2 | 307 ± 3.4 |
| Stress-ED-Bac1.25 | 8.8 ± 0.2 | 290 ± 6.7 |
| Stress-ED-Bac5 | 9.0 ± 0.3 | 307 ± 6.7 |
| F(3, 25) = 2.64; NS | F(4, 35) = 1.99; NS | |
| Experiment 10 | ||
| CD-Veh | NA | 309 ± 4.7 |
| Bac-CD | NA | 305 ± 3.4 |
| Bac-ED | 10.5 ± 0.3 | 307 ± 7.6 |
| ED-Pre Veh stressb | 9.6 ± 0.3 | 316 ± 6.1 |
| ED-Pre Bac stressb | 9.8 ± 0.2 | 314 ± 3.1 |
| F(2, 21) = 3.34, p<0.06 | F(4, 35) = 1.14; NS |
Rats consumed ethanol or control diets for three 5-d cycles with prophylactic drug treatments during periods of withdrawal at the end of the first 2 of 3 cycles or acute treatments at the conclusion of a continuous 23-d ethanol exposure.
Note, after their first social interaction test, animals in these 2 groups were abstinent for 3 d, and then treated with Baclofen for the first time 30 min before administering 45 min of restraint stress and testing with a new partner for social interaction (SI) 30 min later. Social interaction and locomotor data from both the behavioral tests are shown in Fig. 10.
NA, not applicable; NS, not significant; Bac, baclofen; BacA, acute baclofen; BacP, prophylactic baclofen; CGS, CGS19755; Diaz, diazepam; NLX, naloxone; Veh, vehicle; CD, control diet; ED, ethanol diet; Flum, flumazenil; Lamot, lamotrigine; MK, MK-801.
DISCUSSION
The results of these experiments demonstrate that 2 medications with demonstrated utility in alcoholic patients, baclofen, and acamprosate, also have the capacity to limit the development of an emotional response to repeated withdrawals from ethanol in rats and suggest that these agents modulate underlying sensitization. Further, whereas these drugs and the positive controls flumazenil and diazepam (Knapp et al., 2004, 2005) were active, MPEP, dizocilpine, CGS19755, lamotrigine, ifenprodil, and naloxone were not. While it is conceivable that doses of these drugs other than the often-reported ones that were also chosen here could demonstrate differential efficacy, the aggregate profile of these drug actions (or lack thereof) suggests that potentially important events related to acamprosate and baclofen mechanisms occurred in this model and deserve further study. Baclofen was active acutely against the expression and prophylactically against the sensitization of the emotional response. In the stress/withdrawal sensitization studies, baclofen was active against both stress sensitization of a future withdrawal and the acute consequences of stress during abstinence after a history of repeated withdrawals. As with its acute and prophylactic actions against the behavioral expression and sensitization in the withdrawal-only studies, baclofen exerted analogous acute actions against stress-elicited anxiety-like behavior during abstinence as well as prophylactic actions against stress-sensitized withdrawal anxiety-like behavior.
As reported previously (Overstreet et al., 2003, 2004), SI was largely independent of locomotor activity in these tests. Progressive increases in control over locomotor deficits were obtained through successive modifications of the intensity of ethanol treatments across Experiments 1–5. Although SI deficits and locomotor deficits were not wholly dissociable in the current studies, the observation of SI deficits with little or no effects on locomotion or other physical signs of withdrawal (mostly seen with the 4.5% cycled withdrawal experiments) in the studies reported here and in previous studies is a noteworthy feature of the model. Furthermore, the behavior of 1 member of a SI pair is largely independent of the other (Overstreet et al., 2002).
Several considerations should be borne in mind in assessing the potential relevance of these results to withdrawal symptoms in human alcoholic subjects. First, while the phenomenon of progressive worsening of withdrawal severity with increasing duration of alcoholism is widely acknowledged, there are relatively few reports that directly demonstrate this phenomenon in humans, and fewer still for the anxiety phenotype. In fact, data from small studies have been mixed on this point (Duka et al., 2002; Malcolm et al, 2000). While Malcolm et al. did in fact provide evidence for Ballenger and Post’s (1978) idea that symptoms other than seizures might be sensitized in multiply detoxed individuals (based on the CIWA-Ar), little other systematic clinical investigation has been reported. Similarly, in the animal literature, Borlikova et al. (2006) failed to find a sensitized anxiety-like response in rats following repeated withdrawal. Thus, careful consideration must be given to the conditions under which these assessments were carried out and the potential relevance of the models to the human condition. For the model versus the clinic, many alcoholic patients may potentially already be sensitized by the time they enter a trial. The model presented here might best be applied to studying the development of sensitization in early phases of alcoholism or the possible progression of anxiety between detoxes instead of being applied across the entire alcoholic spectrum. The putative sensitization of emotional symptoms in alcoholic subjects over decades (e.g., 15–23 years of problem drinking/alcoholism in the Duka et al., 2002; Malcolm et al., 2000 reports, for example) might not be modeled optimally by 3 weeks of cycled ethanol exposure in rats. The repeated alcohol exposures and “withdrawals” accomplished in these rat studies may be more relevant to shorter periods of alcoholic progression that may occur between detoxes. Studies addressing the early phases of multiple-withdrawal sensitization in humans may be completely absent in that no clinical investigators have addressed the relevant early stage in alcoholic development or progression between detoxifications. The practice in clinical studies of selecting subjects according to whether they meet DSM-IV or similar criteria likely excludes patients experiencing this early stage of multiple-withdrawal sensitization that studies in the present report may best model.
Between-study differences in methodology also make it difficult to compare results from human and animal studies such as the current experiments. For example, all of the data in the Duka et al. (2002) and Malcolm et al. (2000) reports were from detoxed individuals (after at least 2 weeks of abstinence), while all the data from rats in the current studies were collected during acute withdrawal. In the Borlikova et al. (2006) report, the rats in that study had alcohol intakes nearly twice (up to 20 g/kg/d) that of the present report for nearly twice as many days, inconsistencies that make between-study comparisons difficult. With such intense alcohol exposure, sensitization may well have been masked.
These considerations notwithstanding, anxiety disorders are widely and excessively represented in alcoholic subjects, more than 40% of whom have a comorbid anxiety-related disorder (Schneider et al., 2001). This anxiety generally develops over time, and it both is affected by and affects the course of alcoholism. The model in the present experiments is applicable to studying withdrawal-associated anxiety and has shown some promise in generating data relevant to alcoholism. For example, recent results of human trials with baclofen (Addolorato et al., 2000, 2002; Flannery et al., 2004) are predicted from the acute and prophylactic studies reported here. Overall, it seems clear that more work is warranted to firm up the models and to understand their generalizability and applicability to predicting pharmacological efficacy in alcoholic subjects.
These results complement and extend a number of related studies reported previously. Among them are comparable studies in which drug actions on serotonin, GABA/BZD, or CRF receptors were found to exert prophylactic effects on the withdrawal-sensitized anxiety phenotype (Knapp et al., 2005; Overstreet et al., 2003, 2004) and/or to block the sensitizing effect of stress on future exposure to ethanol and withdrawal (Breese et al., 2004). These observations suggest that the mechanisms of action of these drugs at least partially mediate the process of sensitization due to repeated withdrawals and stress. Although the mechanisms and pathways that mediate these actions are not fully understood, the results here support the idea that the process engaged by these agents has multiple access points that provide a promising variety of targets for continued drug development.
Naloxone, while capable of reducing drinking in some animal models (Overstreet et al., 1999; Wegelius et al., 1994) and widely used clinically (e.g., in opioid overdose), did not appear to engage mechanisms associated with anxiety in this experimental paradigm or others (Alvarez et al., 1998). The lack of action in either the multiple-withdrawal or multiple stress-enhanced withdrawal designs suggests this interpretation. This dissociation indicates that systems medicating the rewarding effects of ethanol and the adaptive changes associated with ethanol exposure and withdrawal may be different. There is good evidence for opiates being involved in the rewarding effects of ethanol (Anton and Swift, 2003; Overstreet et al., 1999; Wegelius et al., 1994), but the present findings suggest that they are not involved in the adaptive changes.
Acamprosate showed an anxiolytic effect in these studies. While the effect of acamprosate was observed in the context of an accumulating effect of chronic ethanol, whether cycling per se accounted for the effect cannot be determined from this research. The anxiolytic effect of acamprosate in this model is fairly novel in that little direct other evidence for its anxiolytic action has appeared to date. Further, clinical reports have failed to strongly associate anxiety dimensions with the efficacy of acamprosate in relapse (Tempesta et al., 2000; Verheul et al., 2005). The current results suggest that if acamprosate is acting to antagonize withdrawal-induced actions of glutamate on anxiety, then it likely does so in ways not shared by the competitive and noncompetitive NMDA receptor blockers CGS19755 and dizocilpine, the glutamate release blocker lamotrigine, or the indirect NMDA receptor antagonist, ifenprodil. It should be cautioned, though, that despite the negative results of various repeated attempts to find a glutamate connection with reasonable drug doses, ruling out glutamate would be premature based on the single-dose design of the studies. Furthermore, although another suggested mechanism for acamprosate action is at mGLuR5 metabotropic glutamate receptors (Harris et al., 2003), the mGluR5 receptor antagonist MPEP was not active in the repeated withdrawal model, and thus this mechanism too may not be central to the sensitized anxiety effect. Alternatively, the relevant actions of acamprosate may be elsewhere, perhaps through influences on known anxiomodulatory systems through an unknown mechanism. One candidate mechanism proposed by Berton et al. (1998) involves blockade of presynaptic GABAB receptor-mediated inhibition of GABA release; however, this mechanism would seem contrary to the antianxiety-like action of acamprosate found here.
As a GABAB autoreceptor agonist, 1 action of baclofen should be to limit neurotransmitter release (see Ariwodola and Weiner, 2004; Yamada et al., 1999). Indeed, evidence suggests that, for example, GABAB receptors on dopamine neurons in the ventral tegmental area may function to limit dopamine release from these neurons in projection sites such as the accumbens (e.g., Kalivas et al., 1990). However, the physiology of these receptors is quite complicated as suggested by their presynaptic and postsynaptic location, multiple effector mechanisms and receptor subtypes, and widespread distribution in the brain (Bowery and Enna, 2000). For example, Yamada et al. (1999) showed that synaptic activation of GABAB hetero-receptors elicits presynaptic inhibition of glutamatergic excitatory transmission in sites such as the basolateral amygdala—a finding that might predict results on anxiety other than those found with a number of glutamate inhibitors in the present report. If presynaptic regulation of neurotransmitter release is mediating the action of baclofen on anxiety in the present report, then one might presume that it is limiting the release of known proanxiety neurotransmission in brain regions known to regulate anxiety. One candidate scenario would be GABAB receptor inhibition of CRF or 5-HT release in the amygdala [e.g., a demonstrated mechanism within the raphe (Bagdy et al., 2000) and cortex (Gray and Green, 1987; see also Menzaghi et al., 1994)], which might explain the 5-HT and CRF influence over development of sensitized anxiety in the repeated withdrawal model (Overstreet et al., 2003, 2004). A report linking all 3 of these anxiomodulatory receptor systems found that baclofen inhibited 5-HT-induced CRH secretion, at least in the hypothalamus (Calogero et al., 1988). Further study of baclofen and GABAB receptor antagonists in the amygdala and other sites relevant to anxiety resulting from ethanol withdrawal cycling and/or ethanol withdrawal/stress cycling might help to address this question.
As recently reported experiments demonstrated, two 60-minute periods of stress can substitute for withdrawal experiences to sensitize anxiety, while a single 45-minute period of stress re-elicited anxiety in animals during abstinence (Breese et al., 2004, 2005a, 2005b). In contrast, such stress exposure did not sensitize anxiety in rats not exposed to ethanol (Breese et al., 2004). Further, stress is arguably a critical factor in alcoholic relapse (e.g., Breese et al., 2005a; Sinha, 2001). Thus, given the consistently strong action of baclofen against the repeated withdrawal effect reported here, baclofen was further assessed for its potential action to block stress/withdrawal interactions. First, baclofen was administered before each stress, which in turn preceded exposure to ethanol and withdrawal. Second, baclofen was administered after cycled chronic ethanol had ceased (i.e., during abstinence) to ascertain the action of the drug against stress effects at this time of relative vulnerability in alcoholic subjects. The results show that baclofen was active in both designs; therefore, it appears that baclofen exerts fairly broad actions against sensitization regardless of whether it is caused by multiple withdrawals or stress-enhanced withdrawal.
In addition to its acute and prophylactic actions against withdrawal and stress-sensitized anxiety reported here and in other models (Colombo et al., 2000; File et al., 1991, 1992), baclofen reportedly alters a number of alcohol-related phenotypes that underscore its potential utility in treating alcoholism. For example, baclofen reduced behavioral activation induced by acute alcohol injections (Broadbent and Harless, 1999; Chester and Cunningham, 1999) and reduced ethanol intake (Colombo et al., 2003), particularly in limited access paradigms (Anstrom et al., 2003) and in dependent rats (Walker and Koob, 2007). The suppression of alcohol self-administration by baclofen is consistent with other preclinical literature in which the drug reduced self-administration of cocaine (Campbell et al., 1999), amphetamines (Phillis et al., 2001), heroin (Xi and Stein, 1999), and nicotine (Corrigall et al., 2001). Because baclofen inhibits dopaminergic neurons (Erhardt et al., 2002; Olpe et al., 1977) and inhibits cocaine self-administration after intrategmental injections (Brebner et al., 2000), it is possible that baclofen’s effects on self-administration are mediated by its inhibition of dopaminergic activity. However, Anstrom et al. (2003) have suggested an interesting alternative: that baclofen disrupts the corticothalamic circuits that mediate the compulsive drive to drink. For withdrawal syndromes, baclofen attenuated the severity of ethanol withdrawal (Colombo et al., 2000; File et al., 1991, 1992) and ethanol withdrawal sensitization (data herein), demonstrated efficacy in humans that rivaled that of the gold standard diazepam (Addolorato et al., 2006), and may demonstrate anxiolytic effects during ethanol withdrawal as well (Flannery et al., 2004). Baclofen also attenuates opiate withdrawal in humans (Akhondzadeh et al., 2000), rats (Bexis et al., 2001) and mice (Diaz et al., 2001), and attenuates BZD withdrawal in rats (File et al., 1991). Overall, these effects suggest that baclofen directly and broadly interacts with the reward systems and withdrawal-related processes associated with drugs of abuse.
The mechanisms that mediate these various actions of baclofen may be distinct for each action. However, a clearly important consideration is that baclofen is an agonist at GABAB receptors, which are distributed throughout the brain (e.g., Charles et al., 2003) and function to inhibit chemical signaling both presynaptically and postsynaptically (Luscher et al., 1997; Thompson and Gahwiler, 1992). This inhibition is likely responsible for its muscle-relaxing properties (Roussan et al., 1985). There have also been suggestions that these inhibitory properties of baclofen may contribute to its beneficial effects on self-administration of cocaine (Ling et al., 1998) and alcohol (Addolorato et al., 2002). Regardless of the mechanism(s) that underlie its effects, it is clear that baclofen suppresses responding for and withdrawal from drugs of abuse including alcohol. The fact that baclofen was previously shown to reduce the positive rewarding effects of alcohol, and was found here to reduce acutely the expression of aversive effects of alcohol withdrawal or stress during abstinence, and to prevent the sensitization of withdrawal anxiety caused by either multiple withdrawals or multiple stresses before withdrawal, all support the view that baclofen might be a useful therapeutic agent in the treatment of alcoholism. Initial clinical efforts are promising (Addolorato et al., 2002, 2006; Flannery et al., 2004).
ACKNOWLEDGMENT
We wish to thank Lara Marr, Mili Senapati, and Qi Yu for technical assistance.
This work was supported by Grants AA-00214, AA-11605, AA-14284, and AA-14949 from the NIAAA.
REFERENCES
- Addolorato G, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G. Ability of baclofen in reducing alcohol craving and intake: II—Preliminary clinical evidence. Alcohol Clin Exp Res. 2000;24:67–71. [PubMed] [Google Scholar]
- Addolorato G, Caputo E, Capristo E, Domenicali M, Bernardi M, Janin L, Agabio A, Colombo G, Gessa GL, Gasbarrini G. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind, randomized controlled study. Alcohol Alcohol. 2002;37:504–508. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Abenavoli L, Agabio R, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G. Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam. Am J Med. 2006;119:276.e13–18. doi: 10.1016/j.amjmed.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S, Ahmadi-Abhari SA, Assadi SM, Shabestari OL, Kashani AR, Farzanehgan ZM. Double-blind randomized controlled trial of baclofen vs. clonidine in the treatment of opiate withdrawal. J Clin Pharm Ther. 2000;25:347–353. doi: 10.1046/j.1365-2710.2000.00295.x. [DOI] [PubMed] [Google Scholar]
- Alvarez C, Prunell M, Boada J. Effect of naloxone on behavioral changes induced by subchronic administration of ethanol in rats. Pharmacol Biochem Behav. 1998;59:961–965. doi: 10.1016/s0091-3057(97)00505-4. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Cromwell HC, Markowski T, Woodward DJ. Effect of baclofen on alcohol and sucrose self-administration in rats. Alcohol Clin Exp Res. 2003;27:900–908. doi: 10.1097/01.ALC.0000071744.78580.78. [DOI] [PubMed] [Google Scholar]
- Anton RF, Swift RM. Current pharmacotherapies of alcoholism: a U.S. perspective. Am J Addict. 2003;12(suppl 1):S53–S68. doi: 10.1111/j.1521-0391.2003.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABAB receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdy E, Kiraly I, Harsing LG., Jr. Reciprocal innervation between serotonergic and GABAergic neurons in raphe nuclei of the rat. Neurochem Res. 2000;25:1465–1473. doi: 10.1023/a:1007672008297. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Berton F, Francesconi WG, Madamba SG, Zieglgansberger W, Siggins GR. Acamprosate enhances N-methyl-D-apartate receptor-mediated neurotransmission but inhibits presynaptic GABA(B) receptors in nucleus accumbens neurons. Alcohol Clin Exp Res. 1998;22:183–191. [PubMed] [Google Scholar]
- Bexis S, Ong J, White J. Attenuation of morphine withdrawal signs by the GABA(B) receptor agonist baclofen. Life Sci. 2001;70:395–401. doi: 10.1016/s0024-3205(01)01485-0. [DOI] [PubMed] [Google Scholar]
- Borlikova GG, Le Merrer J, Stephens DN. Previous experience of ethanol withdrawal increases withdrawal-induced c-fos expression in limbic areas, but not withdrawal-induced anxiety and prevents withdrawal-induced elevations in plasma corticosterone. Psychopharmacology. 2006;185:188–200. doi: 10.1007/s00213-005-0301-3. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Enna SJ. Gamma-aminobutyric acid(B) receptors: first of the functional metabotropic heterodimers. J Pharmacol Exp Ther. 2000;292:2–7. [PubMed] [Google Scholar]
- Brebner K, Phelan R, Roberts DC. Intra-VTA baclofen attenuates cocaine self-administration on a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 2000;66:857–862. doi: 10.1016/s0091-3057(00)00286-0. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Le DA, O’Dell LE, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005a;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF1 and benzodiazepine receptor antagonists and a 5-HT1A receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005b;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent J, Harless WE. Differential effects of GABA(A) and GABA(B) agonists on sensitization to the locomotor stimulant effects of ethanol in DBA/2 J mice. Psychopharmacology (Berlin) 1999;141:197–205. doi: 10.1007/s002130050825. [DOI] [PubMed] [Google Scholar]
- Busse CS, Brodkin J, Tattersall D, Anderson JJ, Warren N, Tehrani L, Bristow LJ, Varney MA, Cosford ND. The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[(2-methyl-1, 3-thiazol-4-yl)ethynyl]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology. 2004;29:1971–1979. doi: 10.1038/sj.npp.1300540. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Gallucci WT, Chrousos GP, Gold PW. Interaction between GABAergic neurotransmission and rat hypothalamic corti-cotropin-releasing hormone secretion in vitro. Brain Res. 1988;463:28–36. doi: 10.1016/0006-8993(88)90523-9. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Lac ST, Carroll ME. Effects of baclofen on maintenance and reinstatement of intravenous cocaine self-administration in rats. Psychopharmacology (Berlin) 1999;143:209–214. doi: 10.1007/s002130050937. [DOI] [PubMed] [Google Scholar]
- Charles KJ, Calver AR, Jourdain S, Pangalos MN. Distribution of a GABAB-like receptor protein in the rat central nervous system. Brain Res. 2003;989:135–146. doi: 10.1016/s0006-8993(03)03163-9. [DOI] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. Baclofen alters ethanol-stimulated activity but not conditioned place preference or taste aversion in mice. Pharmacol Biochem Behav. 1999;63:325–331. doi: 10.1016/s0091-3057(98)00253-6. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabia R, Carai MAM, Lobnia C, Pani M, Reali R, Addolorato G, Gessa GL. Ability of baclofen in reducing alcohol intake and withdrawal severity: I—Preclinical evidence. Alcohol Clin Exp Res. 2000;24:58–66. [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Vacca G, Carai MA, Gessa GL. Suppression by baclofen of alcohol deprivation effect in Sardinian alcohol-preferring (sP) rats. Drug Alcohol Dep. 2003;70:105–108. doi: 10.1016/s0376-8716(02)00333-2. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Zhang J, Adamson KL. GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self-administration selectively in the rat. Psychopharmacology (Berlin) 2001;158:190–197. doi: 10.1007/s002130100869. [DOI] [PubMed] [Google Scholar]
- Diaz SL, Kemmling AK, Rubio MC, Balerio GN. Lack of sex-related differences in the prevention by baclofen of the morphine withdrawal syndrome in mice. Behav Pharmacol. 2001;12:75–79. doi: 10.1097/00008877-200102000-00009. [DOI] [PubMed] [Google Scholar]
- Drake RG, Davis LL, Cates ME, Jewel ME, Ambrose SM, Love JS. Baclofen treatment of chronic posttraumatic stress disorder. Ann Pharmacother. 2003;37:1177–1181. doi: 10.1345/aph.1C465. [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN. Kindling of withdrawal: a study of craving and anxiety after multiple detoxifications in alcoholic inpatients. Alcohol Clin Exp Res. 2002;26:785–795. [PubMed] [Google Scholar]
- Erhardt S, Mathe JM, Chergui K, Engberg G, Svensson TH. GABA(B) receptor-mediated modulation of the firing pattern of ventral tegmental area dopamine neurons in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:173–180. doi: 10.1007/s00210-001-0519-5. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- File SE, Zharkovsky A, Gulati K. Effects of baclofen and nitrendipine on ethanol withdrawal responses in the rat. Neuropharmacology. 1991;30:183–190. doi: 10.1016/0028-3908(91)90202-m. [DOI] [PubMed] [Google Scholar]
- File SE, Zharkovsky A, Hitchcot PK. Effects of nitrendipine, chlordiazepoxide, flumazenil and baclofen on the increased anxiety resulting from alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:87–93. doi: 10.1016/0278-5846(92)90011-3. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Garbutt JC, Cody MW, Renn W, Grace K, Osborne M, Crosby K, Morreale M, Trivette A. Baclofen for alcohol dependence: a preliminary open-label study. Alcohol Clin Exp Res. 2004;28:1517–1523. doi: 10.1097/01.alc.0000141640.48924.14. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, West SL, Carey TS, Lohr KN, Crews FT. Pharmacological treatment of alcohol dependence: a review of the evidence. JAMA. 1999;281:1318–1325. doi: 10.1001/jama.281.14.1318. [DOI] [PubMed] [Google Scholar]
- Gasparini F, Lingenhohl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, Varney MA, Johnson EC, Hess SD, Rao SP, Sacaan AI, Santori EM, Velicelebi G, Kuhn R. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Gray JA, Green AR. GABAB-receptor mediated inhibition of potassium-evoked release of endogenous 5-hydroxytryptamine from mouse frontal cortex. Br J Pharmacol. 1987;91:517–522. doi: 10.1111/j.1476-5381.1987.tb11244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BR, Gibson DA, Prendergast MA, Blanchard JA, Holley RC, Hart SR, Scotland RL, Foster TC, Pedigo NW, Littleton JM. The neurotoxicity induced by ethanol withdrawal in mature organotypic hippocampal slices might involve cross-talk between metabotropic glutamate type 5 receptors and N-methyl-D-aspartate receptors. Alcohol Clin Exp Res. 2003;27:1724–1735. doi: 10.1097/01.ALC.0000093601.33119.E3. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Eberhardt H. Modulation of A10 dopamine neurons by gamma-aminobutyric acid agonists. J Pharmacol Exp Ther. 1990;253:858–866. [PubMed] [Google Scholar]
- Knapp DJ, Duncan GE, Crews FT, Breese GR. Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res. 1998;22:481–493. [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Modulation of ethanol withdrawal-induced anxiety-like behavior during later withdrawals by treatment of early withdrawals with benzodiazepine/GABA ligands. Alcohol Clin Exp Res. 2005;29:553–563. doi: 10.1097/01.alc.0000158840.07475.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. New Engl J Med. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Ling W, Shoptaw S, Majewska D. Baclofen as a cocaine anti-craving medication: a preliminary clinical study. Neuropsychopharmacology. 1998;18:403–404. doi: 10.1016/S0893-133X(97)00128-0. [DOI] [PubMed] [Google Scholar]
- Littleton J, Zieglgansberger W. Pharmacological mechanisms of naltrexone and acamprosate in the prevention of relapse in alcohol dependence. Am J Addiction. 2003;12(suppl 1):S3–S11. doi: 10.1111/j.1521-0391.2003.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Roberts JS, Wang W, Myrick H, Anton RF. Multiple previous detoxifications are associated with less responsive treatment and heavier drinking during an index outpatient detoxification. Alcohol. 2000;22:159–164. doi: 10.1016/s0741-8329(00)00114-2. [DOI] [PubMed] [Google Scholar]
- Mayer S, Harris BR, Gibson DA, Blanchard JA, Prendergast MA, Holley RC, Littleton J. Acamprosate, MK-801 and ifenprodil inhibit neurotoxicity and calcium entry induced by ethanol withdrawal in organotypic slice cultures from neonatal rat hippocampus. Alcohol Clin Exp Res. 2002;26:1468–1478. doi: 10.1097/01.ALC.0000033261.14548.D2. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Breese GR. Multiple withdrawals from chronic ethanol “kindles” inferior collicular seizure activity: evidence for kindling of seizures associated with alcoholism. Alcohol Clin Exp Res. 1990;14:394–399. doi: 10.1111/j.1530-0277.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–242. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Rassnick S, Heinrichs S, Baldwin H, Pich EM, Weiss F, Koob GF. The role of corticotropin-releasing factor in the anxiogenic effects of ethanol withdrawal. Ann NY Acad Sci. 1994;739:176–184. doi: 10.1111/j.1749-6632.1994.tb19819.x. [DOI] [PubMed] [Google Scholar]
- Messenheimer JA. Lamotrigine. Epilepsia. 1995;36(suppl 2):S87–S94. doi: 10.1111/j.1528-1157.1995.tb06002.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Criswell HE, Breese GR. Flumazenil blockade of anxiety following ethanol withdrawal in rats. Psychopharmacology. 1997;131:354–360. doi: 10.1007/s002130050303. [DOI] [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Duncan GE, Breese GR. Enhanced ultrasonic vocalization and Fos protein expression following ethanol withdrawal: effects of flumazenil. Psychopharmacology. 2000;152:208–215. doi: 10.1007/s002130000507. [DOI] [PubMed] [Google Scholar]
- National Research Council Guide for the Care and Use of Laboratory Animals. 1996 http://www.nap.edu/readingroom/books/labrats/
- Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Koella WP, Wolf P, Haas HL. The action of baclofen on neurons of the substantia nigra and of the ventral tegmental area. Brain Res. 1977;134:577–580. doi: 10.1016/0006-8993(77)90834-4. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Braun C, Bartus RT, Crews FT. Suppression of alcohol intake by chronic naloxone treatment in P rats: tolerance development and elevation of opiate receptor binding. Alcohol Clin Exp Res. 1999;23:1761–1771. [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decreases in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. A 5-HT1A agonist and a 5-HT2C antagonist reduce social interaction deficits induced by multiple ethanol withdrawals in rats. Psychopharmacology. 2003;167:344–352. doi: 10.1007/s00213-003-1425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelc I, Verbanck P, Le Bon O, Gavrilovic M, Lion K, Lehert P. Efficacy and safety of acamprosate in the treatment of detoxified alcohol-dependent patients. A 90-day placebo-controlled dose-finding study. Br J Psychiatry. 1997;171:73–77. doi: 10.1192/bjp.171.1.73. [DOI] [PubMed] [Google Scholar]
- Phillis BD, Ong J, White JM, Bonnielle C. Modification of d-amphetamine-induced responses by baclofen in rats. Psychopharmacology (Berlin) 2001;153:277–284. doi: 10.1007/s002130000562. [DOI] [PubMed] [Google Scholar]
- Roussan M, Terrence C, Fromm G. Baclofen versus diazepam for the treatment of spasticity and long-term follow-up of baclofen therapy. Pharmatherapeutica. 1985;4:278–284. [PubMed] [Google Scholar]
- Schneider U, Altmann A, Baumann M, Bernzen J, Bertz B, Bimber U, Broese T, Broocks A, Burtscheidt W, Climander KF, Degkwitz P, Driessen M, Ehrenreich H, Fischbach E, Folkerts H, Frank H, Gruth D, Havemann-Reinecke U, Heber W, Heuer J, Hingsammer A, Jacobs S, Krampe H, Lange W, Lay T, Leimbach M, Lemke MR, Leweke M, Mangholz A, Massing W, Meyenberg R, Porzig J, Quattert T, Redner C, Ritzel G, Rollnik JD, Sauvageoll R, Schlafke D, Schmid G, Schroder H, Schwichtenberg U, Schwoon D, Seifert J, Sickelmann I, Sieveking CF, Spiess C, Stiegemann HH, Stracke R, Straetgen HD, Subkowski P, Thomasius R, Tretzel H, Verner LJ, Vitens J, Wagner T, Weirich S, Weiss I, Wendorff T, Wetterling T, Wiese B, Wittfoot J. Comorbid anxiety and affective disorder in alcohol-dependent patients seeking treatment: the first multicentre study in Germany. Alcohol Alcohol. 2001;36:219–223. doi: 10.1093/alcalc/36.3.219. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Snell LD, Claffey DJ, Ruth JA, Valenzuela CF, Cardoso R, Wang Z, Levinson SR, Sather WA, Williamson AV, Ingersoll NC, Ovchinnikova L, Bhave SV, Hoffman PL, Tabakoff B. Novel structure having antagonist actions at both the glycine site of the N-methyl-D-aspartate receptor and neuronal voltage-sensitive sodium channels: biochemical, electrophysiological, and behavioral characterization. J Pharmacol Exp Ther. 2000;292:215–227. [PubMed] [Google Scholar]
- Tempesta E, Janiri L, Bignamini A, Chabac S, Potgieter A. Acamprosate and relapse prevention in the treatment of alcohol dependence: a placebo-controlled study. Alcohol Alcohol. 2000;35:202–209. doi: 10.1093/alcalc/35.2.202. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Gahwiler BH. Comparison of the actions of baclofen at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheul R, Lehert P, Geerlings PJ, Koeter MW, van den Brink W. Predictors of acamprosate efficacy: results from a pooled analysis of seven European trials including 1485 alcohol-dependent patients. Psychopharmacology (Berlin) 2005;178:167–173. doi: 10.1007/s00213-004-1991-7. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist balcofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegelius K, Honkanen A, Korpi ER. Benzodiazepine receptor ligands modulate ethanol drinking in alcohol-preferring rats. Eur J Pharmacol. 1994;263:141–147. doi: 10.1016/0014-2999(94)90534-7. [DOI] [PubMed] [Google Scholar]
- Wilde MI, Wagstaff AJ. Acamprosate: a review of its pharmacology and clinical potential in the management of alcohol dependence after detoxification. Drugs. 1997;53:1038–1053. doi: 10.2165/00003495-199753060-00008. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Baclofen inhibits heroin self-administration behavior and mesolimbic dopamine release. J Pharmacol Exp Ther. 1999;290:1369–1374. [PubMed] [Google Scholar]
- Yamada J, Saitow F, Satake S, Kiyohara T, Konishi S. GABA(B) receptor-mediated presynaptic inhibition of glutamatergic and GABAergic transmission in the basolateral amygdala. Neuropharmacology. 1999;38:1743–1753. doi: 10.1016/s0028-3908(99)00126-4. [DOI] [PubMed] [Google Scholar]