Abstract
Previous work with Sprague–Dawley (SD) rats indicated that subjecting these rats to multiple episodes of ethanol diet could provoke anxiety-like responses. Because alcohol-preferring P rats have been reported to have neurochemical alterations in many systems shown to modulate anxiety-like responses, P rats were compared to SD rats. Rats were subjected to one or three cycles of 5 days’ exposure to 4.5% or 7% ethanol diet to assess anxiety-like behavior. The social interaction test was conducted 5 h after ethanol was removed. Other groups of P and SD rats were injected with flumazenil (5 mg/kg), a benzodiazepine (BZD) receptor antagonist, CP-154,526 (10 mg/kg), CRF1 receptor antagonist, SB243,213, a 5-HT2C receptor inverse agonist, or vehicle during the 1st and 2nd withdrawals but not the third. After a single 5-day cycle of ethanol exposure, SD rats did not exhibit a change in social interaction, but P rats exhibited a decrease after exposure to the 7% ethanol. Both strains of rats exhibited anxiety-like behavior following three cycles of exposure to ethanol and the concentration of ethanol in the diet did not influence the response. It was confirmed that flumazenil, CP-154,523, and SB243,213 had prophylactic effects on anxiety-like behavior in the SD rats. Neither flumazenil nor SB243,213 was as effective in the P rats, while the CRF1 receptor antagonist completely counteracted the reduced social interaction in repeatedly withdrawn P rats. A small study showed that buspirone, a 5-HT1A agonist, also had prophylactic effects in P rats. These findings show that alcohol-preferring P rats exhibit anxiety-like behavior more readily following exposure to ethanol-containing diets and that this behavior is counteracted more readily by pretreatment with a CRF1 receptor antagonist than with BZD or 5-HT2C receptor antagonists.
Keywords: Repeated ethanol withdrawals; Sprague–Dawley rats; Alcohol-preferring P rats; Social interaction test; Anxiety-like behavior; Flumazenil; CP-154,526; CRF1 receptor antagonist; SB243,213; 5-HT2C receptor inverse agonist; Buspirone; 5-HT1A receptor agonist
1. Introduction
Previous work from our laboratory showed that Sprague–Dawley (SD) rats will exhibit anxiety-like behavior (reductions in social interaction behavior) when exposed to repeated cycles of ethanol-containing diet (Overstreet et al., 2002), providing further evidence for a sensitization process in animals and humans chronically exposed to ethanol (e.g., Holter et al., 1998; Malcolm et al., 2000; McCown and Breese, 1990). Because of the nature of our repeated cycle protocol, with three cycles of 5 days’ exposure to ethanol being separated by two 2-day periods of withdrawal, it was possible to examine the prophylactic effects of drugs known or hypothesized to modulate anxiety. For example, flumazenil, an benzodiazepine (BZD) receptor antagonist, acutely reduced the anxiety-like behavior of ethanol-withdrawn rats (File et al., 1989; Moy et al., 1997, 2000; Knapp et al., 2004) and had the prophylactic effect of reducing the anxiety-like behavior after the 3rd withdrawal when given only during the 1st and 2nd withdrawals (Knapp et al., 2005). Serotonin (5-HT) and corticotropin releasing factor (CRF) also appear to be involved because a 5-HT2C receptor antagonist, a 5-HT1A receptor agonist, and a CRF1 receptor antagonist also reduced the anxiety-like behavior in rats subjected to repeated withdrawals from ethanol (Overstreet et al., 2003, 2004a). The counteraction of anxiety in alcohol-withdrawn rats by these drugs suggests that they might play a therapeutic role in the management of anxiety in alcoholics (See Breese et al., 2004b; Potokar et al., 1997; Seymour et al., 2003).
The alcohol-preferring P rat was developed by selectively breeding from Wistar rats that drank high volumes of ethanol solution voluntarily (e.g., Li et al., 1993; Murphy et al., 2002). An extensive amount of behavioral and physiological data have accumulated on P rats (Murphy et al., 2002). Of particular interest to the present project is the realization that the P rat has a number of neurotransmitter abnormalities found to modulate ethanol withdrawal-induced anxiety, including GABA (Hwang et al., 1990), BZD (Thielen et al., 1993,1997, 1998), 5-HT (McBride et al., 1997; Murphy et al., 1987; Pandey et al., 1996; Zhou et al., 1991), and CRF (Ehlers et al., 1992; Hwang et al., 2001) mechanisms. It is, possible therefore, that these alterations could affect the response of the P rat to chronic and repeated exposures to ethanol and/or to the effects of these agents.
The purpose of the present investigation was threefold: (1) to assess whether the P rat would react to ethanol diet manipulations in a manner similar to the SD rat; (2) to determine whether compounds known to counteract anxiety-like behavior induced by repeated ethanol withdrawal in SD rats would be equally effective in alcohol-preferring P rats; (3) to evaluate whether known neurochemical alterations in the P rat might account for the differential responses.
2. Materials and methods
2.1. Animals
Male Sprague–Dawley (SD) rats (Charles-River, Raleigh) were purchased at 40 days of age (160–180 g). The alcohol-preferring inbred P rats were selected from breeding colonies maintained at the UNC Bowles Center for Alcohol Studies at similar weights and ages. After 5 days to adapt to new conditions (22 °C, 50% humidity, 12:12 light–dark cycle with lights on between 0900 and 2100), they were placed on a nutritionally complete diet used previously in our laboratory (e.g., Frye et al., 1983; Knapp et al., 1998; Moy et al., 2000; Overstreet et al., 2002). Intakes of the liquid diet were recorded daily and body weights were measured weekly. These experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NRC, 1996) and were approved by the UNC Institutional Animal Care and Use Committee.
2.2. Liquid diet
Briefly, the diet was a lactalbumin/dextrose-based, nutritionally complete diet (with concentrations of vitamins, minerals and other nutrients derived from ICN Research Diets). Dextrose calories in the control diet (CD) were equated with ethanol calories in the ethanol diet (ED, 4.5 or 7%, w/v).
A modified pair-feeding design was used in all of the diet studies. The rats maintained on the CD were given a volume of diet equivalent to the average volume consumed the previous day by the rats maintained on the ED. The rats were weighed at weekly intervals and volumes of diet were adjusted to insure that the groups had similar body weights. Behavioral assessments were conducted after 5 or 15 days of exposure to the ED between 5 and 6 h after the removal of the ethanol. This time point was selected on the basis of previous observations of anxiety-like behavior in our laboratory (e.g., Knapp et al., 1998; Moy et al., 1997, 2000; Overstreet et al., 2002).
2.3. Social interaction test
The social interaction test involves placing a pair of animals in an arena and measuring the amount of time engaged in such behaviors as grooming, sniffing, crawling over or under, and boxing; locomotor activity is simultaneously recorded and provides a measure that is independent of social interaction (File and Seth, 2003; Overstreet et al., 2002, 2003). Social interaction has been repeatedly validated as an index of anxiety-related behavior (See File and Seth, 2003). Of particular relevance to the present study is the observation that social interaction behavior is decreased following withdrawal from drugs of abuse, including ethanol (Andrews et al., 1997; Costall et al., 1990; File et al., 1989; Irvine et al., 2001; Kampov-Polevoy et al., 2000; Overstreet et al., 2002). This task has been used to monitor changes in anxiety-like behavior in rats subjected to repeated withdrawals from ethanol as well as injected with drugs to modify the consequences of ethanol withdrawal (Breese et al., 2004a; Overstreet et al., 2002, 2003, 2004a; Knapp et al., 2005).
A modification of the standard social interaction test was used to reduce the number of animals needed for experiments. Pairs of rats with the same treatment and similar body weights were placed in the arena and the social interactions initiated by each member of the pair were recorded, thereby requiring fewer rats. Previous studies have shown that the time spent in social interaction of one member of the pair does not influence the social interaction of the other member (Breese et al., 2004a; Overstreet et al., 2002, 2003, 2004a).
Experienced observers who were blind to the experimental condition carried out the social interaction test in a square open field (60 cm by 60 cm, with 16 squares marked out on the floor). The rats were unfamiliar with the open field and the lighting conditions were low (30 lx) in order to generate an intermediate level of anxiety-related behavior. Rat pairs were matched on the basis of ethanol intakes, body weights, and treatment conditions and placed simultaneously in the open field. During the 5-min session, line crosses (by two forepaws) and time spent in social interaction (grooming, sniffing, following, crawling over/under) were scored individually for each rat (Kampov-Polevoy et al., 2000; Overstreet et al., 2002).
2.4. Procedure
Several distinct experiments were performed, as described in Table 1. The concentration of ethanol in the diet was 7% for some experiments to allow comparison with our previous studies (e.g., Knapp et al., 2004; Overstreet et al., 2002). However, 4.5% ethanol was used in other studies because of the realization that locomotor activity was not affected (Overstreet et al., 2002), thereby providing a clearer interpretation of the manipulations on social interaction.
Table 1.
Experimental design for the studies
| Experiment | Diet features | Drug treatments |
|---|---|---|
| 1 | CD vs. 7% ED | None |
| 1 cycle vs. 3 cycles | ||
| 2 | CD vs. 4.5% ED | None |
| 1 cycle vs. 3 cycles | ||
| 3 | CD vs. 7% ED | Flumazenil, benzodiazepine antagonist |
| 3 cycles | ||
| 4 | CD vs.4.5% ED | SB-243,213, 5-HT2C inverse agonist |
| 3 cycles | ||
| 5 | CD vs. 4.5% ED | CP-154,526, CRF1 receptor antagonist |
| 3 cycles; 2 stresses. 1 cycle |
Abbreviations: CD = control diet; ED = ethanol diet; CRF = corticotropin releasing factor.
2.4.1. Repeated exposures to 7% ethanol
Rats were exposed to CD throughout the study or to ED (7%, w/v) for either 5 or 15 days. Those given 15 days of ED were given 3 cycles of 5 days’ exposure with two withdrawal days between each cycle, during which time they received CD. Between 5 and 6 h after removal of the ethanol, pairs of rats were placed in the open field arena for the recording of social interaction behavior and line crosses over 5 min. During this withdrawal period, the rats had free access to CD. There were 8–10 rats from each strain in each of three treatment groups: control diet, one 5-day cycle of 7% ethanol diet, three 5-day cycles of 7% ethanol diet.
2.4.2. Repeated exposures to 4.5% ethanol
Rats were exposed to CD throughout the study or to ED (4.5%, w/v) for either 5 or 15 days. Those given 15 days of ED were given 3 cycles of 5 days’ exposure with two withdrawal days between each cycle, during which time they received CD. Between 5 and 6 h after removal of the ethanol, pairs of rats were placed in the open field arena for the recording of social interaction behavior and line crossings over 5 min. During this withdrawal period, the rats had free access to CD. There were 8–10 rats from each strain in each of three treatment groups: control diet, one 5-day cycle of 4.5% ethanol diet, three 5-day cycles of 4.5% ethanol diet.
2.4.3. Prophylactic treatment with flumazenil
Rats were exposed to CD throughout or to 15 days of ED (7%, w/v) in three cycles of 5 days. During the 1st and 2nd withdrawals, the rats were injected i.p. with either 5 mg/kg flumazenil or carboxymethylcellulose (CMC, 0.5%) vehicle 4 h after the ethanol was removed. Thus, there were three treatment groups: control diet (CD); ethanol diet given vehicle (EDV), and ethanol diet given flumazenil (EDF). There were 8–10 rats per group.
2.4.4. Prophylactic treatment with SB243,213
The SD and P rats were exposed to 3 cycles of 4.5% ethanol diet or control diet. At about 4 h into the 1st and 2nd withdrawals, the ethanol-exposed rats were injected with CMC vehicle or SB243,213 (3 mg/kg). The social interaction test was carried out 5 h after the final ethanol exposure was withdrawn. Thus, there were three treatment groups for each strain: control diet (CD), ethanol diet given vehicle (EDV), and ethanol diet given SB243,213 (EDSB). There were 6–8 rats per group.
2.4.5. Prophylactic treatment with CP-154,526
This study took advantage of the fact that rats subjected to 4.5% ED exhibited a reduction in social interaction during the third withdrawal (Overstreet et al., 2002; Experiment 2). Rats were maintained on control diet or 15 days of 4.5% ED, in three 5-day cycles. The rats exposed to ED were injected with either CMC vehicle or CP-154,526 (10 mg/kg, i.p.), a CRF1 receptor antagonist, 4 h after the ethanol was removed during the 1st and 2nd cycles. There were 8 rats in the CD group and 5 in each of the ED groups. This study was conducted in P rats only. A similar study has recently been published in the SD rats (Overstreet et al., 2004a).
A second study took advantage of the observation that exposure to restraint stress can potentiate the effects of ethanol withdrawal (Breese et al., 2004a). The P rats were either exposed to CD throughout or to one cycle of 5 days of 4.5% ED. The rats exposed to ED were either unstressed, stressed after being given vehicle, or stressed after being given CP-154,526 (10 mg/kg). The 1-h restraint sessions occurred 30 min after the injections and 1 and 6 days before being exposed to ED. The social interaction test was carried out between 5 and 6 h after the ethanol was removed. There were 8 rats per treatment group: control diet (CD), ethanol diet (ED), ethanol diet stressed and given Vehicle (EDSTV), and ethanol diet stressed and given CP-154,526 (EDSTC).
2.5. Statistical analysis
The time spent in social interaction was summarized as mean sec±S.E.M., while activity was summarized as mean number of line crosses±S.E.M. The data were analyzed by two-way or one-way analyses of variance (ANOVA), depending on the characteristics of the data set. Follow-up analyses were carried out with Tukey’s protected t tests.
3. Results
3.1. Exposure to 7% ethanol
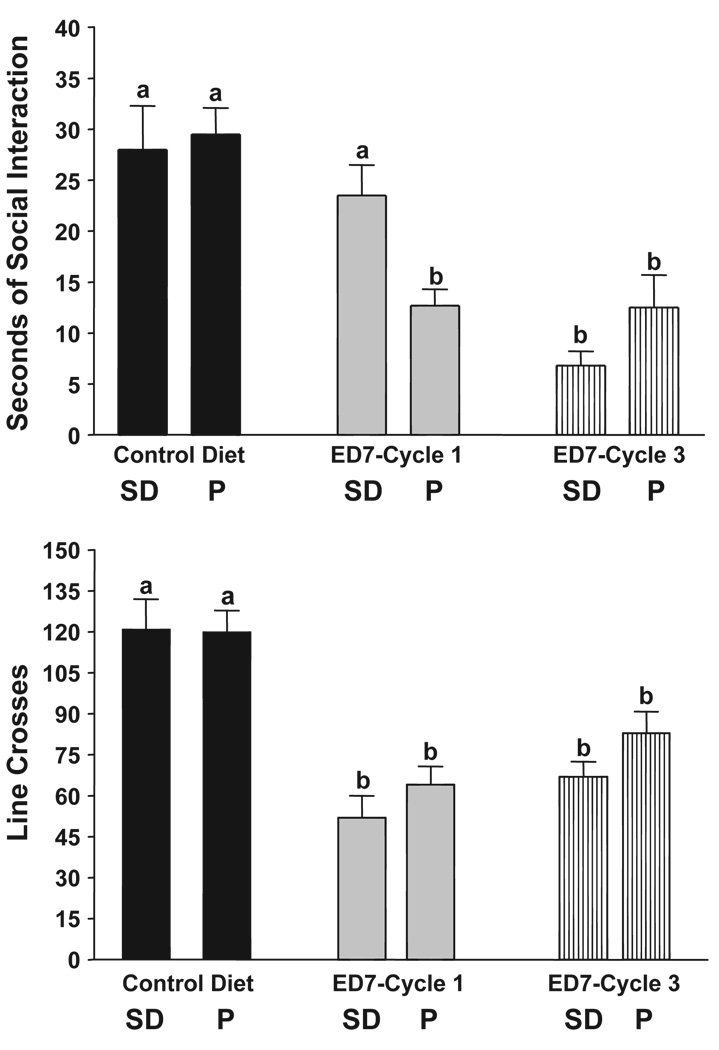
The data for time spent in social interaction in P and SD rats exposed to 7% ED are illustrated in Fig. 1, upper panel. The groups exposed to CD are similar, suggesting that P rats are not innately more anxious in the social interaction test. The P rats exhibited an anxiety-like behavior after exposure to a single cycle of 5 days’ exposure, but the SD rats did not (Fig. 1). However, both groups exhibited much less time spent in social interaction after 3 cycles of 5 days’ exposure to ED (Fig. 1). Analysis with two-way ANOVA confirmed these impressions. There were significant treatment (F[2,42] = 36.32, p < 0.0001), strain (F[1,42] = 5.53 p < 0.01), and interaction (F[2,42] = 13.56, p < 0.0001) effects. Thus, P rats are more sensitive to the anxiety-like behavior induced by ethanol withdrawal. For line crosses, both P and SD rats exhibited reductions after either one or three cycles of exposure (Fig. 1, lower panel). There was a significant treatment effect (F[2,42] = 16.54, p < 0.001) but no significant strain or interaction effects (p > 0.1).
Fig. 1.
Repeated exposures to 7% ethanol diet in P and SD rats. Rats were exposed to control liquid diet continuously or 7% ethanol diet (ED) in three cycles of 5 days, with 2 days of withdrawal after each cycle. The social interaction test was carried out after the 1st or 3rd cycles. Upper panel: data represent the mean sec±S.E.M. for 8–10 rats; lower panel: data represent the mean line crosses±S.E.M. Groups with different letters are significantly different, p < 0.01, according to 2-way ANOVA and Tukey’s protected t tests.
3.2. Exposure to 4.5% ethanol
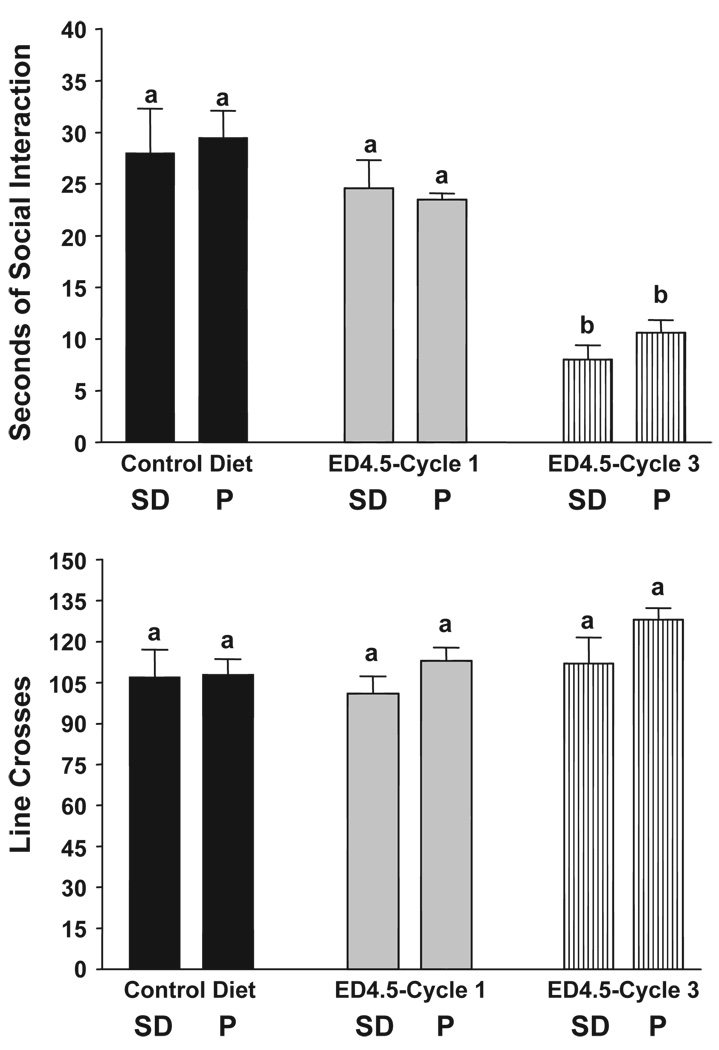
As illustrated in the upper panel of Fig. 2, the pattern for social interaction behavior in P and SD rats after exposure to 4.5% ED was different from that after exposure to 7% ED (Fig. 1). Now, neither strain was affected by exposure to 5 days of ED, but both exhibited anxiety-like behavior following exposure to 3 cycles of ED. Thus, the 2-way ANOVA showed that there was a significant treatment effect (F[2,42] = 25.32, p < 0.0001) but no significant strain (F[1,42] = 1.25, NS) or interaction (F[2,42] = 0.79, NS) effects. Exposure to 4.5% ED did not significantly affect locomotor activity in either strain (all p > 0.1; Fig. 2, lower panel).
Fig. 2.
Repeated exposures to 4.5% ethanol diet in P and SD rats. Rats were exposed to control liquid diet continuously or 4.5% ethanol diet (ED) in three cycles of 5 days, with 2 days of withdrawal after each cycle. The social interaction test was carried out after the 1st or 3rd cycles. Upper panel: data represent the mean sec±S.E.M. for 8–10 rats. Lower panel: data represent the mean line crosses±S.E.M. Groups with different letters are significantly different, p < 0.01, according to 2-way ANOVA and Tukey’s protected t tests.
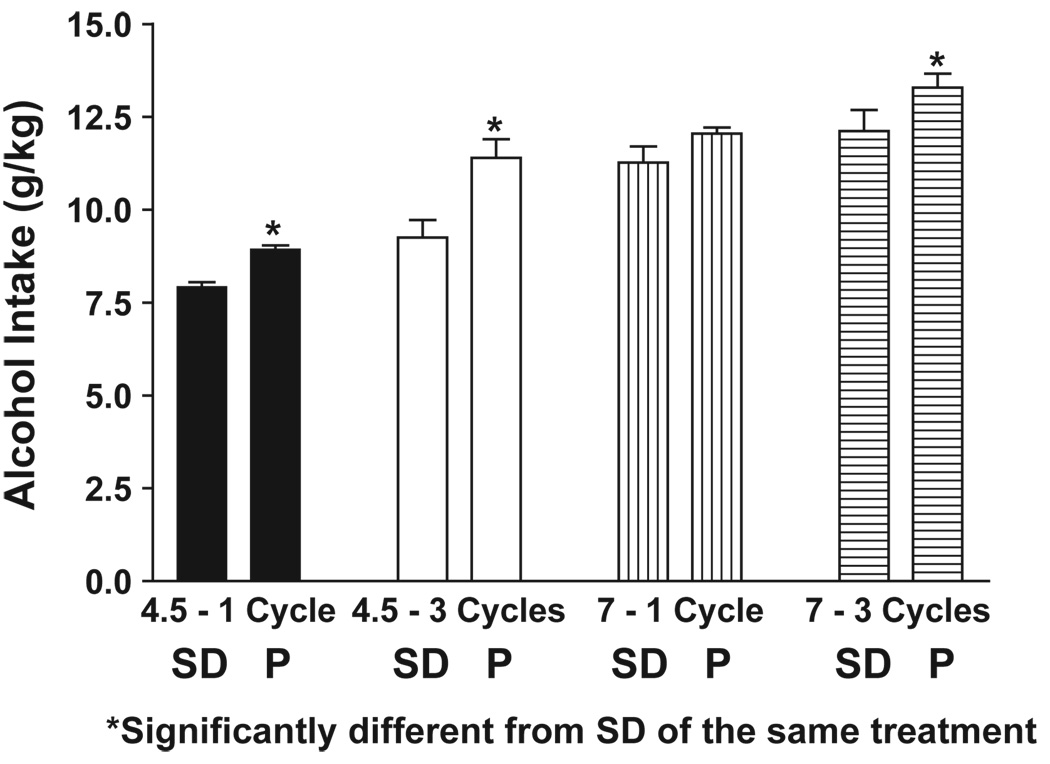
Alcohol intakes during the 1st and last cycles of exposure to 4.5% or 7% ED are illustrated in Fig. 3. Both groups had very high ethanol intakes. However, on 3 of the 4 comparisons, the P rat drank significantly more ethanol than the SD rat. It should be stressed that the ethanol is in the liquid diet and the rat cannot avoid drinking it. Nevertheless, it appears that the P rat drinks more ethanol under these forced conditions than does the SD rat. Blood ethanol levels were not recorded in this study, but earlier experiments in SD rats showed that the levels in rats exposed to 7% ED were 100–125 mg% during the first two cycles and about 200 mg% at the end of the third cycle (Overstreet et al., 2002).
Fig. 3.
Alcohol intake in P or SD rats exposed to three cycles of 4.5 or 7% ethanol diets. Data represent the mean g/kg±S.E.M. ethanol intake. *Significant difference, p < 0.01, from SD rats of the same condition, t test.
3.3. Treatment with flumazenil
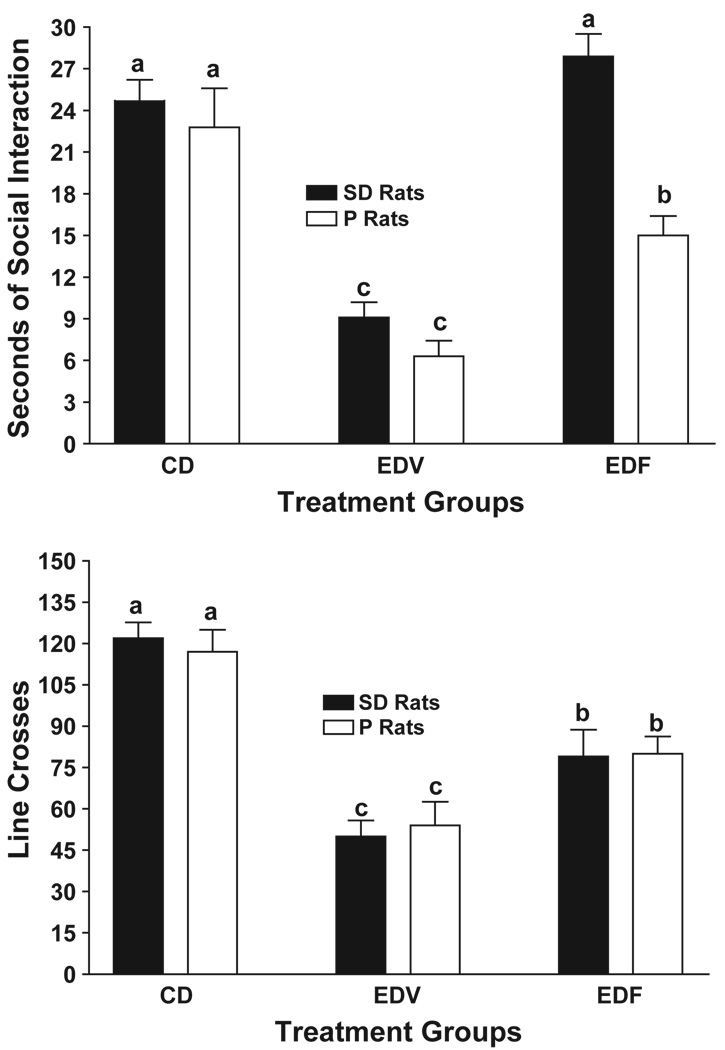
Rats of both strains that were exposed to 3 cycles of 5 days 7% ED exhibited large reductions in time spent in social interaction (Fig. 4, upper panel), as noted in Fig. 1. As previously reported (Knapp et al., 2005), flumazenil pretreatment during the 1st and 2nd withdrawals significantly counteracted this anxiety-like behavior in the SD rats. However, in the P rats, flumazenil treatment was only partially effective. Consequently, the 2-way ANOVA revealed significant treatment (F[2,42] = 41.84, p < 0.0001), strain (F[1,42] = 24.67, p < 0.001), and interaction (F[2,42] = 3.24, p < 0.05) effects.
Fig. 4.
Prophylactic effects of flumazenil in P or SD rats subjected to three cycles of exposure to 7% ethanol diet. Rats were exposed continuously to control diet or to three cycles of 5 days’ exposure to 7% ethanol, with 2 days of withdrawal between cycles. Flumazenil (5 mg/kg) or CMC vehicle were injected i.p. 4 h into the 1st and 2nd withdrawals only. The social interaction test was conducted 5 h into the 3rd withdrawal. Upper panel: data represent the mean±S.E.M sec of social interaction for 8–10 rats. Lower panel mean line crosses±S.E.M. Groups with different letters are significantly different, p < 0.01, according to 2-way ANOVA and Tukey’s protected t tests.
As illustrated in the lower panel of Fig. 4, the pattern of the prophylactic effects of flumazenil on locomotor activity was different from that on social interaction. Both strains exhibited reduced line crosses after exposure to 3 cycles of 7% ED and both showed partial recovery when pretreated with flumazenil. Therefore, the two-way ANOVA revealed only a significant treatment effect (F[2,42] = 30.87, p < 0.0001). The strain (F[1,42] = 0.62, NS) and interaction (F[2,42] = 0.15, NS) effects were not significant.
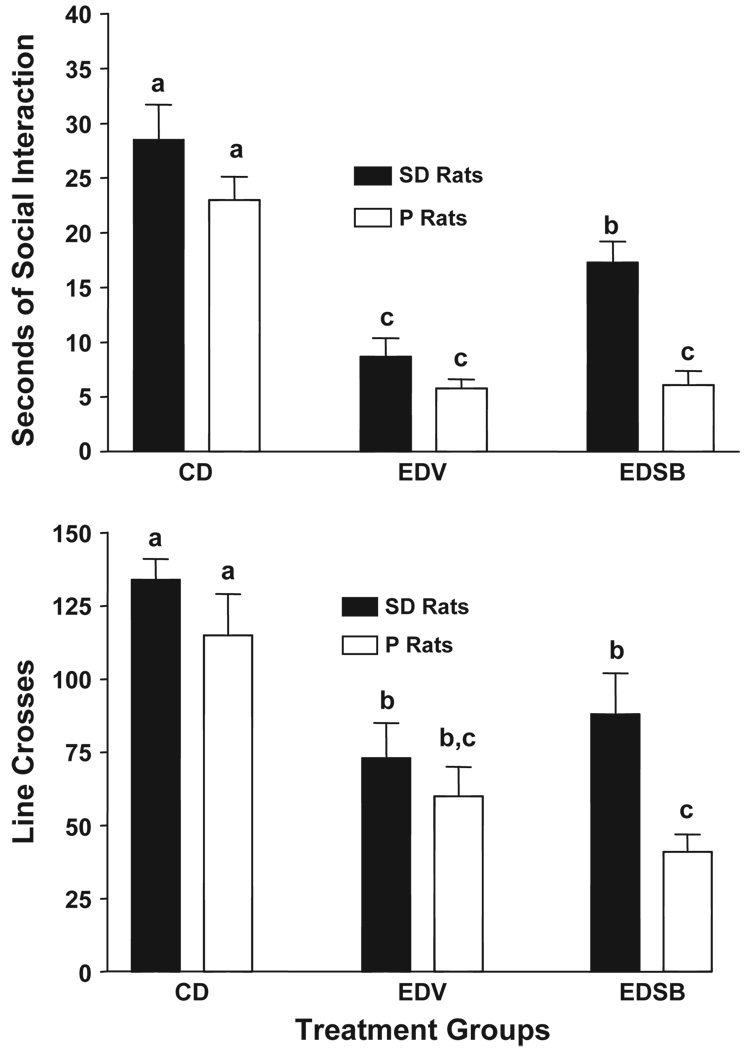
3.4. Treatment with SB243,213
The effects of SB243,213 on social interaction behavior and line crosses in repeatedly withdrawn P and SD rats were fairly straight forward (Fig. 5). By far the most noteworthy finding was the significant treatment effect (F[2,34] = 58.48, p < 0.0001 for social interaction; F[2,34] = 24.11, p < 0.0001 for line crosses). There were also significant strain effects (F[2,34] = 9.16, p < 0.01 for social interaction; F[2,34] = 4.38, p < 0.05 for line crosses) but there were no significant interaction effects for either social interaction (F[2,34] = 2.80, p = 0.08) or line crosses (F[2,34] = 0.32, NS). The simple conclusion is that SB243,213 was partially effective in counteracting the reduced social interaction in the SD rats but not in the P rats (Fig. 5, upper panel). The reduced activity was unaffected by treatment with SB243,213 (Fig. 5, lower panel).
Fig. 5.
The prophylactic effects of SB243,213, 5-HT2C receptor inverse agonist, on anxiety-like behavior in P and SD rats induced by repeated ethanol withdrawals. Rats were exposed to control diet (CD) continuously or to three cycles of 5 days’ exposure to 4.5% ethanol, with two days of withdrawal between cycles. Injections of CMC vehicle (EDV) or 3 mg/kg SB243,213 (EDSB) were given at 4 h into withdrawal from the 1st and 2nd cycles. The social interaction test was conducted 5 h into the 3rd withdrawal. Upper panel: data represent the mean±S.E.M. sec of social interaction for 6 – 8 rats. Lower panel: mean line crosses±S.E.M. Groups with different letters are significantly different, p < 0.01, according to 2-way ANOVA and Tukey’s protected t tests.
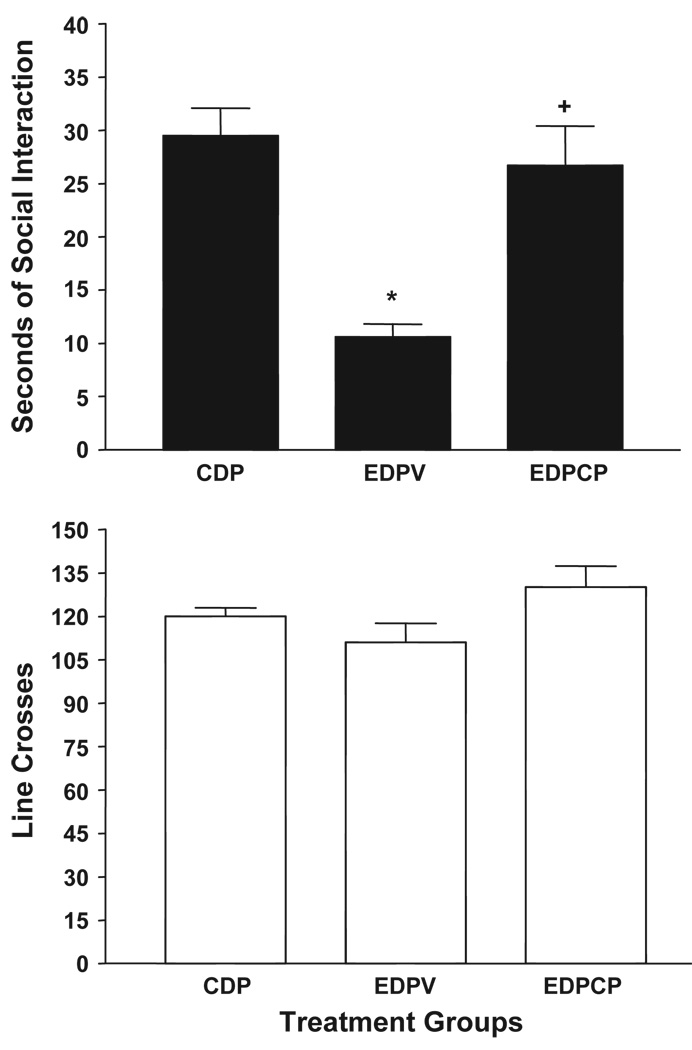
3.5. Treatment with CP-154,526
As was previously found in SD rats (Overstreet et al., 2004a), pretreatment with the CRF1 receptor antagonist CP154,526 counteracted the anxiety-like behavior in P rats induced by 3 cycles of exposure to 4.5 ED, as illustrated in the upper panel of Fig. 6. A one-way ANOVA confirmed the significant group differences (F[2,21] = 36.73, p < 0.001) and follow-up Tukey’s tests indicated that the group pretreated with CP154,526 engaged in significantly more social interaction than the group given vehicle. There were no differences (F[2,21] = 2.63, p > 0.05) in line crossings (Fig. 6, lower panel).
Fig. 6.
Prophylactic effects of CP-154,526, CRF1 receptor antagonist, on anxiety-like behavior of P Rats exposed to repeated cycles of ethanol exposure. Rats were exposed to control diet (CD) continuously or three cycles of 5 days’ exposure to 4.5% ethanol diet (EDV or EDCP). Injections of the vehicle CMC or CP-154,526 (10 mg/kg) were given 4 h into the 1st and 2nd withdrawals only. The social interaction test was conducted 5 h into the 3rd withdrawal. Upper panel: data represent the mean±S.E.M. sec of social interaction for 8 – 10 rats. Lower panel: data represent the mean line crosses±S.E.M. *Significantly different from CD group, p < 0.01, Tukey’s protected t test. +Significantly different from EDV group, p < 0.01, Tukey’s protected t test. Groups: CDP—control diet in P rats; EDPV—ethanol diet in P rats given vehicle; EDPCP—ethanol diet in P rats given CP-154526.
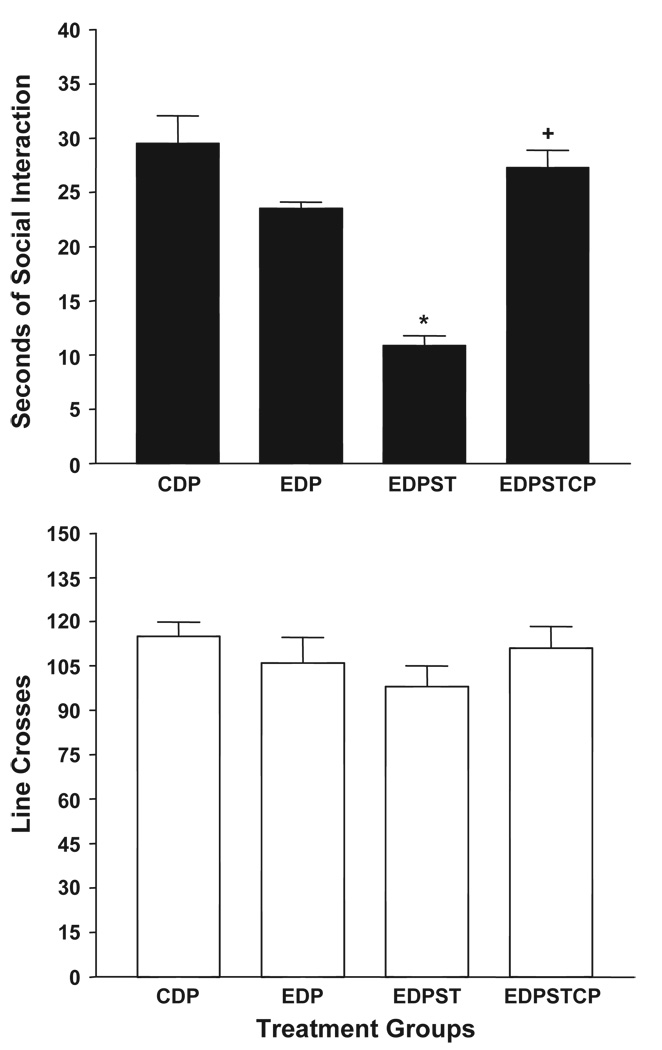
Fig. 7 shows that, as with SD rats, prior exposure to restraint stress in P rats results in anxiety-like behavior following exposure to a single 5-day cycle of 4.5% ED (Breese et al., 2004a). Pretreatment with CP154,526 30 min prior to each of these stresses prevented the anxiety-like behavior (Fig. 7, upper panel). Analyses with one-way ANOVA (F[3,28] = 42.46, p < 0.0001) and subsequent Tukey’s tests indicated that the stressed group exhibited less social interaction than the rats exposed to CD or one cycle of ED and that the rats pretreated with CP154,526 exhibited a significantly higher time spent in social interaction than the group given vehicle. Once again, there were no significant effects on line crosses (F[3,28] = 2.47, p > 0.05; Fig. 7, lower panel).
Fig. 7.
Counteraction of restraint stress facilitation of anxiety-like behavior induced by ethanol withdrawal in P rats by CP-154,526, CRF1 receptor antagonist. Rats were exposed to control diet (CDP) throughout or to a final period of 5 days of 4.5% ethanol diet (ED). The rats exposed to ED were either unstressed (EDP) or subjected to 1 h of restraint stress 1 and 6 days prior to being exposed to ethanol. One group received CMC vehicle (EDPST) 30 prior to restraint and the other received CP-154,526 (EDPSTCP, 10 mg/kg). Upper panel: data represent the mean±S.E.M. sec of social interaction for 5–8 rats. Lower panel: data represent the mean line crosses±S.E.M. *Significantly different from CD group, p < 0.01, Tukey’s protected t test. +Significantly different from EDV group, p < 0.01, Tukey’s protected t test.
4. Discussion
The alcohol-preferring P rats exhibit similar anxiety-related behavior in the social interaction test to the SD rats following one or three cycles of exposure to 4.5% ED (Fig. 2) or three cycles of exposure to 7% ED (Fig. 1). They differed only in their response to a single cycle of exposure to 7% ED (Fig. 1). This pattern of results suggests that the P rat is more sensitive to the withdrawal effects of ethanol.
A fairly straightforward explanation for this difference could be differences in alcohol intake, with the P rats generally having higher alcohol intakes than the SD rats (Fig. 3). However, a closer examination of our data indicates that this explanation is not adequate. The exposure condition which differentiated the behavior of the P and SD rats was a single cycle of 5 days exposure to 7% ED, but the strains did not differ in alcohol intake in this condition (Fig. 3). On the other hand, the P and SD rats exhibited differences in alcohol intake after the other exposure conditions, but they did not exhibit differences in social interaction behavior.
Flumazenil was less effective in counteracting ethanol withdrawal-induced anxiety in the P rat (Fig. 4). Differences in BZD receptor function in the P rat (Thielen et al., 1993, 1997, 1998) could account for this reduced effectiveness of flumazenil. Compared to nonalcohol-preferring (NP) rats, the P rats have elevated BZD receptors (Thielen et al., 1997) and exhibit a greater response to a BZD agonist (Thielen et al., 1993). Such enhanced BZD function in the P rats would make it more difficult for flumazenil to block these BZD receptors and, consequently, less effective in counteracting the anxiety-like behavior. However, there is a caveat to this apparently straightforward explanation. There are no data indicating that the BZD receptor function in SD rats is similar to the NP rats and, therefore, less than that of the P rats. The changes in GABA (A) receptor function observed in the P rat after isolation (Thielen et al., 1993) would not appear to be a contributing factor to the results observed here because comparable changes have been reported in Sprague–Dawley and other outbred rats (Dong et al., 1999; Insel, 1989; Ojima et al., 1997; Serra et al., 2000).
One of the key findings in the P rats is a reduction in 5-HT content in many limbic regions (Murphy et al., 1987). An interbreeding study has confirmed that low 5-HT content and high alcohol intake are associated in the F2 crosses (McBride et al., 1995). Of particular relevance to this study, however, is a report that the 5-HT2C receptors are elevated in the amygdala of the P rats compared to the NP rats (Pandey et al., 1996). We have recently reported that alcohol withdrawal induced anxiety-like behavior can be counteracted by injections of the 5-HT2C receptor inverse agonist, SB-243,213, directly into the amygdala (Overstreet et al., 2004b). If alcohol-preferring P rats have elevated 5-HT2C receptors relative to SD rats, then one might expect them to be resistant to the prophylactic effects of the compound, as was found to be the case (Fig. 5).
The CRF1 receptor antagonist, CP-154,526, was very effective in counteracting both multiple withdrawal- and stress-induced increases in anxiety-like behavior (Figs. 6 and 7). It appears, therefore, that the compound is as effective in P rats as it is in SD rats (Overstreet et al., 2004a), unlike flumazenil and SB-243,213. Although there are reports of lower CRF levels in the P rat relative to the NP rat (Ehlers et al., 1992; Hwang et al., 2001), it is not clear what impact these differences might have on CRF receptors and there appears to be no data on this measure. If the receptors had increased in response to the low levels of CRF, it would have been predicted that the CRF1 receptor antagonist would not be as effective in the P rats. Clearly, that is not the case, and it is likely that the CRF receptors in the P rats are functioning normally.
These findings support the view that the neurochemical background of the alcohol-preferring P rats does influence its response to drugs that have been reported to have a prophylactic effect in the repeated ethanol withdrawal paradigm. Importantly, the anxiety-like behavior of the P rats was ameliorated very well by a CRF1 receptor antagonist, a finding that provides further support for the potential use of this class of compounds as anxiolytic agents (Heinrichs et al., 1997; Kehne and De Lombaert, 2002; Seymour et al., 2003).
These strain-dependent prophylactic effects also have important clinical implications. Because each of these agents has both acute and prophylactic anxiolytic effects, they might prove useful in treating alcoholics during the detoxification phase and thereby lessen the impact of anxiety on relapse (e.g., Sloan et al., 2003). To the extent that alcoholics have neurochemical abnormalities similar to those shown by the P rat, they may be resistant to the anxiolytic effects of certain classes of drugs (See Breese et al., 2004b). The fact that the CRF1 receptor antagonist CP-154,526 counteracted the anxiety-like behavior of both P (Figs. 6 and 7) and SD rats (Breese et al., 2004a; Overstreet et al., 2004a) supports the view that these drugs deserve further attention as potential therapeutic agents for alcoholism.
Another drug that has been successful in reducing anxiety-like behavior in SD rats repeatedly exposed and withdrawn from ethanol is buspirone, a partial 5-HT1A agonist (Overstreet et al., 2003). To determine whether a similar prophylactic effect could be seen in P rats, 4 animals were exposed to 3 cycles of 5 days’ exposure to 4.5% alcohol diet and treated with 0.6 mg/kg buspirone at 4 h into the first and second withdrawals. The time spent in social interaction (18.8 ± 2.4 s) was not significantly different from that of a group of 8 rats maintained on control diet (25.3 ± 1.9; t = 2.11, p > 0.05). Thus, buspirone appears to have similar prophylactic effects in P and SD rats. These effects are consistent with the early reports of the effectiveness of buspirone in managing alcohol detoxification (Dougherty and Gates, 1990; Tollefson et al., 1992), even though later studies indicated that buspirone was relatively ineffective in preventing relapse in alcoholics (e.g., Malec et al., 1996). Thus, buspirone and the other drugs used in this study may play a role in treatment of alcoholics, but not as traditional anti-relapse agents (see Breese et al., 2004b). Indeed, they might be most effective if given in conjunction with naltrexone or acamprosate.
Acknowledgements
This work was supported, in part, by funding from NIAAA (2 P60 AA011605-06, AA-014073, AA-014284). We acknowledge Pfizer for providing CP-154,526 and Glaxo-Smith-Kline for providing SB-243,213. We thank T-K Li and Lawrence Lumeng of the Indiana Alcohol Research Center for sending us breeding pairs of the inbred P rats to establish our own colonies. We wish to thank the following for technical assistance: Lara Marr, Mili Senapati, Qi Yu.
References
- Andrews N, File SE, Fernandes C, Gonzalez LE, Barnes NM. Evidence that the median raphe nucleus–dorsal hippocampal pathway mediates diazepam withdrawal-induced anxiety. Psychopharmacology. 1997;130:228–234. doi: 10.1007/s002130050233. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DK, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF1 and benzodiazepine receptor antagonists and a 5-HT1A receptor agonist. Neuropsychopharmacology. 2004a;29:470–482. doi: 10.1038/sj.npp.1300282. [online publication 10 July, 2003 at http://www.acnp.org/citations/Npp07100303159/default.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiolgy of alcoholism: a “kindling”/stress hypothesis. Psychopharmacology. 2004b doi: 10.1007/s00213-004-2016-2. [Electronic publication ahead of print] PMID: 15517196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B, Jones BJ, Kelly ME, Naylor RJ, Onaivi ES, Tyers MB. Ondansetron inhibits a behavioural consequence of withdrawing from drugs of abuse. Pharmacol Biochem Behav. 1990;36:339–344. doi: 10.1016/0091-3057(90)90414-d. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Tohda M, Kaneko Y, Watanabe H. Diazepam binding inhibitor (DBI) gene expression in the brains of socially isolated and group-housed mice. Neurosci Res. 1999;33:171–177. doi: 10.1016/s0168-0102(99)00010-3. [DOI] [PubMed] [Google Scholar]
- Dougherty RJ, Gates RR. The role of buspirone in the management of alcohol withdrawal: a preliminary investigation. J Subst Abuse Treat. 1990;7:189–192. doi: 10.1016/0740-5472(90)90021-h. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI, Wall TL, Lumeng L, Li TK, Owens MJ, et al. Corticotropin releasing factor (CRF): studies in alcohol-preferring and -nonpreferring rats. Psychopharmacology. 1992;106:359–364. doi: 10.1007/BF02245418. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- File SE, Baldwin HA, Hitchcot PK. Flumazenil but not nitrendipine reverses the increased anxiety during alcohol withdrawal in the rat. Psychopharmacology. 1989;98:262–264. doi: 10.1007/BF00444702. [DOI] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Differential sensitivity of alcohol withdrawal signs in the rat to γ-aminobutyric acid (GABA) mimetics: blockade of audiogenic seizures but not forelimb tremors. J Pharmacol Exp Ther. 1983;226:720–725. [PubMed] [Google Scholar]
- Heinrichs DT, Lapansky SC, Lovenberg J, DeSouza TW, Chalmers EB. Corticotropin releasing factor CRF1 but not CRF2, receptors mediate anxiogenic-like behavior. Regul Pept. 1997;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- Holter SM, Engelmann M, Kirschke C, Liebsch G, Landgraf R, Spanagel R. Long-term ethanol self-administration with repeated ethanol deprivation episodes changes ethanol drinking pattern and increases anxiety-related behaviour during ethanol deprivation in rats. Behav Pharmacol. 1998;9:41–48. [PubMed] [Google Scholar]
- Hwang BH, Lumeng L, Wu JY, Li TK. Increased number of GABAergic terminals in the nucleus accumbens is associated with alcohol preference in rats. Alcohol Clin Exp Res. 1990;14:503–507. doi: 10.1111/j.1530-0277.1990.tb01188.x. [DOI] [PubMed] [Google Scholar]
- Hwang BH, Stewart RB, Lumeng L, Li T-K. The down-regulation of corticotropin releasing factor gene expression in the central nucleus of the amygdala is associated with high alcohol preference and anxiety in alcohol-preferring rats. Abstr-Soc Neurosci. 2001;27 [program no. 732-11] [Google Scholar]
- Insel TR. F Decreased in vivo binding to brain benzodiazepine receptors during social isolation. Psychopharmacology. 1989;97:142–144. doi: 10.1007/BF00442234. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Bagnalasta M, Marcon C, Motta C, Tessari M, File SE, et al. Nicotine self-administration and withdrawal: modulation of anxiety in the social interaction test in rats. Psychopharmacology. 2001;153:315–320. doi: 10.1007/s002130000586. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol Clin Exp Res. 2000;24:278–284. [PubMed] [Google Scholar]
- Kehne J, De Lombaert S. Non-peptide CRF1 receptor antagonists for the treatment of anxiety, depression and stress disorders. Curr Drug Target CNS Neurol Disord. 2002;1:467–493. doi: 10.2174/1568007023339049. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Duncan GE, Crews FT, Breese GR. Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res. 1998;22:481–493. [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet, Breese GR. Modulation of ethanol withdrawal-induced anxiety-like behavior during later withdrawals by treatment of early withdrawals with benzodiazepine/GABA ligands. Alcohol Clin Exp Res. 2005;29:553–563. doi: 10.1097/01.alc.0000158840.07475.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Lumeng L, Doolittle DP. Selective breeding for alcohol preference and associated responses. Behav Genet. 1993;23:163–170. doi: 10.1007/BF01067421. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Roberts J-S, Wang W, Myrick H, Anton RF. Multiple previous detoxifications are associated with less responsive treatment and heavier drinking during an index outpatient detoxification. Alcohol. 2000;22:159–164. doi: 10.1016/s0741-8329(00)00114-2. [DOI] [PubMed] [Google Scholar]
- Malec TS, Malec EA, Dongier M. Efficacy of buspirone in alcohol dependence: a review. Alcohol Clin Exp Res. 1996;20:853–858. doi: 10.1111/j.1530-0277.1996.tb05263.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Bodart B, Lumeng L, Li T-K. Association between low contents of dopamine and serotonin in the nucleus accumbens and high alcohol preference. Alcohol Clin Exp Res. 1995;19:1420–1422. doi: 10.1111/j.1530-0277.1995.tb01001.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Chernet E, Russell RN, Wong DT, Guan X-M, Lumeng L, et al. Regional CNS densities of monoamine receptors in alcohol-naive alcohol preferring P and -nonpreferring NP rats. Alcohol. 1997;14:141–148. doi: 10.1016/s0741-8329(96)00117-6. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Breese GR. Multiple withdrawals from chronic ethanol “kindles” inferior collicular seizure activity: evidence for kindling of seizures associated with alcoholism. Alcohol Clin Exp Res. 1990;14:394–399. doi: 10.1111/j.1530-0277.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Criswell HE, Breese GR. Flumazenil blockade of anxiety following ethanol withdrawal in rats. Psychopharmacology. 1997;131:354–360. doi: 10.1007/s002130050303. [DOI] [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Duncan GE, Breese GR. Enhanced ultrasonic vocalization and Fos protein expression following ethanol withdrawal: effects of flumazenil. Psychopharmacology. 2000;152:208–215. doi: 10.1007/s002130000507. [DOI] [PubMed] [Google Scholar]
- Murphy JM, McBride WJ, Lumeng L, Li T-K. Contents of monoamines in forebrain regions of alcohol-preferring (P) and -nonpreferring (NP) lines of rats. Pharmacol Biochem Behav. 1987;26:389–392. doi: 10.1016/0091-3057(87)90134-1. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Ojima K, Matsumoto K, Watanabe H. Flumazenil reverses the decrease in the hypnotic activity of pentobarbital by social isolation stress: are endogenous benzodiazepine receptor ligands involved? Brain Res. 1997;745:127–133. doi: 10.1016/s0006-8993(96)01136-5. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decreases in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1269. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. A 5-HT1A agonist and a 5-HT2C antagonist reduce social interaction deficit induced by multiple ethanol withdrawals in rats. Psychopharmacology. 2003;167:344–352. doi: 10.1007/s00213-003-1425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004a;78:459–464. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of alcohol withdrawal-induced anxiety by drugs that interact with 5-HT2C receptors in the amygdala; Presented at Meeting of World Federation of Societies of Biological Psychiatry; Australia: Sydney; 2004b. Feb 9–13, Presentation #7206. [Google Scholar]
- Pandey SC, Lumeng L, Li T-K. Serotonin2C receptors and serotonin2C receptor-mediated phosphoinositide hydrolysis in the brain of alcohol-preferring and alcohol-nonpreferring rats. Alcohol Clin Exp Res. 1996;20:1038–1042. doi: 10.1111/j.1530-0277.1996.tb01944.x. [DOI] [PubMed] [Google Scholar]
- Potokar J, Coupland N, Glue P, Groves S, Malizia A, Biley J, et al. Flumazenil in alcohol withdrawal: a double-blind placebo-controlled study. Alcohol Alcohol. 1997;32:605–611. doi: 10.1093/oxfordjournals.alcalc.a008302. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, et al. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Seymour PA, Schmidt AW, Schultz DW. The pharmacology of CP-154,526, a non-peptide antagonist of the CRH1 receptor A review. CNS Drug Rev. 2003;9:57–96. doi: 10.1111/j.1527-3458.2003.tb00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan TB, Roache JD, Johnson BA. The role of anxiety in predicting drinking behaviour. Alcohol Alcohol. 2003;38:360–363. doi: 10.1093/alcalc/agg090. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, McBride WJ, Lumeng L, Li T-K. Housing conditions alter GABA (A) receptor of alcohol-preferring and -nonpreferring rats. Pharmacol Biochem Behav. 1993;46:723–727. doi: 10.1016/0091-3057(93)90568-e. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, McBride WJ, Chernet E, Lumeng L, Li T-K. Regional densities of benzodiazepine sites in the CNS of alcohol-naïve P and NP rats. Pharmacol Biochem Behav. 1997;57:875–882. doi: 10.1016/s0091-3057(96)00464-9. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, McBride WJ, Lumeng L, Li T-K. GABA (A) receptor function in the cerebral cortex of alcohol naive P and NP rats. Pharmacol Biochem Behav. 1998;59:209–214. doi: 10.1016/s0091-3057(97)00418-8. [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Montagu-Clouse J, Tollefson SF. Treatment of comorbid generalized anxiety in a recently detoxified alcoholic population with a selective serotonergic drug (buspirone) J Clin Psychopharmacol. 1992;12:19–25. doi: 10.1097/00001573-199202000-00004. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Bledsoe S, Lumeng L, Li T-K. Immunostained serotonergic fibers are decreased in selected brain regions of alcohol-preferring rats. Alcohol. 1991;8:425–431. doi: 10.1016/s0741-8329(91)90034-t. [DOI] [PubMed] [Google Scholar]