Abstract
Favorable health outcomes at 2 years postbariatric surgery have been reported. With exception of the Swedish Obesity Subjects (SOS) study, these studies have been surgical case series, comparison of surgery types, or surgery patients compared to subjects enrolled in planned nonsurgical intervention. This study measured gastric bypass effectiveness when compared to two separate severely obese groups not participating in designed weight-loss intervention. Three groups of severely obese subjects (N = 1,156, BMI ≥ 35 kg/m2) were studied: gastric bypass subjects (n = 420), subjects seeking gastric bypass but did not have surgery (n = 415), and population-based subjects not seeking surgery (n = 321). Participants were studied at baseline and 2 years. Quantitative outcome measures as well as prevalence, incidence, and resolution rates of categorical health outcome variables were determined. All quantitative variables (BMI, blood pressure, lipids, diabetes-related variables, resting metabolic rate (RMR), sleep apnea, and health-related quality of life) improved significantly in the gastric bypass group compared with each comparative group (all P < 0.0001, except for diastolic blood pressure and the short form (SF-36) health survey mental component score at P < 0.01). Diabetes, dyslipidemia, and hypertension resolved much more frequently in the gastric bypass group than in the comparative groups (all P < 0.001). In the surgical group, beneficial changes of almost all quantitative variables correlated significantly with the decrease in BMI. We conclude that Roux-en-Y gastric bypass surgery when compared to severely obese groups not enrolled in planned weight-loss intervention was highly effective for weight loss, improved health-related quality of life, and resolution of major obesity-associated complications measured at 2 years.
INTRODUCTION
Several studies have shown bariatric surgery as a successful long-term treatment for weight-reduction for people with extreme (class III) obesity, especially when measured 2 years postoperatively (1–4). More recently, three prospective randomized controlled trials have compared health outcomes between bariatric surgery and nonsurgical treatment intervention (5–8). Importantly, none of these trials included gastric bypass procedures, only one study included exclusively severely obese subjects (6), one study limited participants to the overweight BMI range (a range that does not now qualify a patient for bariatric surgery) (5,8), and one study limited BMI to <40 kg/m2 (7). Further, none of these studies included control groups not enrolled in weight-loss intervention treatment.
Mingrone et al. (6) randomly assigned 79 severely obese patients to biliopancreatic diversion, a malabsorptive surgery, or a low-calorie diet (~1,200 kcal/day) and followed all subjects for 1 year. O’Brien and Dixon and colleagues (5,8) randomized 80 overweight participants (BMI 30–35 kg/m2) to a program of very-low-calorie diets, pharmacotherapy, and lifestyle change for 24 months or to placement of an adjustable gastric band. In addition, Dixon et al. (7) randomized 60 patients recently diagnosed with diabetes (BMI, >30 kg/m2 and <40 kg/m2) to primarily lifestyle modification (diet and exercise) with very-low-calorie diet and weight-loss medications prescribed as appropriate or to placement of an adjustable gastric band. The Swedish Obesity Subjects (SOS) study (4), a prospective nonrandomized intervention trial, enrolled over 4,000 surgical and nonsurgical severely obese participants from 25 surgical departments and 480 primary health-care centers (9). In this study, only 13% of the surgical patients had gastric bypass surgery, while 19% had adjustable or nonadjustable banding, and the remaining majority, 68%, underwent vertical banded gastroplasty, a procedure that results in less weight loss than gastric bypass and is now performed on a very limited basis (9). The nonsurgical control patients received usual care for weight loss at the center where they registered for the study. Finally, MacDonald et al. studied 232 severely obese patients, all with type 2 diabetes. In total, 154 had gastric bypass and the remaining 78 did not have bariatric surgery due to personal preference or because their health insurance would not cover the procedure. Control patients received usual care during the follow-up period.
In view of the rapid increase in bariatric surgery (1,10–12), with Roux-en-Y gastric bypass representing the greater majority of these surgeries in the United States, and in response to the limited data comparing bariatric surgery to nonsurgical treatment (1), this study was undertaken. This research represents the only study to date that has exclusively included Roux-en-Y patients not limited by a specific disease (i.e., diabetes) and that has included comparative groups not enrolled in a specific, nonsurgical weight-loss treatment program. Using this design, the primary hypothesis was to determine health outcomes following gastric bypass surgery in comparison to severely obese subjects, seeking and not seeking gastric bypass surgery. The unique inclusion of the two comparative groups provided the opportunity to test what happens to the health status of severely obese individuals if they are left to their own devices regarding weight-loss treatment and directly addresses the beneficial effects of gastric bypass surgery vs. no intervention.
METHODS AND PROCEDURES
Subjects
A total of 1,156 participants comprised the three study groups (Table 1). Two groups included patients seeking gastric bypass surgery from a partnership of three bariatric surgeons in Utah. The surgical group (N = 420) underwent gastric bypass surgery while the first comparative group (N = 415) sought but did not have surgery, primarily as a result of denial of insurance coverage, and in a few cases due to personal preference. The second comparative group was population-based, consisting of severely obese participants who were not seeking bariatric surgery (N = 321). These participants were recruited from the Utah Health Family Tree program based in high schools (13,14) a database representing over 1 million first-degree relatives from 151,188 families (15). These families had previously given consent for future contact in regard to research activities. From this database, there were 16,482 individuals who had reported being at least 100 pounds overweight. These potential severely obese individuals were contacted by telephone to determine whether (i) they were severely obese (BMI ≥ 35 kg/m2), (ii) they had not had bariatric surgery, and (iii) they were willing to participate in this study. Exclusion criteria for all study participants included: previous gastric surgery for weight loss; gastric or duodenal ulcers in the previous 6 months; active cancer within the past 5 years (except for nonmelanoma skin cancer); myocardial infarction in the previous 6 months; and history of alcohol or narcotic abuse.
Table 1.
Cumulative participation (N): General Clinical Research Center, Cardiovascular Genetics Clinic, and Endpoints Questionnaire
| Gastric bypass surgery patients | Comparative group: seeking but did not have bypass surgery | Comparative group: not seeking bariatric surgery | |
|---|---|---|---|
| Baseline participation | |||
| GCRC, N | 307 | 285 | 282 |
| Clinic, N | 113 | 129 | 39 |
| Total, N | 420 | 415 | 321 |
| Follow-up participation | |||
| GCRC or CVG clinic or medical chart abstraction or Disease Endpoints Questionnaire | 402 (96%) | 386 (93%) | 315 (98%) |
| Only GCRC, N | 220 | 173 | 225 |
| Only CVG clinic, N | 98 | 97 | 54 |
| Only medical chart abstraction or Disease Endpoints Questionnaire, N | 84 | 116 | 36 |
| Comparative subjects going on to have GBP surgery | NA | 56 | 12 |
| Refused follow-up participation | 4 | 1 | 1 |
| Lost-to-follow-upa | 12 | 26 | 4 |
| Deaths | 2 | 2 | 0 |
GCRC, general clinical research center; CVG, cardiovascular genetics; GBP, gastric bypass; NA, not applicable.
Represents participants who at the time of follow-up could not be found.
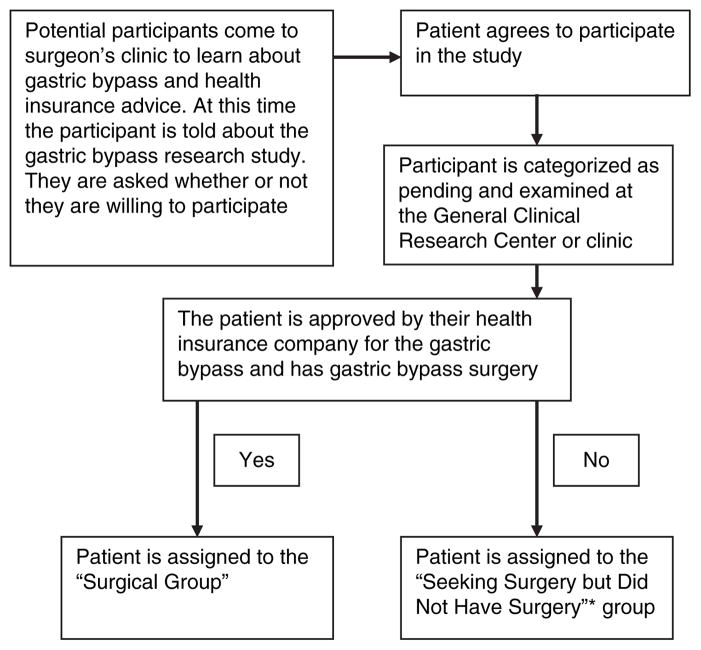
Of note, gastric bypass patients seeking surgery quite often face an extended application process of many months before hearing whether or not their surgery is approved, denied or put on hold pending more clinical data. This situation, coupled with the time-line set forth for this study, compelled us to recruit all patients visiting the surgical center seeking information about gastric bypass. Often, subjects expressed willingness to participate in the study before they knew whether or not health insurance would cover surgical costs. Therefore, many patients agreed to be in our study, participated in the initial testing and were categorized as “pending-surgery” subjects. Once the participant found out surgery approval status, they were further classified as “surgery group” or “seeking surgery but did not have surgery group” (see Figure 1). The primary factor determining group assignment (surgery or seeking surgery but did not have surgery) was whether or not the insurance company would cover the procedure.
Figure 1.
Recruitment scheme for gastric bypass surgical patients and patients seeking gastric bypass but did not have gastric bypass surgery. *In a few instances, patients chose not to have gastric bypass surgery.
The study protocol was approved by the University of Utah Institutional Review Board, and signed consent was obtained from all participants.
Surgery technique and study protocol
The surgical group underwent either open or laparoscopic Roux-en-Y gastric bypass, by one of three surgeons, performed as previously described (16,17). Participants of all three groups underwent a baseline evaluation, which was performed in the surgical group before surgery. For most participants (Table 1) the baseline evaluation was performed in the University of Utah General Clinical Research Center (GCRC) during an overnight stay in which they completed a series of standardized tests and questionnaires as described elsewhere (18). For the remaining participants (Table 1) baseline data were obtained in our outpatient clinic where identical questionnaires but an abbreviated clinical protocol were administered (18). Subjects visiting the clinic did so if they preferred not to stay overnight in the GCRC or if the GCRC was already full for that evening.
All participants were invited to return to the GCRC or our clinic for a 2-year follow-up exam. For subjects who had moved out of state or who chose not to return, telephone contact was attempted to obtain self-reported medical endpoint data. Also, release of medical information forms were mailed to them to attempt to obtain medical records from their primary care physician or bariatric surgeon to ascertain information on study endpoints. As previously described (18), coronary heart disease was defined as a myocardial infarction, coronary bypass surgery, or percutaneous transluminal coronary angioplasty. Hypertension was defined as a blood pressure of ≥140/90 mm Hg (average of three measurements sitting) or use of antihypertensive medications. Diabetes was defined as a fasting glucose level of ≥126 mg/dl or use of antidiabetic medication. Dyslipidemia was defined as low-density lipoprotein-cholesterol ≥160 mg/dl, high-density lipoprotein-cholesterol <40 mg/dl, or triglycerides ≥200 mg/dl, or use of lipid-lowering medication. In addition to these outcome variables, in all subjects seen in the GCRC we also obtained a resting metabolic rate (RMR) (indirect calorimetry), measured the time required to reach 80% of maximal calculated heart rate using a modified Bruce treadmill protocol, and performed overnight sleep studies to obtain the apnea/hypopnea index, percent of oxygen saturation readings <90%, and average night-time oxygen saturation (18).
Due to variation in measurement technique, we specifically describe methods used for measurement of RMR and body fat. The RMR was measured in the GCRC before the subject getting out of bed the morning after an overnight stay and 12-h fast. RMR was measured with open circuit indirect calorimetry using a portable metabolic cart (TrueMax 2400; Parvo Medics, Salt Lake City, UT) and a plastic ventilated hood. Before data collection, the metabolic system was calibrated with standard gases of known concentration and participants were familiarized with the ventilation hood. Participants were asked to remain motionless and not to sleep during the procedure. Once steady state was obtained, the test was continued for at least 10 min. Percent body fat was calculated from measurement of resistance and reactance to an electrical current using bioelectrical impedance equipment (RJL Systems Analyzer; Quantum II, Clinton, MI). Participants were asked to comply with the following criteria before the impedance analysis: fasting overnight or for a minimum of 4–5 h; no exercise for at least 12-h; no alcohol for at least 24-h; and balanced hydration. All participants were asked to lie in a supine position for at least 5 min before the examination.
Statistical methods
To test the main hypothesis that Roux-en-Y gastric bypass surgery reduces adverse effects of morbid obesity on disease, our primary comparison was between the surgical and the seeking but did not have surgery groups, because these two groups were most similar at baseline and this comparison provided the most valid test of surgery effectiveness. Additional comparisons were performed between the surgical and population comparative groups to infer whether the risks and benefits of gastric bypass surgery were replicated when a general population sample of severely obese subjects were used as comparative subjects. A general linear model was used to regress the continuous biological variables on a categorical variable representing study group. Covariates included in the model were gender, age, and baseline BMI. For changes from baseline, the baseline level of the variable being analyzed was also included as a covariate. A one-degree-of-freedom P value is reported for the comparison of the surgical group with each of the comparative groups separately. Because there are two multiple comparisons for each variable, we considered a P value <0.025 as evidence of statistical significance. Only nominal P values are reported in the text and tables.
Disease prevalence, incidence, and resolution were analyzed by logistic regression. Resolution of disease that was present at baseline was defined as normal levels of the variable without medication. We also tested the hypothesis that the amount of reduction of BMI or waist circumference 2 years following gastric bypass surgery would correlate with the improvement in variables of obesity comorbidity such as lipid levels and blood pressure. Partial correlation coefficients were estimated adjusting for age, gender, baseline BMI, and baseline levels of the variable being analyzed, using only gastric bypass subjects who were not on medications for the variable being analyzed at either exam. Subjects were excluded from analysis of change if follow-up measurements were not available. All analyses of change were performed both with and without subjects from the two comparative groups who went on to have bariatric surgery following baseline testing and before testing at 2-year follow-up. Because the results of these analyses showed minimal differences in effect size and significance when including subjects who went on to have bariatric surgery following baseline testing, these subjects were not included in the reported results.
Results
Baseline results
At baseline, the surgical group was generally well-matched for age, sex, and anthropometric measurements with the seeking but did not have surgery comparative group (Table 2). The surgical group compared with the population comparative group was significantly younger, had greater weight, BMI, percent body fat, and waist circumference (all P < 0.001 or <0.0001). Although there were minor differences in certain baseline clinical measurements (Table 2), there were no differences in prevalence of baseline endpoints (i.e., diabetes, hypertension, and dyslipidemia) between the surgical and population-based comparative group. Prevalence of disease also did not differ between the surgical group and the comparative group who sought surgery but did not have surgery.
Table 2.
Baseline mean and s.e. of study variables
| Study variables | Gastric bypass patients (N = 420) | Comparative group: seeking but did not have bypass surgery (N = 415) | Comparative group: not seeking bariatric surgery (N = 321) |
|---|---|---|---|
| Age, years (1,156) | 43.4 (0.61) | 43.6 (0.62) | 49.4 (0.65)**** |
| Sex, % female (1,156) | 83.8 | 85.1 | 76.0 |
| Weight, kg (1,156) | 144.0 (1.24) | 140.3 (1.26)* | 133.2 (1.37)**** |
| BMI, kg/m2 (1,156) | 47.7 (0.41) | 46.8 (0.41) | 44.3 (0.45)**** |
| Waist circumference, cm (1,155) | 139.4 (0.92) | 138.4 (0.93) | 133.7 (1.02)**** |
| Percent body fat, % (1,141) | 45.7 (0.24) | 45.4 (0.24) | 44.6 (0.26)*** |
| Systolic blood pressure, mm Hg (1,146) | 129.3 (0.97) | 128.9 (0.97) | 128.8 (1.06) |
| Diastolic blood pressure, mm Hg (1,146) | 73.5 (0.59) | 73.7 (0.59) | 73.1 (0.65) |
| Resting heart rate, bpm (1,140) | 72.8 (0.84) | 73.5 (0.84) | 73.9 (0.92) |
| Glucose, mg/dl (1,152) | 104.1 (1.92) | 109.6 (1.93)* | 107.5 (2.10) |
| Insulin, μU/ml (1,150) | 19.8 (0.82) | 18.6 (0.83) | 15.1 (0.90)**** |
| HOMA-IR (1,148) | 5.3 (0.24) | 5.1 (0.24) | 4.1 (0.26)*** |
| HbA1c, % (929) | 5.9 (0.07) | 6.1 (0.07)* | 6.0 (0.07) |
| Total cholesterol, mg/dl (1,154) | 186.5 (2.01) | 182.0 (2.02) | 185.1 (2.20) |
| LDL-cholesterol (measured), mg/dl (1,154) | 107.1 (1.52) | 104.6 (1.53) | 108.1 (1.67) |
| HDL-cholesterol, mg/dl (1,154) | 44.5 (0.59) | 42.1 (0.59)** | 43.8 (0.64) |
| VLDL-cholesterol, mg/dl (1,154) | 36.1 (1.23) | 37.0 (1.24) | 34.1 (1.35) |
| Triglycerides, mg/dl (1,154) | 194.7 (7.51) | 201.4 (7.58) | 185.6 (8.24) |
| IWQOL–Lite total score (1,057) | 65.7 (1.05) | 68.5 (1.05) | 87.9 (1.14)**** |
| SF-36, Physical component score (1,118) | 35.9 (0.34) | 36.2 (0.34) | 40.0 (0.37)**** |
| SF-36, Mental component score (1,118) | 41.3 (0.38) | 41.4 (0.38) | 44.1 (0.41)**** |
| Apnea/hypopnea index, per h (705) | 27.0 (1.56) | 27.0 (1.55) | 27.1 (1.55) |
| % Sleep time SpO2 <90%, sin−1, % (705) | 0.3159 (0.0229) | 0.3143 (0.0233) | 0.3189 (0.0227) |
| Average night-time SpO2, % O2 (705) | 90.74 (0.23) | 90.74 (0.23) | 90.69 (0.22) |
| Treadmill time, s (782) | 631 (15.00) | 602 (15.18) | 611 (15.36) |
| RMR, kcal/day (851) | 2,391 (31.83) | 2,393 (33.09) | 2,274 (32.28)** |
Weight, BMI, and % fat adjusted for age and gender only; age adjusted for gender only. All other variables adjusted for age, gender, and BMI.
HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; IWQOL–Lite, impact of weight quality of life–lite; LDL, low-density lipoprotein; RMR, resting metabolic rate; VLDL, very low-density lipoprotein.
P < 0.05,
P <0.01;
P < 0.001,
P < 0.0001 vs. surgical cases.
Follow-up results
Table 1 includes follow-up participation results for each exam by group and the type of exam (GCRC, clinic, telephone contact for disease endpoints and body weight, and clinic data obtained from physician’s office). Follow-up time was 2.3 years for each group. There were four subjects who died during the follow-up period. From the two comparative groups, 68 subjects went on to have bariatric surgery (67 had gastric bypass and 1 had adjustable lap banding) following baseline but before their follow-up exam. All but two of these participants returned for the 2-year follow-up testing. At the 2-year follow-up, participants not returning for the overnight GCRC or cardiovascular genetics clinic visit were phoned to obtain medical endpoints information as well as self-reported body weight over the telephone and to arrange for obtaining medical information from their primary care physicians. Of participants eligible for follow-up (N = 1,156), the total percentage of participants who were followed-up at the GCRC, cardiovascular genetics clinic, or by telephone for medical endpoint collection and physician-based medical records was 96, 93, and 98% of the surgery group, seeking surgery but did not have surgery comparative group, and the population-based comparative group, respectively. We were unable to contact only 3, 6, and 1% of the three groups. The number of participants who absolutely refused to visit with us further about follow-up was only 4, 1, and 1 participants of the surgery, seeking surgery but did not have surgery, and population comparative groups, respectively. Of the two deaths in the surgical group, neither occurred perioperatively. Of the two deaths in the comparative groups, one subject went on to have bariatric surgery and died ~11 months following their surgery.
Baseline clinical measurements did not differ significantly between subjects who returned for follow-up at the GCRC or at our clinic compared with those who did not complete a follow-up examination, except for lower percent body fat (P = 0.04), better short form (SF-36) health survey mental component score (P = 0.01), and better impact of weight quality of life–lite (P = 0.04) in those who returned.
Table 3 shows baseline and 2-year change results of subjects who had both baseline and follow-up exams, were not receiving medications (at either exam) that could influence the variable being analyzed, and who did not participate in bariatric surgery following baseline and before 2-year follow-up testing if they were in a comparative group. The improvements were highly significant for all major study variables for the gastric bypass surgery group when compared with the seeking but did not have surgery comparative subjects or the population-based comparative subjects (Table 3; P < 0.0001 for all variables except diastolic blood pressure and the SF-35 mental component score, which were significant at P < 0.01). When participants on medications were included in the 2-year change analysis, the significance of the improvements in the surgery group compared to the two comparative groups remained the same as the above reported results without medication with the exception of diastolic blood pressure P < 0.01 rather than P < 0.0001 for surgery compared to population-based comparative subjects.
Table 3.
Baseline and 2-year change, means and s.e. by study group (comparative group subjects going on to have bariatric surgery not included in analyses)
| Study variables (total N) | Gastric bypass surgery patients |
Comparative group: seeking but did not have bypass surgery |
Comparative group: not seeking bariatric surgery |
|||
|---|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | Baseline | Change | |
| Age, years (816) | 45.2 (0.47) | 2.35 (0.02) | 46.5 (0.52) | 2.27 (0.02) | 47.7**** (0.47) | 2.28 (0.02) |
| Weight, kg (816) | 144.0 (1.39) | −44.83 (0.88) | 139.3** (1.61) | −2.23**** (0.97) | 132.8**** (1.44) | −0.29**** (0.87) |
| BMI, kg/m2 (816) | 47.97 (0.46) | −15.77 (0.29) | 46.49** (0.53) | −0.70**** (0.34) | 44.20**** (0.47) | −0.16**** (0.30) |
| Waist circumference, cm (816) | 139.4 (1.0) | −32.09 (0.83) | 137.7 (1.2) | −0.26**** (0.95) | 133.7**** (1.09) | 1.86**** (0.86) |
| Percent body fat, % (795) | 45.62 (0.27) | −14.23 (0.42) | 45.00* (0.32) | −2.98**** (0.48) | 44.41*** (0.29) | −2.18**** (0.44) |
| Systolic blood pressure, mm Hg (501) | 126.0 (1.34) | −12.88 (1.14) | 126.5 (1.52) | −0.14**** (1.31) | 127.4 (1.45) | 0.55**** (1.24) |
| Diastolic blood pressure, mm Hg (503) | 71.9 (0.85) | −2.72 (0.74) | 72.9 (0.98) | 0.81**** (0.85) | 72.3 (0.91) | 0.18** (0.80) |
| Resting heart rate, bpm (493) | 72.4 (0.97) | −13.84 (0.74) | 73.8 (1.11) | −3.84**** (0.85) | 71.5 (1.06) | −5.92**** (0.81) |
| Glucose, mg/dl (638) | 96.9 (1.15) | −14.86 (1.03) | 95.6 (1.36) | −0.60**** (1.22) | 96.9 (1.21) | 2.63**** (1.08) |
| Insulin, μU/ml (641) | 19.79 (1.04) | −10.69 (0.70) | 18.85 (1.24) | 3.82**** (0.83) | 15.19*** (1.11) | 2.92**** (0.74) |
| HOMA-IR (635) | 4.89 (0.27) | −2.78 (0.20) | 4.47 (0.32) | 0.85**** (0.24) | 3.66*** (0.28) | 0.99**** (0.21) |
| HbA1c, % (455) | 5.65 (0.05) | −0.10 (0.03) | 5.61 (0.06) | 0.15**** (0.04) | 5.56 (0.05) | 0.24**** (0.04) |
| Total cholesterol, mg/dl (666) | 187.0 (2.39) | −26.3 (1.93) | 182.3 (2.72) | −0.32**** (2.20) | 183.2 (2.47) | 3.23**** (1.99) |
| Measured LDL-cholesterol, mg/dl (666) | 109.3 (1.93) | −18.98 (1.70) | 106.3 (2.20) | 4.87**** (1.93) | 109.9 (2.00) | 8.44**** (1.75) |
| HDL-cholesterol, mg/dl (666) | 44.2 (0.77) | 9.02 (0.69) | 41.4** (0.87) | −4.76**** (0.86) | 44.2 (0.79) | −3.67**** (0.71) |
| Measured VLDL-cholesterol, mg/dl (666) | 34.9 (1.32) | −13.59 (1.20) | 36.1 (1.50) | 0.77**** (1.36) | 30.1** (1.36) | −1.44**** (1.24) |
| Triglycerides, mg/dl (666) | 189.97 (6.90) | −68.87 (8.85) | 199.2 (7.85) | 13.49**** (10.10) | 163.2** (7.12) | −6.00**** (9.14) |
| IWQOL–Lite total score (771) | 64.80 (1.27) | 58.90 (1.22) | 68.16 (1.47) | 7.47**** (1.38) | 89.40**** (1.31) | 11.51**** (1.38) |
| SF-36, Physical component score (743) | 36.05 (0.39) | 9.39 (0.39) | 35.9 (0.44) | 1.04**** (0.44) | 40.0**** (0.39) | 2.30**** (0.41) |
| SF-36, Mental component score (743) | 41.9 (0.43) | 2.82 (0.44) | 42.5 (0.49) | −0.69**** (0.50) | 44.3**** (0.44) | 1.06** (0.45) |
| Apnea/hypopnea index, per (401) | 30.2 (1.90) | −13.91 (1.30) | 29.7 (2.34) | −1.05**** (1.45) | 25.0* (1.91) | −1.34**** (1.22) |
| % Sleep time SpO2 <90%, sin−1 (%) (401) | 0.33 (0.03) | −0.19 (0.02) | 0.30 (0.03) | 0.01**** (0.02) | 0.27 (0.03) | 0.02**** (0.02) |
| Average night-time SpO2, (401) | 90.7 (0.28) | 2.28 (0.20) | 90.7 (0.32) | −0.22**** (0.23) | 91.2 (0.26) | −0.43**** (0.19) |
| Treadmill time, s (397) | 625.1 (23.3) | 291.5 (13.7) | 628.6 (30.1) | 39.3**** (17.3) | 622.4 (23.6) | 2.9**** (13.7) |
| RMR, kcal/day (517) | 2,136 (27.3) | −424 (17.9) | 2,202 (34.9) | 44.6**** (22.9) | 2,298 (28.0) | −6.6**** (18.4) |
Data for blood pressure, lipid, and diabetes-related variables were excluded if participant was taking medication affecting that variable at either exam. Follow-up changes are adjusted for age, sex, baseline BMI, and the baseline level of the variable being analyzed. Baseline means are adjusted for age, sex, and baseline BMI (except for the anthropometric variables).
HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; IWQOL–Lite, impact of weight quality of life–lite; LDL, low-density lipoprotein; RMR, resting metabolic rate; VLDL, very low-density lipoprotein.
Significance of change between baseline and follow-up.
P < 0.05,
P < 0.01,
P < 0.001,
P < 0.0001 vs. surgical cases.
Table 4 includes the association of change in BMI or waist circumference with change in quantitative biological measures and psychological inventories (impact of weight quality of life–lite and SF-36) among the subset of gastric bypass subjects who were unmedicated at both exams for that clinical variable. BMI reduction correlated significantly with changes in all variables, except diastolic blood pressure and the mental component of the SF-36 questionnaire. Waist circumference reductions were generally weaker predictors of most variable changes than were BMI reductions.
Table 4.
Association of 2-year change in physiologic measurements and psychological assessments with change in BMI or waist circumference for surgical group subjects
| 2-Year change variables (N) | BMI partial correlation coefficienta | P value | Waist circumference partial correlation coefficienta | P value |
|---|---|---|---|---|
| Waist circumference (315) | 0.56 | <0.0001 | — | — |
| Percent body fat (311) | 0.76 | <0.0001 | 0.54 | <0.0001 |
| Systolic blood pressure (212) | 0.26 | 0.078 | 0.14 | 0.037 |
| Diastolic blood pressure (212) | 0.06 | 0.35 | 0.10 | 0.15 |
| Resting heart rate (209) | 0.19 | 0.008 | 0.15 | 0.03 |
| Glucose (265) | 0.24 | 0.0001 | 0.15 | 0.015 |
| Insulin (265) | 0.31 | <0.0001 | 0.25 | <0.0001 |
| HOMA-IR (259) | 0.34 | <0.0001 | 0.26 | <0.0001 |
| HbA1c (183) | 0.27 | 0.0002 | 0.89 | 0.23 |
| Total cholesterol (271) | 0.29 | <0.0001 | 0.31 | <0.0001 |
| LDL-cholesterol, measured (271) | 0.31 | <0.0001 | 0.32 | <0.0001 |
| HDL-cholesterol (267) | −0.28 | <0.0001 | −0.30 | <0.0001 |
| VLDL-cholesterol measured (267) | 0.44 | <0.0001 | 0.44 | <0.0001 |
| Triglycerides (271) | 0.48 | <0.0001 | 0.45 | <0.0001 |
| IWQOL–Lite total score (298) | −0.56 | <0.0001 | −0.45 | <0.0001 |
| SF-36, Physical component score (285) | −0.14 | 0.019 | −0.19 | 0.0016 |
| SF-36, Mental component score (285) | −0.11 | 0.08 | −0.06 | 0.30 |
| Apnea/hypopnea index (148) | 0.21 | 0.012 | 0.13 | 0.13 |
| % Sleep time SpO2 <90%, sin−1 (%) (148) | 0.29 | 0.0005 | 0.29 | 0.0004 |
| Average night-time SpO2 (148) | −0.39 | <0.0001 | −0.37 | <0.0001 |
| Treadmill time, s (157) | −0.35 | <0.0001 | −0.45 | <0.0001 |
| RMR, kcal/day (225) | 0.39 | <0.0001 | 0.39 | <0.0001 |
Analysis limited to only those surgical subjects not taking medications at either baseline or follow-up that affect the variable being analyzed.
HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; IWQOL–Lite, impact of weight quality of life–lite; LDL, low-density lipoprotein; RMR, resting metabolic rate; VLDL, very low-density lipoprotein.
Adjusted for age, gender, baseline BMI or waist circumference, and baseline levels of the variable analyzed.
Table 5 shows the baseline disease endpoint prevalence and the change in disease status (incidence and resolution) during the 2-year follow-up period. There were no significant differences in disease prevalence between the surgical and comparative groups. The surgical group experienced significantly lower rates of incident disease and greater disease resolution as compared with each comparative group. Resolution of diabetes occurred in about 80% of the surgical group, and hypertension and dyslipidemia resolved in ~40–50% of surgical patients while <1–14% not receiving surgery had resolution of these problems. There were no significant differences in incidence of cardiovascular events among the three groups; however, the number of patients with heart disease was small, given the relatively short, 2-year follow-up period and the relatively young age of the subjects.
Table 5.
Group comparison of prevalence at baseline, 2-year incidence, and 2-year resolution of clinical parameters
| Study variables and clinical endpoints (N) | Gastric bypass surgery patients |
Comparative group: seeking but did not have bypass surgery |
Comparative group: not seeking bariatric surgery |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalence, % (N) | Incidence, % (N) | Resolved, % (N) | Prevalence, % (N) | Incidence, % (N) | Resolved, %, (N) | Prevalence, % (N) | Incidence, % (N) | Resolved, % (N) | |
| Hypertension | 31.9 (101/317) | 1.4 (3/216) | 37.6 (38/101) | 34.1 (78/229) | 7.3 (11/151)** | 1.3 (1/78)*** | 36.9 (101/274) | 9.8 (17/173)** | 3.7 (3/101)**** |
| Dyslipidemia | 57.8 (190/329) | 5.1 (7/139) | 54.2 (103/190) | 64.3 (153/238) | 35.3 (30/85)**** | 7.2 (11/153)**** | 51.8 (144/278) | 39.6 (53/134)**** | 13.9 (20/144)**** |
| Diabetes | 19.5 (61/313) | 0 (0/252) | 78.7 (48/61) | 22.0 (50/227) | 5.6 (10/177)*** | 0 (0/50)**** | 23.4 (64/274) | 9.5 (20/210)**** | 4.7 (3/64)**** |
| CHD | 1.3 (5/399) | 0.8 (3/394) | NA | 2.7 (9/329) | 0 (0/320) | NA | 2.3 (7/305) | 0 (0/298) | NA |
| Cerebrovascular disease | 0.5 (2/399) | 0.8 (3/397) | NA | 1.4 (5/330) | 0.6 (2/325) | NA | 1.6 (5/305) | 0.3 (1/300) | NA |
For hypertension, dyslipidemia, and diabetes, both physiologic measurements and endpoints questionnaire data were used to determine prevalence, incidence, and resolution. For other endpoints, only questionnaire data from both exams were used.
CHD, coronary heart disease; NA, not applicable.
P < 0.05,
P < 0.01;
P < 0.001,
P < 0.0001 vs. surgical cases.
DISCUSSION
The prevalence of severe obesity has risen sharply in the past decade and has shown an ever-increasing economic cost and disease toll in the United States (12). Due to the poor success rate of medical treatments for severe obesity, bariatric surgery is increasingly used to treat this disorder and its many comorbidities (10–12,19,20). In fact, the frequency of bariatric surgery increased sevenfold (from 3.5 to 24.0 per 100,000 population) between 1996 and 2002 (10), and an estimated 170,000 bariatric surgeries were performed in the United States in 2005 (11,12). The largest portion of this increase was in gastric bypass procedures (12,21). Although analyzing bariatric surgery outcome data has certain limitations (1), bariatric surgery has been shown to be an effective treatment for obesity-related comorbidities in individuals who are severely obese (2–4). In addition, two large studies have recently shown a reduction in total mortality following bariatric surgery of about one-third (9,22). However, despite the sharp rise in bariatric surgery and the reported improvement in health outcomes following surgery, limited research comparing currently employed bariatric surgical procedures to nonsurgical weight-loss treatments has been reported (1).
This study represents the first prospective study to exclusively follow gastric bypass patients whose study inclusion was not limited by disease (i.e., diabetes) and which included for comparison two nonsurgical severely obese groups, who did not have a planned nonsurgical weight-loss intervention. Following baseline testing, the nonsurgical subjects were free to decide whether or not to pursue weight-loss treatment on their own volition. After 2-year follow-up, surgical patients had significantly greater weight loss, lower incidence and greater resolution of major obesity-related comorbidity and improved levels of health-related quality of life compared with the two comparative groups.
In particular, degree of obesity, blood pressure, lipids, glucose, and insulin levels, sleep apnea, RMR, aerobic capacity, and health-related quality of life all improved significantly after surgery compared to comparative subjects. These improvements were associated with resolution of ~80% of diabetes, 40% of hypertension, and 50% of dyslipidemia. High-density lipoprotein-cholesterol increased by an average of 20% (9.0 mg/dl) in the surgery group, a greater increase than can be obtained with all but the most aggressive multidrug regimens. This change in high-density lipoprotein alone would predict about a 20% reduction in cardiovascular disease risk. Although, the major risk factors for cardiovascular disease were markedly improved following surgery, the numbers of cardiovascular and cancer events were too small to evaluate the effectiveness of surgery in reducing such events. In addition to greater disease resolution, incidence of new hypertension, diabetes, and dyslipidemia was significantly lower in the postsurgical subjects compared with nonsurgical participants. Furthermore, the amount of weight lost among surgical patients was significantly associated with the degree of improvement in most biological measures.
The analysis presented in Table 3 was repeated including all medicated subjects while adjusting for variable-specific medication use at baseline and at follow-up exams (e.g., adjustment for diabetes medication use when analyzing glucose, HbA1c, and insulin). Inclusion of medicated subjects resulted in only minor differences in means and no differences in significance levels, except for less significance for diastolic blood pressure change in the surgery vs. comparative subjects not seeking surgery. When those participants from the two comparative groups who went on to have bariatric surgery after the initial visit were included within the analyses, the mean values of measures listed in Table 3 changed, but all parameters remained significantly improved for the surgery group compared to the comparative groups. Although virtually all study variables improved following surgery, not all risks associated with gastric bypass surgery were evaluated. In particular, osteoporosis could not be assessed at baseline using dual-energy X-ray absorptiometry scans due to equipment-related weight restrictions. Also, anemia and micronutrient deficiency were not assessed.
The degree of postsurgical weight loss by BMI predicted improvements in all clinical variables (Table 4) except diastolic blood pressure and the mental component of the SF-36, despite the fact that diastolic blood pressure and the SF-36 score improved in the surgery group. The degree of post-surgical weight loss by waist circumference also predicted improvement in most clinical variables. Although studies have shown that diabetes and abnormal glucose, insulin, HbA1c, and homeostasis model assessment of insulin resistance resolve very early after surgery, even before observable weight loss (23,24), improvements in these variables at 2 years still correlated strongly with amount of weight lost at that time point.
Recent review articles and large meta-analyses have focused on bariatric surgery outcomes, such as cardiovascular disease and diabetes (1–3,25). Data from these reports suggest that bariatric surgery, especially gastric bypass surgery (1,26), is the most effective treatment for severely obese patients, at least with regard to short-term weight loss. However, there are key limitations in the information available from these prior studies. In a recent review, Kushner and Noble (1) identified significant methodological concerns including inconsistencies in surgical technique, definitions, and ascertainment of outcome variables, and incompleteness in follow-up (1). In addition, very few bariatric surgery studies have included non-surgical comparative groups.
Two recent randomized control trials of bariatric surgery have demonstrated that bariatric surgery is far more effective than nonsurgical dietary treatment for the severely obese population (5,6). In a study by Mingrone et al., obese subjects (n = 79) were randomly assigned to a low-calorie diet (~1,200 cal) or biliopancreatic surgery. After 1-year follow-up, the women and men undergoing biliopancreatic surgery lost on average 28 and 34% of their initial weight, respectively, while the average initial weight loss with diet alone was 5.8% for women and 6.2% for men (6). More recently, O’Brien et al. randomly assigned patients (n = 80) with mild to moderate obesity (BMI = 30–35 kg/m2) to either a very-low-calorie diet that included pharmacotherapy and lifestyle change or to surgery for placement of an adjustable gastric band (5). After 2 years, the surgery group lost an average of 21.6% initial weight compared with an average weight loss of 5.5% in the nonsurgical group.
The ongoing SOS study included a nonsurgical control group closely matched to bariatric surgical patients (27). After 10-year follow-up, the average initial weight loss among the surgical group was 23.4% compared to an increase of 0.1% in the controls (4). However, only 13% of the cases had a gastric bypass procedure. Interestingly, long-term (10 years) results in the SOS study differed according to surgical procedure type, with gastric banding providing 13.2 ± 13% weight loss, vertical banded gastroplasty 16.5 ± 11%, and gastric bypass 25 ± 11% (4). In addition, type of surgical procedure demonstrated greater long-term (10 years) reductions in blood pressure for gastric bypass compared with other surgery types. Changes in systolic blood pressure in the gastric banding, vertical banded, and gastric bypass surgery groups were 2.1, 0.4, and −4.7 mm Hg, respectively, and changes in diastolic pressure were −1.4, −2.5, and −10.4 mm Hg, respectively. Given the more favorable blood pressure outcomes with gastric bypass, additional long-term research with this procedure is warranted, especially with broader clinical and outcome measurements, such as those performed in this study.
Our recruitment of two comparative groups allowed us to explore additional questions. Before surgical intervention, the two surgery-seeking groups were very comparable despite minor differences in weight (but not BMI), glucose, HbA1c, and high-density lipoprotein-cholesterol. The comparability of these two groups facilitated direct testing of the effectiveness of gastric bypass surgery itself in subjects seeking bariatric surgery and with similar health and obesity. The population-based severely obese comparative subjects who were not seeking weight-loss surgery group, allowed for comparisons of characteristics of severely obese subjects in the general population with surgical patients potentially self-selected for significant comorbidities and other conditions associated with seeking surgery. The population comparative group was older and had lower BMI compared to the two seeking gastric bypass surgery groups, although disease prevalence for diabetes, hypertension, and dyslipidemia was not different between the three groups. Consistent with prior studies (28,29), the health-related quality of life was higher in population comparative subjects than the other seeking surgery groups, even after adjustment for age, gender, and BMI. This finding may have suggested that the population-based comparative subjects were more content with their current state of obesity and perhaps felt they were healthier. Even though the population-based comparative group did not exactly match the gastric bypass group, the improvements in the surgical group were far greater than the baseline differences, and the 2-year follow-up means were much better in absolute terms than the population-based comparative group. Finally, differences in age and BMI shown at baseline between the three groups were readily controlled for in multivariate analysis and did not make a significant impact upon the major clinical changes that occurred in the surgical group compared to the two comparative groups, and therefore did not affect the validity of the results. For every parameter, the changes from baseline to follow-up were very large in the surgical group (P < 0.0001) in relation to the two comparative groups. The exception to this finding was a less significant difference (P < 0.01) in diastolic blood pressure and SF-36 mental component score between the surgical and the comparative group who were not seeking surgery. Similar clinical benefits using two different comparative groups demonstrate how robust the study is to differences in makeup of the comparative groups. We believe that the use of multiple comparative groups is a unique strength of the study.
A recognized limitation of the Utah Obesity Study is that it is not a randomized comparison of gastric bypass surgery to nonsurgery. However, significant potential barriers to randomization studies of bariatric surgery have been identified (30–32). Due to differences in safety and quality-of-life outcomes, randomizing severely obese patients to a nonmedical treatment arm for long-term follow-up may be “inappropriate” (31), because of the high mortality risks associated with untreated severe obesity. A further cited barrier includes the likely unwillingness for patients seeking bariatric surgery to be randomized. These patients may contend that their entire life has been spent participating in the “nonintervention arm” (30).
Another potential limitation of this study is that all gastric bypass surgical patients and patients seeking but not having surgery were recruited from a single high-volume surgical practice of three surgeons, potentially interfering with the opportunity to generalize the findings to other locals. Short-term complication rates and even long-term efficacy may differ by procedure and by subtle variance in surgical technique and other factors between surgeons and surgical centers. The three surgeons who performed all the operations used the same procedure (Roux-en-Y gastric bypass) by approximately the same method. Utilizing a single, very experienced center removes potential differences between centers, provides an excellent test of the effectiveness of the surgery itself and enhances the validity of the results. Due to improved patient outcome associated with patient surgery volume (33), bariatric centers are now required to perform a certain number of specific bariatric surgeries (i.e., ≥100 per year) to qualify as a center of excellence. The Utah Surgical Center, having performed over 13,000 gastric bypass procedures, exemplifies this center of excellence requirement. In addition, the mortality in our surgical group was very low (0.5% at 2 years) supporting the findings that high-surgical volume strongly reduces short-term mortality (33).
We believe that the size of the study and strength of the findings suggest that results will be generalizable to a mostly white, female, premenopausal population—the very population which most commonly seeks this treatment approach in the United States. Lack of sufficient minority patients participating in this study represents a limitation. The large SOS (4) and the randomized trials performed in Australia (5,7,8) appear also to be limited by minority representation. Another limitation of this report is that it provides only intermediate, 2-year follow-up data.
In summary, despite these limitations, this unique comparative cohort study demonstrates that gastric bypass surgery, when compared to severely obese subjects not enrolled in structured nonsurgical weight loss treatment, effectively improves major risk factors for cardiovascular disease 2 years after surgery. Improvements include dramatically reduced obesity, increased health-related quality of life, and improvements in other weight-related clinical health outcomes. Further, at 2-year follow-up, gastric bypass was shown to significantly lower rates of incident disease and result in greater disease resolution for diabetes, hypertension, and dyslipidemia.
Acknowledgments
This work was supported by a grant (DK-55006) from the National Institute of Diabetes and Digestive and Kidney Diseases and a grant (M01-RR00064) from the National Center for Research Resources. Acknowledgments are also expressed for clinical and technical support from the staff of the Cardiovascular Genetics Division (including Sara Frogley, Sawsan Ibrahim, Loni Gardner, Jeff Yancey, and Kristie Reid), the Huntsman General Clinical Research Center and their very capable staff, and the University of Utah School of Medicine Cardiology and Pulmonary Divisions.
Footnotes
DISCLOSURE
S.C.S. receives a lecture fee from Covidien. For all other authors, no other potential conflicts of interest relevant to this article exist.
References
- 1.Kushner RF, Noble CA. Long-term outcome of bariatric surgery: an interim analysis. Mayo Clin Proc. 2006;81:S46–S51. doi: 10.1016/s0025-6196(11)61180-4. [DOI] [PubMed] [Google Scholar]
- 2.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 3.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547–559. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 4.Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien PE, Dixon JB, Laurie C, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med. 2006;144:625–633. doi: 10.7326/0003-4819-144-9-200605020-00005. [DOI] [PubMed] [Google Scholar]
- 6.Mingrone G, Greco AV, Giancaterini A, et al. Sex hormone-binding globulin levels and cardiovascular risk factors in morbidly obese subjects before and after weight reduction induced by diet or malabsorptive surgery. Atherosclerosis. 2002;161:455–462. doi: 10.1016/s0021-9150(01)00667-0. [DOI] [PubMed] [Google Scholar]
- 7.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 8.Dixon JB, Strauss BJ, Laurie C, O’Brien PE. Changes in body composition with weight loss: obese subjects randomized to surgical and medical programs. Obesity (Silver Spring) 2007;15:1187–1198. doi: 10.1038/oby.2007.639. [DOI] [PubMed] [Google Scholar]
- 9.Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 10.Davis MM, Slish K, Chao C, Cabana MD. National trends in bariatric surgery, 1996–2002. Arch Surg. 2006;141:71–4. doi: 10.1001/archsurg.141.1.71. discussion 75. [DOI] [PubMed] [Google Scholar]
- 11.American Society for Bariatric Surgery web site. [Accessed 6 November 2006];Medicare expands coverage for lifesaving obesity surgery (press release) 2006 February 21; < www.asbs.org/html/about/ncd_release.html>.
- 12.Hensrud DD, Klein S. Extreme obesity: a new medical crisis in the United States. Mayo Clin Proc. 2006;81:S5–10. doi: 10.1016/s0025-6196(11)61175-0. [DOI] [PubMed] [Google Scholar]
- 13.Hunt SC, Williams RR, Barlow GK. A comparison of positive family history definitions for defining risk of future disease. J Chronic Dis. 1986;39:809–821. doi: 10.1016/0021-9681(86)90083-4. [DOI] [PubMed] [Google Scholar]
- 14.Williams RR, Hunt SC, Barlow GK, et al. Health family trees: a tool for finding and helping young family members of coronary and cancer prone pedigrees in Texas and Utah. Am J Public Health. 1988;78:1283–1286. doi: 10.2105/ajph.78.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams RR, Hunt SC, Heiss G, et al. Usefulness of cardiovascular family history data for population-based preventive medicine and medical research (the Health Family Tree Study and the NHLBI Family Heart Study) Am J Cardiol. 2001;87:129–135. doi: 10.1016/s0002-9149(00)01303-5. [DOI] [PubMed] [Google Scholar]
- 16.Smith SC, Edwards CB, Goodman GN, Halversen RC, Simper SC. Open vs laparoscopic Roux-en-Y gastric bypass: comparison of operative morbidity and mortality. Obes Surg. 2004;14:73–76. doi: 10.1381/096089204772787329. [DOI] [PubMed] [Google Scholar]
- 17.Smith SC, Goodman GN, Edwards CB. Roux-en-Y Gastric Bypass: A 7-year Retrospective Review of 3,855 Patients. Obes Surg. 1995;5:314–318. doi: 10.1381/096089295765557700. [DOI] [PubMed] [Google Scholar]
- 18.Adams TD, Avelar E, Cloward T, et al. Design and rationale of the Utah obesity study. A study to assess morbidity following gastric bypass surgery. Contemp Clin Trials. 2005;26:534–551. doi: 10.1016/j.cct.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004;350:1075–1079. doi: 10.1056/NEJMp048029. [DOI] [PubMed] [Google Scholar]
- 20.The ASBS Consensus Conference on the State of Bariatric Surgery and Morbid Obesity: Health Implications for Patients, Health Professionals and Third-Party Payors. ASBS Consensus Conference; 6–7 May 2004; Washington, DC: Georgetown University; 2004. [DOI] [PubMed] [Google Scholar]
- 21.Trus TL, Pope GD, Finlayson SR. National trends in utilization and outcomes of bariatric surgery. Surg Endosc. 2005;19:616–620. doi: 10.1007/s00464-004-8827-8. [DOI] [PubMed] [Google Scholar]
- 22.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 23.Lefevre M, Aronson N. Special report: the relationship between weight loss and changes in morbidity following bariatric surgery for morbid obesity. TEC Bull (online) 2003;18:1–25. [PubMed] [Google Scholar]
- 24.Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg. 2002;236:554–559. doi: 10.1097/00000658-200211000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colquitt J, Clegg A, Loveman E, Royle P, Sidhu MK. Surgery for morbid obesity. Cochrane Database Syst Rev. 2005:CD003641. doi: 10.1002/14651858.CD003641.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793–2796. doi: 10.1001/jama.288.22.2793. [DOI] [PubMed] [Google Scholar]
- 27.Sjöström L, Larsson B, Backman L, et al. Swedish obese subjects (SOS). Recruitment for an intervention study and a selected description of the obese state. Int J Obes Relat Metab Disord. 1992;16:465–479. [PubMed] [Google Scholar]
- 28.Kolotkin RL, Crosby RD, Williams GR. Health-related quality of life varies among obese subgroups. Obes Res. 2002;10:748–756. doi: 10.1038/oby.2002.102. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson J, Sjöström L, Sullivan M. Swedish obese subjects (SOS)–an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord. 1998;22:113–126. doi: 10.1038/sj.ijo.0800553. [DOI] [PubMed] [Google Scholar]
- 30.Courcoulas AP, Flum DR. Filling the gaps in bariatric surgical research. JAMA. 2005;294:1957–1960. doi: 10.1001/jama.294.15.1957. [DOI] [PubMed] [Google Scholar]
- 31.Kral JG, Dixon JB, Horber FF, et al. Flaws in methods of evidence-based medicine may adversely affect public health directives. Surgery. 2005;137:279–284. doi: 10.1016/j.surg.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien P, Laurie C, Skinner S, Proietto J, Strauss B. A randomized controlled trial of medical versus surgical therapy in the management of obesity. Obes Res. 2004;12:A33. [Google Scholar]
- 33.Flum DR, Salem L, Elrod JA, et al. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA. 2005;294:1903–1908. doi: 10.1001/jama.294.15.1903. [DOI] [PubMed] [Google Scholar]