Abstract
Coronary heart disease (CHD) is the single largest cause of death in the developed countries and is one of the leading causes of disease burden in developing countries. In 2001, there were 7.3 million deaths due to CHD worldwide. Three-fourths of global deaths due to CHD occurred in the low and middle-income countries. The rapid rise in CHD burden in most of the low and middle and income countries is due to socio-economic changes, increase in life span and acquisition of lifestyle related risk factors. The CHD death rate, however, varies dramatically across the developing countries. The varying incidence, prevalence, and mortality rates reflect the different levels of risk factors, other competing causes of death, availability of resources to combat CVD, and the stage of epidemiologic transition that each country or region finds itself. The economic burden of CHD is equally large but solutions exist to manage this growing burden.
INTRODUCTION
Coronary heart disease (CHD) is the single largest cause of death in the developed countries and is one of the leading causes of disease burden in developing countries as well. In 2001, there were 7.3 million deaths and 58 million disability adjusted life years (DALYs) lost due to CHD worldwide [1]. Three-fourths of global deaths and 82% of the total DALYs due to CHD occurred in the low and middle-income countries.
The CHD death rate however varies dramatically across the developing countries both as a proportion of cardiovascular disease (CVD) deaths and as a proportion of all deaths. CHD is the leading cause of CVD death throughout the world. Forty-three percent of all CVD deaths are attributable to CHD according to the global burden of disease estimates from 2001. Globally CVD deaths represent about 30% of all deaths. However, the death rates and patterns vary between developed or high-income countries and the low and middle income countries. High-income countries have CVD deaths rates of approximately 38%. While the overall rate of CVD deaths (28%) in low and middle-income countries collectively is less, there is a great range from as high as 58% in Eastern Europe to as low as 10% in Sub Saharan Africa. CVD is the largest cause of death in all the developing regions with the exception of Sub-Saharan Africa where it is the leading cause of death in those over the age of 45.
The variability in disease prevalence in the various regions is likely a result of multiple factors. First, the countries are in various phases of the epidemiologic transition described in detail below. As countries progress from agrarian to industrial to post-industrial states there are a series of environmental, social, and structural changes that occur, some that lead to increase longevity, others that result in exposure to risk factors for chronic diseases. With the increased level of development comes increased risk factor levels as well as improved public health and medical access for a greater proportion of the population. The balance of the two can lead to varying levels of coronary heart disease incidence, prevalence and coronary heart disease mortality. Second, certain additional pressures in some regions such as war or infectious diseases (HIV/AIDS) in Sub Saharan Africa can limit an aging population and hence CHD mortality has not risen like other regions. Third, different regions may have genetic predisposition for coronary heart disease risk factors such as possibly the metabolic syndrome in South Asia.
In this paper, we review the features of the epidemiologic transition and attempt to evaluate the status of each of the different economic and geographic regions of the world as defined by the World Bank in regards to the transition. We review key economic and social indicators such as per capita income and expenditure on health. Further, we review data from select countries in each region. The main criterion for country selection is based on the availability of complete and accurate data. Reliable data for most countries is scarce. There are significant costs and infrastructure limitations that prohibit most countries from having complete nationally representative demographic surveys, vital registration systems or disease registries. Other criteria include the size of a country, such as China and India, and whether something distinguishes a country from others in the same region.
The Epidemiologic Transition
The overall increase in the global burden and the distinct patterns in the various regions can be explained in part by the epidemiologic transition. The epidemiological transition has been divided into four basic stages [2–4]: Pestilence and Famine, Receding Pandemics, Degenerative and Man-made Diseases, and Delayed Degenerative Diseases. Movement through these stages has resulted in a dramatic shift in the cause of death from infectious diseases and malnutrition in the first stage to CVD and cancer in most high-income countries over the last two centuries.
During the first stage of Pestilence and Famine, cardiovascular disease, which accounts for less than 10% of deaths, takes the form of rheumatic heart disease and cardiomyopathies due to infection and malnutrition. Per capita income and life expectancy increase during the stage of Receding Pandemics as the emergence of public health systems, cleaner water supplies, and improved nutrition combine to drive down deaths from infectious disease and malnutrition. Rheumatic valvular disease, hypertension, coronary heart disease and stroke are the predominant forms of CVD. CHD is often seen at an equivalent or lower prevalence rate compared to stroke. During the stage of Degenerative and Man-made Diseases CHD and stroke are predominant and between 35% and 65% of all deaths can be traced to CVD. Typically, the rate of CHD deaths exceeds that of stroke by a ratio of 2:1 to 3:1. In the stage of Delayed Degenerative Diseases, CVD and cancer remain the major causes of morbidity and mortality, with CVD accounting for 30–40% of all deaths. However, age-adjusted CVD mortality rate declines, aided by preventive strategies such as smoking cessation programs and effective blood pressure control, acute hospital management, and technological advances such as the availability of bypass surgery. CHD, stroke, and congestive heart failure are the primary forms of CVD with CHD remaining a significantly greater cause of death and congestive heart failure dramatically increases with improved survival from myocardial infarction. Japan was an exception to this transition with rates of CHD never exceeding those of stroke. A further characteristic of the coronary heart disease transition in the developed countries is that members of higher socioeconomic classes tended to pass through them first with a certain lag for those of lower socioeconomic status.
Most developing regions appear to be following a similar pattern; but the transition has occurred at a more compressed rate. Between 1990 and 2020, coronary heart disease alone is anticipated to increase by 120% for women and 137% for men in developing countries [5]. However, there remain distinct differences in how severe the burden is affecting the various populations. Table 1 data displays where CHD ranks as a cause of death in each geographic region of the world.
Table 1.
Regional CHD mortality rank (2001)
| World Bank Region | Rank of CHD within the leading causes of mortality in the region |
|---|---|
| East Asia and Pacific | 3 |
| Europe and Central Asia | 1 |
| Latin America and Caribbean | 1 |
| Middle East and North Africa | 1 |
| South Asia | 1 |
| Sub-Saharan Africa | 8 |
| High Income | 1 |
WHO Global Burden of Disease and Risk Factors 2006 [6]
Differing Trends
In developed countries, despite the overall increase in CHD burden, the age-adjusted death rates for CHD are declining. This age-adjusted decline is driven largely by preventive interventions that allow people to avert disease, treatments to prevent death during an acute manifestation of disease (particularly stroke or myocardial infarction) and interventions that prolong survival once CVD is manifest. Thus the average age of death from CVD continues to climb and as a result affects a larger population in retirement. Nearly eighty percent of deaths in the high-income countries occur among those over the age of 60 compared to forty-two percent in low and middle-income countries [6].
In the developing countries the increase in CVD burden is largely a result of an increase in the prevalence of the risk factors and a relative lack of access to the above-mentioned interventions. As a result the age-adjusted death rates for coronary heart disease are increasing in some developing regions; and a relatively younger population is afflicted by CHD in developing regions. Thus the increase number of deaths in the working age population. For some of the developing countries, the severity of the epidemiologic transition has appeared to follow a reverse social gradient with members of lower socioeconomic groups suffering the largest rates of CHD and levels of various risk factors [7]. Another large difference between developed and developing countries is the amount of resources devoted to CVD care. For example, there is about a fifty fold difference in what the United States and South Africa spends on CVD care [8–10].
REGIONAL PATTERNS
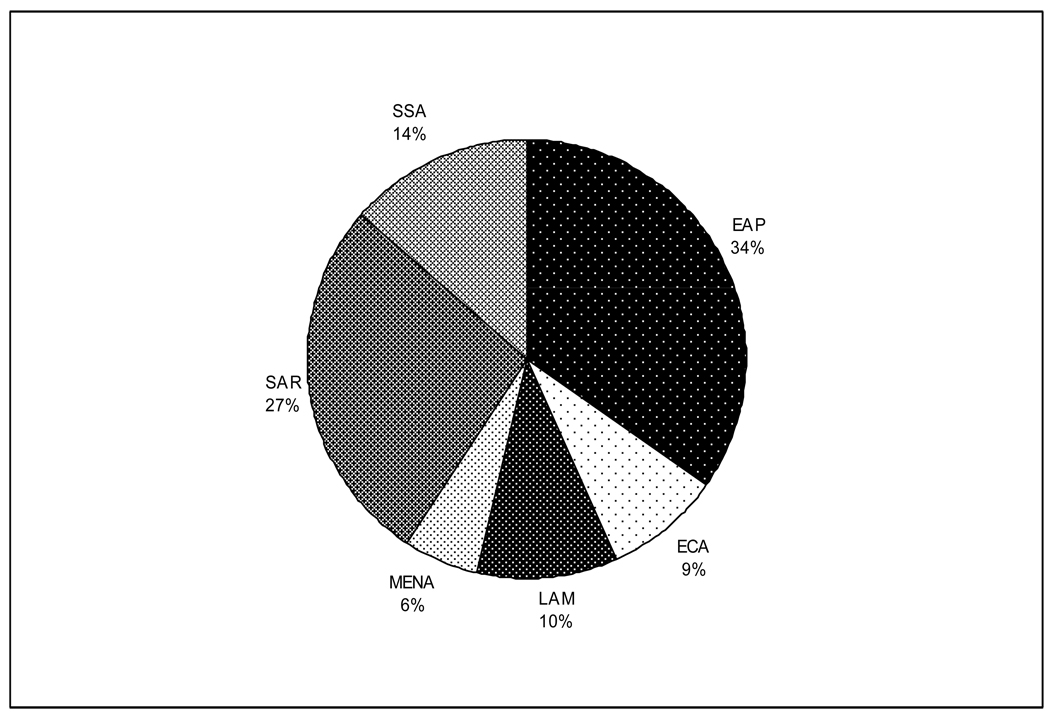
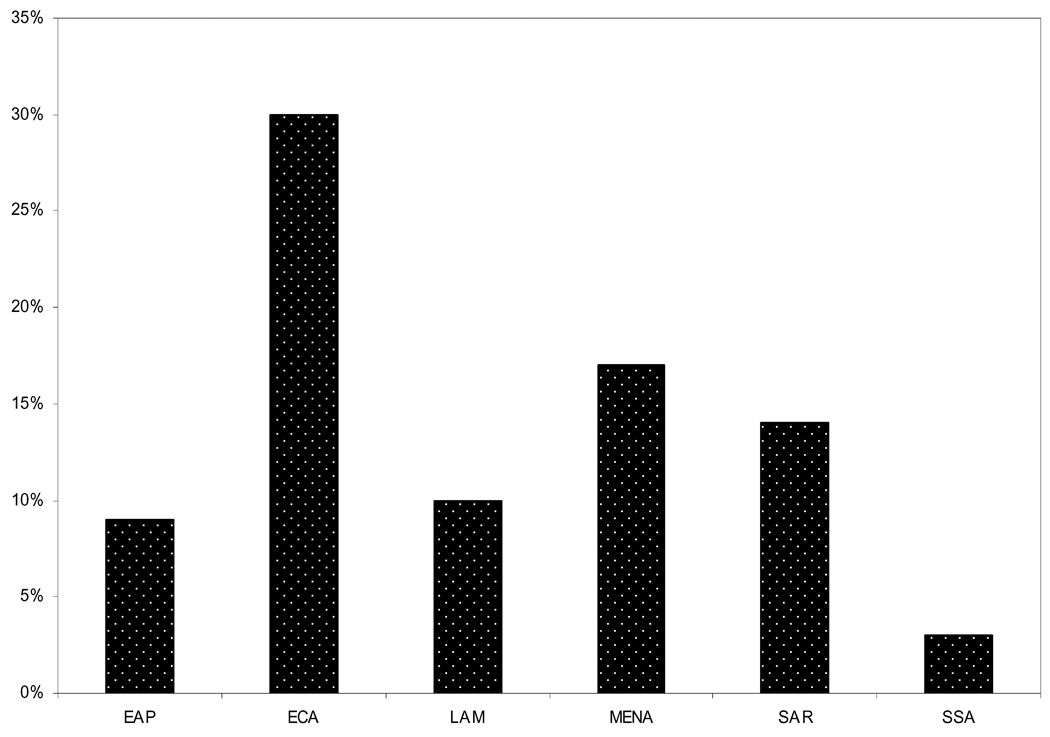
The World Bank places countries in regions based on both geography and income level. Therefore, there are six low and middle-income geographically defined regions and the remaining high-income countries which are not geographically distinct. For example, the “Europe and Central Asia” region is made up of low and middle income countries from eastern Europe while the wealthier western European countries are part of the “High Income” region as defined by the World Bank. Figure 1 shows the absolute numbers and the relative proportion of the population living in each developing region. We describe the burden of disease and treatment patterns for CHD in each of the regions below. Figure 2 shows the proportion of total deaths attributable to CHD for each of the regions. We describe demographic indices, burden of CHD, and treatment patterns in each region below.
Figure 1.
Population of each developing region (millions), 2005. (World Bank Development Indicators 2007 [12])
Figure 2.
Percentage of total mortality attributable to CHD in 2001 by developing region. (WHO Global Burden of Disease and Risk Factors 2006 [6])
East Asia and Pacific
Demographic and Social Indices
The East Asia and Pacific region (EAP) is the most populated low and middle income region with 1,885 million people. China is by far the most populated country with almost 70% of the entire region. The Pacific islands, many of which are among the least developed in the world [11], account for barely 18% [12].
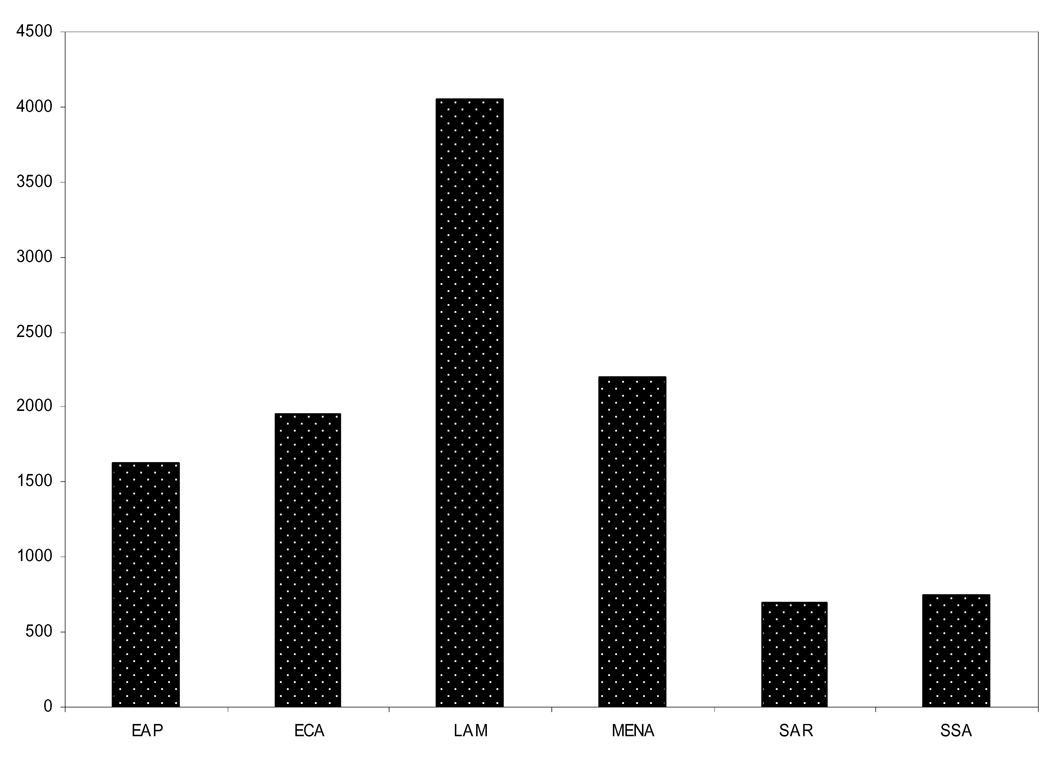
The World Bank, 2005 (Figure 3) indicates the gross national income (GNI) per capita is $1,630 ($$5,914 PPP). The range of GNI per capita in all countries in the region is $2,720 ($8,440 PPP) in Thailand to $430 in both Laos ($2,020 PPP) and Cambodia ($2,490 PPP). China sits in between with $1,740 per capita ($6,600 PPP) [12].
Figure 3.
Gross National Income ($US) per capita 2005 (World Bank Development Indicators 2007 [12])
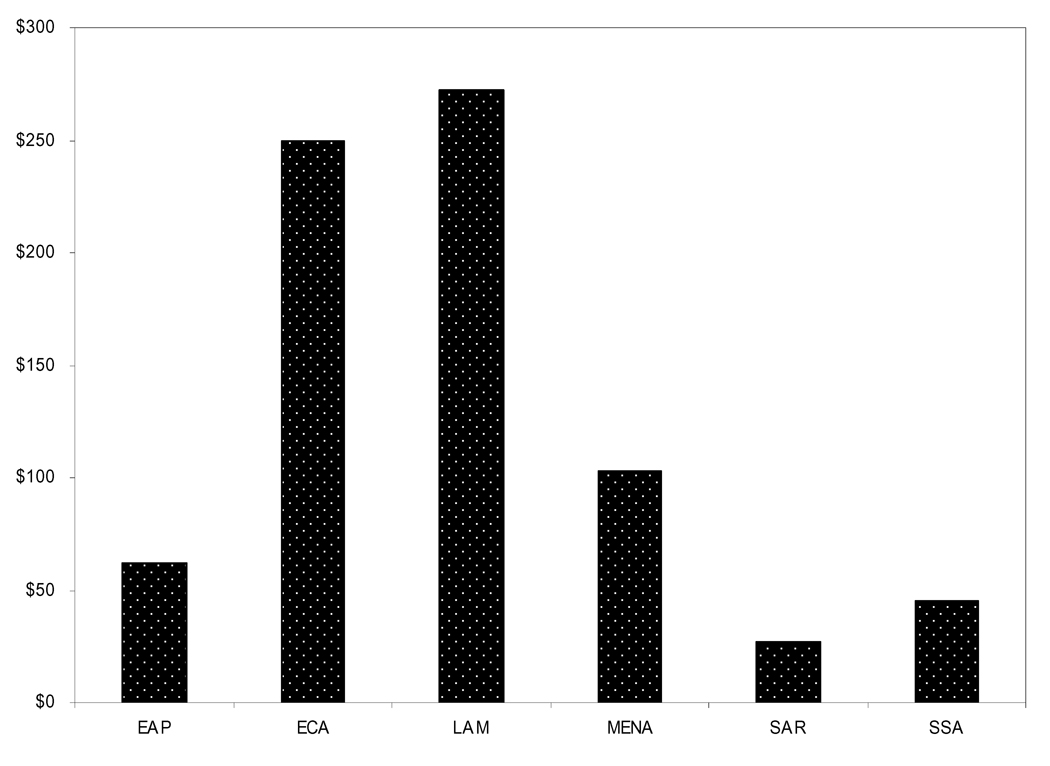
In 2004 total health expenditure was 4.4% of total gross domestic product (GDP), or $62 per capita (Figure 4). Malaysia spends the most per capita on health care with $180 per capita and Myanmar spends the least with $5 per capita. China contributes 4.7% of total GDP, or $71 per capita to health care [12].
Figure 4.
Health Care Expenditure ($US) per capita, 2004 (World Bank Development Indicators 2007 [12])
Life expectancy has risen quickly across the EAP region. Nowhere is this more evident than in China, which saw its life expectancy increase from 37 years in the mid 1950’s to 71 years in 2000[13]. The increase in life expectancy has been accompanied by a large rural to urban migration pattern, rapid urban modernization, aging of the population, decreased birthrates, major dietary changes, increasing tobacco use, and a transition toward work involving physical inactivity. These interlinked forces explain much of the rise in coronary heart disease in this region.
Burden of Disease
According to the WHO Global Burden of Disease Project, cardiovascular diseases were responsible for more than 4 million deaths in the Western Pacific region in 2004, approximately 1 million from CHD and 2.1 million from cerebrovascular diseases[14]. The prevalence of angina and cerebrovascular diseases was 8.2 and 9.1 million people, respectively[14]. The number of Disability Adjusted Life Years (DALYs) lost for CHD was 11.8 million and 24.2 million for cerebrovascular diseases[14].
Stroke and coronary heart disease are the most prevalent forms of cardiovascular disease in the EAP region. Together they account for between 60 and 77% of cardiovascular disease mortality in China[15]. In contrast to North America and Europe, stroke is the leading cause of cardiovascular disease in most areas of EAP[16]. Among men aged 35 to 64 in China, stroke death rates per 100,000 are 217–243 per 100,000 versus CHD death rates of 64–106 per 100,000[15].
Even with high stroke rates, coronary heart disease is emerging as a large and growing burden in East Asia. Data from the largest death registration and classification study in China showed that CHD accounted for 13–22% of overall CVD deaths and 4–9% of total deaths, with the higher percentages seen in urban areas[16]. In 2004, the WHO estimated that nearly 400,000 people died in China from CHD and 652,000 cases were diagnosed[16]. The age-adjusted mortality from CHD was 80 to 128 per 100,000 for men and 57 to 98 per 100,000 for women[16]. Higher rates were seen in urban versus rural areas (by a factor of six), higher income compared to lower income areas, and northeastern parts of China compared to southern parts[16]. In China acute coronary events occur 5 years earlier than in Caucasian and Latin American countries[17].
CHD rates grew quickly over the past two decades. Age-adjusted CHD mortality increased 39% in women and 41% in men aged 35 to 74 years between 1984 to 1999[18]. The incidence of CHD increased by 2.7% annually in men and 1.2% annually in women[18]. While rates are higher, hospitalizations are somewhat low. Acute MI accounted for 4.1% of all hospital discharges in 2004 in large cities, and 2.1% of discharges in smaller cities and rural areas[16].
The data for the burden of CHD in the Pacific Islands is much more limited. However, the WHO Global Burden of Disease Project estimates suggest that Pacific Island age-standardized CHD rates are at least 2 to 3 times higher than China[14]. CHD age-standardized death rates range from 110 per 100,000 in the Federated States of Micronesia, to 125 per 100,000 in Samoa, and up to 181 per 100,000 in Nauru[14].
Treatment
Unlike most other regions, a number of large, well-conducted randomized controlled trials (RCT) of CHD interventions have been conducted in China, yielding results that are or may affect treatment not only in China but throughout the world. The Chinese Cardiac Study 2 (CCS-2/COMMIT) randomized 45,852 patients with AMI in the prior 24 hours to aspirin plus clopidogrel and/or intravenous metoprolol in a 2×2 factorial design[19]. Addition of clopidogrel caused a reduction in death and recurrent events in 9 out of 1000 patients treated[19]. Early use of IV metoprolol showed no overall benefit due to mixed effects on shock and coronary events early and late in the course [20].
Other studies looked at the effects of captopril on early mortality in AMI. The Chinese Cardiac Study (CCS-1) randomized 14,962 patients with suspected AMI within 36 hours of onset to receive captopril or placebo[21]. The overall mortality at 4 weeks was not changed, but the incidence of heart failure was lower in the treatment group (17%) versus placebo (18.7%, P = 0.01)[21]. At two years of follow up on approximately half of the original study sample, the death rate in the captopril group was 16% versus 17.9% in the placebo group (p=0.03)[22].
Finally, Xuezhikang is a Chinese medicine made of red yeast that may have lipid-lowering properties due to the natural occurrence of statin-like compounds in its extract. A recent trial of Xuezhikang of 4870 randomized patients found decreases in total cholesterol, triglycerides, LDL, and total coronary events[23, 24]. These encouraging early trials need replication and verification, but may represent another avenue of CHD prevention.
Europe and Central Asia
Demographic and Social Indices
Low- and middle- income areas in the Europe and Central Asia region (ECA) include countries west of Poland, the Czech Republic, and Croatia, the most populated of which is Russia, with 30% of the total inhabitants. The rest of Central Asia makes up 12% of the total population for the region of 472 million, leaving 58% of inhabitants in Europe. The average Gross National Income (GNI) for the region is $1,954.7 ($9,152 PPP). GNI ranges from $330 ($1,260 PPP) in Tajikistan to $11,220 ($20,140 PPP) in the Czech Republic. Russia has a GNI of $4,460 ($10,640 PPP) [12].
According to the World Bank Indicators from 2004, the ECA region spends an average of 6.6% of total GDP on public and private health care. The average health expenditure per capita is $250. Tajikistan spent the least with $14 per capita and Hungary spent the most with $800 per capita. Russia spent $245 per capita, or 6% of their GDP [12].
Burden of Disease
According to the Global Burden of Disease (GBD) study, coronary heart disease (CHD) is responsible for 1.685 million deaths annually (roughly 30% of all deaths) in the (ECA) developing region, and 18.510 million disability-adjusted life-years (DALYs) are lost to CHD in this region [6]. Although the GBD provides a common estimate for the whole ECA region, an analysis of country-level information reveals important differences in CHD profiles between countries in this region. In the 1980’s, at a time when CHD mortality decreased in Western European countries, Eastern European countries experienced increases in deaths due to CHD. Percent increases in CHD mortality between 1980 and 1992 ranged from 8.3 for males and 7.8 for females in Hungary, to 57.4 and 45.7 in Romania [25]. However, since the early 1990s, CHD death rates have declined remarkably in some ECA countries but not in others. Specifically, in countries that experienced economic and market transformations in the early 1990s, notably Poland, Slovenia, Hungary, the Czech Republic and Slovakia, CHD rates declined dramatically throughout the decade, across both sexes, all ages, residential and educational groups [26]. In the meantime, in formerly Soviet Republics (FSRs), where economic and market transformations were delayed, CHD mortality continued to increase throughout the early 1990s, and has since then only experienced modest declines.
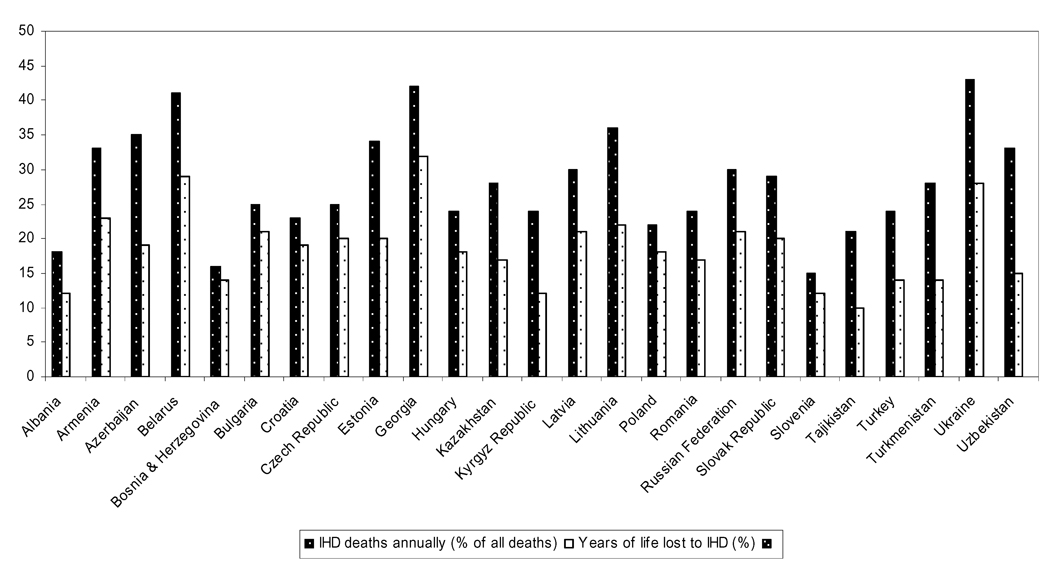
By 2002, CHD mortality in all ECA countries was still much higher than that of Western Europe of North America; however, the highest rates were seen in FSRs, with the Russian Federation claiming the highest rates of CHD deaths in the world [27]. Figure 5 shows the 2002 mortality due to CHD in select ECA countries, as estimated by the World Health Organization. Importantly, deaths due to CHD in these countries are not restricted to the elderly. The GBD study estimates that 601 000 (35.7%) of all CHD deaths in ECA occur in the working age population (ages 15–69). Applying the GBD death rates for ECA to the Russian population figures from the UN Demographic Yearbook, results in an estimate of approximately 204 860 people aged 15–69 dying annually from CHD; in Ukraine, where CHD mortality is almost as high, this number is roughly 74 788. High rates of CHD are especially troublesome in Ukraine and other FSRs in transition, where health systems are not sufficiently financed to respond to a high demand for chronic disease treatment, and out-of-pocket health care expenditures incurred by patient households are often catastrophic [28].
Figure 5.
CHD Mortality in Select ECA, 2002. (WHO Global Infobase [122])
Treatment
Regional differences in use of medical treatments for CHD have been identified as one factor that may have a role in the high CHD mortality observed in ECA. Observational evidence regarding treatment of angina and secondary prevention of CHD events in ECA is scarce; that which does exist suggests some variation in preferred treatments between ECA and more developed regions, as well as disparities in patient outcomes. One study that looked specifically at treatment of acute myocardial infarction (AMI) patients in Bulgaria found that quality of care for these patients was far from evidence-based recommendations. Only 18% were admitted to hospital within the first hour after experiencing chest pain and administration of ASA, beta-blockers and ACE inhibitors was lower during hospitalization compared to Western European countries, as was the use of these drugs as well as lipid-lowering medications after discharge. Only 6 of the 134 patients in the study received percutaneous coronary intervention treatments within one month of AMI, the suggested reason for which was deficient financing for these procedures in the Bulgarian health care system. [29] On the other hand, another study, comparing patients with a variety of acute coronary symptoms from Russia, Poland, Hungary and the Czech Republic with similar patients from mostly Western Europe, North America and Latin America, found that the Eastern European countries made more use of medications for acute coronary syndrome (such as ASA and beta-blockers) both before and after hospitalization, but relied less on adjunctive medications (such as lipid-lowering medications) and percutaneous interventions than did countries in other regions. Eastern European patients in the study had worse outcomes at 30 days and 10 months after presentation [30].
Intra-regional variations in CHD treatment have also been observed in ECA. Table 2 shows results from a study that implemented the Angina Treatment Patterns survey in Russia, Hungary, the Czech Republic and Slovakia (as well as other non-ECA countries) The authors found that the standard of angina treatment in these countries does not meet established guidelines and that these guidelines should be analyzed for their applicability in various developing country settings [31].
Table 2.
Current Pharmacological treatment for IHD in select ECA countries
| Treatment (% of angina patients receiving) |
Czech Republic |
Hungary | Russia | Slovak Republic |
|---|---|---|---|---|
| Beta blocker + calcium channel blocker |
3.7 | 9.3 | 1 | 3.4 |
| Beta blocker+nitrate | 36.8 | 29.2 | 43.4 | 31.2 |
| Calcium channel blocker+nitrate |
11.7 | 8 | 16.4 | 15 |
| Lipid -lowering therapy | 53.1 | 52.2 | 17.7 | 44.4 |
| Anti-thrombotic therapy | 90.3 | 82.9 | 89.9 | 90.5 |
Eastaugh ATP Survey Results 2005 [31]
Latin America and Caribbean
Demographic and Social Indices
The Latin America and Caribbean region (LAM) comprises of Central America, South America and most island nations in the Caribbean, and has a total population of about 560 million [17]. Brazil, the region’s most populous country, houses a third of the population, with Argentina, Colombia, Mexico, Peru and Venezuela making up another third. The Caribbean nations, including Dominican Republic, Jamaica and Haiti account for less than 10% of the population in the region.
Average GNI in the region is around $5500 dollars per capita (PPP $9321) [32]. All countries in the region spend less than 10% of their GDP on health care with the exception of Argentina which spends exactly 10% [33]. This level of spending translates into a range of health care expenditure from $28 (Haiti) to $775 (Barbados) per capita—and matches the relative GNI per capita of the two countries at $560 and $8480 respectively. Brazil spends around $371 per capita on health care with a corresponding GNI of $5910 per capita. It is estimated that severe cases of CVD (i.e. those requiring hospitalization at least once during the year) cost Brazil 8% of its total health care expenditure [34].
Burden of Disease
The WHO Global Burden of Disease study ranked CHD as the single leading cause of mortality in the region, estimating it to be 11% of all deaths in 2001. Overall CVD causes 28% of all deaths. Based on regional 2004 statistics from the Pan American Health Organization (PAHO), the burden of CHD could be updated to 10% of total mortality [35]. Data available from PAHO also indicate that circulatory diseases—a category which includes both cardiovascular and pulmonary diseases—accounted for 29% of all deaths in the region in 2004. In contrast all communicable diseases such as TB, malaria and HIV/AIDS account for 10% of deaths. Unlike the High Income regions where CHD dominates among circulatory diseases, CHD and cerebrovascular disease are equivalent contributors to mortality from circulatory diseases, at 35% and 29% respectively—pointing to relatively higher rates of untreated hypertension in this region. No regional data on morbidity from CHD are readily available. But a household survey conducted in Brazil found that about 3.6% of the population, or around 7 million people, reported having a heart disease [36]. In addition, CHD accounts for about 1% of total hospitalizations in the country [37].
Compared to the prior decade, total regional mortality attributable to circulatory disease and CHD is stable. But age adjusted mortality from circulatory diseases is declining. From 1995 to 2004, there was a 14% decline in age adjusted circulatory disease mortality (the overall circulatory disease mortality grew by 0.1% over the same time period) [35].
Similar regional trends likely apply to age adjusted CHD mortality with some exceptions. For example in Brazil age adjusted circulatory diseases mortality has declined 3.9% annually and age adjusted CHD mortality has declined 3.6% annually [38]. The decline, seen across all age groups and both genders, was most significant for the 44 years or younger population. In another study analyzing trends in age adjusted CHD mortality from 1970 to 2002, Argentina, Brazil, Chile, Colombia and Puerto Rico all experienced declines anywhere from 2 to 68% [39].
However age adjusted CHD mortality trended upwards over the same period in Mexico, Costa Rica and Venezuela. One explanation is that these countries may have been in an earlier stage of development and are likely catching up with the rest of the region. For example, in Mexico, although age adjusted CHD mortality grew 90–94% over the three decade period, the age adjusted mortality is 82 per 100,000 in men and 53 per 100,000 in women in the year 2000, falling within the overall range of 21–136 per 100,000 for the region.
Treatment
Despite this decline in regional age adjusted CHD mortality, approach to treatment of CHD events is more conservative in the region. The PURSUIT trial—for the use of glycoprotein IIb/IIIa inhibitors in patients with NSTEMI—found a significant regional variation in treatment and mortality between Latin American and North American countries [40], [41]. Eight Latin American countries were included in the study: Argentina, Chile, Colombia, El Salvador, Guatemala, Mexico, Venezuela and Uruguay. Here the number of patients undergoing angiography was 47% with 11% undergoing angioplasty, compared to 80% and 18% respectively in North American countries. CABG was pursued in 19% of patients compared to 34% of the North American patients. While there was no difference in the primary end point of death or MI after 30 days between the two regions, death in itself occurred at a significantly higher rate in Latin America (6.8% versus 3.1% in North America).
In terms of secondary prevention after CHD events, data from the WHO PREMISE study found aspirin use in Brazil at 66%, beta blockers and angiotensin enzyme (ACE) inhibitors at around 50% and statins at less than 30% [42]. Similarly, a survey of patients with CHD and hypercholesterolemia, 67% of patients were prescribed statins and only around 41% took the drug [43]. Most patients reported that they were not able to afford the drug. In a group of Mexican patients with acute coronary syndrome enrolled in a prospective registry, the use of secondary prevention was higher but not universal [44]. Aspirin was prescribed to 89%, beta blockers to 51%, ACE inhibitors to 59% and statins to 13% of patients. Overall mortality was 7%. Therefore it is likely that basic preventive measures are likely at play for the overall regional decline in age adjusted CHD mortality: government policy aimed at reducing tobacco and salt consumption, nutritional labeling campaigns, and greater access to primary prevention.
Middle East and North Africa
Demographic and Social Indices
The Middle East and North Africa (MEN) is home to about 6% of the world’s population with 306 million people over 17 countries. Egypt and Iran are the two most populous countries in the region, with Egypt having 24% of the total inhabitants, and Iran with 22%. According to the World Bank indicators from 2005, the GNI per capita for the region is $2,198 ($6,084 PPP) [12]. GNI per capita for individual countries ranges from $600 ($920 PPP) in Yemen to $30,630 ($24,010 PPP) in Kuwait. GNI per capita for Egypt and Iran are $1,260 ($4,440 PP) and $2,300 ($8,050 PP) respectively [12].
According to World Bank data from 2004, approximately 5.6 % of the total GDP for the MEN region is used for public and private health care. The average health expenditure per capita is $103. Egypt spends $64 per capita and Iran spends $158 per capita. Yemen spends the least amount on health care per capita, with $34 and the United Arab Emirates spend the most, with $711[12].
Burden of Disease
Statistics from the WHO Global Burden of Disease study, 2002, show that about 5% of worldwide low- and middle- income country cardiovascular disease deaths come from the MEN region. Over 35% of all deaths in the region are attributable to CVD. CHD, the leading cause of mortality in 2001, accounts for 16.9% of total mortality and almost half of CVD mortality. Cerebrovascular disease causes 6.8% of total deaths and 19% of CVD deaths. This translates into approximately three hundred twenty three thousand deaths in 2001 in the region [6].
Mortality rates for the region have declined over time, while life expectancy has increased from 64.05 in 1990 to 67.35 in 2001. The crude death rate for the region has notably decreased. The newest data shows the crude death rate was 7.55% in 1990 and in 2001 was 6.15%. In 2001 the number of deaths attributed to non-communicable diseases in MEN was 1.235 million [6]. For CVD specifically, the number of deaths was 671 thousand in MEN [45]. However, with the increase in life expectancy due to reductions in infectious causes, there is an expectation for increases in CHD in the region. One study done on the global burden of CVD predicts that by 2020 rates of CHD will have increased by 161% [4].
Individual country surveys show that Iran possibly has a higher burden than other countries including Saudi Arabia and Jordan. A study done on a random sample of 3723 people in Iran found that 11.3% had coronary symptoms and an additional 1.4% had a myocardial infarction (MI). The age adjusted prevalence was therefore 12.7% [32]. Another study done in Saudi Arabia on 17,232 people from the general population found that 5.5% were diagnosed with CHD. The data also showed that prevalence was higher, 6.2% compared to 4%, in urban vs. rural Saudi Arabia [46]. In Jordan, a study showed a total of 5.9% out of 3083 participants were told they had an MI [47]. A Tunisian study in 2001 covering a fifth of the population of men only found age-standardized rates of myocardial infarcition of 163.8 per 100,000 in Tunis, 161.9 in Ariana, and 170.5 in Ben Arous [48].
Treatment
The WHO-PREMISE study was carried out to discover obstacles in secondary prevention of CVD in low-and middle- income countries. For the MEN region, Egypt, Iran, and Tunisia were covered. Aspirin use was the largest form of prevention, prescribed to 82.7% of Egyptians, 81.3% of Iranians, and 77.6% of Tunisians. Beta blockers were second, taken by 35.2%, 66%, and 59.6% in Egypt, Iran and Tunisia, respectively. ACE-inhibitors were used by 22.6%, 27.9% and 41.6% respectively. And statins were used the least in most cases, prescribed to 8.6%, 28.1%, and 5.84% respectively [42].
South Asia Region
Demographic and Social Indices
The South Asia Region (SAR), one of the most densely populated regions in the world, comprises about 20% of the world’s population, with a total of 1,470 million residents. India is the largest country in the region, making up nearly 75% of inhabitants. Average gross national income per capita for the region is $692 ($3,142 PPP), according to World Bank Indicators in 2005. GNI per capita ranges from $270 ($1,530 PPP) in Nepal to $2,560 in Maldives. India sits near the average with a GNI per capita of $730 ($3,460 PPP). Numbers from 2004 illustrate that all countries spend an average of 4.6% of the total GDP, or $27 per capita, on health care. Maldives spends the most on health care per capita with $208 and India spends $31, or 5% of their GDP. The lowest expenditure for healthcare is $14 per capita in Pakistan, Nepal and Bhutan [12].
Burden of Disease
Based on statistics from Global Burden of Disease, 2001, over 25% of worldwide low- and middle- income country CVD deaths come from SAR. Similarly, CVD accounts for over 25% of all deaths for the SAR region. This translates into for a total CVD of 3.4 million deaths. In particular coronary heart disease, the leading cause of mortality in 2001, makes up 13.6% of total mortality, or 1.8 million deaths, and more than half of CVD mortality. Cerebrovascular disease accounts for 6.8% of all deaths and 27% of CVD deaths. By comparison, communicable diseases account for 43.4% of total mortality [6].
Over time regional mortality rates have declined as life expectancy rates have increased from 57.15 in 1990 to 60.7 in 2001. The crude death rate for the region has decreased significantly. In 1990 the crude death rate was 11.45% for men and women and in 2001 was 9.75% [6]. However, deaths from CHD in India are increasing. In 1990 there were 1.17 million deaths and in 2000 the number of deaths rose to 1.59 million. It is predicted that deaths from CHD will be around 2.03 million by 2010 [49]. Similarly overall CVD burden is expected to increase as well. By 2020 compared with 1990 there will be a 111% increase in CVD deaths [17].
Several studies in India and Pakistan suggest that the morbidity of CHD is significant in this region. There are an estimated 31.8 million people living with CHD in India alone [17]. This is an increase 10 times that of 40 years ago, and translates into an overall prevalence in 2001 of about 11% in urban India and an age adjusted prevalence of 9% [50]. Additional evidence suggests there are a greater number of women in India with CVD in proportion to men [51]. IC Health’s National Cardiovascular Disease Database cited a study in India which found prevalence among men was over 6% and women over 10% [52]. Most recently, a study on CHD in Pakistan found a CHD prevalence of about 6% in men and 4% in women, but active ischemia was two times greater in women. They suggest that one in five adults in urban parts of Pakistan have CHD [53].
Of those who have CHD, it is estimated that only a fourth are aware of their disease and are seeking medical care. Despite this, a survey done on hospital data in Dehli revealed nearly 25% of all medical admissions are due to CHD. Patients who do not seek treatment die at a rate of 7–8% per year [17].
Contrary to the epidemiologic transition in developed countries, there is recent evidence to suggest that rather than those in high income brackets, SAR individuals with a lower socioeconomic status are developing a higher burden of CHD first. One reason for this could be the fact that a higher proportion of the poor use tobacco products [49].
Another demographic trend is the considerable increase in urban residents, normally associated with increased rates of CHD. Currently, about 30% of all inhabitants in the region live in an urban setting. This number is estimated to reach 43% by 2021 [54]. Consequently there are a larger number of people in urban settings affected by CHD. Prevalence has increased from 7% to 9.7% to 10.5% in 1980, 1990, and 2000 respectively. CHD prevalence in rural areas is on the rise as well, from 2.5% to 4% to 4.5% in 1980, 1990, and 2000, respectively [17]. However, more recent data from the rural region of Andhra Pradesh in South India suggests an increased prevalence that is higher than many rural regions [55]. CHD death rates were over 15% in that study. This either means that the rural/urban protection no longer exists or the urban rates if more carefully accounted for could be much higher.
The rise in CHD mortality contributes to the economic burdens in the Indian subcontinent. It has been found that unlike Caucasian and Latin American Countries, symptoms of CHD arise a whole 10 years earlier [17]. In India, 52% of CVD deaths occur among those under 70 years [54]. This results in a considerable burden of CHD on working class citizens [17].
Treatment
According to the WHO-Premise study, the number of patients receiving indicated treatments varies quite widely for CHD and cerebrovascular disease. For the SAR region, data for India, Pakistan, and Sri Lanka was recorded. Aspirin had the highest rate of appropriate use, prescribed to 94.5% of Indians, 96.1% of Pakistani’s and 66.3% of Sri Lankan’s. Beta blockers were used by 46.2%, 60.5%, and 8.7% of India, Pakistan, and Sri Lanka respectively. ACE inhibitors were used by 41.3%, 45.1%, and 10.8% respectively. Lastly, statins were used the least, prescribed to 38.4%, 15.6%, and 3.81% respectively [42].
Sub-Saharan Africa
Demographic and Social Indices
Sub-Saharan Africa (SSA), as defined by the World Bank, is one of six world regions and comprises 31 island and continental nations. Approximately 782 million people lived in SSA in 2006 with Nigeria being the most populous nation (145 million) and both Mauritius and Cape Verde having the smallest populations (1 million). The average annual population growth rate for 2000–2006 (4.7%) is almost twice the rate for 1990–2000 (2.5%). The average gross national income per capita was 830 dollars (US) and the spread of this income was from $100 (Burundi) to $5,570 (Botswana). Overall, the SSA region also had the lowest average life expectancy at birth (50 years) [56].
Average public and private health care expenditures for the region is 6.3% of the total GDP and an average of $45 per capita according to 2004 World Bank indicators. The range of health care expenditures per capita for the region is similar to the GDP range for this region, from $3 in Burundi to $511 in Seychelles. Nigeria spends $23 per capita, or 4.6% of the total GDP [12].
Burden of Disease
CHD was the leading cause of death in low and middle income countries in 2001, accounting for 11.8% (5.7 million) of all deaths, and in SSA, CHD accounted for 3.2% of all deaths[57]. In SSA in 2001, cardiovascular disease accounted for 46% of all deaths due to non-communicable diseases (1,048,000) and CHD accounted for 33% of all cardiovascular diseases (343,000) [57]. Stroke was responsible for 4.5% of the global burden of disease and 9.5% of the regional mortality in low and middle income countries in 2001. The burden of HIV/AIDS was 5.1% globally, with middle and low income countries only contributing 5.3% of mortality in the region.
Treatment
Given the paucity of resources in SSA, screening, interventions, and treatments for CVD need to be cost-effective. Investigators demonstrated that pharmacotherapy can be cost effective in low resource settings if appropriate regimens are used[58]. The authors estimated that death from CVD could be reduced by up to 50% and life expectancy could be increased by up to two years if the suggested, cost-effective drug regimens were used. In addition to pharmacotherapy approaches, the control and prevention of CVD through modification of certain lifestyle factors are essential. Smoking reduction has received a fair amount of attention and the WHO Framework Convention on Tobacco Control (FCTC) focuses resources and prevention efforts in the African region [59]. The WHO CVD Risk Management package for low resource settings focuses on opportunistic screening for high blood pressure but the package itself can be adapted to screening for diabetes, tobacco use, diabetes and dyslipidemia [60].
HIV-CHD Interaction
Given the double-burden of disease due to HIV/AIDS and CVD in SSA, the potential interaction between the two diseases is of growing concern. Premature vascular disease is an emerging problem, especially in those on antiretroviral therapy. HIV-positive men over the age of 50 were also found to have a greater prevalence of dyslipidemia, diabetes and peripheral artery disease (50% of the cases were asymptomatic), compared to their non-infected counterparts[61]. Of note is the fact that 55% of the HIV-infected men were prior smokers and they were also more likely to use antihypertensive drugs, lipid lowering agents and anti-diabetic medications. A recent study of 95 patients initiating antiretroviral drugs indicated that patients who had high baseline lipid levels showed marked increased in lipoprotein(a)[62]. Grover et al conducted a randomized control trial comparing lipid level changes after 32 weeks between patients on two different HAART therapy regimens: Atazanavir and Nelfinavir [63]. Levels of total cholesterol and LDL increased significantly more among patients using Nelfinavir (+24%, +28%) compared to those using Atazanavir (+4%, +1%), increasing the 10-year risk of coronary disease by 50% in the former patients. All of these data indicate that the interaction of positive HIV status, HAART therapy and risk for cardiovascular disease warrants continued attention.
RISK FACTORS
Table 3 displays the population attributable fraction (PAF) of deaths due to CHD for leading risk factors. Elevated levels of blood pressure and cholesterol remain the leading causes of CHD while tobacco, obesity, and physical inactivity remain important contributors. Diabetes is not listed as the GBD project lists it as a disease and not a risk factor. The PAFs sum to more than a 100% as there is interaction in the risk factors. Unique features regarding some CHD risk factors in the developing countries are described below.
Table 3.
Risk factor PAF's* for mortality from CHD
| World Bank Region | High Blood Pressure (%) |
Cholesterol (%) |
Tobacco (%) |
Overweight and Obesity (%) |
Physical Inactivity (%) |
|---|---|---|---|---|---|
| East Asia and Pacific | 41 | 32 | 6 | 10 | 19 |
| Europe and Central Asia | 61 | 55 | 17 | 24 | 20 |
| Latin America and Caribbean | 47 | 49 | 10 | 23 | 20 |
| Middle East and North Africa | 48 | 47 | 11 | 22 | 20 |
| South Asia | 39 | 43 | 10 | 5 | 19 |
| Sub-Saharan Africa | 43 | 15 | 5 | 8 | 20 |
| Low- and Middle-Income | 47 | 43 | 11 | 14 | 20 |
| High-Income | 49 | 52 | 15 | 19 | 19 |
PAF’s may sum to > 100%
WHO Global Burden of Disease and Risk Factors 2006 [6]
Tobacco
By many accounts, tobacco use is the most preventable cause of death in the world. Over 1.3 billion people use tobacco worldwide [64], more than 1 billion of whom smoke [65] and the rest who use oral or nasal tobacco. Over 80% of tobacco use occurs in low and middle income countries [64], and if current trends continue unabated there will be more than 1 billion deaths due to tobacco by the end of the 21st Century [66].
The use of tobacco varies greatly across the world. In general, more men than women smoke, and smoking is now more common in the developing world than in the developed, where it is on the decline. Smoking and tobacco use is most common in Russia (>60% male prevalence), Indonesia (>60% male prevalence), and China (approximately 60% male prevalence) [65]. Together, these three countries account for nearly half of the world’s users of tobacco. China alone has an estimated 311 million smokers [65]. Tobacco use is also quite prevalent in Latin America, the Pacific Islands (which have some of the highest female smoking rates in the world) and the Middle East [65]. Sub-Saharan Africa is currently a relatively low-prevalence tobacco using area with the exception of South Africa and parts of East Africa.
The clear and linear risk between cigarette smoking and CHD in the developed world is well-established [67, 68]. Ezzati and colleagues studied the global burden of cardiovascular disease attributable to smoking using data from the WHO global burden of disease studies as well as American Cancer Society data [69]. They calculated that in 2000, more than 1.62 million cardiovascular deaths in the world, or 11% of the total, were due to smoking [69].Of the overall total, 1.17 million were among men and 670,000 deaths occurred in the developing world [69]. Smoking-related CHD deaths globally made up approximately 890,000 deaths compared to 420,000 smoking-related cerebrovascular deaths [69]. The PAR of smoking for CHD in this global sample was 13%[69], significantly lower than the INTERHEART estimates[70] possibly due to the older nature of the data used in the Ezzati study. Smoking-related CHD deaths in the developing world totaled 360,000 compared to 200,000 cerebrovascular deaths [69].
Other forms of tobacco use beyond cigarette smoking increase risk for CHD. Bidis (hand-rolled cigarettes common in South Asia), Kreteks (clove and tobacco cigarettes), hookah (flavored tobacco smoked through a water pipe), and smokeless tobacco are all linked to increased risk for CHD[66, 68]. The combined use of different forms of tobacco is associated with a higher risk of AMI than use one type alone [68].
Secondhand smoke (SHS) also has now been well-established as a cause of CHD. Barnoya and Glantz performed a conservative random effects model and found that SHS was associated with a 1.31 increased risk of CHD (95% CI 1.21–1.41) [71]. Their review of the biological and epidemiological literature on SHS concluded that the effects of chronic SHS exposure on increased CHD risk are substantial, rapid, and nearly as large (80–90%) as those of active smoking [71]. These observations may explain the large and immediate drop seen in many communities such as Helena, Montana and Scotland that implemented smoke-free laws and found 20–40% decreases in admissions for AMI, controlling for time, locality, and other variables [72, 73].
Management: WHO Efforts - Tobacco
Tobacco control can be conceptualized in terms of strategies that reduce supply of, or demand for tobacco. Most public health and clinical strategies to date focus on reducing demand through economic disincentives (taxes), health promotion (media and packaging efforts), restricted access (to advertising and tobacco), or clinical assistance for cessation. The WHO effort to catalyze the creation of a global treaty against tobacco use was a key milestone in tobacco control. In May 2003, the WHO World Health Assembly unanimously adopted the WHO Framework Convention in Tobacco Control (FCTC), the first global tobacco treaty [66]. The FCTC has been ratified by 164 countries as of April 2009, making it one of the most widely embraced treaties in the United Nations [66]. The FCTC has spurred efforts for tobacco control across the globe by providing both rich and poor nations with a common framework of evidence-based legislation and implementation strategies known to reduce tobacco use.
To highlight the potential of the FCTC and promote further implementation of its articles, the WHO introduced the MPOWER package of policies in 2008 [66]. MPOWER stands for the following six key evidence-based tobacco control strategies that each signatory to the FCTC should implement [66]:
Monitor tobacco use and prevention policies
Protect people from tobacco smoke
Offer help to quit tobacco use
Warn about the dangers of tobacco
Enforce bans of tobacco advertising, promotion, and sponsorship
Raise taxes on tobacco
While each of these strategies is crucial, the recent 2008 WHO MPOWER report demonstrated that their implementation is lacking significantly. Currently half of countries worldwide, and two thirds of developing countries, do not have minimal information on tobacco use or monitoring capacity [66]. Only 5% of the world’s population is protected from tobacco smoke by national smoke-free legislation[66]. Comprehensive treatment services to help users quit tobacco use are available in only 9 countries representing 5% of the world population, and none of these countries is in the developing world [66]. Only 5 countries representing 4% of the global population mandate pack warnings that meet the highest known effectiveness standards, including pictorial warnings [66].More than half of children in the world live in countries that do not ban free tobacco product distribution, and only 5% of the global population lives in countries with comprehensive national advertising bans [66]. Tobacco taxation is considered the most effective way to reduce tobacco use quickly; a 10% tax increase decreases consumption an average of 8% in developing countries [66]. However, only 4 countries have tax rates greater than the accepted benchmark of 75% of retail price [66]. In developing countries, even the current low tobacco tax revenues are 4000 to 9000 times higher than spending on tobacco control [66].
Hypertension
Elevated blood pressure is an early indicator of the epidemiological transition. Rising mean population blood pressure is apparent as populations industrialize and move from rural to urban settings. Among urban-dwelling men and women in India, for example, the prevalence of hypertension is 25.5% and 29.0%, respectively, whereas it is just 14.0% and 10.8%, respectively, in rural communities. One major concern in developing countries is the high rate of undetected, and therefore untreated, hypertension. This may explain, at least in part, the higher stroke rates in these countries in relation to CHD rates during the early stages of the transition.
Worldwide, approximately 62% of strokes and 49% of cases of coronary heart disease are attributable to suboptimal (<115 mm Hg systolic) blood pressure, and it is thought to account for more than 7 million deaths annually. In a recent publication [74], the authors report that 14% percent of deaths and 6% of disability adjusted life-years (DALYs) lost globally were estimated to be due to non-optimal levels of blood pressure. While most societies define hypertension as a systolic blood pressure greater than 140 mm Hg, Lawes, et al. [74] found that just over half of the attributable CVD burden occurs among those with a systolic blood pressure less than 145 mm Hg.
The high rates of hypertension, especially undiagnosed hypertension, throughout Asia likely contribute to the high prevalence of hemorrhagic stroke. Approximately 30% of CHD in Chinese adults is due to hypertension, translating into more than 220,000 cases of CHD [16]. Data from the Asia-Pacific Cohort Studies Collaboration (APCSC) suggests that the age-adjusted male prevalence of hypertension in East Asia and the Pacific ranges from 6% in Fiji to 49% in Mongolia [75]. According to the Burden of Cardiovascular Disease report, data from 2004 show that hypertension affects 20–40% of urban residents and 12–17% of rural residents in SAR. This is an estimated total of 118 million people in India alone [54]. In addition, in an urban study on hypertension and socioeconomic status in 2000 showed rates of 54% in low income groups and 40% in high income groups. Another IC Health 2002 study reported an even higher number of 61% prevalence in Assam, India [52]. INTER-HEART found a prevalence for hypertension of 19.3% attributable to MI [70].
The PAF for mortality and years of life lost due to hypertension is higher in ECA than in any other region of the world. For example, the PAF for hypertension and CHD mortality is 61% in ECA, whereas the PAF for these same risk factors are below 50% in all other regions of the world.
Lipids
Worldwide, high cholesterol levels are estimated to cause 56% of ischemic heart disease and 18% of strokes, amounting to 4.4 millions deaths annually. Unfortunately, most developing countries have limited data on cholesterol levels and where they do, often only total cholesterol values are collected. In the high-income countries, mean population cholesterol levels are generally falling, but in the low- and middle-income countries, there is wide variation in these levels. Generally, the ECA region has the highest levels with the EAP and SSA regions having the lowest levels[76]. As countries move through the epidemiological transition, mean population plasma cholesterol levels tend to rise. Social and individual changes that accompany urbanization clearly play a role because plasma cholesterol levels tend to be higher among urban residents than among rural residents. This shift is largely driven by greater consumption of dietary fats—primarily from animal products and processed vegetable oils—and decreased physical activity.
Obesity
Rates of obesity are increasing throughout the world, particularly in developing countries—where the trajectories are steeper than those experienced by the developed countries. According to the latest WHO data, this is equivalent to about 1.1 billion overweight adults in the world, with 115 million of them known to be living with obesity related problems in the developing world [77]. A 2005 compilation of population based surveys updates this number to about 1.3 billion and estimates that 23% of adults over age 20 are overweight (BMI>25) and an additional 10% are obese (BMI>30) [78, 79]. In developing countries such as Egypt, Mexico and Thailand, rates of the overweight population are increasing at two to five times those in the U.S. In China over an eight year period, the prevalence of BMI>25 increased over 50% in both men and women [78].
Explanations for this rapid trajectory are complex but include changes in dietary patterns, physical activity and urbanization. Popkin and Garden-Larsen report that use of edible oils, caloric sweeteners and animal source foods is increasing [78]. Annual animal food consumption has tripled in China from 1950s to 1990s. Physical activity levels are expected to decline as urbanization leads to increased use of motorized vehicles and change to more sedentary occupations.
Unlike prior data from the 1980s which showed that obesity was a problem of the higher income group in developing countries, a recent analysis shows that the poor are relatively more vulnerable to obesity as a developing country’s GNP approaches the middle income range [80, 81]. For example, in this analysis once a country reaches $2,500 per capita of GNP (about the median GNP for lower-middle income countries), the probability of being obese was higher among women living in the lower income group than in the higher income one.
Two groups are spotlighted in the literature. Women are more affected than men, with the number of overweight women generally exceeding underweight women based on data from 36 developing countries [82]. In the same survey, prevalence of overweight women exceeded 20% in more than 90% of surveyed countries. These rates were reached even in rural areas in half of the countries surveyed. Adolescents are at particular risk as well, with one in ten children estimated to be overweight currently [78, 83]. The number of overweight children is increasing in countries as diverse as China, Brazil, India, Mexico and Nigeria. In Brazil there was alarming rise from 4% to 14% over a two decade period.
Aging Demographics
Average life expectancy will reach 73 years by 2025 according to WHO statistics [84]. This rise is related to a decline in overall infant mortality and fertility rates. Although the elderly will represent a greater percentage of the developed world’s population—over 20% of the population here will be over 65 years old by 2025—developing regions such as Asia and Latin America will nearly double their relative proportion of elderly to 10% of their populations [11].
The time of transition to an older population is sharply shorter in developing countries. For example while it took the US and Canada over 65 years to double its over 65 population, China will do so in 26 years, Tunisia in 24 and Brazil in 21 years [85]. In fact currently 77% of the growth in the elderly population is occurring in developing regions [86]. Such acute changes in the population structure leave less time to expand an already overburdened health infrastructure to address the diseases of the elderly which are predominantly non communicable and more specifically cardiovascular.
Diabetes
About 180 million people are living with diabetes mellitus (DM) in the world right now, and the number is expected to double by 2030 [87]. Ninety percent of those with DM have DM type 2. About 80 percent of this population currently lives in low and middle income countries. Future growth in affected people will be highest in developing regions such as Asia, Latin America and Caribbean, and Sub Saharan Africa where growth rates will exceed 104–162%, compared to about 72% in the U.S. and 32% in Europe [88, 89]. In addition a majority of cases are and will remain within the 45–64 years age group in developing countries whereas an older population (over 65 years) is the major group affected in developed countries.
Rising rates of obesity as well as aging and urbanization of the population have been linked to the DM epidemic. Nearly 90% of DM type 2 cases are estimated to be related to obesity. And in reverse, DM and its related complications are the costliest consequence of obesity. Mortality from DM is also on the rise. About 1.1 million people died due to DM in 2005 and the number is estimated to increase by 50% in 10 years [87].
Interestingly Asian countries face a relatively larger burden of DM (compared to ECA or LAM). For example, India and China house the largest number of diabetics at 32 million and 21 million respectively in the world [89]. As well, Indonesia, Pakistan and Bangladesh are in the top ten in terms of high absolute number of diabetics. It is speculated that Asian populations may have a higher risk for developing DM even at a lower BMI due to a greater tendency towards abdominal obesity. In addition this population may experience both under-nutrition (during perinatal period) and rapid weight gain (during childhood), a combination which increases the risk for insulin resistance [90].
ECONOMIC BURDEN
There are at least three ways to measure the economic burden associated with coronary heart disease and they are somewhat overlapping[91]. The first source of financial burden is defined by the costs incurred in the health care system itself and reported in “cost-of-illness” studies. In these studies, the cost of coronary heart disease includes the costs of hospitalizations for angina and myocardial infarction as well as heart failure attributable to coronary heart disease. In addition, there are the costs of specific procedures related to CHD such as catheterization and PCI. Further, there are costs associated with the outpatient management and secondary prevention, both in terms of office visits and pharmaceutical costs. In addition there is nursing home, rehabilitation (inpatient and outpatient), and home nursing costs associated with this. An additional cost not often accounted for is the intangible loss of welfare associated with pain disability or suffering by the individual. These indirect costs are often accounted for by “willingness-to-pay” analyses, asking generally how much would an individual pay to avert suffering or dying prematurely from coronary heart disease. The gains are not merely improved work performance but also enjoying activities beyond production.
The second economic assessment is based on microeconomic studies that look at the household impact on catastrophic health care events such as myocardial infarction. These studies look at out of pocket expenses incurred by the individual or family that might have other downstream economic impacts such as loss of savings or sale of property to cover health costs. Given that in many developing countries without an extensive insurance scheme, health care costs are almost entirely borne by individuals[92]. The microeconomic studies to date have not been exclusively related to coronary heart disease and have more generally looked at chronic diseases overall. Furthermore, the limited data to date does not confirm the causality between chronic disease and individual or household poverty [91]. However, expenditures for coronary disease or its addictive risk factors such as tobacco could lead to substantial and even impoverishing costs.
The third type of financial burden from coronary heart disease is based on a macroeconomic analysis. These assessments look at lost worker productivity or economic growth that is lost by having adults with CHD or their caregivers partially or completely out of the work as a result of their illness. The data for the impact of chronic diseases on labor supply and productivity is more robust.
Studies in the United States suggest that as much as 1–3% of GDP is attributable to CVD with almost half of that related to coronary heart disease[93]. In China, annual direct costs of CVD are estimated at more than 40 billion US dollars or roughly four percent of its gross national income. In South Africa 2–3% of the country’s gross national income was devoted to the direct treatment of CVD or roughly 25% of the South African health care expenditures.[10] The indirect costs were estimated to being more than double that of the direct costs. Fewer cost-of-illness studies have been done in other regions for coronary heart disease. However, other cost-of-illness studies report on financial burdens attributed to the risk factors for coronary heart disease. For example the direct costs due to diabetes in the Latin American and Caribbean countries was estimated at $US 10 billion. Indirect costs were estimated at over $US 50 billion [94] in the year 2000. Studies are limited, but suggest that obesity-related diseases are responsible for 2 to 8 percent of all health care expenditures in developed countries. In India and China the costs for obesity are about 1.1% and 2.1% of GDP respectively [95].
The macroeconomic impact in developing countries is highlighted by the fact that such a high proportion of cardiovascular disease burden occurs earlier among adults of working age. Under current projections developing countries such as South Africa will have CVD strike 40% of adults between the ages of 35–64 compared to 10% in the US.[5] India and China will have death rates in the same age group that are two and three times that of most developed countries. Given the large populations in these two rapidly growing economies, this could have profound economic effects over the next 25 years as workers in their prime succumb to cardiovascular disease.
This can lead to a large impact on a developing countries economic viability. In the recent report A Race Against Time [5], the authors evaluated the potential loss due to early CVD. In five countries surveyed (Brazil, India, China, South Africa and Mexico) conservative estimates indicated that at least 21 million years of future productive life are lost because of CVD each year.
COST-EFFECTIVE SOLUTIONS
In this section we review a series of interventions focused both at the individual level for those at with established CHD or at near term high risk for CHD as well as the general population in order to reduce the future burden of CHD. The section is not exhaustive but highlights the variety of types of interventions. Much work is still needed to be done in developing countries to determine the best strategies given the limited resources but the interventions reviewed if implemented could go a long way to reduce a large proportion of the burden. Table 4 lists the cost-effectiveness ratios for many of the high-yield interventions that could or have been adopted in many developing regions.
Table 4.
Cost-Effectiveness for a Selection of CHD Interventions in Developing Regions.
| Cost-effectiveness ratio ($US/DALY)* |
|
|---|---|
| DRUG TREATMENTS | |
| Acute Myocardial Infarction | |
| ASA, BB | 11 – 22 |
| ASA, BB, SK | 634 – 734 |
| ASA, BB,TPA | 15,860 – 18,893 |
| Secondary Treatment (CHD†) | |
| Multidrug regimen (ASA, BB, ACEI, Statin) | 1,686 – 2,026 |
| Coronary Artery Bypass Graft (CABG) | 24,040 – 72,345 |
| POLICY INTERVENTIONS | |
| Tobacco | |
| Price increase of 33% | 2 – 85 |
| Non-policy interventions | 33 –1,432 |
| Salt Reduction‡ | |
| 2 – 8 mmHg Reduction | Cost saving -250 |
| Fat-related interventions§ | |
| Reduced saturated fat intake | Cost saving – 2,900 |
| Trans fat replacement - 7% reduction in CHD | 50 – 1,500 |
ASA indicates aspirin; BB, beta-blocker; SK, streptokinase; ACEI, Angiotensin converting enzyme inhibitor; tPA tissue plasminogen activator.
Across six World Bank regions.
CHD is coronary heart disease.
Range includes different estimates of cost of interventions, as well as blood pressure reduction (<$0.50 – $1.00).
Range includes estimates of cost of interventions (<$0.50 – $6.00).
CHD Management
Those at highest risk are those suffering an AMI or stroke with as many as half dying before they ever reach medical attention. For those who do make it to a hospital, standard medical therapies were analyzed in a cost-effectiveness analysis in the Disease Control Priorities Project in Developing Countries [58, 96, 97].
Four incremental strategies were evaluated for the treatment of AMI and compared to a strategy of no treatment as a base case for the six World Bank low and middle income regions. The four strategies compared were: aspirin; aspirin and atenolol; aspirin, atenolol, and streptokinase; and aspirin, atenolol, and tissue plasminogen activator (t-PA). The incremental cost per QALY gained for both the aspirin and beta-blocker interventions were under $25 for all 6 regions. Costs per QALY gained for streptokinase were between $630–730 across the regions. ICERs for t-PA were around $16000/QALY gained compared to streptokinase. Minor variations occurred between regions due to small differences in follow up care based on regional costs.
Secondary prevention strategies are equally cost-effective in developing country regions. Studies show that a combination of aspirin, ACE-inhibitor, beta-blocker and statin for secondary prevention can lead to acceptable cost-effectiveness ratios in all developing regions[97]. Use of currently available generic agents even in the absence of the so-called “polypill” could be highly cost-effective on the order of $300–400 per person [58].
Risk Assessment
Besides those with overt CHD, there are a large number at high risk for CHD for whom primary prevention is important. Given the limited resources, finding low-cost prevention strategies is a top priority. Using prediction rules or risk scores to identify those at higher risk in order to target specific behavioural or drug interventions is a well-established primary prevention strategy and proven to be cost-effective in developing countries [98]. Most have included age, sex, hypertension, smoking status, diabetes mellitus, lipid values or family history[99–102]. Recently, many investigators have been attempting to see if additional lab-based risk factors can add to predictive discrimination of the risk factors used in the Framingham Heart Study. The recent analyses in the Atherosclerosis Risk in Communities (ARIC) Study [103], and the Framingham Offspring Study [104, 105], suggested that little additional information was gained when other blood based novel risk factors were added to the traditional risk factors. The Reynolds [106] score for women which added hsCRP and hemoglobin A1c only had a marginally higher C-statistic (0.808) than the Framingham covariates (0.791). However, there was important reclassification of patients that may have important implications for guidelines.
Less attention has been directed at developing risk scores that would be easier to use in clinical practice without loss of predictive discrimination in resource poor countries. In developed countries, a prediction rule that requires a lab test is an inconvenience; but in low-income countries, with limited testing facilities, it may make its use too expensive or preclude its use altogether. In response to this real concern, the WHO recently released risk prediction charts for the different regions of the world with and without cholesterol [107, 108]. A study based on the US NHANES follow-up cohort demonstrated that a non-lab based risk tool that uses information obtained in a single encounter (age, systolic blood pressure, BMI, diabetes status, and smoking status) can predict CVD outcomes as well as one that requires lab testing with C-statistics of 0.79 for men and 0.83 for women that were no different from the Framingham based risk tool [109]. Further, the results of the goodness-of-fit tests suggest that the non lab-based model is well calibrated across a wide range of absolute risk levels and without changes in classification of risk.
Tobacco
Jha and colleagues presented a landmark analysis in 2006 of tobacco control cost effectiveness [110]. They calculated the reductions in future tobacco deaths due to a range of tax, treatment, and non-price interventions among smokers alive in 2000. They found that a 33% price increase would reduce between 19.7 and 56.8 million (5.4–15.9% of total) deaths in smokers from the developing world alive in 2000 [110]. Nicotine replacement therapy (NRT) was calculated to reduce between 2.9 and 14.3 million deaths (0.8–4.0% of total) in the 2000 cohort [110]. A range of non-price interventions such as advertising bans, health warnings, and smoke-free laws would reduce between 5.7 and 28.6 million deaths (1.6–7.9% of total) in that cohort[110]. These reductions would translate into developing world cost effectiveness values of between $3 and $42 dollars per QALY saved for tax increases (not including tax revenue), $55 – $761 per QALY for NRT, and $54 to $674 per QALY for non-price measures [110].
Of critical importance for patients who have had a coronary event, smoking cessation saves lives at a greater rate than any individual medical treatment. Mohiuddin et al conducted a randomized controlled trial of a behavioral and medication smoking cessation program for smokers who were hospitalized with an coronary event in the critical care unit [111]. They were able to nearly triple quit rates and decrease all-cause mortality at one year by an absolute risk of 9% (77% reduction in relative risk). This reduction corresponded with a number needed to treat (NNT) of 11 for smoking cessation to prevent 1 death in the year following a major coronary cardiac event[111]. This NNT for secondary prevention is more favorable than statins, beta blockers, or even aspirin[112].
Policy and Community Interventions
The cost-effectiveness analyses on salt reduction as result of public education are quite favourable[113]. The intervention ranges from being cost-saving to two hundred dollars per DALY averted across the range of estimates of the cost of the intervention as well as its effectiveness. The results of a campaign for reducing saturated fat and replacing it with polyunsaturated fat is also likely cost-effective. In the base case, a 3% decline in cholesterol and a $6 per capita education costs was assumed. This resulted in a cost as low as $1800 per DALY averted in the South Asian region up to $4000/DALY in the Middle East and North Africa region. However, if the cost for the education plan were halved the ratio is approximately $900/DALY and would be cost-saving if the reduction could be achieved for under $0.50 per capita which may be possible in areas where media is much less expensive.
Several community intervention studies where risk factors for CHD have been reduced have been conducted in developing countries, including those in China[114, 115], Mauritius[116], and South Africa[117]. However, a significant reduction in mortality has not been shown nor has the cost-effectiveness of such interventions been evaluated. The Tianjin Project showed reductions in hypertension and obesity. The Mauritius project among other interventions resulted in a government led program that changed the prime cooking oil from a predominantly saturated fat palm oil to a soybean oil that is high in unsaturated fatty acids. Overall total cholesterol levels fell 14% during the five year study period from 1987 to 1992[118]. Changes in other risk factors were mixed with declines in blood pressure and smoking rates and increases in obesity and diabetes. The Coronary Risk Factor Study in South Africa compared a control community to two communities receiving two different levels of intensity of interventions. The interventions included mass media, group sponsored educational sessions, and blood pressure screening and follow-up with the health sector when appropriated. Both high and low intensity interventions showed improvements in blood pressure, smoking rates, and HDL to total cholesterol ratio over the control community, but with little difference between the two intervention communities.
One other significant reduction of CHD came not through a concerted community intervention but through changes in fiscal policy. In Poland, reductions in subsidies for animal products such as butter and lard led to a switch from saturated to polyunsaturated fats[119, 120]. The increased consumption in polyunsaturated fats was mainly through rapeseed and soybean based oils. There was a resultant decrease in CHD mortality of greater than 25%[119, 120] between 1991 and 2002 that could not be explained by increased fruit consumption or declines in smoking.
SUMMARY
Coronary heart disease remains a significant and growing problem in most of the developing regions of the world. The increase in prevalence and mortality associated with the large burden of coronary heart disease is a reflection of the epidemiological transition that has accompanied economic and social development. The challenge for developing economies, unlike developed economies, is that this economic and social transformation is occurring much more rapidly in a post-industrial world with rapid globalization. Therefore the changes in risk factors and incidence rates are much more rapid in many cases outpace the development of health care networks, human resources, and the infrastructure needed to manage such an important cause of chronic disease.
The different regions of the world face different stages of the epidemic. Currently, the Eastern European countries and members of the former Soviet Republic are facing enormous burdens with over half of all deaths attributed to cardiovascular disease. Meanwhile, countries in Sub Saharan Africa are just beginning to see increases in these chronic illnesses while still grappling with HIV/AIDS. The trends in risk factors suggest the problem is only going to continue to grow in the near term. Nonetheless, viable solutions to curbing if not reversing the epidemic exist. The reduction in the disease burden will require changes at the policy level as well as at the personal level. From societal perspective efforts to improve lifestyle choices such as tobacco control strategies will be paramount. At the personal level strategies to assess risk will need to be simplified as well as the treatment modalities employed. Further, alternative uses of allied health workers such as community health workers will need to be evaluated given the reduce human resources in most developing countries.
ACKNOWLEDGEMENT
We would like to acknowledge Gail Robinson for her tremendous support, in editing and preparing the final document prior to submission.
Dr. Gaziano is supported by a grant from the Fogarty International Center, NIH. Grant # 2K01TW007141-05.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
References
- 1.WHO, The World Health Report 2002. Geneva: World Health Organization; Reducing Risks, Promoting Healthy Life. 2002 doi: 10.1080/1357628031000116808. [DOI] [PubMed]
- 2.Olshansky SJ, Ault AB. The fourth stage of the epidemiologic transition: the age of delayed degenerative diseases. Milbank Memorial Fund Quarterly. 1986;64(3):355–391. [PubMed] [Google Scholar]
- 3.Omran AR. The epidemiologic transition: a theory of the epidemiology of population change. Milbank Memorial Fund Quarterly. 1971;49(4):509. [PubMed] [Google Scholar]
- 4.Yusuf S, et al. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 5.Leeder S, et al. A Race Against Time: The Challenge of Cardiovascular Disease in Developing Countries. New York: Trustees of Columbia University; 2004. [Google Scholar]
- 6.Lopez AD, Mathers CD, Ezzati M, Jamison DT, editors. Global Burden of Disease and Risk Factors. ed. D.C.P. Project. New York, NY: The World Bank and Oxford University Press; 2006. p. 552. [Google Scholar]
- 7.Reddy KS. Cardiovascular disease in non-Western countries. N Engl J Med. 2004;350(24):2438–2440. doi: 10.1056/NEJMp048024. [DOI] [PubMed] [Google Scholar]
- 8.World Bank. Washington, DC: The World Bank; World Development Indicators. 2002
- 9.American Heart Association. [cited July 13, 2003];Heart Disease and Stroke Statistics - 2004 Update. 2003 Available from: http://www.americanheart.org.
- 10.Pestana JA, et al. The direct and indirect costs of cardiovascular disease in South Africa in 1991. South African Medical Journal. 1996;86(6):679–684. [PubMed] [Google Scholar]
- 11.UN Office of the High Representative for the Least Developed Countries. [cited 2009 April 16, 2009];Landlocked Developing Countries and Small Island Developing States. 2009 Available from: http://www.un.org/special-rep/ohrlls/ldc/list.htm.
- 12.Bank TW, editor. Development Data Group. World Development Indicators. Washington, D.C: International Bank; 2007. p. 402. [Google Scholar]
- 13.Geneva: World Health Organization; World Health Report 2003: Shaping the Future. 2003
- 14.WHO. World Health Organization; The Global Burden of Disease: 2004 Update. 2008:160.
- 15.Liu L. Cardiovascular diseases in China. Biochem Cell Biol. 2007;85(2):157–163. doi: 10.1139/O07-004. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XH, Lu ZL, Liu L. Coronary heart disease in China. Heart. 2008;94(9):1126–1131. doi: 10.1136/hrt.2007.132423. [DOI] [PubMed] [Google Scholar]
- 17.Gupta R. Burden of coronary heart disease in India. Indian Heart J. 2005;57(6):632–638. [PubMed] [Google Scholar]
- 18.Wu Z, et al. Sino-MONICA project: a collaborative study on trends and determinants in cardiovascular diseases in China, Part i: morbidity and mortality monitoring. Circulation. 2001;103(3):462–468. doi: 10.1161/01.cir.103.3.462. [DOI] [PubMed] [Google Scholar]
- 19.Chen ZM, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366(9497):1607–1621. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- 20.Chen ZM, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366(9497):1622–1632. doi: 10.1016/S0140-6736(05)67661-1. [DOI] [PubMed] [Google Scholar]
- 21.Oral captopril versus placebo among 13,634 patients with suspected acute myocardial infarction: interim report from the Chinese Cardiac Study (CCS-1) Lancet. 1995;345(8951):686–687. [PubMed] [Google Scholar]
- 22.Liu L. Long-term mortality in patients with myocardial infarction: impact of early treatment with captopril for 4 weeks. Chin Med J (Engl) 2001;114(2):115–118. [PubMed] [Google Scholar]
- 23.Ye P, et al. Effect of xuezhikang on cardiovascular events and mortality in elderly patients with a history of myocardial infarction: a subgroup analysis of elderly subjects from the China Coronary Secondary Prevention Study. J Am Geriatr Soc. 2007;55(7):1015–1022. doi: 10.1111/j.1532-5415.2007.01230.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhao SP, et al. Xuezhikang, an extract of cholestin, reduces cardiovascular events in type 2 diabetes patients with coronary heart disease: subgroup analysis of patients with type 2 diabetes from China coronary secondary prevention study (CCSPS) J Cardiovasc Pharmacol. 2007;49(2):81–84. doi: 10.1097/FJC.0b013e31802d3a58. [DOI] [PubMed] [Google Scholar]
- 25.La Vecchia C, et al. Trends in mortality from major diseases in Europe, 1980–1993. Eur J Epidemiol. 1998;14(1):1–8. doi: 10.1023/a:1007440201137. [DOI] [PubMed] [Google Scholar]
- 26.Kesteloot H, Sans S, Kromhout D. Dynamics of cardiovascular and all-cause mortality in Western and Eastern Europe between 1970 and 2000. Eur Heart J. 2006;27(1):107–113. doi: 10.1093/eurheartj/ehi511. [DOI] [PubMed] [Google Scholar]
- 27.Mirzaei M, et al. Coronary Heart Disease (CHD) epidemics: not all the same. Heart. 2008 [Google Scholar]
- 28.Xu K, et al. Household catastrophic health expenditure: a multicountry analysis. Lancet. 2003;362(9378):111–117. doi: 10.1016/S0140-6736(03)13861-5. [DOI] [PubMed] [Google Scholar]
- 29.Ganova-Iolovska M, Kalinov K, Geraedts M. Quality of care of patients with acute myocardial infarction in Bulgaria: a cross-sectional study. BMC Health Serv Res. 2009;9:15. doi: 10.1186/1472-6963-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurjeva OS, et al. Treatment and outcomes of eastern Europeans with coronary syndromes in OPUS-TIMI 16. Int J Cardiol. 2005;100(1):101–107. doi: 10.1016/j.ijcard.2004.08.075. [DOI] [PubMed] [Google Scholar]
- 31.Eastaugh JL, Calvert MJ, Freemantle N. Highlighting the need for better patient care in stable angina: results of the international Angina Treatment Patterns (ATP) Survey in 7074 patients. Fam Pract. 2005;22(1):43–50. doi: 10.1093/fampra/cmh711. [DOI] [PubMed] [Google Scholar]
- 32.Nabipour I, et al. The metabolic syndrome and nonfatal ischemic heart disease; a population-based study. Int J Cardiol. 2007;118(1):48–53. doi: 10.1016/j.ijcard.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Health, nutrition and population statistics. World Bank Economic Indicators; 2007. [cited 2009 3/27]. Available from: http://go.worldbank.org/N2N84RDV00. [Google Scholar]
- 34.Azambuja MI, et al. Economic burden of severe cardiovascular diseases in Brazil: an estimate based on secondary data. Arq Bras Cardiol. 2008;91(3):148, 163–155. doi: 10.1590/s0066-782x2008001500005. [DOI] [PubMed] [Google Scholar]
- 35. [cited 2009 4/1];Regional Core Health Data Initiative. 2004 Available from: www.paho.org/English/SHA/coredata/tabulator/newTabulator.htm. [PubMed]
- 36.Balbinotto Neto G, Silva EN. The costs of cardiovascular disease in Brazil: a brief economic comment. Arq Bras Cardiol. 2008;91(4):198, 217–199. doi: 10.1590/s0066-782x2008001600002. [DOI] [PubMed] [Google Scholar]
- 37.Laurenti R, Buchalla CM, Caratin Vde S. Ischemic heart disease. Hospitalization, length of stay and expenses in Brazil from 1993 to 1997. Arq Bras Cardiol. 2000;74(6):483–492. doi: 10.1590/s0066-782x2000000600001. [DOI] [PubMed] [Google Scholar]
- 38.Curioni C, et al. The decline in mortality from circulatory diseases in Brazil. Rev Panam Salud Publica. 2009;25(1):9–15. doi: 10.1590/s1020-49892009000100002. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez T, et al. Trends in mortality from coronary heart and cerebrovascular diseases in the Americas: 1970–2000. Heart. 2006;92(4):453–460. doi: 10.1136/hrt.2004.059295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. The PURSUIT Trial Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy. N Engl J Med. 1998;339(7):436–443. doi: 10.1056/NEJM199808133390704. [DOI] [PubMed] [Google Scholar]
- 41.Akkerhuis KM, et al. Geographic variability in outcomes within an international trial of glycoprotein IIb/IIIa inhibition in patients with acute coronary syndromes. Results from PURSUIT. Eur Heart J. 2000;21(5):371–381. doi: 10.1053/euhj.1999.1743. [DOI] [PubMed] [Google Scholar]
- 42.Mendis S, et al. WHO study on Prevention of REcurrences of Myocardial Infarction and StrokE (WHO-PREMISE) Bull World Health Organ. 2005;83(11):820–829. [PMC free article] [PubMed] [Google Scholar]
- 43.Mansur AP, et al. Prescription and adherence to statins of patients with coronary artery disease and hypercholesterolemia. Arq Bras Cardiol. 2001;76(2):111–118. doi: 10.1590/s0066-782x2001000200002. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Castillo A, et al. Mexican Registry of Acute Coronary Syndromes. Arch Cardiol Mex. 2005;75 Suppl 1:S6–S32. [PubMed] [Google Scholar]
- 45.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349(9061):1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 46.Al-Nozha MM, et al. Coronary artery disease in Saudi Arabia. Saudi Med J. 2004;25(9):1165–1171. [PubMed] [Google Scholar]
- 47.Nsour M, et al. Prevalence and predictors of nonfatal myocardial infarction in Jordan. East Mediterr Health J. 2008;14(4):818–830. [PubMed] [Google Scholar]
- 48.Ben Romdhane H, et al. The first Tunisian cardiovascular diseases register: processes and results. Rev Epidemiol Sante Publique. 2004;52(6):558–564. doi: 10.1016/s0398-7620(04)99094-3. [DOI] [PubMed] [Google Scholar]
- 49.Ghaffar A, Reddy KS, Singhi M. Burden of non-communicable diseases in South Asia. BMJ. 2004;328(7443):807–810. doi: 10.1136/bmj.328.7443.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohan V, et al. Prevalence of coronary artery disease and its relationship to lipids in a selected population in South India: The Chennai Urban Population Study (CUPS No. 5) J Am Coll Cardiol. 2001;38(3):682–687. doi: 10.1016/s0735-1097(01)01415-2. [DOI] [PubMed] [Google Scholar]
- 51.Ahmad N, Bhopal R. Is coronary heart disease rising in India? A systematic review based on ECG defined coronary heart disease. Heart. 2005;91(6):719–725. doi: 10.1136/hrt.2003.031047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ajay VS, Gupta R, Panniyammakkal J, et al. National Cardiovascular Disease Database. Geneva: World Health Organization; Delhi Ministry of Health and Family Welfare, Government of India. 2002
- 53.Jafar TH, Qadri Z, Chaturvedi N. Coronary artery disease epidemic in Pakistan: more electrocardiographic evidence of ischaemia in women than in men. Heart. 2008;94(4):408–413. doi: 10.1136/hrt.2007.120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goyal A, Yusuf S. The burden of cardiovascular disease in the Indian subcontinent. Indian J Med Res. 2006;124(3):235–244. [PubMed] [Google Scholar]
- 55.Joshi R, et al. Fatal and Nonfatal Cardiovascular Disease and the Use of Therapies for Secondary Prevention in a Rural Region of India. Circulation. 2009;119(14):1950–1955. doi: 10.1161/CIRCULATIONAHA.108.819201. [DOI] [PubMed] [Google Scholar]
- 56.World Bank. [cited April 16, 2009];World Development Indicators. 2008 Available from: http://siteresources.worldbank.org/DATASTATISTICS/Resources/reg_wdi.pdf.
- 57.Lopez AD, et al., editors. Global Burden of Disease and Risk Factors. New York: Oxford University Press; 2006. Measuring the Global Burden of Disease and Risk Factors, 1990–2001. [Google Scholar]
- 58.Gaziano TA, Opie LH, Weinstein MC. Cardiovascular disease prevention with a multidrug regimen in the developing world: a cost-effectiveness analysis. Lancet. 2006;368(9536):679–686. doi: 10.1016/S0140-6736(06)69252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mensah GA. Ischaemic heart disease in Africa. Heart. 2008;94(7):836–843. doi: 10.1136/hrt.2007.136523. [DOI] [PubMed] [Google Scholar]
- 60.World Health Organization. Bengoa Rafael, Yach D., editors. Integrated management of cardiovascular risk: report of a WHO meeting, Geneva, 9–12 July 2002. New York: World Health Organization; Cardiovascular Disease Program. 2002
- 61.Palacios R, et al. Peripheral Arterial Disease in HIV Patients Older than 50 Years of Age. AIDS Research and Human Retroviruses. 2008;24(8):1043–1046. doi: 10.1089/aid.2008.0001. [DOI] [PubMed] [Google Scholar]
- 62.Mauss S, et al. Lipoprotein(a) in patients initiating antiretroviral therapy. HIV Med. 2008;9(6):415–420. doi: 10.1111/j.1468-1293.2008.00574.x. [DOI] [PubMed] [Google Scholar]
- 63.Grover SA, et al. Impact of dyslipidemia associated with Highly Active Antiretroviral Therapy (HAART) on cardiovascular risk and life expectancy. The American journal of cardiology. 2005;95(5):586–591. doi: 10.1016/j.amjcard.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 64.Thun M, da Costa e Silva V. Introduction and Overview of Global Tobacco Surveillance. Geneva: World Health Organization; Tobacco Control Country Profiles. 2003
- 65.Shafey O, et al. The Tobacco Atlas, Third Edition. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 66.Geneva: World Health Organization; WHO Report on the Global Tobacco Epidemic, 2008: The MPOWER Package. 2008
- 67.Parish S, et al. Cigarette smoking, tar yields, and non-fatal myocardial infarction: 14,000 cases and 32,000 controls in the United Kingdom. The International Studies of Infarct Survival (ISIS) Collaborators. Bmj. 1995;311(7003):471–477. doi: 10.1136/bmj.311.7003.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teo KK, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368(9536):647–658. doi: 10.1016/S0140-6736(06)69249-0. [DOI] [PubMed] [Google Scholar]
- 69.Ezzati M, et al. Role of smoking in global and regional cardiovascular mortality. Circulation. 2005;112(4):489–497. doi: 10.1161/CIRCULATIONAHA.104.521708. [DOI] [PubMed] [Google Scholar]
- 70.Yusuf S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 71.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111(20):2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 72.Sargent RP, Shepard RM, Glantz SA. Reduced incidence of admissions for myocardial infarction associated with public smoking ban: before and after study. Bmj. 2004;328(7446):977–980. doi: 10.1136/bmj.38055.715683.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pell JP, et al. Smoke-free legislation and hospitalizations for acute coronary syndrome. N Engl J Med. 2008;359(5):482–491. doi: 10.1056/NEJMsa0706740. [DOI] [PubMed] [Google Scholar]
- 74.Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371(9623):1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 75.Martiniuk AL, et al. Hypertension: its prevalence and population-attributable fraction for mortality from cardiovascular disease in the Asia-Pacific region. J Hypertens. 2007;25(1):73–79. doi: 10.1097/HJH.0b013e328010775f. [DOI] [PubMed] [Google Scholar]
- 76.Ezzati M, et al. Comparative Quantification of Mortality and Burden of Disease Attributable to Selected Risk Factors. In: M.C. Lopez AD, Ezzati M, Jamieson DT, Murray CJL, editors. Global Burden of Disease and Risk Factors. New York: Oxford University Press; 2006. pp. 241–396. [PubMed] [Google Scholar]
- 77.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S9–S30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 78.Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes Relat Metab Disord. 2004;28 Suppl 3:S2–S9. doi: 10.1038/sj.ijo.0802804. [DOI] [PubMed] [Google Scholar]
- 79.Kelly T, et al. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32(9):1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 80.Raymond SU, Leeder S, Greenberg HM. Obesity and cardiovascular disease in developing countries: a growing problem and an economic threat. Curr Opin Clin Nutr Metab Care. 2006;9(2):111–116. doi: 10.1097/01.mco.0000214568.52192.91. [DOI] [PubMed] [Google Scholar]
- 81.Monteiro CA, et al. Socioeconomic status and obesity in adult populations of developing countries: a review. Bull World Health Organ. 2004;82(12):940–946. [PMC free article] [PubMed] [Google Scholar]
- 82.Mendez MA, Monteiro CA, Popkin BM. Overweight exceeds underweight among women in most developing countries. Am J Clin Nutr. 2005;81(3):714–721. doi: 10.1093/ajcn/81.3.714. [DOI] [PubMed] [Google Scholar]
- 83.Poskitt EM. Countries in transition: underweight to obesity non-stop? Ann Trop Paediatr. 2009;29(1):1–11. doi: 10.1179/146532809X401971. [DOI] [PubMed] [Google Scholar]
- 84.Seale C. Changing patterns of death and dying. Soc Sci Med. 2000;51(6):917–930. doi: 10.1016/s0277-9536(00)00071-x. [DOI] [PubMed] [Google Scholar]
- 85. [cited 2009 4/15];Speed of population aging in selected countries. 2009 Available from: http://www.prb.org/Home/Publications/GraphicsBank/Aging.aspx.
- 86. [cited 2009 4/15];The demographic transition. 1998 Available from: http://www.unfpa.org/swp/1998/pressumary2.htm.
- 87. [cited 2009 4/20];Fact sheet: Diabetes. 2008 Available from: http://www.who.int/mediacentre/factsheets/fs312/en/.
- 88.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356(3):213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 89.Wild S, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 90.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 91.Nugent Suhrcke M, Stuckler Rachel A, Lorenzo Rocco David. Chonic Disease: an economic perspective. The Oxford Health Alliance; 2006. p. 60. [Google Scholar]
- 92.Schieber GJ, et al. Financing global health: mission unaccomplished. Health Aff (Millwood) 2007;26(4):921–934. doi: 10.1377/hlthaff.26.4.921. [DOI] [PubMed] [Google Scholar]
- 93.Thom T, et al. Heart Disease and Stroke Statistics--2006 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 94.Barcelo A, et al. The cost of diabetes in Latin America and the Caribbean. Bull World Health Organ. 2003;81(1):19–27. [PMC free article] [PubMed] [Google Scholar]
- 95.Popkin BM, et al. Trends in diet, nutritional status, and diet-related noncommunicable diseases in China and India: the economic costs of the nutrition transition. Nutr Rev. 2001;59(12):379–390. doi: 10.1111/j.1753-4887.2001.tb06967.x. [DOI] [PubMed] [Google Scholar]
- 96.Gaziano T, Reddy KS, Paccaud F, Horton S. Cardiovascular Disease. In: Jamison D BJ, Measham A, Alleyne G, Cleason M, Evans D, Jha P, Mills A, Musgrove P, editors. Disease Control Priorities in Developing Countries. New York: Oxford University Press and The World Bank; 2006. pp. 645–662. [Google Scholar]
- 97.Gaziano TA. Cardiovascular Disease in the Developing World and Its Cost-Effective Management. Circulation. 2005;112(23):3547–3553. doi: 10.1161/CIRCULATIONAHA.105.591792. [DOI] [PubMed] [Google Scholar]
- 98.Gaziano TA, et al. Cost-Effectiveness Analysis of Hypertension Guidelines in South Africa: Absolute Risk Versus Blood Pressure Level. Circulation. 2005;112(23):3569–3576. doi: 10.1161/CIRCULATIONAHA.105.535922. [DOI] [PubMed] [Google Scholar]
- 99.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105(3):310–315. doi: 10.1161/hc0302.102575. [erratum appears in Circulation 2002 Feb 19;105(7):900] [DOI] [PubMed] [Google Scholar]
- 100.Conroy RM, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 101.Ferrario M, et al. Prediction of coronary events in a low incidence population. Assessing accuracy of the CUORE Cohort Study prediction equation. Int J Epidemiol. 2005;34(2):413–421. doi: 10.1093/ije/dyh405. [DOI] [PubMed] [Google Scholar]
- 102.Wilson PW, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 103.Folsom AR, et al. An Assessment of Incremental Coronary Risk Prediction Using C-Reactive Protein and Other Novel Risk Markers: The Atherosclerosis Risk in Communities Study. Arch Intern Med. 2006;166(13):1368–1373. doi: 10.1001/archinte.166.13.1368. [DOI] [PubMed] [Google Scholar]
- 104.Wang TJ, et al. Multiple Biomarkers for the Prediction of First Major Cardiovascular Events and Death. N Engl J Med. 2006;355(25):2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 105.Ware JH. The Limitations of Risk Factors as Prognostic Tools. N Engl J Med. 2006;355(25):2615–2617. doi: 10.1056/NEJMp068249. [DOI] [PubMed] [Google Scholar]
- 106.Ridker PM, et al. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 107.Lindholm LH, Mendis S. Prevention of cardiovascular disease in developing countries. Lancet. 2007;370(9589):720–722. doi: 10.1016/S0140-6736(07)61356-7. [DOI] [PubMed] [Google Scholar]
- 108.Mendis S, et al. World Health Organization (WHO) and International Society of Hypertension (ISH) risk prediction charts: assessment of cardiovascular risk for prevention and control of cardiovascular disease in low and middle-income countries. J Hypertens. 2007;25(8):1578–1582. doi: 10.1097/HJH.0b013e3282861fd3. [DOI] [PubMed] [Google Scholar]
- 109.Gaziano TA, et al. Laboratory-based versus non-laboratory-based method for assessment of cardiovascular disease risk: the NHANES I Follow-up Study cohort. Lancet. 2008;371(9616):923–931. doi: 10.1016/S0140-6736(08)60418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jha P, et al. Tobacco Addiction, in Disease Control Priorities in the Developing Countries, 2nd Edition. New York: Oxford University Press; 2006. [Google Scholar]
- 111.Mohiuddin SM, et al. Intensive smoking cessation intervention reduces mortality in high-risk smokers with cardiovascular disease. Chest. 2007;131(2):446–452. doi: 10.1378/chest.06-1587. [DOI] [PubMed] [Google Scholar]
- 112.Ong HT. Beta blockers in hypertension and cardiovascular disease. Bmj. 2007;334(7600):946–949. doi: 10.1136/bmj.39185.440382.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jamison D, et al. Disease Control Priorities in Developing Countries. 2nd ed. New York: Oxford University Press and The World Bank; 2006. [PubMed] [Google Scholar]
- 114.Dong Y. Community health services in China: the experience of Tianjin City. Aust Health Rev. 2001;24(1):176–182. [PubMed] [Google Scholar]
- 115.Yu Z, et al. Changes in Blood Pressure, Body Mass Index, and Salt Consumption in a Chinese Population. Preventive Medicine. 1999;29(3):165–172. doi: 10.1006/pmed.1999.0530. [DOI] [PubMed] [Google Scholar]
- 116.Hodge AM, et al. Incidence, increasing prevalence, and predictors of change in obesity and fat distribution over 5 years in the rapidly developing population of Mauritius. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1996;20(2):137–146. [PubMed] [Google Scholar]
- 117.Rossouws JE, et al. Community-based intervention: the Coronary Risk Factor Study (CORIS) International Journal of Epidemiology. 1993;22(3):428–438. doi: 10.1093/ije/22.3.428. [DOI] [PubMed] [Google Scholar]
- 118.Uusitalo U, et al. Fall in total cholesterol concentration over five years in association with changes in fatty acid composition of cooking oil in Mauritius: cross sectional survey. Bmj. 1996;313(7064):1044–1046. doi: 10.1136/bmj.313.7064.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zatonski WA, McMichael AJ, Powles JW. Ecological study of reasons for sharp decline in mortality from ischaemic heart disease in Poland since 1991. BMJ. 1998;316(7137):1047–1051. doi: 10.1136/bmj.316.7137.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zatonski WA, Willett W. Changes in dietary fat and declining coronary heart disease in Poland: population based study. BMJ. 2005;331(7510):187–188. doi: 10.1136/bmj.331.7510.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gaziano TA, Galea G, Reddy KS. Scaling up interventions for chronic disease prevention: the evidence. Lancet. 2007;370(9603):1939–1946. doi: 10.1016/S0140-6736(07)61697-3. [DOI] [PubMed] [Google Scholar]
- 122.World Health Organization. WHO Global InfoBase. [cited; Available from: http://www.who.int/infobase.