Abstract
Background
Adjuvant endocrine treatment with aromatase inhibitors improves disease-free survival compared with tamoxifen in postmenopausal women with estrogen receptor–positive breast cancer. This difference could be due to differences in tamoxifen metabolism because levels of endoxifen, the active tamoxifen metabolite, vary with the number of mutant alleles, including the *4 allele, of the gene encoding cytochrome P450 2D6 (CYP2D6).
Methods
We created a Markov model to determine whether tamoxifen or aromatase inhibitor monotherapy maximized 5-year disease-free survival for patients with the wild-type CYP2D6 genotype (wt/wt). Annual risks of recurrence with aromatase inhibitors and tamoxifen in breast cancer patients who were not selected by CYP2D6 genotype were derived from the Breast International Group 1–98 trial. Genotype frequencies and the hazard ratio for cancer recurrence on tamoxifen among patients with the *4/*4 genotype relative to the wt/wt or wt/*4 genotypes (HR*4/*4 = 1.86) were based on data from an analysis of the North Central Cancer Treatment Group trial of adjuvant tamoxifen. We explored the impact of CYP2D6 (*4) heterozygosity on disease-free suvival for wt/wt patients by studying a range of effect (ie, recurrence on tamoxifen) estimates, from no effect of the single mutation (Effwt/*4 = 0, recurrence rate in wt/*4 patients same as that in wt/wt patients) to complete effect (Effwt/*4 = 1, recurrence rate in wt/*4 patients same as that in *4/*4 patients).
Results
With HR*4/*4 = 1.86 and Effwt/*4 = 0.5, the 5-year disease-free survival of tamoxifen-treated patients with no mutations (wt/wt) was 83.9%, that is, essentially the same as that (84.0%) for genotypically unselected patients who were treated with aromatase inhibitors . With greater HR*4/*4 estimates, disease-free survival with tamoxifen exceed that with aromatase inhibitors in wt/wt patients, even at lower assumed Effwt/*4 ratios.
Conclusions
Modeling suggests that among patients who are wild type for CYP2D6, 5-year disease-free survival out-comes are similar to or perhaps even superior with tamoxifen than with aromatase inhibitors. Endocrine therapy tailored to CYP2D6 genotype could be considered for women who are newly diagnosed with breast cancer, particularly those who have with concerns about either the relative toxicity or the increased cost of aromatase inhibitors.
Adjuvant endocrine therapy reduces the risk of recurrence and improves survival among women with hormone receptor–positive breast cancer (1). Because most breast cancers, especially those among postmenopausal women, are hormone receptor positive, hundreds of thousands of women worldwide initiate adjuvant endocrine treatment each year. Historically, the standard recommendation for such patients has been 5 years of therapy with the selective estrogen receptor modulator tamoxifen (1).
Two large randomized clinical trials—the Breast International Group Trial 1–98 (BIG 1–98) (2) and the Arimidex, Tamoxifen Alone or in Combination trial (3)—have shown that initial adjuvant endocrine treatment with aromatase inhibitors yields improved disease-free survival compared with tamoxifen in postmenopausal women with estrogen receptor–positive breast cancer. These findings have led many oncologists to adopt aromatase inhibitors as their preferred initial adjuvant therapy for postmenopausal women with hormone receptor–positive breast cancer (4). However, interpretation of these trial results is complicated by new pharmacogenomic data that suggest that the clinical benefit of tamoxifen may vary according to the cytochrome P450 (CYP) 2D6 genotype of the patient (5–7).
CYP2D6 is the cytochrome P450 isoform that is mainly responsible for catalyzing the conversion of tamoxifen to endoxifen (8), the tamoxifen metabolite that is thought to be the primary mediator of estrogen-dependent suppression of cell proliferation (9). The CYP2D6 gene has multiple allelic variants, some of which, including the *4 allele, result in the loss of CYP2D6 enzyme function. Population genetic studies (10–12) have revealed that variations in the CYP2D6 gene sequence correspond to pharmacogenomic variations in endoxifen levels among women taking tamoxifen. For example, in a study of 80 newly diagnosed breast cancer patients who were beginning tamoxifen treatment, Jin et al. (10) found that those who carried either the homozygous (*4/*4) or the heterozygous (wt/*4) variant genotype of CYP2D6 had statistically significantly lower mean plasma endoxifen levels (20.0 and 43.1 nM, respectively) than those who carried the homozygous wild-type (wt/wt) genotype (78.0 nM). Additional studies have also supported the finding of a CYP2D6 gene dose effect on plasma concentrations of endoxifen (11,12). These data prompted a Food and Drug Administration advisory panel to recommend in October 2006 that a warning label be added to tamoxifen; the panel cited the new pharmacogenomic studies (5,12) indicating that the drug is less effective in women who carry a mutation in one of the enzymes used to metabolize tamoxifen than in those who do not.
Both of the landmark clinical studies (2,3) that revealed improved disease-free survival outcomes for up-front adjuvant treatment with aromatase inhibitors vs tamoxifen accrued patients irrespective of their CYP2D6 gene mutation status. Given the link between CYP2D6 mutation status and compromised clinical outcomes among mutation carriers on tamoxifen, it is likely that aromatase inhibitors would be the preferred treatment for a woman with deficiencies in tamoxifen metabolism, such as those heterozygous (wt/*4) or homozygous (*4/*4) for the *4 mutation of CYP2D6. However, for women who do not carry a CYP2D6 gene mutation that affects tamoxifen metabolism, the decision about which type of adjuvant endocrine therapy to undergo is not as clear. To date, no genotype analysis of patients who participated in the randomized trials comparing adjuvant tamoxifen with aromatase inhibitors has been performed. However, it is reasonable to infer that if these trials had been restricted to women with wild-type tamoxifen metabolism, the women in the tamoxifen arms would have had better outcomes than those that were reported. To study the magnitude of difference between reported outcomes on tamoxifen and the estimated efficacy of tamoxifen in patients with-out CYP2D6 gene mutations, we constructed a decision-analytic model using data from CYP2D6 pharmacogenomic studies and used the model to evaluate whether an aromatase inhibitor or tamoxifen is the optimal initial treatment choice for the large majority of postmenopausal women who do not carry a mutation that affects the function of the CYP2D6 enzyme.
Methods
Model Design
We developed a Markov decision model (13) to simulate the clinical histories of hypothetical cohorts of postmenopausal women with hormone receptor–positive invasive breast cancer. The model simulated the transition between two health states—from being well with no evidence of cancer recurrence to having recurrent local or regional disease or being diagnosed with a new primary breast cancer—for women who are newly treated for breast cancer. In these simulations, women newly treated for breast cancer start in the “well” or “without disease” state and each month face a probability of experiencing a recurrence. The model was run 60 times using monthly cycles to calculate the 5-year disease-free survival probability. The model was designed and analyzed using TreeAge Pro 2005 software (TreeAge Software, Williamstown, MA).
Data Sources and Assumptions
Model estimates for recurrence probabilities by initial treatment—aromatase inhibitor or tamoxifen—were derived from the annual hazard rates from the BIG 1–98 (2) (Table 1 ; R. Gelber PhD, November, 2006, BIG 1–98 Steering Committee: personal communication). These estimates apply to all breast cancer patients in the study population regardless of CYP2D6 genotype. We assumed that the recurrence probabilities during a given year were constant.
Table 1.
Model parameters*
| Variable | Value | Source (reference) |
|---|---|---|
| Annual hazard rate of recurrence with aromatase inhibitors |
||
| Year 0–1 | 0.0243 | |
| Year 1–2 | 0.0268 | |
| Year 2–3 | 0.0415 | BIG 1–98 (2) |
| Year 3–4 | 0.0414 | |
| Year 4–5 | 0.0401 | |
| Annual hazard rate of recurrence with tamoxifen |
||
| Year 0–1 | 0.0264 | |
| Year 1–2 | 0.0460 | |
| Year 2–3 | 0.0469 | BIG 1–98 (2) |
| Year 3–4 | 0.0481 | |
| Year 4–5 | 0.0397 | |
|
CYP2D6(*4) genotype frequencies (%) |
||
| Wild type (wt/wt) | 72.1 | |
| Heterozygous (wt/*4) | 21.1 | Goetz et al. (5) |
| Homozygous mutant (*4/*4) | 6.8 | |
| Hazard ratio for disease-free survival in *4/*4 patients |
||
| Adjusted | 1.86 | Goetz et al. (5) |
BIG 1–98 = Breast International Group Trial 1–98.
We then used the model to estimate recurrence probabilities for each CYP2D6 (*4) genotype, that is, wild-type (wt/wt), heterozygous mutant (wt/*4), and homozygous mutant (*4/*4). Base case values for genotype-specific hazard ratios for recurrence were derived from a study by Goetz et al. (5), who analyzed the geno-types of postmenopausal women who were enrolled on the tamoxifen-only arm of a randomized North Central Cancer Treatment Group (NCCTG) trial (NCCTG 89-30-52). Of the 256 eligible patients who were assigned to this arm, 223 were genotyped (213 via paraffin-embedded tumor blocks and 10 via blocks of normal [ie, buccal] tissue). Clinical outcomes by CYP2D6 (*4) mutation status were assessed for the 190 patients for whom the CYP2D6 (*4) allele was successfully amplified. We assigned a hazard ratio for recurrence on tamoxifen among *4/*4 mutation carriers relative to the wild-type (wt/wt) and heterozygous (wt/*4) carriers (HR*4/*4) of 1.86, that is, the hazard ratio with adjustment for clinical factors that was reported by Goetz et al. (5). In addition, we used the CYP2D6 (*4) genotype frequency values reported by Goetz et al. (5) for the breast cancer patients in their study population: 72.1% for wt/wt, 21.1% for wt/*4, and 6.8% for *4/*4 (Table 1). We assumed that neither the hazard rate for recurrence on aromatase inhibitors nor the tumor stage varied by CYP2D6 genotype.
We designed the model so that when the recurrence probabilities for each genotype were adjusted using the weight of their genotypic frequency and then combined, they produced a recurrence probability that was consistent with that of the genotypically unselected population on tamoxifen. The model was also calibrated to account for slight changes in genotypic frequencies within the disease-free patient population that occur over time as the relatively greater percentage of mutation carriers experience a recurrence and their representation among the “well” population declines. We then examined which of the two treatment strategies—tamoxifen or aromatase inhibitor monotherapy—optimized 5-year disease-free survival in the wild-type subgroup.
We defined the effect of having a single chromosome affected by the CYP2D6 mutation in heterozygous patients as Effwt/*4. The increased hazard ratio of recurrence among heterozygous patients was defined by the following formula:
Data suggest an intermediate clinical phenotype for heterozygous (wt/*4) patients taking tamoxifen (6,7)—that is, outcomes for heterozygotes were between those for wild-type (wt/wt) and double mutant (*4/*4) patients—but small numbers have precluded precise estimates for the hazard ratio in heterozygous patients. Therefore, we varied the effect of a single CYP2D6 (*4) mutation from 0 (ie, no effect of carrying a single mutation, where the hazard rate for wt/*4 equals that of wt/wt) to 1 (ie, complete effect, where the hazard rate for wt/*4 equals that of *4/*4 mutation carriers). In the base case analysis, we assumed that Effwt/*4 = 0.5, which corresponds to a hazard ratio for recurrence among wt/*4 patients that is midway between that in patients who carried the *4/*4 mutation and that in wt/wt patients. Note that the formula for the recurrence rate in heterozygotes is dependent on HR*4/*4 when Effwt/*4 is not equal to zero; because HR*4/*4 is defined as a hazard ratio for recurrence on tamoxifen among *4/*4 mutation carriers relative to both the wild-type (wt/wt) and heterozygous (wt/*4) carriers, the calculation of HR*4/*4 was determined by a formula that solved for both equations as well as adjusting for the change in genotype frequency over time.
Sensitivity Analyses
We performed a two-way sensitivity analysis to test the robustness of the results to variations in model parameters. We simultaneously varied HR*4/*4 (the increased hazard ratio for recurrence on tamoxifen among *4/*4 patients relative to wt/wt and wt/*4 patients) from 1.0 (ie, no increased recurrence) to 3.0 and Effwt/*4 (the increased hazard ratio for recurrence on tamoxifen among wt/*4 patients relative to wt/wt patients) from 0 (ie, no increased recurrence) to 1.0 (ie, recurrence rate equals that of *4/*4 patients). This sensitivity analysis compared 5-year disease-free survival in wt/wt patients by treatment strategy for all possible combinations of HR*4/*4 and Effwt/*4.
We also reran the model using data from a more recent analysis of the Goetz et al. cohort (7) that included information about the use of selective serotonin reuptake inhibitors (SSRIs)—agents that inhibit the function of the CYP2D6 enzyme—so that patients who had received SSRIs could be excluded to allow for a more accurate assessment of the true effect of CYP2D6 mutation on outcomes. With these patients excluded, NCCTG trial data revealed a hazard ratio for recurrence on tamoxifen for homozygous mutation carriers relative to both wild-type and heterozygous patients (HR*4/*4) of 2.6 (95% confidence interval [CI] = 1.34 to 5.07) and for homozygous mutation carriers relative to wild-type patients alone of 2.9 (95% CI = 1.47 to 5.73); the hazard ratio for recurrence among heterozygous patients relative to wild-type patients was 1.60 (95% CI = 0.94 to 2.71) (M. Goetz MD, V. Suman PhD, June, 2007: personal communication). We examined the effect of using these estimates on outcomes for wild-type patients treated with tamoxifen in the two-way sensitivity analysis as described above.
CONTEXT AND CAVEATS
Prior knowledge
In two clinical trials, adjuvant endocrine treatment with aromatase inhibitors yielded improved disease-free survival compared with tamoxifen in postmenopausal women with estrogen receptor–positive breast cancer. Recent pharmacogenomic data suggest that the clinical benefit of tamoxifen may vary with the number of mutant alleles the patient carries in the gene that encodes CYP2D6, the cytochrome P450 isoform that is mainly responsible for catalyzing the conversion of tamoxifen to its functional metabolite endoxifen.
Study design
A decision-analytic model was constructed using data from CYP2D6 pharmacogenomic studies and used to evaluate whether an aromatase inhibitor or tamoxifen is the optimal initial treatment choice for postmenopausal women who do not carry a mutation that affects tamoxifen metabolism.
Contribution
Modeling suggests that among patients who are wild type for CYP2D6, adjuvant treatment with tamoxifen appears to provide 5-year disease-free survival outcomes that are similar or perhaps even superior to those achieved with aromatase inhibitors.
Implications
Endocrine therapy tailored to CYP2D6 genotype could be considered for women who are newly diagnosed with breast cancer.
Limitations
The model relies on the assumptions and estimates used. The model cannot be used to study the question of whether or how to sequence therapy. The findings apply to only postmenopausal women.
Results
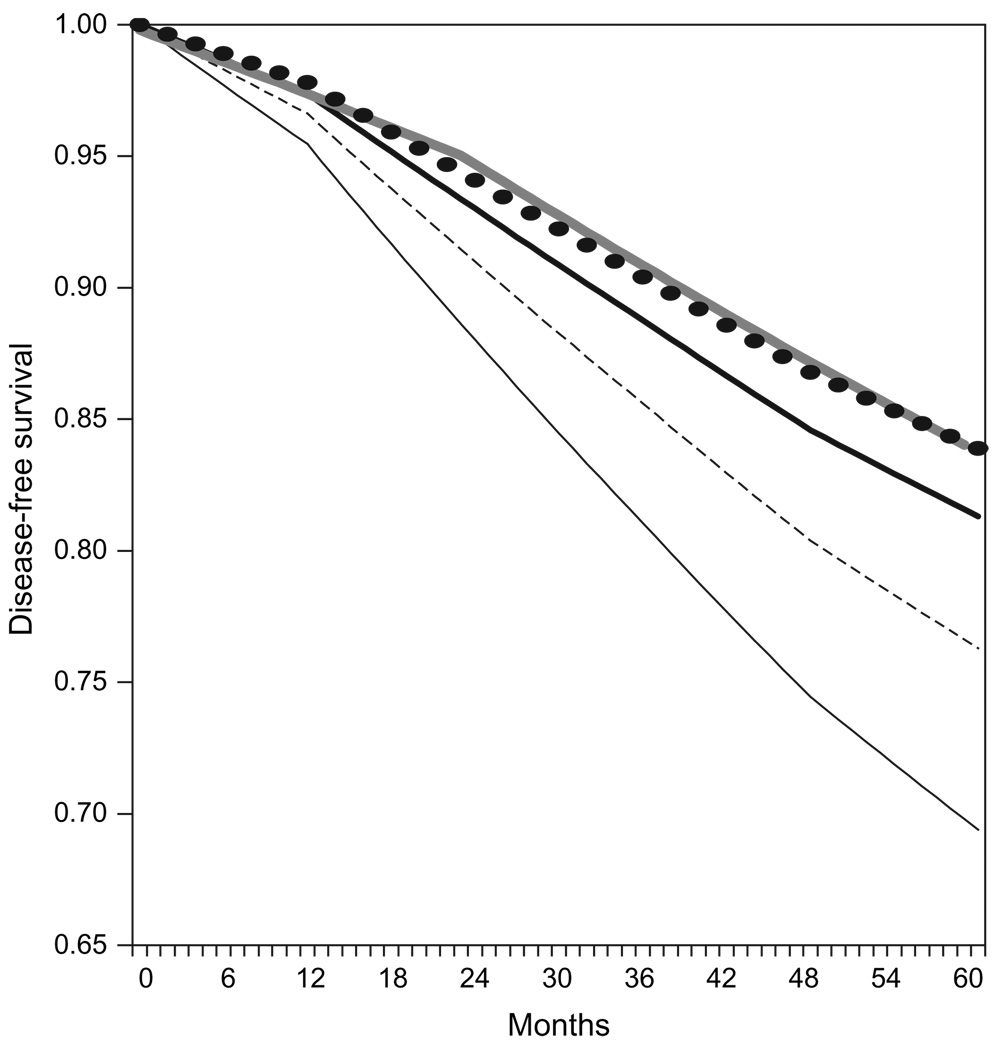
Our model, which was designed to reproduce the recurrence rates among patients in the BIG 1–98 trial (who were not selected by genotype), yielded a 5-year disease-free survival of 84.0% for those receiving aromatase inhibitors and 81.3% for those receiving tamoxifen. We used this model to examine disease-free survival by CYP2D6 genotype. In the base case analysis, we assumed an increased hazard ratio for recurrence on tamoxifen for homozygous mutation carriers (*4/*4; 6.8% of patients) of 1.86 relative to wt/wt and wt/*4 patients. In the base case analysis, we also assigned heterozygous patients (wt/*4; 21.1% of patients) a hazard ratio of recurrence on tamoxifen halfway between that of the homozygous mutation carriers and wild-type patients (Effwt/*4 = 0.5). With these parameters, the 5-year disease-free survival of tamoxifen-treated patients with no mutations (wt/wt) was 83.9%, that is, essentially the same as that for genotypically unselected patients who were treated with aromatase inhibitors. Figure 1 displays the disease-free survival curves for patients treated with aromatase inhibitors and tamoxifen in the genotypically unselected population as well as those for patients in each genotype subgroup (wt/wt, wt/*4, and *4/*4), as modeled using the basic assumptions of HR*4/*4 = 1.86 and Effwt/*4 = 0.5.
Figure 1.
Model results for disease-free survival in the unselected population and in each genotypic subgroup. For these analyses, we assumed a hazard ratio of recurrence in homozygous mutation carriers (*4/*4) of 1.86 (HR*4/*4 = 1.86) and that the increased hazard ratio in heterozygotes (wt/*4) was half that in *4/*4 homozygotes. The thick gray line represents the aromatase inhibitor strategy in the unselected population. The thick black line represents in the tamoxifen strategy in the unselected population. The tamoxifen strategy in the wild-type (wt/wt) subgroup is shown as black circles, in the wt/*4 subgroup as a thin dashed black line, and in the *4/*4 subgroup as a thin solid black line.
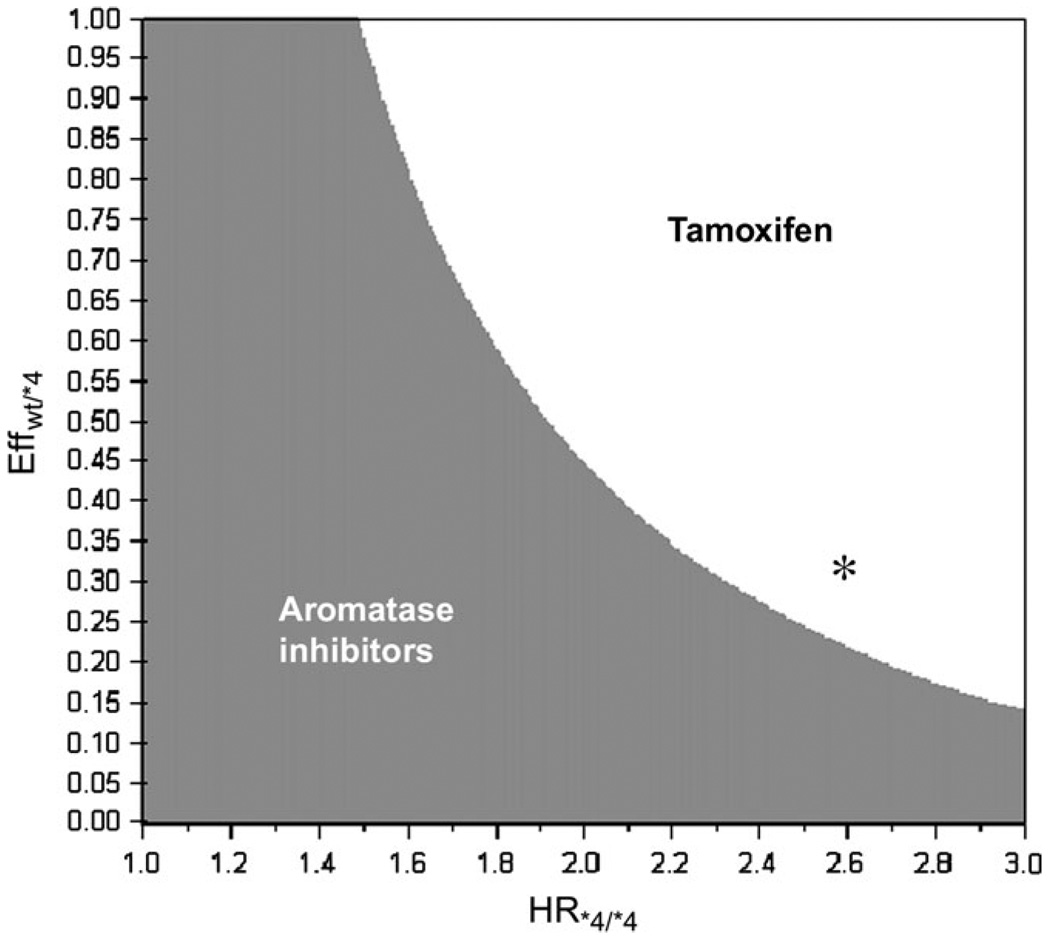
To examine the robustness of our findings across a range of assumptions for the impact of heterozygosity at the CYP2D6 locus and hazard ratios for recurrence among homozygous mutation carriers, we conducted a two-way sensitivity analysis that simultaneously varied HR*4/*4 and Effwt/*4 (Table 2 and Figure 2). The highest value of HR*4/*4 tested in the sensitivity analysis—3.0—was the one at which 5-year disease-free survival estimate for homozygous mutation carriers approximated that for patients not receiving any hormonal therapy (1).
Table 2.
Five-year disease-free survival for homozygous wild-type patients receiving tamoxifen obtained by varying the hazard ratio of recurrence among the homozygous mutation carriers (HR*4/*4) and the proportion of the HR*4/*4 that was applicable to heterozygotes (Effwt/*4)*
| HR*4/*4 |
|||||
|---|---|---|---|---|---|
| Effwt/*4 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
| 0.0 | 81.3 | 81.8 | 82.3 | 82.7 | 83.1 |
| 0.25 | 81.3 | 82.3 | 83.2 | 84.1 | 84.8 |
| 0.50 | 81.3 | 82.9 | 84.3 | 85.6 | 86.7 |
| 0.75 | 81.3 | 83.5 | 85.4 | 87.1 | 88.7 |
| 1.0 | 81.3 | 84.1 | 86.6 | 88.7 | 90.6 |
Five-year disease-free survival is expressed as a percentage. Combinations of HR*4/*4 and Effwt/*4 for which 5-year disease-free survival for wt/wt patients on tamoxifen is greater than that for wt/wt patients on aromatase inhibitors (ie, >84%) are shown in bold.
Figure 2.
Two-way sensitivity analysis for the wild-type (wt/wt) subgroup. The hazard ratio of recurrence among the homozygous mutation carriers (HR*4/*4) is plotted on the x-axis, and proportion of HR*4/*4 attributed to heterozygotes (Effwt/*4) is plotted on the y-axis. The gray zone represents the combinations of Effwt/*4 and HR*4/*4 for which aromatase inhibitors optimize 5-year disease-free survival for wt/wt patients. The white zone represents the combinations of Effwt/*4 and HR*4/*4 for which tamoxifen optimizes 5-year disease-free survival for these patients. The asterisk marks the combination of HR*4/*4 and calculated Effwt/*4—2.6 and 0.316, respectively—that corresponds to updated but unadjusted estimates from the North Central Cancer Treatment Group trial data.
The data shown in Table 2 and Figure 2 are for wild-type patients only. Although the HR*4/*4 and Effwt/*4 parameters directly influence the recurrence rates in mutation carriers only, outcomes among wild-type patients are also an indirect function of these parameters because the weighted sum of the genotype-specific curves is a fixed value. Increases in the HR*4/*4 and Effwt/*4 values (ie, moving to the right and down, respectively, in Table 2) resulted in a higher recurrence rate among mutation carriers and thereby increased the 5-year disease-free survival probability for wild-type patients.
Figure 2 displays results of the two-way sensitivity analysis in graphical form. Each point on this figure can be described by an (x,y) coordinate. The x-axis plots HR*4/*4, and the y-axis plots Effwt/*4. Each (x,y) point represents a unique HR*4/*4 and Effwt/*4 combination. The shading of an (x,y) point corresponds to the treatment strategy that maximizes 5-year disease-free survival in wt/wt patients—gray for upfront treatment with an aromatase inhibitor, white for upfront treatment with tamoxifen—for that particular combination of HR*4/*4 and Effwt/*4. The white “zone” therefore depicts the combinations of HR*4/*4 and Effwt/*4 parameters for which tamoxifen optimizes 5-year disease-free survival in the wt/wt subgroup, whereas the gray zone depicts those for which aromatase inhibitors optimize 5-year disease-free survival in this subgroup.
This sensitivity analysis allows for the rapid interpretation of results when further relevant data become available. For example, by using the updated but unadjusted estimates from the NCCTG trial data (HR*4/*4 = 2.6 and hazard ratio for recurrence for wt/*4 patients relative to wt/wt of 1.60; M. Goetz MD, V. Suman PhD, June, 2007: personal communication), we calculated an Effwt/*4 of 0.316. We then used our two-way sensitivity analysis to examine the optimal treatment strategy among wt/wt patients with these updated estimates and found that with an HR*4/*4 of 2.6 and a calculated Effwt/*4 of 0.316 wt/wt patients who receive upfront treatment with tamoxifen should have better 5-year disease-free survival than those who receive upfront treatment with aromatase inhibitors (asterisk in Figure 2).
Likewise, our base case analysis used a hazard ratio of recurrence on tamoxifen for heterozygous patients that was halfway between that for the homozygous mutation carriers and that for wild-type patients (Effwt/*4 = 0.5). However, Table 2 reveals that with an HR*4/*4 of 2.0, even with a smaller difference between the effect of tamoxifen in heterozygous and wild-type patients, wild-type patients treated with tamoxifen would have disease-free survival that was similar to that of patients treated with an aromatase inhibitor (eg, 83.2% vs 84.0% at HR*4/*4 = 2.0 and Effwt/*4 = 0.25).
Discussion
The effect of genotypic variation in CYP2D6 on tamoxifen metabolism is one of the best characterized and most clinically important examples of pharmacogenomics in cancer. We used decision-analytic modeling of the available data to explore how CYP2D6 variation might influence adjuvant therapy recommendations for postmenopausal women with breast cancer. We found that among patients who are homozygous wild type for CYP2D6, adjuvant treatment with tamoxifen appears to provide 5-year disease-free survival outcomes that are similar or perhaps even superior to those achieved with aromatase inhibitors. This finding differs from the results of clinical trials in unselected populations, in which aromatase inhibitors have demonstrated statistically significant improvements in disease-free survival over tamoxifen (2,3).
A limitation inherent to all modeling studies, including ours, is the reliance of the model on the assumptions and estimates used. The validity of our findings therefore rests on the soundness of the estimated hazard ratios for recurrence for tamoxifen-treated patients with homozygous or heterozygous mutations at the CYP2D6 locus used in the model. Our base case model assumed an HR*4/*4 of 1.86, which was derived from the adjusted hazard ratio for recurrence on tamoxifen among CYP2D6 *4/*4 mutation carriers that was seen in NCCTG trial (5). Emerging data from other groups support this hazard ratio estimate. For example, a German study (6) estimated a hazard ratio for event-free survival of 1.89 (95% CI = 1.10 to 3.25) among a pooled sample of women homozygous or heterozygous for either the *4 mutation or the much less frequent CYP2D6 *5 mutation. A revised analysis of the NCCTG trial that defined patients on SSRIs as well as those who were homozygous mutation carriers as “poor metabolizers” found an adjusted hazard ratio for disease-free survival on tamoxifen of 2.2 (P = .02) (14). As shown by our two-way sensitivity analysis (Figure 2), with this hazard ratio for homogygous mutation carriers, as long as the Effwt/*4 (proportion of HR*4/*4 attributed to wt/*4) is as little as 0.34, tamoxifen would be the preferred treatment choice for wt/wt patients. If we used the hazard ratios estimated after excluding SSRI-treated patients (HR*4/*4 of 2.6 and a calculated Effwt/*4 of 0.316), wild-type patients who receive initial treatment with tamoxifen would have better 5-year disease-free survival than those who receive initial treatment with aromatase inhibitors. However, the size of the cohort after exclusion of the SSRI-treated patients was too small to allow the investigators to generate hazard ratio estimates adjusted for potential confounding factors. We chose to use the adjusted estimates from their full cohort in our base case analysis, thus introducing an intentional conservative bias.
In addition, our model results are heavily dependent on the estimates we used for the efficacy of tamoxifen in heterozygous patients. Our base case analysis used a hazard ratio of recurrence on tamoxifen for heterozygous patients that was halfway between that for the homozygous mutation carriers and that for wild-type patients (Effwt/*4 = 0.5). Although some clinical data support an intermediate phenotype, or gene–dose effect, for heterozygous patients on tamoxifen (6,7), we do not currently have precise estimates for the hazard ratio of recurrence in this subgroup. We therefore studied the range of tamoxifen effects in heterozygotes, including the assumptions that an intermediate level of endoxifen is completely inactive (ie, Effwt/*4 = 1) and that it is maximally active (Effwt/*4 = 0). However, even when we assumed a much smaller difference between the effect of tamoxifen in heterozygous and wild-type patients than halfway between that for *4/*4 and wt/wt patients (ie, Effwt/*4 = 0.5), disease-free survival for wild-type patients on tamoxifen appeared to be similar to that of patients treated with an aromatase inhibitor (eg, 83.2% vs 84.0% at HR*4/*4 = 2.0 and Effwt/*4 = 0.25; Table 2).
Other key assumptions of our model were that cancer type, cancer stage, and the efficacy of treatment with aromatase inhibitors do not vary by CYP2D6 genotype. A growing body of literature supports these assumptions. For example, genotype analysis revealed no statistically significant association between CYP2D6 mutation status and tumor size, nodal status, or histologic grade in women who were or were not treated with tamoxifen (6). The same study found no differences in clinical outcome by CYP2D6 genotype among women who did not receive tamoxifen (6). Finally, aromatase inhibitors do not undergo conversion to active metabolites (15), and there are no in vitro data to suggest the metabolism of these agents by the CYP2D6 enzyme. Nevertheless, it is possible that unknown factors may contribute directly to the worse clinical outcomes experienced by CYP2D6 mutation carriers.
Our model was designed to examine the initial use of either tamoxifen or aromatase inhibitors. However, postmenopausal breast cancer patients may receive sequential therapy with tamoxifen followed by an aromatase inhibitor (16). There is the possibility that sequential tamoxifen followed by aromatase inhibitor treatment might improve disease-free survival more than aromatase inhibitor therapy alone, as suggested by meta-analyses of clinical trials (17) and by our previous modeling efforts (18,19). Because the hazard ratios for recurrence on aromatase inhibitors after tamoxifen therapy are not currently available for CYP2D6 genotype subgroups, we could not use this model to study the question of whether or how to sequence therapy. In addition, because the data informing our model estimates were derived from trials in postmenopausal women and because aromatase inhibitors are not effective in premenopausal women (20), our findings apply to only postmenopausal women.
What are the implications of our findings for tailored therapy based on CYP2D6 genotype? Currently, there is no widespread testing for CYP2D6 gene mutations in breast cancer patients, and the role for such testing to guide endocrine therapy decisions is not well established. Although our findings were based on a model with embedded assumptions and estimates, the model itself was simple and incorporated values for genotype frequencies and only two parameters (HR*4/*4 and Effwt/*4) along with the results of a large randomized clinical trial that compared treatment with adjuvant tamoxifen vs aromatase inhibitors. Our model had no embedded assumptions regarding the natural history of breast cancer; instead, it used the direct patient outcome data provided by a randomized trial. Confirmatory pharmocogenomic analyses of CYP2D6 genotype and clinical outcomes (6,7) suggest that any bias in the estimates used in our base case model would have caused us to underestimate rather than overestimate the effectiveness of tamoxifen in wild-type patients. Nevertheless, our model is still only a model that used estimates for key parameters that were based on analyses of small numbers of women. The most direct method of establishing these parameters would be through genotype analysis of women who are enrolled in the large randomized trials of tamoxifen vs aromatase inhibitors. In the absence of such information, we cannot make any definite recommendations regarding whether or which patients should undergo genetic testing.
However, while we await direct comparisons of outcomes by genotype, decisions about adjuvant endocrine treatment are being made for thousands of postmenopausal women who are newly diagnosed with breast cancer. Our model raises the possibility that tailored therapy based on pharmacogenomics could be considered for such women. In particular, women who are concerned about the relative toxicity or cost of an aromatase inhibitor as initial treatment might consider CYP2D6 genetic testing and pursue treatment with tamoxifen if found to be wild type at CYP2D6. To obtain definitive recommendations about genetic testing, studies that explore associations among CYP2D6 genotype, endoxifen levels, and efficacy of tamoxifen vs aromatase inhibitors are of tremendous interest and importance. Because the vast majority of breast cancers in postmenopausal women are estrogen receptor positive and the majority of women who are diagnosed with breast cancer do not carry a CYP2D6 gene mutation, results of such studies could have important implications for the treatment of breast cancer.
Acknowledgments
Funding
National Institutes of Health (1K07 CA118629 to R.S.P.); American Society Clinical Oncology (Career Development Award to R.S.P.).
We would like to acknowledge Dr Richard Gelber (Dana-Farber Cancer Institute) and the BIG 1–98 Steering Committee; Drs Matthew Goetz and Vera Suman (Mayo Clinic College of Medicine); and Drs Werner Schroth, Michel Eichelbaum, and Hiltrud Brauch (Dr Margarete Fischer-Bosch Institute of Clinical Pharmacology, Stuttgart, and University Tuebingen) for providing their data prior to publication to inform our modeling efforts.
The study sponsors had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/2.0/uk/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol. 2007;25(5):486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 3.Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer. 2003;98(9):1802–1810. doi: 10.1002/cncr.11745. [DOI] [PubMed] [Google Scholar]
- 4.Carlson RW, Hudis CA, Pritchard KI. Adjuvant endocrine therapy in hormone receptor-positive postmenopausal breast cancer: evolution of NCCN, ASCO, and St Gallen recommendations. J Natl Compr Canc Netw. 2006;4(10):971–979. doi: 10.6004/jnccn.2006.0082. [DOI] [PubMed] [Google Scholar]
- 5.Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23(36):9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 6.Schroth W, Anotniadou L, Fritz P, et al. Breast cancer treatment outcome with adjuvant tamoxifen in relation to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol. 2007;25(33):5187–5193. doi: 10.1200/JCO.2007.12.2705. [DOI] [PubMed] [Google Scholar]
- 7.Goetz MP, Knox SK, Suman VJ, et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101(1):113–121. doi: 10.1007/s10549-006-9428-0. [DOI] [PubMed] [Google Scholar]
- 8.Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310(3):1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- 9.Lee KH, Ward BA, Desta Z, Flockhart DA, Jones DR. Quantification of tamoxifen and three metabolites in plasma by high-performance liquid chromatography with fluorescence detection: application to a clinical trial. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;791(1–2):245–253. doi: 10.1016/s1570-0232(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97(1):30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 11.Borges S, Desta Z, Li L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80(1):61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95(23):1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 13.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13(4):322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 14.Knox SK, Ingle JN, Suman VJ, et al. Cytochrome P450 2D6 status predicts breast cancer relapse in women receiving adjuvant tamoxifen (Tam) J Clin Oncol. 2006;24(18):4s. [Google Scholar]
- 15.Brueggemeier RW. Update on the use of aromatase inhibitors in breast cancer. Expert Opin Pharmacother. 2006;7(14):1919–1930. doi: 10.1517/14656566.7.14.1919. [DOI] [PubMed] [Google Scholar]
- 16.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349(19):1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 17.Ciccarese M, Bria E, Cuppone F, et al. Disease-free survival (DFS) as surrogate end point for overall survival (OS) in adjuvant aromatase inhibitors (AIs) trials for breast cancer (BC): meta-analysis of 10 randomized clinical trials (RCTs) exploring the magnitude of the benefit. J Clin Oncol. 2007;25(18):539. [Google Scholar]
- 18.Punglia RS, Kuntz KM, Winer EP, Weeks JC, Burstein HJ. Optimizing adjuvant endocrine therapy in postmenopausal women with early-stage breast cancer: a decision analysis. J Clin Oncol. 2005;23(22):5178–5187. doi: 10.1200/JCO.2005.02.964. [DOI] [PubMed] [Google Scholar]
- 19.Punglia RS, Kuntz KM, Winer EP, Weeks JC, Burstein HJ. The impact of tumor progesterone receptor status on optimal adjuvant endocrine therapy for postmenopausal patients with early-stage breast cancer: a decision analysis. Cancer. 2006;106(12):2576–2582. doi: 10.1002/cncr.21919. [DOI] [PubMed] [Google Scholar]
- 20.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor- positive breast cancer: status report 2004. J Clin Oncol. 2005;23(3):619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]