Abstract
The targeted brain dysfunction that accompanies aging can have a devastating effect on cognitive and intellectual abilities. A significant proportion of older adults experience precipitous cognitive decline that negatively impacts functional activities. Such individuals meet clinical diagnostic criteria for dementia, which is commonly attributed to Alzheimer's disease (AD). Structural neuroimaging, including magnetic resonance imaging (MRI), has contributed significantly to our understanding of the morphological and pathology-related changes that may underlie normal and disease-associated cognitive change in aging. White matter hyperintensities (WMH), which are distributed patches of increased hyperintense signal on T2-weighted MRI, are among the most common structural neuroimaging findings in older adults. In recent years, WMH have emerged as robust radiological correlates of cognitive decline. Studies suggest that WMH distributed in anterior brain regions are related to decline in executive abilities that is typical of normal aging, whereas WMH distributed in more posterior brain regions are common in AD. Although epidemiological, observational, and pathological studies suggest that WMH may be ischemic in origin and caused by consistent or variable hypoperfusion, there is emerging evidence that they may also reflect vascular deposition of (β-amyloid, particularly when they are distributed in posterior areas and are present in patients with AD. Findings from the literature highlight the potential contribution of small-vessel cerebrovascular disease to the pathogenesis of AD, and suggest a mechanistic interaction, but future longitudinal studies using multiple imaging modalities are required to fully understand the complex role of WMH in AD.
Keywords: Alzheimer's disease, MRI, white matter hyperintensity, beta amyloid
Abstract
La disfunción cerebral objetivable que acompaña el envejecimiento puede tener un efecto devastador en las capacidades cognitivas e intelectuales. Un porcentaje significativo de adultos mayores experimentan una marcada declinación cognitiva que afecia negativamente las actividades funcionales. Esos individuos cumplen los criterios diagnósticos clínicos para demencia, la cual se atribuye comúnmente a la Enfermedad de Alzheimer (EA). La neuroimagenología estructural, incluida la resonancia magnética (RM), ha contribuido significativamente a la comprensión de los cambios morfológicos y aquéllos relacionados con alguna patologia que pueden estar a la base del cambio cognitivo normal y el asociado con alguna enfermedad durante el envejecimiento. Las hiperintensidades de la sustancia blanca (HSB), que se distribuyen en parches de hiperintensidad de señal en las secuencias potenciadas en 72 están entre los hallazgos más frecuentes de la neuroimagenología estructural en los adultos mayores. En los últimos años, la HSB se ha considerado un potente correlato radiológico de la declinación cognitiva. Los estudios sugieren que la HSB distribuida en las regiones cerebrales anteriores se relaciona con la declinación de las habilidades ejecutivas y es típica del envejecimiento normal; en cambio, la HSB distribuida en regiones cerebrales más posteriores es común en la EA. Aunque los estudios epidemiológicos, observacionales y patológicos sugieren que la HSB puede tener un origen isquémico y ser causada por hipoperfusión constante o variable, ha aparecido evidencia que sugiere que también puede reflejar depósito vascular de β-amiliode, particularmente cuando está distribuida en áreas posteriores y se presenta en pacientes con EA. Los hallazgos de la Iiteratura destacan la potencial contribución de la enfermedad cerebro vascular de pequeño vaso a la patogénesis de la EA y sugieren una interacción mecánistica, pero se requiere a futuro de estudios longitudinales que utilicen varias formas de neuroimágenes para una total comprensión del complejo papel de la HSB en la EA.
Abstract
Les dysfonctions cérébrales objectivées liées à l'âge peuvent avoir un effet dévastateur sur les capacités cognitives et intellectuelles. Un pourcentage significatif d'adultes âgés subissent un déclin cognitif brutal qui retentit défavorablement sur les activités fonctionnelles. Ces personnes présentent des critères diagnostiques cliniques de démence, habituellement rapportés à la maladie d'Aizheimer (MA). La neuro-imagerie anatomique, dont l'imagerie par résonance magnétique (IBM), nous a beaucoup aidés à comprendre les modifications morphologiques et pathologiques qui pourraient sous-tendre les changements cognitifs du vieillissement normal et pathologique. Les hyperdensités de la substance blanche (HSB), qui se présentent sous la forme de petites taches éparses apparaissant à l'IRM en hypersignal en séquence pondérée en T2, sont très fréquemment observées en neuro-imagerie anatomique chez les sujets âgés. Ces dernières années, les HSB ont été reconnues comme étant des signes radiologiques fiables du déclin cognitif. Des études suggèrent que les HSB localisées dans la région antérieure du cerveau seraient liées au déclin des aptitudes exécutives typique du vieillissement normal, alors que les HSB situées dans les régions plus postérieures sont fréquentes dans la MA. Bien que des études épidémiologiques, observationnelles et pathologiques suggèrent que les HSB puissent être d'origine ischémique et provoquées par une hypoperfusion permanente ou variable, il semble maintenant qu'elles puissent aussi être le signe d'un dépôt vasculaire de substance bêta-amyloïde, surtout lorsqu'il est localisé dans les régions postérieures et présent chez les patients atteints de MA. Les résultats de la littérature soulignent l'éventuelle contribution de la pathologie cérébrovasculaire des petits vaisseaux à la pathogenèse de la MA et suggèrent une interaction mécanistique. Mais il faudra encore d'autres études longitudinales aux modalités d'imagerie multiples pour comprendre parfaitement le rôle complexe des HSB dans la MA.
With advancing age comes inevitable decline in most biological systems. Perhaps among the most devastating is the targeted brain dysfunction that accompanies aging, and its negative impact on cognitive and intellectual abilities. Descriptively, cognitive aging across individuals is heterogeneous. Some experience a precipitous and universal decline in cognitive abilities, while others experience more subtle downward cognitive trajectories in certain cognitive domains, with preservation or even improvement in others. From a taxonomic perspective, when age-associated cognitive decline is severe enough to impact functional abilities, we define the syndrome as “dementia” and assign the most likely etiology. By far, probable Alzheimer's disease (AD) is the most commonly diagnosed cause of dementia. Other commonly diagnosed causes include dementia due to cerebrovascular disease (ie, “vascular dementia”) and dementia due to Lewy bodies. The concept of mild cognitive impairment (MCI) first gained popularity in the 1990s to categorize older adults who evidence some degree of cognitive decline but not enough to impact functional abilities and meet formal criteria for dementia. Mild cognitive impairment and its variants are often considered to be “transition states” between normal cognitive functioning and dementia. Thus, cognitive aging can be described as comprising heterogeneous trajectories across domains or by categories, including “normal,” “MCI,” and “dementia.”
The clinical diagnosis of probable AD is made by analyzing the neuropsychological profile and history of a patient and after ruling out other potential causes of the dementia syndrome. In clinical neuroscience, our reliance on a taxonomic system for the characterization of ageassociated cognitive syndromes suggests, at least implicitly, that there is a unitary disease or pathology that accounts for the clinical or cognitive presentation. Indeed, pathologically, AD is defined by the presence of of amyloid plaques and neurofibrillary tangles, which emerge in the hippocampal formation and spread throughout posterior and anterior cortex. However, accumulating evidence indicates that, in addition to the pathological features that define the disease, factors associated with poor cognitive aging (in the absence of frank dementia) may play a primary role in the pathogenesis and progression of AD. At the top of the list of these factors are small-vessel cerebrovascular disease and its antecedent modifiable risk factors. Epidemiological studies, for example, confirm that hypertension, diabetes, insulin resistance, obesity/overweight, and hyperlipidemia increase the risk of AD.1-6
The role of neuroimaging in cognitive aging
While associative epidemiological studies play an important role by identifying correlates of poor cognitive aging or AD, they tell us little about directional causality or brain mechanisms involved in pathogenesis. Over the past three decades, the field ol biomedical engineering has infused the clinical neurosciences with powerful neuroimaging instruments equipped to study directly morphological and functional properties of the aging brain in vivo. Advances in the acquisition, visualization, and analysis of neuroimaging data continue to evolve rapidly, with ongoing development of hardware, software, and conceptual statistical approaches that have already made tremendous scientific contributions. Structural magnetic resonance imaging (MRI) in particular can be used to examine macrostructural changes-gross differences in tissue volume that reflect volume variability, parenchymal atrophy, or frank pathology (eg, large-vessel inlarct, tumor); or microstructural changes-fiber tract integrity and pathology that can be altered due to subtle changes in myelin-associated pathology. Several studies have highlighted the importance of gross structural or volumetric changes in cognitive aging and dementia (for example, see refs 7-11; see ref 12 for review). Small-vessel cerebrovascular disease, visualized as white matter hyperintensities (WMH), has emerged as a particularly strong correlate of cognitive aging (for review, see ref 13) and is the focus of our discussion here.
Characterization and quantification of white matter hyperintensities
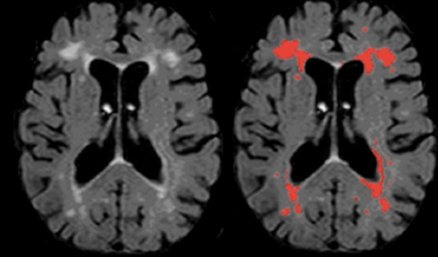
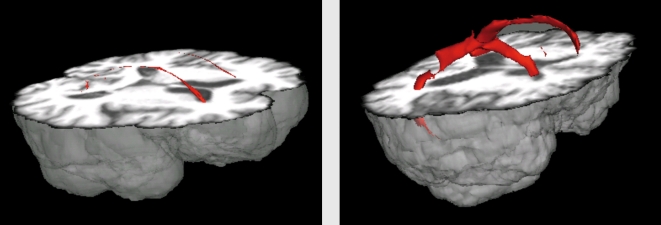
White matter hyperintensities, sometimes referred to as leukoaraiosis or leukoencephalopathy, are areas of increased lucency visualized on T2-weighted images. They have enjoyed a rich, albeit capricious, history in clinical practice and in the aging literature, at points considered incidental with little clinical significance and at points considered a central source of cognitive, motoric, and emotional dysfunction. Initially, WMH were described as “unidentifiable bright objects,” confounding radiologists as either artifact ual or adventitious companions of aging. Indeed, chronological age is the strongest correlate of WMH severity14-16 and most older adults have some degree of WMH burden. (Figure 1) displays a typical example of distributed WMH and (Figure 2) shows examples of two elderly individuals, one with mild WMH and one with more severe WMH reconstructed in three dimensions. White matter hyperintensities usually appear in the white matter confluent to the lateral ventricles (ie, “periventricular” WMH), often projecting deep into cortical white matter and grey matter nuclei (ie, “deep” WMH), or as circumscribed punctate spheres in deep cortical tissue. Of note, punctate WMH often appear as isolated lesions on two-dimensional MRI axial slices, but with three-dimensional reconstruction it often becomes evident that they are contained within the same process stemming off the lateral ventricles.
Figure 1. Typical distribution of white matter hyperintensities on a single subject's axial T2 -weighted FLAIR raw image (left) and labeled with an intensity threshold (right). FLAIR, fluid attenuated inverse recovery.
Figure 2. Three-dimensional reconstruction of white matter hyperintensities (WMH) superimposed on a T1 -weighted high resolution anatomical image. Patient on the left has relatively mild distribution of periventricular WMH; patient on the right has more severe distribution with WMH extending into deep cortical areas.
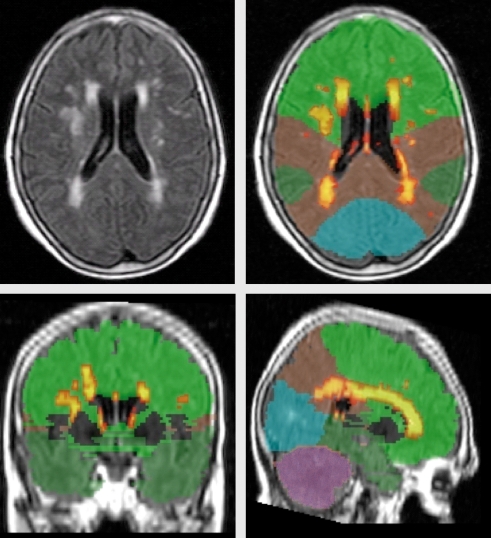
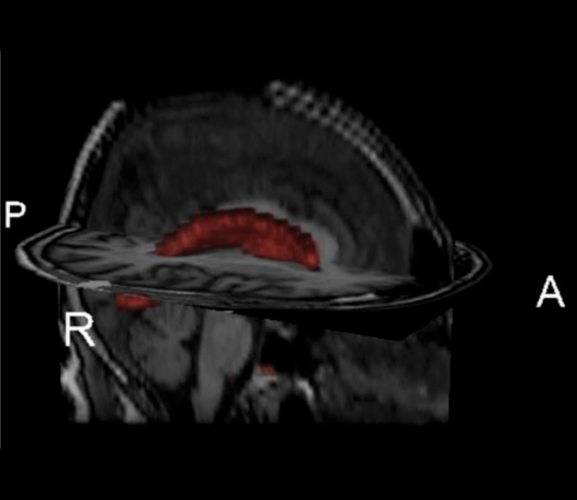
Optimal characterization of the severity of WMH among older adults has been a matter of some debate. Some authors have argued that periventricular WMH are clinically less important than deep WMH. Others have stressed the importance of regional, or lobar, distribution of WMH. These characteristics are reflected in many visual rating scales, such as the Scheltens Scale, 17 which are commonly used to evaluate the severity and distribution of WMH. Our laboratory has developed a quantitative approach for regional WMH severity analysis. Briefly, by considering the distribution of voxel intensities on individual fluid attenuated inverse recovery (FLAIR) images, we fit Gaussian curves to each cerebral hemisphere and derive the mean and standard deviation for each hemisphere. White matter hyperintensity seeds are defined as greater than or equal to 2.5 standard deviations above the mean. The left and right seeds are combined, and each seed is then passed into a mean intensity-based region-growing algorithm. The algorithm uses the seed voxel intensity as its starting mean and, applying a 10-point connectivity scheme (x-y plane, and 1 up in z and 1 down in z-plane) it searches for and labels voxels that fall within 5% of the seed mean. Neighboring voxels that fall within 5% are added to the image and a new mean is created. This process continues iteratively until all seeds have been included in the final WMH image. The summation of the number of voxels labeled as WMH multiplied by voxel dimensions yields the total WMH volume. By spatially normalizing an anatomical atlas18 to each image, we are able to derive WMH volumes in each of the major anatomical lobes, basal ganglia, and cerebellum. (Figure 3) illustrates three orthogonal views of a FLAIR image with WMH labeled and regionally parcellated. Furthermore, through segmentation of the lateral ventricles (Figure 4) we are able to calculate the distance in three dimensions of each voxel from the ventricular wall. Thus, our quantitative processing approach can be used to derive total WMH volume, regional WMH volume, and periventricular vs deep regional WMH volumes.
Figure 3. Example of regional white matter hyperintensity (WMH) quantification for one subject. Upper left: raw T2-weighted FLAIR image. Upper right and lower two: WMH labeled with “hottest” colors indicating most hyperintense voxels. Colors correspond to cerebral lobes (green: frontal, brown: parietal, dark green: temporal, blue: occipital, mauve: cerebellum). FLAIR, fluid attenuated inverse recovery.
Figure 4. Segmented ventricular volume (in red) superimposed on axial and sagittal orthogonal images from high resolution T1weighted anatomical scan. By segmenting the ventricular system, we are able to calculate the distance from the ventricular walls of each white matter hyperintensity voxel.
Correlates of white matter hyperintensities
Despite the ubiquity of WMH among older adults, they are a uniquely radiological phenomenon. That is, when examining grossly the brain regions underlying WMH, there is no obvious pigmentation abnormality. Our current understanding of the nature, clinical importance, and cognitive consequences of WMH has come from a number of careful clinicopathological correlates and observational studies among clinical and epidemiological samples. A prevailing view is that WMH are a surrogate marker of small-vessel vascular disease19 resulting from ischemic damage due to chronic hypoperfusion. White matter hyperintensities tend to develop in regions that are considered “watershed” areas, which extend up to 13 mm beyond the ventricular walls.20-22 Indeed, most of the major risk factors for ischemia have been shown to be associated with the severity of WMH distribution.23-27 Further evidence for an ischemic origin comes from postmortem pathological examination of tissue that appears as WMH during life. Areas most vulnerable to development of WMH receive blood supply primarily from ventriculofugal vessels, which originate from the subependymal arteries.28,29 These vessels have relatively few anastomoses and are particularly vulnerable to injurydue to systemic hypoperfusion.29-30 Clinico-pathological correlate studies have shown that smooth periventricular WMH are associated with subependymal gliosis and disruption of the ependymal lining, whereas deep white matter punctate WMH or irregularly shaped periventricular WMH are associated with disruption in fibers secondary to ischemic/arteriosclerotic changes.31,32 In general, WMH are related to diminished pallor or rarefaction and gliosis33 and myelin or axonal loss.34
By combining structural neuroimaging data with measures of cerebral blood flow, as measured by arterial spin labeling (ASL), we showed that areas appearing as WMH on FLAIR images had diminished blood flow relative to normal appearing white matter and grey matter.35 The finding complements recent observations that the spatial frequency of WMH among healthy older adults is greater in regions with lower normative perfusion values.36 We also showed that, among an epidemiological cohort of nondemented older adults, WMH were associated with chronological age and vascular risk factors37 and were most severe among adults with the highest absolute blood pressure and blood pressure fluctuation over a 3-year period (Brickman et al, unpublished). These observations lend further support that diminished perfusion and perhaps compromised cerebral autoregulation increase the risk for WMH development.
The role of white matter hyperintensities in cognitive aging
Consistent observations of increasing WMH severity and variability with aging supports ongoing interest in their clinical or cognitive correlates (see ref 13). Most investigations of the cognitive correlates of WMH burden have been cross-sectional analyses among nondemented older adults, and few have considered regional specificity. Based on these studies, there is emerging evidence that the severity or volume of WMH is one source of the cognitive decline that is typical of normal aging.38 In one of the earlier syntheses of the cognitive correlates of WMH in aging, a quantitative review showed that the extent of WMH is associated particularly with poorer performance on tasks of executive functioning and processing speed, but not with fluid or crystallized intelligence or fine motor functioning.39 The results are consistent with a more recent quantitative meta-analysis, which also showed that the severity of WMH burden is associated with poorer performance on speeded tasks of executive function in both healthy elderly and in individuals with a history of cardiovascular disease.40
WMH may affect cognitive functioning through disruption of intracerebral connectivity, compromising efficient neuronal communication.41 Thus, regional specificity of the distribution of these lesions may be associated with unique cognitive profiles. The prefrontal cortex and its extensive cortical-cortical and cortical-subcortical connectivity is thought to play a central role in executive functioning,10,11,42 and damage to these areas may account for the predominant pattern of executive functioning decline in aging. Indeed, the age-associated changes in executive functioning appear to be partially mediated byincreased burden of WMH distributed in frontal lobe regions43,44 and WMH distribution in prefrontal regions among older adults negatively impacts functional activity in the same region.45 Despite cross-sectional observations of associations between frontal WMH and executive functioning, there has been a paucity of studies examining the longitudinal progression of WMH and associated changes in cognitively normal elderly. Studies have found that increasing global WMH ewer a 4- or 5-year period, but not lacunar infarcts, are associated with worsening executive abilities and speeded abilities.46-48 Taken together, the culmination of findings establish that WMH are common in normal aging, progress substantially, and suggest that this progression, particularly in anterior regions, may partially account for typical ageassociated decline in executive abilities.
The role of white matter hyperintensities in Alzheimer's disease
More recently, the question of whether WMH play a unique role in the presentation or pathogenesis of AD has emerged. WMH are more prevalent and severe in AD patients compared with nondemented, but demographically similar older adults.17,49-51 Studies that have examined regional distribution of WMH show more posterior involvement, including posterior periventricular regions and posterior corpus callosum20,52 and increasing caudal involvement with more severe cognitive impairment.20 Interestingly, brain regions where WMH are most severe in AD colocalize to the distribution of AD pathology and areas showing the greatest metabolic dysfunction in AD.53 In our community-based study, we showed a selective association between WMH burden and diagnosis of amnestic mild cognitive impairment (MCI)-those at greatest risk for development of AD-but not nonamnestic MCI.54 Preliminary examination of the regional distribution showed that WMH burden in parietal lobes discriminated best among those with amnestic MCI, non amnestic MCI, and controls, again suggesting that a posterior distribution may be specific to or linked pathologically to AD.
Whether evaluation of neuroimaging data at one point in time has prognostic value for future clinical course or progression to AD remains an important question. Older adults who are not demented but who have increased WMH burden are at higher risk for the development of AD55-57 and MCI.58 We sought to determine whether baseline measurement of WMH severity and global atrophy, as a proxy of overall disease burden, predict future cognitive decline among patients with AD.59 Using a series of generalized estimating equation models, we demonstrated that the degree of baseline atrophy, the severity of WMH, and their interaction predicted the rate of cognitive decline. That is, greater severity of baseline atrophy and greater severity of baseline WMH were associated with faster rates of cognitive decline in AD and the interaction of the two variables suggest synergy between cerebrovascular disease and overall disease burden. These findings are consistent with others showing that the presence of both elevated amounts of atrophy and high WMH burden is more associated with AD than either measure alone.60,61 Results have been somewhat mixed, however, as neither Smith and colleagues59 nor DeCarli and colleagues62 found that variability in baseline measures of total WMH burden predicted future conversion from cognitively normal or MCI to AD.
The association of vascular risk factors, brain perfusion abnormalities, and increased WMH burden with AD suggests that vascular disease plays an important role in the pathogenesis of AD. Vascular disease may increase risk or lower a clinical threshold for the expression of the disease even in the absence of a mechanistic link or, alternatively, may be mechanistically related. Prevailing hypotheses on the pathogenesis of AD implicate abnormal deposition of parenchymal Aβ protein,63 and research shows that having high levels of plasma Aβ42 that decrease over time elevates risk for development of AD, presumably reflecting deposition and oligomerization of Aβ peptides in senile plaques in the brain.64 However, recent literature suggests that vascular deposition of Aβ, primarily comprising the Aβ40 species, may also be a primary pathological feature of the disease. For example, in vitro studies show that the severity and frequency of white matter, but not gray matter, perivascular spaces is greater in AD and correlates with the amount of Aβ deposition in overlying cortex and associated arteries.65 As vascular Aβ may interfere with the ability of the blood vessel walls to shunt deposited Aβ peptides through the periarterial spaces in the brain vasculature66-69 and white matter in AD contains 4 times more soluble Aβ than among controls,70 it is possible that the increased WMH burden among patients with AD, to some degree, reflects the pathological accumulation of vascular Aβ. Plasma Aβ40 concentrations have been shown to be associated with WMH burden among patients with AD and MCF71 and among members of the Rotterdam cohort with the APOE-E4 allele.72 These cross-sectional efforts provide evidence that increases in circulating Aβ40 may cause white matter microvascular damage, or, alternatively, that the accumulation of microvascular white matter disease causes pathological release of cerebral Aβ40 into the blood plasma. Longitudinal studies are critical to define whether increases in plasma Aβ40 are a biomarker of cerebrovascular disease or a risk factor for the development of cerebrovascular disease.71
Direct examination of the association between centrallydeposited Aβ and WMH provides another approach towards understanding a link between WMH or microvascular disease and AD pathology, and two general classes of studies have begun to address this issue precisely. First, cerebral amyloid angiopathy (CAA) is present in the vast majority of patients with AD at autopsy. Cerebral amyloid angiopathy reflects the deposition of Aβ in cerebral arterioles and is manifested as lobar cerebral microbleeds, best visualized in vivo on T2*-weighted gradient-echo MRI. Importantly, WMH are more frequent in the presence ol microbleeds or clinical CAA36,73 and those with clinical CAA show a progressive increase in WMH, suggesting that CAA may cause progressive white matter changes.74 A recent report75 noted that microbleeds had a lobar distribution in 92% of patients with AD and were predominantly distributed in posterior regions. The presence and frequency of microbleeds among AD patients predicted the severity of WMH, which was colocalized in parieto-occipital distributions. Given the studies showing colocalization among WMH, microbleeds, and the pathological distribution of AD, it is possible that the greater posterior distribution of WMH in AD could reflect the specific contribution of CAA, but future studies will need to address this possibility specifically.
Second, one of the most exciting developments in neuroimaging has been the ability to label in vivo central amyloid depositions using a carbon-11-labeled, lipophilic derivative of thioflavin-T, termed “Pittsburgh Compound B” or simply “PIB.”76,77 PIB can detect amyloid pathology even among nondemented individuals78 and has been associated with Aβ42 levels in cerebrospinal fluid.79 More recently, two reports demonstrated that PIB also reliably labels vascular deposition of Aβ and is able to discriminate patients with clinically diagnosed cerebral amyloid angiopathy from those with AD.80,81 Thus, while the culmination of studies reviewed above suggest that WMH are purely ischemic, resulting from systemic or variable hypoperfusion, multimodal neuroimaging, and pathological examination would suggest a more heterogeneous profile, perhaps with an amyloidogenic source of WMH distributed in posterior cortex among individuals with and at risk for AD. The studies highlight the potential importance of both parenchymal and vascular β amyloid in the pathogenesis of AD and suggest that the two are mechanistically linked. It will be critical to extend this line of research and determine the association between regional distribution of WMH, cerebral microbleeds, and PIB uptake among individuals with and without AD, and future studies should undertake this effort among large samples of community-based individuals.
Current status of white matter hyperintensities and future directions
Structural neuroimaging studies of aging and dementia have highlighted the importance of WMH in normal ageassociated cognitive loss and in AD. The prevailing view of WMH is that they represent small-vessel ischemic cerebrovascular disease secondary to perfusion abnormalities. Recent work implicates their involvement in the presentation and pathogenesis of AD and points to a potential amyloidogenic source, particularly when they are distributed in posterior cortex. There are several consistent findings regarding cerebrovascular disease in the context of AD that have emerged, with several etiological possibilities. First, the presence of small-vessel cerebrovascular disease among patients with AD is the norm, not the exception.60 Second, patients who have coexisting AD and small-vessel cerebrovascular disease have more severe cognitive impairment than those having either alone82-84 and brain imaging markers of each seem to interact synergistically to impact longitudinal cognitive course.59 Third, cerebrovascular disease and AD share common risk factors.87 From an etiological perspective, AD and cerebrovascular disease may be independent, but share common risk factors. Similarly, cerebrovascular disease may represent an independent pathology that lowers the threshold for clinical expression of AD or contributes independently to cognitive dysfunction. On the other hand, cerebrovascular disease may be in the causal pathway lor development of AD or interact synergistically with AD pathology. These possibilities are not mutually exclusive, but given the overlap in risk factors, prevalence of cerebrovascular disease in AD, involvement of both vascular and parenchymal forms of p amyloid, and interactions between the two on clinical presentation, there is preliminary evidence of etiological or mechanistic overlap.
It is clear that future work should focus on disentangling these etiological possibilities in order to better inform treatment and prevention strategies. Longitudinal studies comprising community samples and incorporating multimodal neuroimaging modalities will help establish cause-effect relationships. For example, while associations between WMH burden and AD have been observed, the questions of whether the progression or accumulation of WMH leads to AD needs to be addressed. Similarly, as acquisition and analytic techniques continue to evolve, investigators need to follow suit and become more precise in the questions being asked and the nature of the neuroimaging signal under study. WMH are important radiological correlates of cognitive aging, but most likely represent heterogeneous pathology that requires further elucidation through advanced imaging techniques and combined methodological approaches.
Acknowledgments
This work was supported in part by NIH grants AG029949, AG024708, AG007232, and Alzheimer's Association grant 05-14586 awarded to AMB and a Clinical and Translational Science Award Imaging Pilot Grant (NIH through Columbia University).
Selected abbreviations and acronyms
- AD
Alzheimer's disease
- CAA
cerebral amyloid angiopathy
- FLAIR
fluid attenuated inverse recovery
- MCI
mild cognitive impairment
- MRI
magnetic resonance imaging
- WMH
white matter hyperintensities
Contributor Information
Adam M. Brickman, Taub Institute for Research on Alzheimer's Disease and the Aging Brain, College of Physicians and Surgeons, Columbia University, New York, NY, USA.
Jordan Muraskin, Taub Institute for Research on Alzheimer's Disease and the Aging Brain, College of Physicians and Surgeons, Columbia University, New York, NY, USA.
Molly E. Zimmerman, Saul R. Korey Department of Neurology, Albert Einstein College of Medicine, Bronx, NY, USA.
REFERENCES
- 1.Elkins JS., O'Meara ES., Longstreth WT., Jr, Carlson MC., Manolio TA., Johnston SC. Stroke risk factors and loss of high cognitive function. Neurology. 2004;63:793–799. doi: 10.1212/01.wnl.0000137014.36689.7f. [DOI] [PubMed] [Google Scholar]
- 2.Kilander L., Nyman H., Boberg M., Hansson L., Lithell H. Hypertension is related to cognitive impairment: a 20- year follow-up of 999 men. Hypertension. 1998;31:780–786. doi: 10.1161/01.hyp.31.3.780. [DOI] [PubMed] [Google Scholar]
- 3.Kivipelto M., Helkala EL., Hanninen T., et al. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology. 2001;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 4.Kivipelto M., Helkala EL., Laakso MP., et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ (Clinical research ed). 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knopman D., Boland LL., Mosley T., et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 6.Swan GE., DeCarli C., Miller BL., Reed T., Wolf PA., Jack LM., Carmelli D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51:986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- 7.Brickman AM., Buchsbaum MS., Shihabuddin L., et al. Age-associated change in orbital, cingulate, and dorsolateral frontal lobe gray and white matter volume [abstract], J Int Neuropsych Soc. 2005;11(suppl 1):176. [Google Scholar]
- 8.Brickman AM., Habeck C., Ramos MA., Scarmeas N., Stern Y. A forward application of age associated gray and white matter networks. Hum Brain Map. 2008;29:1139–1146. doi: 10.1002/hbm.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brickman AM., Habeck C., Zarahn E., Flynn J., Stern Y. Structural MRI covariance patterns associated with normal aging and neuropsychological functioning. Neurobiol Aging. 2007;28:284–295. doi: 10.1016/j.neurobiolaging.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Brickman AM., Zimmerman ME., Paul RH., et al. Regional white matter and neuropsychological functioning across the adult lifespan. Biol Psychiatry. 2006;60:444–453. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman ME., Brickman AM., Paul RH., et al. The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. Am J Geriatr Psychiatry. 2006;14:823–833. doi: 10.1097/01.JGP.0000238502.40963.ac. [DOI] [PubMed] [Google Scholar]
- 12.Brickman AM., Buchsbaum MS. Alzheimer's disease and normal aging: Neurostructures. In: Byrne JH, ed. Learning and Memory: a Comprehensive Reference. Vol 3. New York, NY: Elsevier; 2008:601–621. [Google Scholar]
- 13.Gunning-Dixon FM., Brickman AM., Cheng JC., Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeCarli C., Massaro J., Harvey D., et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Pfefferbaum A., Mathalon DH., Sullivan EV., Rawles JM., Zipursky RB., Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 16.Coffey CE., Wilkinson WE., Parashos IA., et al. Quantitative cerebral anatomy of the aging human brain: a cross-sectional study using magnetic resonance imaging. Neurology. 1992;42:527–536. doi: 10.1212/wnl.42.3.527. [DOI] [PubMed] [Google Scholar]
- 17.Scheltens P., Barkhof F., Valk J., et al. White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer's disease. Evidence for heterogeneity. Brain. 1992;115:735–748. doi: 10.1093/brain/115.3.735. [DOI] [PubMed] [Google Scholar]
- 18.Admiraal-Behloul F., Olofesen H., Van den Heuvel DM., Schmitz N., Reiber JH., Van Buchem MA. Fully automated lobe delineation for regional white matter lesion load quantification in a large scale study. Proc Int Soc Magn ResonMed. 2004:138. [Google Scholar]
- 19.Pantoni L., Poggesi A., Inzitari D. The relation between white-matter lesions and cognition. Curr Opin Neurol. 2007;20:390–397. doi: 10.1097/WCO.0b013e328172d661. [DOI] [PubMed] [Google Scholar]
- 20.Yoshita M., Fletcher E., Harvey D., et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moody DM., Bell MA., Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. Am J Neuroradiol. 1990;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson MD., Jr Gonzalez-Gomez I, Gilles FH. Dyke Award. The search for human telencephalic ventriculofugal arteries. Am J Neuroradiol. 1991;12:215–222. [PMC free article] [PubMed] [Google Scholar]
- 23.Awad IA., Spetzler RF., Hodak JA., Awad CA., Carey R. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke. 1986;17:1084–1089. doi: 10.1161/01.str.17.6.1084. [DOI] [PubMed] [Google Scholar]
- 24.DeCarli C., Miller BL., Swan GE., Reed T., Wolf PA., Carmelli D. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol. 2001;58:643–647. doi: 10.1001/archneur.58.4.643. [DOI] [PubMed] [Google Scholar]
- 25.Inzitari D., Diaz F., Fox A., et al. Vascular risk factors and leuko-araiosis. Arch Neurol. 1987;44:42–47. doi: 10.1001/archneur.1987.00520130034014. [DOI] [PubMed] [Google Scholar]
- 26.Longstreth WT., Jr, Manolio TA., Arnold A., et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 27.Streifler JY., Eliasziw M., Benavente OR., Hachinski VC., Fox AJ., Barnett HJ. Lack of relationship between leukoaraiosis and carotid artery disease. The North American Symptomatic Carotid Endarterectomy Trial. Arch Neurol. 1995;52:21–24. doi: 10.1001/archneur.1995.00540250025008. [DOI] [PubMed] [Google Scholar]
- 28.Rowbotham GF., Little E. A new concept of the circulation and the circulations of the brain, the discovery of surface arteriovenous shunts. Br J Surg. 1965;52:539–542. doi: 10.1002/bjs.1800520714. [DOI] [PubMed] [Google Scholar]
- 29.Pantoni L., Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 30.De Reuck J. The human periventricular arterial blood supply and the anatomy of cerebral infarctions. Eur Neurol. 1971;5:321–334. doi: 10.1159/000114088. [DOI] [PubMed] [Google Scholar]
- 31.Fazekas F., Kleinert R., Offenbacher H., et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 32.Thomas AJ., O'Brien JT., Davis S., et al. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002;59:785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- 33.Englund E. Neuropathology of white matter changes in Alzheimer's disease and vascular dementia. Dement Geriatr Cogn Disord. 1998;9(suppl 1):6–12. doi: 10.1159/000051183. [DOI] [PubMed] [Google Scholar]
- 34.Udaka F., Sawada H., Kameyama M. White matter lesions and dementia: MRI-pathological correlation. Ann N Y Acad Sci. 2002;977:411–415. doi: 10.1111/j.1749-6632.2002.tb04845.x. [DOI] [PubMed] [Google Scholar]
- 35.Brickman AM., Zahra A., Muraskin J., et al. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psych Res: Neuroimaging. 2009;172:117–120. doi: 10.1016/j.pscychresns.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holland CM., Smith EE., Csapo I., et al. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39:1127–1133. doi: 10.1161/STROKEAHA.107.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brickman AM., Schupf N., Manly JJ., et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65:1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brickman AM., Siedlecki KL., Muraskin J., et al. White matter hyperintensities and cognition: testing the reserve hypothesis (abstract). J Int Neuropsych Soc. 2009;15 (suppl S1):53. doi: 10.1016/j.neurobiolaging.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunning-Dixon FM., Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- 40.Oosterman JM., Sergeant JA., Weinstein HC., Scherder EJ. Timed executive functions and white matter in aging with and without cardiovascular risk factors. RevNeurosci. 2004;15:439–462. doi: 10.1515/revneuro.2004.15.6.439. [DOI] [PubMed] [Google Scholar]
- 41.Albert M. Neuropsychological and neurophysiological changes in healthy adult humans across the age range. Neurobiol Aging. 1993;14:623–625. doi: 10.1016/0197-4580(93)90049-h. [DOI] [PubMed] [Google Scholar]
- 42.Royall DR., Lauterbach EC., Cummings JL., et al. Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatrie Association. J Neuropsychiatry Clin Neurosci. 2002;14:377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- 43.Oosterman JM., van Harten B., Weinstein HC., Scheltens P., Sergeant JA., Scherder EJ. White matter hyperintensities and working memory: an explorative study. Neuropsychol Dev Cogn. 2008;15:384–399. doi: 10.1080/13825580701879998. [DOI] [PubMed] [Google Scholar]
- 44.Paul RH., Gunstad J., Poppas A., et al. Neuroimaging and cardiac correlates of cognitive function among patients with cardiac disease. Cerebrovasc Dis (Basel, Switzerland). 2005;20:129–133. doi: 10.1159/000086803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordahl CW., Ranganath C., Yonelinas AP., et al. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci. 2006;18:418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kramer JH., Mungas D., Reed BR., et al. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Longstreth WT., Jr, Arnold AM., Beauchamp NJ., Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 48.van den Heuvel DM., ten Dam VH., de Craen AJ., et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry. 2006;77:149–153. doi: 10.1136/jnnp.2005.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalaria RN. The role of cerebral ischemia in Alzheimer's disease. Neurobiol Aging. 2000;21:321–330. doi: 10.1016/s0197-4580(00)00125-1. [DOI] [PubMed] [Google Scholar]
- 50.Meyer JS., Kawamura J., Terayama Y. White matter lesions in the elderly. JNeurSci. 1992;110:1–7. doi: 10.1016/0022-510x(92)90002-3. [DOI] [PubMed] [Google Scholar]
- 51.Rezek DL., Morris JC., Fulling KH., Gado MH. Periventricular white matter lucencies in senile dementia of the Alzheimer type and in normal aging. Neurology. 1987;37:1365–1368. doi: 10.1212/wnl.37.8.1365. [DOI] [PubMed] [Google Scholar]
- 52.Leys D., Pruvo JP., Parent M., et al. Could Wallerian degeneration contribute to “leuko-araiosis” in subjects free of any vascular disorder? J Neurol Neurosurg Psychiatry. 1991;54:46–50. doi: 10.1136/jnnp.54.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 54.Luchsinger JA., Brickman AM., Reitz C., et al. Subclinical cerebrovascular disease in mild cognitive impairment. Neurology. In press. doi: 10.1212/WNL.0b013e3181b1636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prins ND., van Dijk EJ., den Heijer T., et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61:1531–1534. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- 56.Vermeer SE., Prins ND., den Heijer T., Hofman A., Koudstaal PJ., Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 57.Wolf H., Ecke GM., Bettin S., Dietrich J., Gertz HJ. Do white matter changes contribute to the subsequent development of dementia in patients with mild cognitive impairment? A longitudinal study. Int J Geriatr Psychiatry. 2000;15:803–812. doi: 10.1002/1099-1166(200009)15:9<803::aid-gps190>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 58.Smith EE., Egorova S., Blacker D. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol. 2008;65:94–100. doi: 10.1001/archneurol.2007.23. [DOI] [PubMed] [Google Scholar]
- 59.Brickman AM., Honig LS., Scarmeas N., et al. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Arch Neurol. 2008;65:1202–1208. doi: 10.1001/archneur.65.9.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Flier WM., Barkhof F., Scheltens P. Shifting paradigms in dementia: toward stratification of diagnosis and treatment using MRI. Ann N Y AcadSci. 2007;1097:215–224. doi: 10.1196/annals.1379.013. [DOI] [PubMed] [Google Scholar]
- 61.van der Flier WM., Middelkoop HA., Weverling-Rijnsburger AW., et al. Interaction of medial temporal lobe atrophy and white matter hyperintensities in AD. Neurology. 2004;62:1862–1864. doi: 10.1212/01.wnl.0000125337.65553.8a. [DOI] [PubMed] [Google Scholar]
- 62.DeCarli C., Mungas D., Harvey D., Reed B., Weiner M., Chui H., Jagust W. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Small SA., Duff K. Linking Abeta and tau in late-onset Alzheimer's disease: a dual pathway hypothesis. Neuron. 2008;60:534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schupf N., Tang MX., Fukuyama H., et al. Peripheral Abeta subspecies as risk biomarkers of Alzheimer's disease. Proc Natl Acad Sci U S A. 2008;105:14052–14057. doi: 10.1073/pnas.0805902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roher AE., Kuo YM., Esh C., et al. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer's disease. Moi Med (Cambridge, Mass). 2003;9:112–122. [PMC free article] [PubMed] [Google Scholar]
- 66.Weller RO., Cohen NR., Nicoll JA. Cerebrovascular disease and the pathophysiology of Alzheimer's disease. Implications for therapy. Panminerva Med. 2004;46:239–251. [PubMed] [Google Scholar]
- 67.Niwa K., Carlson GA., ladecola C. Exogenous A beta1-40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab. 2000;20:1659–1668. doi: 10.1097/00004647-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 68.Preston SD., Steart PV., Wilkinson A., Nicoll JA., Weller RO. Capillary and arterial cerebral amyloid angiopathy in Alzheimer's disease: defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol Appl Neurobiol. 2003;29:106–117. doi: 10.1046/j.1365-2990.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 69.Thomas T., Thomas G., McLendon C., Sutton T., Mullan M. beta-Amyloidmediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–171. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- 70.Roher AE., Weiss N., Kokjohn TA., et al. Increased A beta peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in Alzheimer's disease. Biochemistry. 2002;41:11080–11090. doi: 10.1021/bi026173d. [DOI] [PubMed] [Google Scholar]
- 71.Gurol ME., Irizarry MC., Smith EE., et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 72.van Dijk EJ., Prins ND., Vermeer SE., et al. Plasma amyloid beta, apolipoprotein E, lacunar infarcts, and white matter lesions. Ann Neurol. 2004;55:570–575. doi: 10.1002/ana.20050. [DOI] [PubMed] [Google Scholar]
- 73.Maia LF., Vasconcelos C., Seixas S., Magalhaes R., Correia M. Lobar brain hemorrhages and white matter changes: Clinical, radiological and laboratorial profiles. Cerebrovasc Dis (Basel, Switzerland). 2006;22:155–161. doi: 10.1159/000093245. [DOI] [PubMed] [Google Scholar]
- 74.Chen YW., Gurol ME., Rosand J., Viswanathan A., Rakich SM., Groover TR., Greenberg SM., Smith EE. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology. 2006;67:83–87. doi: 10.1212/01.wnl.0000223613.57229.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pettersen JA., Sathiyamoorthy G., Gao FQ., et al. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol. 2008;65:790–795. doi: 10.1001/archneur.65.6.790. [DOI] [PubMed] [Google Scholar]
- 76.Lopresti BJ., Klunk WE., Mathis CA., et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. JNud Med. 2005;46:1959–1972. [PubMed] [Google Scholar]
- 77.Price JC., Klunk WE., Lopresti BJ., et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 78.Mintun MA., Larossa GN., Sheline Yl., et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 79.Fagan AM., Mintun MA., Mach RH., et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 80.Greenberg SM., Grabowski T., Gurol ME., et al. Detection of isolated cerebrovascular beta-amyloid with Pittsburgh compound B. Ann Neurol. 2008;64:587–591. doi: 10.1002/ana.21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson KA., Gregas M., Becker JA., et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 82.Petrovitch H., Ross GW., Steinhorn SC., et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- 83.Riekse RG., Leverenz JB., McCormick W., et al. Effect of vascular lesions on cognition in Alzheimer's disease: a community-based study. J Am Geriatr Soc. 2004;52:1442–1448. doi: 10.1111/j.1532-5415.2004.52405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rockwood K., Davis H., MacKnight C., et al. The Consortium to Investigate Vascular Impairment of Cognition: methods and first findings. Can J Neurol Sci. 2003;30:237–243. doi: 10.1017/s0317167100002663. [DOI] [PubMed] [Google Scholar]
- 85.Luchsinger JA., Honig LS., Tang MX., Devanand DP. Depressive symptoms, vascular risk factors, and Alzheimer's disease. Int J Geriatr Psychiatry. 2008;23:922–928. doi: 10.1002/gps.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hachinski V. Shifts in thinking about dementia. JAMA. 2008;300:2172–2173. doi: 10.1001/jama.2008.525. [DOI] [PubMed] [Google Scholar]