Abstract
Rationale
Two pharmacotherapies are approved for treating alcohol craving (acamprosate and naltrexone), but both have shown mixed findings in animals and humans.
Objectives
The present experiments utilized a “reinforcer blocking” approach (i.e., rats were able to consume ethanol during treatment) to better understand the efficacy of these treatments for ethanol seeking and drinking using ethanol-dependent and nondependent rats.
Materials and methods
In “nondependent” experiments, drugs (acamprosate 50, 100, and 200 mg/kg; naltrexone 0.1, 0.3, and 1.0 mg/kg) were administered over 3-week periods prior to operant sessions with a low response requirement to gain access to reinforcers for 20 min. For “dependent” experiments, rats were made dependent in vapor/inhalation chambers.
Results
Acamprosate and naltrexone had similar effects on intake in nondependent and dependent rats; neither drug was selective for ethanol over sucrose drinking. In nondependent animals, naltrexone was more efficacious at more doses than acamprosate, and acamprosate’s effects were limited to a dose that also had adverse effects on body weight. Both pharmacotherapies showed more selectivity when examining reinforcer seeking. In nondependent rats, acamprosate and naltrexone had response-attenuating effects in ethanol, but not sucrose, groups. In dependent animals, acamprosate had selective effects limited to a decrease in sucrose seeking. Naltrexone, however, selectively decreased ethanol-seeking in nondependent rats.
Conclusions
The naltrexone-induced decreases in seeking suggested a change in incentive motivation which was selective for ethanol in nondependent rats. The “nondependent” paradigm may model early stages of “problem drinking” in humans, and the findings suggest that naltrexone could be a good intervention for this level of alcohol abuse and relapse prevention.
Keywords: Alcohol, Ethanol, Operant, Reinforcement, Self-administration
Introduction
Two pharmacotherapies are currently approved clinically for use in treating alcohol craving: acamprosate (calcium acetyl homotaurinate) and naltrexone (an opiate receptor antagonist) (Bouza et al. 2004; Heilig and Egli 2006; Johnson 2008 for reviews). Acamprosate has been used to treat relapse in detoxified alcoholics (Paille et al. 1995; Tempesta et al. 2000; for review, see Littleton 1995), and it has been shown to decrease alcohol, but not sucrose, food, or water self-administration in rats (Boismare et al. 1984; Czachowski et al. 2001b; Naassila et al. 1998). Clinically, while some studies report that acamprosate treatment decreases the severity of relapse, there are mixed and negative findings related to the effects of acamprosate on measures of alcohol craving and dependence (Anton et al. 2006; Chick et al. 2000; Tempesta et al. 2000). In an animal model of relapse, chronic acamprosate (two daily injections for 5 days) prevented the increase in responding seen after ethanol deprivation, but did not decrease baseline ethanol intake (Heyser et al. 1998). An analysis of the time course of acamprosate’s effects using this model suggested that acamprosate must be experienced together with ethanol intake to cause a long-lasting decrease in ethanol intake (Hölter et al. 1997). A shorter treatment regimen, twice daily acamprosate at 100–200 mg/kg, has also been shown to decrease reinstatement responding for an ethanol-paired cue (Bachteler et al. 2005). We have previously demonstrated in nondependent rats that acamprosate (twice daily at 50–200 mg/kg) specifically decreased ethanol drinking on the second day of administration without affecting sucrose drinking and without attenuating the reinforcer-seeking response for either fluid (Czachowski et al. 2001b). This pattern of results also suggested that drug treatment was most effective following some experience with the ethanol/drug combination (i.e., following the first day of administration of acamprosate). Ideally, however, for an “anti-craving” pharmacotherapy, a decrease in ethanol seeking would accompany the decrease in ethanol drinking. The present experiments examined both a more extended treatment period (14 days as compared to 2 or 5) in nondependent rats and treatment in ethanol-dependent animals after a recovery phase based on the positive clinical findings with detoxified alcoholics.
Naltrexone is an opiate receptor antagonist that has been shown to be useful for attenuating alcohol craving and for postponing relapse in humans (O’Malley et al. 1992; Volpicelli et al. 1992) and to decrease alcohol intake in animal models (for review, see Ulm et al. 1995). Recent studies continue to demonstrate the clinical efficacy of naltrexone treatment for decreased intake and alcohol relapse prevention in alcoholics and heavy drinkers (Anton et al. 2006; Donovan et al. 2008; Morley et al. 2006; Nava et al. 2006; O’Malley et al. 2008), with some conflicting findings regarding a role for genetic influence in naltrexone’s effects (Anton et al. 2008; McGeary et al. 2006) and an unsurprising mitigating effect of medication compliance (Baros et al. 2007). Interestingly, evidence suggests that naltrexone treatment may be more efficacious in patients who continue to drink some alcohol during treatment (Heinälä et al. 2001; Killeen et al. 2004). The interaction between consumed ethanol and naltrexone’s efficacy may be somewhat explained by rodent studies showing that naltrexone interacts directly with the palatability of ethanol (Ferraro et al. 2002) and that a learned, associative component of a “disruption” of ethanol reinforcement while drinking best explains naltrexone-mediated attenuation of responding (Stromberg et al. 1998). Tests of pure ethanol consumption (i.e., non-operant models) using nondependent rats have shown selective attenuation of ethanol versus water (Stromberg 2004; Stromberg et al. 2002a) but not sucrose (Stromberg et al. 2002b) intake using moderate doses (1–3.0 mg/kg) of naltrexone. Using the rodent model of relapse discussed above, naltrexone also decreased the alcohol deprivation effect, and a combination of naltrexone and acamprosate blocked both the deprivation effect and the rebound in intake that occurred following low dose treatment with naltrexone alone (Heyser et al. 2003). The pattern of ethanol intake following naltrexone treatment suggested that blocking opiate receptors does not affect the initial onset of drinking. Therefore, treatment may be specific to ongoing consummatory rather than seeking/appetitive responding for ethanol, again consistent with the idea of associative or “reinforcer blocking” effects of naltrexone mitigating ethanol drinking. Also, in nondependent rats using a model that separates seeking behavior from drinking, our group has previously shown that naloxone, a shorter acting opiate antagonist as compared to naltrexone, decreased ethanol intake at a lower dose (3.0 mg/kg) than it decreased sucrose intake (5.0 mg/kg) with some disruption of reinforcer seeking that was not statistically significant (Sharpe and Samson 2001). In that study, all testing followed only a single drug administration and the seeking response requirement was relatively low (16 lever presses). The present experiments examined a longer treatment period, utilized ethanol-dependent and nondependent rats, and measures of extinction responding for more stringent tests of reinforcer seeking.
The goal of the present study was to determine treatment efficacy for “craving-like” behavior as well as for ongoing alcohol drinking in both alcohol-dependent and nondependent rats. To this end, the operant test sessions employed a behavioral model that procedurally separates seeking from drinking to distinctly assess these two reinforcer-directed responses (e.g., Czachowski and Samson 1999; Samson et al. 2000). Using this approach, rats consume pharmacologically relevant amounts of ethanol (Hodge et al. 2001; Macenski and Shelton 2001) in operant sessions, and previously, we have shown that the behavioral responses are sensitive to pharmacological manipulations that specifically affect either the seeking (Czachowski et al. 2001a, 2002) or the consumption (Czachowski et al. 2001b) component of the reinforced responding. Alcohol dependence was achieved by exposing animals to alcohol vapors for 14 h/day over 14-day cycles, which has been shown to be sufficient to produce mild to moderate dependence (O’Dell et al. 2004). The approach employed for drug administration was conceptualized as a “reinforcer blocking” strategy where the low response requirement during sessions that followed drug injections made it likely that rats would continue to consume ethanol during acamprosate and naltrexone treatment.
Materials and methods
Four separate experiments were conducted, two with non-dependent animals and two with dependent animals given either acamprosate (experiments 1 and 3) or naltrexone (experiments 2 and 4) treatment, each with an ethanol- and a sucrose-reinforced group. Table 1 gives a brief timeline for each experiment, beginning with sessions that followed training and establishment of baseline responding.
Table 1.
Following operant lever press training and ethanol initiation by sucrose fading, an ethanol-reinforced and sucrose-reinforced group in each of four experiments (solution refers to the reinforcer in the operant chamber) underwent the following conditions for the indicated number of days (more detailed descriptions are given in “Materials and methods”)
| Experiment | Number of days/Session type | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acamprosate | 14OpS | EXT | 5 OpS | 9 OpS | EXT | 5 OpS | 14OpS | EXT | 5 OpS | 9 OpS | EXT | 5 OpS | 9OpS | EXT | |||
| Nondependent | RR 2 | ↑RR | RR 2 | ↑RR | RR 2 | ↑RR | RR 2 | ↑RR | RR 2 | ||||||||
| Naltrexone | 14OpS | EXT | 5 OpS | 9 OpS | EXT | 5 OpS | 14OpS | EXT | 5 OpS | 9 OpS | EXT | 5 OpS | 14OpS | EXT | 5 OpS | 9 OpS | EXT |
| Nondependent | RR 2 | ↑RR | RR 2 | ↑RR | RR 2 | ↑RR | RR 2 | ↑RR | RR 2 | ↑RR | RR 2 | ||||||
| Acamprosate | 14VAP | 7Recov | 4 OpS | EXT | 14VAP | 7Recov | 4 OpS | EXT | 14VAP | 7Recov | 4OpS | EXT | |||||
| Dependent | ↑RR | ↑RR | ↑RR | ||||||||||||||
| Naltrexone | 14VAP | 7Recov | 4 OpS | EXT | 14VAP | 7Recov | 4 OpS | EXT | 14VAP | 7Recov | 4OpS | EXT | 5 OpS | EXT | |||
| Dependent | ↑RR | ↑RR | ↑RR | ↑RR | |||||||||||||
Text in bold indicates that drug treatments were given on those days (either acamprosate or naltrexone; three doses in nondependent experiments and one dose in dependent experiments), text in italics indicates that saline injections were given, and normal text indicates that there was no pretreatment. Dependent measures reflecting intake come from operant sessions, while dependent measures reflecting reinforcer seeking come from extinction sessions
OpS operant chamber sessions, RR 2 response requirement of 2, EXT extinction session; ↑RR increasing response requirement to 20, VAP ethanol vapor exposure sessions, Recov recovery/withdrawal sessions
Subjects
The subjects were male Long Evans rats from Harlan Sprague–Dawley, Indianapolis, IN that were randomly divided into sucrose- or ethanol-reinforced groups (n=8/group; 64 total in four experiments). Body weights ranged from 175 to 199 g on arrival and averaged 420±6 g at completion of all training immediately prior to treatment or dependence induction. Ad libitum access to food and water was maintained except as noted below. The rats were housed individually on a 12-h light/dark cycle (6:00 A.M. to 6:00 P.M.), and animal care was in accordance with NIH guidelines (Guide for the Care and Use of Laboratory Animals; NIH Guide 1996) and approved by the Institutional Animal Care and Use Committee.
Apparatus and drugs
Daily sessions were conducted in modular chambers (Med-Associates; St. Albans, VT, USA; 30×30×24.5 cm) equipped with houselight, a retractable lever, and a retractable graduated cylinder tube with rubber stopper and a stainless steel spout with double ball bearings to prevent leakage. The lever was located on the wall opposite to the sipper tube drinking bottle. All chambers were housed in sound-attenuated enclosures equipped with exhaust fans that masked external noise. Electrical inputs and outputs of each chamber were controlled using Med-Associates software (Med-Associates). Ethanol solutions were prepared volume/volume in water from 95% ethanol. For sucrose/ethanol solutions, the sucrose solution was prepared weight/volume and used as a solute. Acamprosate (generously provided by Forest Research Institute, Jersey City, NJ, USA) and naltrexone (Sigma-Aldrich, Inc., St. Louis, MO, USA) were dissolved in saline and injected at a volume of 1.0 ml/kg. Acamprosate injections were administered intraperitoneally at −2 and −21 h relative to the time of operant conditioning sessions, and naltrexone was administered subcutaneously at −30 min. Ethanol dependence induction was conducted using alcohol inhalation systems from La Jolla Alcohol Research Inc., La Jolla, CA, USA. These consisted of 16 separate standard plastic rodent cages in sets of four that allowed for the controlled delivery of alcohol vapor. Briefly, within these four closed systems, alcohol was dripped at a set rate (24–27 mg/l) into a heated flask, and airflow was controlled by vacuum pump to deliver an alcohol vapor/air mixture in order to achieve desired blood alcohol levels.
Training and ethanol initiation
Upon arrival, subjects were weighed and handled for a minimum of 3 days. Daily sessions were conducted 5 days/week at the same time each day during the lights-on cycle. Subjects were initially trained to press the lever on a fixed-ratio-one schedule that resulted in 15 s of access to the sipper tube with 10% sucrose in 30-min sessions. Subjects were water-restricted for the initial two to five sessions only, after which food and water were available ad libitum in the home cage. Over a 3-week period, the training/sucrose-fading (Samson 1986) procedures involved the following: increasing the fixed ratio from 1–4, decreasing the sucrose concentration to 2%, and in the ethanol groups introducing ethanol and increasing the concentration from 2% to 10% while fading the sucrose out completely (final solutions: sucrose groups, 2%; ethanol groups, 10%). The procedural separation between seeking (lever pressing) and consumption was then instituted. Following completion of a single-response requirement, access to the sipper tube was provided for 20 min. Over 4 weeks, the response requirement was increased from four to 20, and then the response requirement of 20 was maintained for four additional weeks. Four different treatment/ethanol exposure paradigms were then instituted; see Table 1 for a brief timeline of each of the treatment portions of the experiments.
Treatment schedule and test sessions (experiments 1 and 2; nondependent subjects)
Daily session cycles of drug (3 weeks/14 sessions from a Tuesday to Friday) or saline (2 weeks/nine sessions from a Tuesday to Friday) treatment were then alternated. For these sessions, the response requirement was decreased to 2 to ensure that all subjects would gain access to the reinforcer solution. At the end of each drug or saline cycle, a single, non-reinforced extinction session was conducted on a Monday (i.e., following 2 days of no injections or reinforcer access). Extinction sessions consisted of 20 min of access to the lever, with no presentation of the sipper tube spout (solutions were present in the sipper tube to control for scent cues). The response requirement then was gradually increased back to 20 for the remainder of that week (no injections) through the following Monday to ensure subjects would continue to reliably respond (i.e., that the prolonged exposure to the response requirement 2 schedule did not cause a decrement in responding), and then the next cycle was initiated.
For naltrexone (experiment 2), the literature provided good evidence for dose choices, so 0.1, 0.3, and 1.0 mg/kg were tested in a balanced/random order by subject across the three dosing cycles with a corresponding saline cycle paired to each dose (so there were a total of three drug cycles and three intervening saline cycles for this experiment; see Table 1). However, for acamprosate (experiment 1), 50, 100, and 200 mg/kg were tested in increasing dose order due to some health concerns about high doses (dose-dependent weight loss problems; Bowers et al., 2007). We had previously observed some problems using a single injection of a 400-mg/kg dose (Czachowski et al. 2001b) and again noted severe weight loss and intestinal issues after repeated injections of the 200-mg/kg dose, so only nine drug sessions were conducted in the final drug cycle with no final saline cycle (i.e., there were a total of three drug cycles and two intervening saline cycles for this experiment; see Table 1).
Dependence induction, treatment schedule, and test sessions (experiments 3 and 4; dependent subjects)
For experiments 3 and 4 to assess treatment in dependent subjects, following training, three cycles of dependence induction, recovery, and operant sessions were instituted (see Table 1). Dependence induction consisted of exposure to ethanol vapor for 14 days at 14 h/day with target blood ethanol levels of 150–200 mg%. Ethanol flow rates were adjusted during vapor exposure based on animal’s individual blood ethanol measures (see below). These parameters were based upon recent validations of the approach (Funk and Koob 2007; O’Dell et at. 2004; Sabino et al. 2006; Zhao et al. 2007) that demonstrate that the intermittent 14-h/day exposure ranging from 2 to 4 weeks in length has better face validity than 24-h exposure and confirming that the 2-week exposure length is sufficient for the “escalation of the allostatic processes responsible for excessive ethanol self-administration” and blood ethanol concentrations (BECs) associated with mild to moderate dependence and mild signs of physical withdrawal (O’Dell et al. 2004). The recovery week for each of the three dependence/treatment cycles involved no handling or access to ethanol. This was followed by five daily sessions; for the first four sessions, the response requirement was increased from 10 to 20, and then a single, non-reinforced extinction session was conducted on Friday. Cycle 1 was “no treatment”; in cycle 2, saline injections preceded the sessions, and drug treatment was conducted during recovery and preceding sessions in cycle 3 (experiment 3, 100 mg/kg acamprosate; experiment 4, 0.3 mg/kg naltrexone). Note that in the nondependent experiments 1 and 2, extinction sessions were conducted in animals with no drug treatment on board (i.e., final injection on Friday with extinction on Monday; see Table 1). Therefore, in experiment 4 only, an additional week of sessions/naltrexone treatments and a final reinforcer-seeking test (extinction) on the following Monday with no drug treatment on board was conducted to compare to the similar session in experiment 2. This final treatment/test was not conducted in experiment 3 with acamprosate due to the weight loss observed and since no effects of acamprosate were observed in experiment 1 that warranted comparison in dependent subjects (see “Results” below).
BEC determination
To measure BECs, samples were collected (100 μl) into heparinized capillary tubes from a nick to the tip of the tail while the rats were restrained briefly for a maximum of 2–4 min. Samples were stored sealed on ice during collection and then immediately centrifuged, and a 5 μl sample of plasma was analyzed using the AM1 Analyzer (Analox Instruments, Lunenburg, MA, USA). Ethanol concentration is determined with an amperometric oxygen electrode that measures oxygen consumption during the enzymatic oxidation of alcohol to acetaldehyde. BECs were monitored at random intervals over the two dependence experiments (three samples on nonconsecutive days in each experiment). Blood samples were taken from all subjects immediately at the end of a 14-h ethanol vapor exposure.
Data analyses and statistics
Total intakes of sucrose and ethanol were determined from the change in fluid volume in the graduated cylinder sipper tube, and grams per kilogram intake was calculated from the intake volume and daily body weight measures. Total lever presses as well as a cumulative record of responding were recorded for each session. In all four experiments, ethanol-reinforced and sucrose-reinforced groups were analyzed separately using repeated measures analysis of variance (RM ANOVA), and post hoc comparisons were performed using Student–Newman–Keuls method when appropriate. For experiments 1 and 2, saline treatment data (two determinations for acamprosate and three for naltrexone) were collapsed for each subject when possible as confirmed by paired t test or RM ANOVA (all cases except acamprosate experiment, extinction responding, see below). For intake data, dose (four: saline plus three drug doses) and day were the main factors over the first 9 days. In addition, mean intake over the nine (saline) or 14 (drug) day treatments were analyzed with dose as the main factor. For the seeking data, dose was the main factor. For experiments 3 and 4, condition (four: pre-dependence, post-dependence, post-dependence/saline, and post-dependence/drug) and day (four) were the main factors for the intake data. For the seeking data, condition was the main factor (experiment 3 had three levels: post-dependence/no injection, post-dependence/saline, and post-dependence/drug; experiment 4 had four levels with the additional extinction test in naltrexone-treated dependent animals with no drug on board).
Results
All groups started with eight subjects, and a total of seven rats were dropped from the initial 64 animals. One animal from each group was dropped in experiment 3 for failing to maintain consistent responding prior to any manipulations, and one subject was dropped from the ethanol group in experiment 4 for failure to respond on the saline extinction day (i.e., failure to provide a reliable control response). Four subjects (two from the sucrose and two from the ethanol group) were removed very early in training in experiment 2 for failing to acquire the lever press response while sucrose was still the reinforcer solution (this is an unusually large number to drop from one experiment using this paradigm, but there was no obvious reason for the failure to train).
Acamprosate in nondependent subjects (experiment 1)
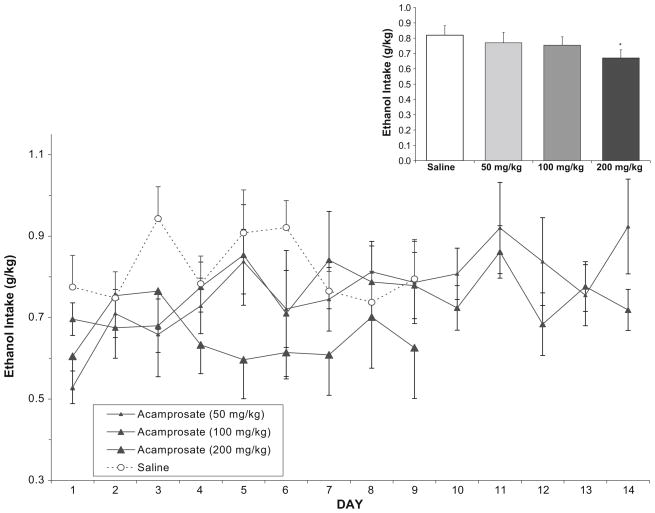
In the ethanol-reinforced group, analysis of ethanol intake (g/kg) following saline or acamprosate (Fig. 1) over days 1–9 showed that there was a main effect of dose [F(3,161)= 7.96, p<0.001] with post hoc analyses indicating that every dose differed from saline but no differences between doses. There was no main effect of day and no day/dose interaction. Analysis of total mean ethanol intake collapsed over all days again showed a main effect of dose [F(3,21)=4.4, p<0.05]; however, post hoc analyses indicated that only the high dose differed from saline. Since this collapsed analysis includes the extra 5 days of drug treatment for the low and medium doses, this suggests that these two lower doses began to lose effectiveness after the initial 9-day treatment.
Fig. 1.
In the ethanol-reinforced group (n=8), mean (±SEM) ethanol intake (g/kg) over days following either saline or acamprosate treatment in nondependent rats. Inset Total mean (±SEM) ethanol intake collapsed over all days. Asterisk indicates a main effect of dose, with the high dose of acamprosate differing from saline
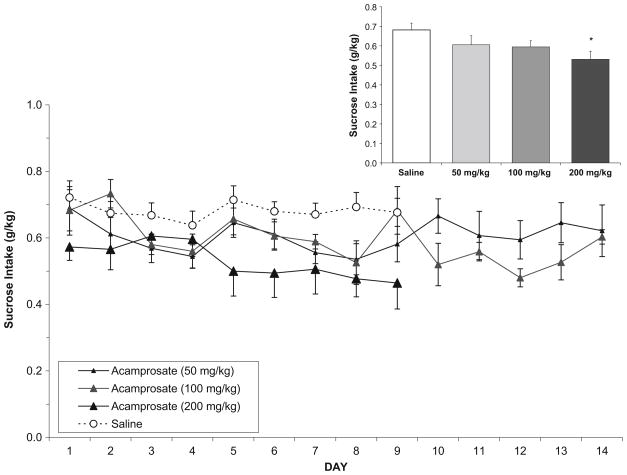
In the sucrose-reinforced group, analysis of sucrose intake (g/kg) following saline or acamprosate (Fig. 2) over days 1–9 also showed a main effect of dose [F(3,144)=6.3, p<0.01], with post hoc analyses indicating that the high dose decreased intake relative to both saline treatment and the middle dose of acamprosate. There was also a main effect of day [F(8,144)=3.2, p<0.01] and a day/dose interaction [F(24,144)=1.6, p<0.05]. Post hoc analyses indicated that the effect of day was due to a slight decrease in intake over days overall (days 1 and 2 vs. 7 and 8), but the day/dose interaction showed no meaningful pattern of differences when days within dose (saline and acamprosate) were examined. Analysis of total mean sucrose intake collapsed over all days again indicated a main effect of dose [F(3,18)=5.5, p<0.01], and post hoc analyses showed that the high dose of acamprosate was decreased as compared to saline.
Fig. 2.
In the sucrose-reinforced group (n=8), mean (±SEM) sucrose intake (g/kg) over days following either saline or acamprosate treatment in nondependent rats. Inset Total mean (±SEM) sucrose intake collapsed over all days. Asterisk indicates a main effect of dose, with the high dose of acamprosate differing from saline
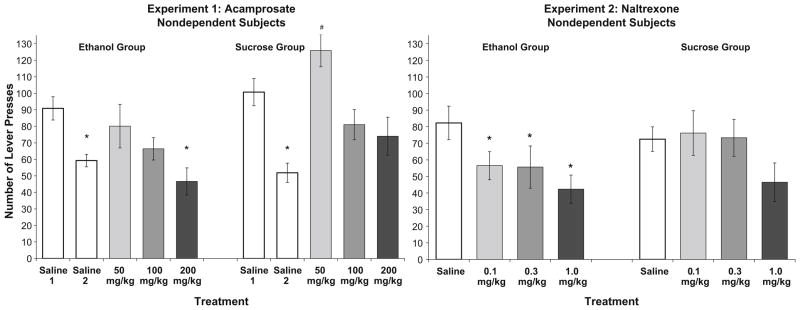
Ethanol seeking and sucrose seeking following saline and acamprosate treatments were assessed using extinction responding (Fig. 5, left panel). The lever press response in this experiment was the only dependent measure in any of the four experiments for which the saline treatment data could not be collapsed over the repeated determinations in either group due to a decrease in responding from the first to the second determination. Specifically, in the ethanol group, there was a main effect of dose [F(4,26)=4.3, p< 0.01], with post hoc analyses showing that both the high dose of acamprosate and the second saline test were decreased relative to the first saline. In the sucrose group, there was also an effect of dose [F(4,26)=10.9, p<.001], with saline treatments again differing from each other and the low dose of acamprosate increasing responding relative to all other treatments. There was no effect of treatment in either group on the latency to initiate lever press responding.
Fig. 5.
Mean (±SEM) lever-press responses during single, non-reinforced extinction sessions in the ethanol and sucrose groups (as indicated) in acamprosate-treated (left panel) or naltrexone-treated (right panel) nondependent rats. Asterisks indicate a significant difference from the first or collapsed saline treatment conditions; also, the low dose of acamprosate increased the sucrose-seeking response (number symbol)
Naltrexone in nondependent subjects (experiment 2)
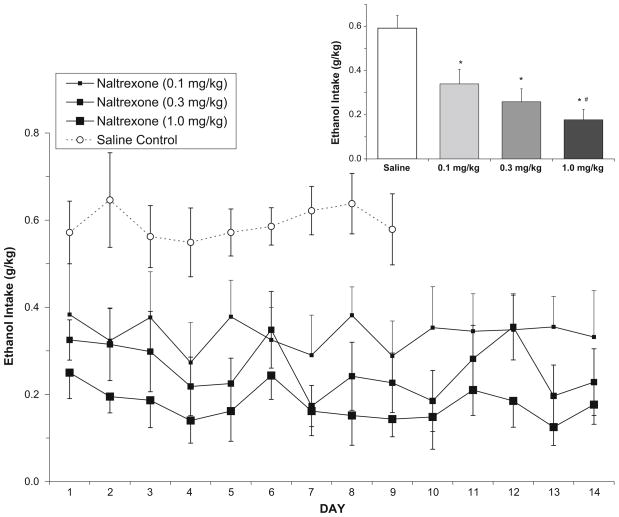
In the ethanol-reinforced group, analysis of ethanol intake (g/kg) following saline or naltrexone (Fig. 3) showed that over days 1–9, there was a main effect of dose [F(3,120)= 23.5, p<0.001], but not day and no day/dose interaction. Post hoc analyses showed a dose-dependent decrease in ethanol intake in that every dose of naltrexone differed from saline, and the low and high doses of naltrexone (0.1 vs. 1.0) were also significantly different from each other. Analysis of the total mean ethanol intake collapsed over all days again showed a main effect of dose [F(3,15)=31.6, p<0.001], with the same pattern of findings.
Fig. 3.
In the ethanol-reinforced group (n=6), mean (±SEM) ethanol intake (g/kg) over days following either saline or naltrexone treatment in nondependent rats. Inset Total mean (±SEM) ethanol intake collapsed over all days. Asterisks indicates that all doses of naltrexone decreased intake relative to saline, and the high and low doses of naltrexone produced significantly different attenuation of ethanol drinking (number symbol)
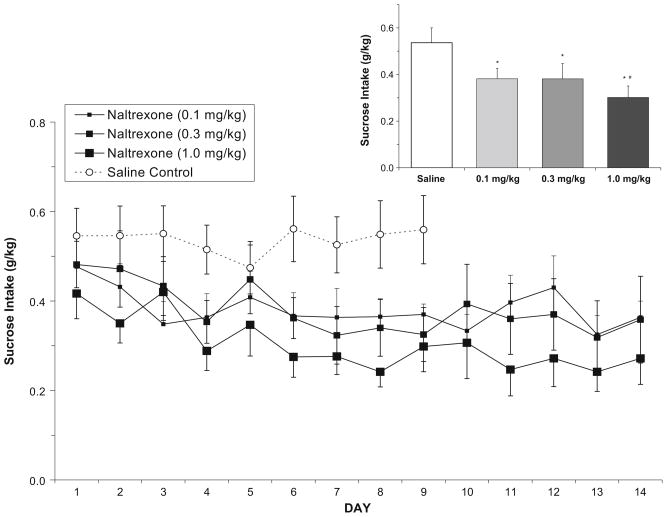
In the sucrose-reinforced group, analysis of sucrose intake (g/kg) following saline or naltrexone (Fig. 4) showed that over days 1–9, there was a main effect of dose [F(3,119)= 21.5, p<0.001] and of day [F(8,119)=0.15, p<0.001] and an interaction between dose and day [F(24,119)=2.5, p<0.001]. Post hoc analyses of the dose effect showed that every dose of naltrexone differed from saline, and all but the low and middle doses of naltrexone (0.1 and 0.3) differed from each other. The dose/day interaction and main effect of day were due to a general decrease in sucrose intake over days in the naltrexone conditions, but not in the saline condition. When collapsed over all days, analysis of the total mean sucrose intake (g/kg) showed that there was again a main effect of dose [F(3,15)=25.8, p<0.001], with the same pattern of findings.
Fig. 4.
In the sucrose-reinforced group (n=6), mean (±SEM) sucrose intake (g/kg) over days following either saline or naltrexone treatment in nondependent rats. Inset Total mean (±SEM) sucrose intake collapsed over all days. Asterisks indicates that all doses of naltrexone decreased intake relative to saline, and the high and low doses of naltrexone produced significantly different attenuation of sucrose intake (number symbol)
Ethanol seeking and sucrose seeking following saline or naltrexone treatments were assessed using extinction responding (Fig. 5, right panel). In the ethanol group, analysis of number of responses showed that naltrexone significantly decreased responding [F(3,15)=5.6, p<0.01], with all doses differing from saline and no differences between naltrexone treatments. In the sucrose group, naltrexone had no effect on reinforcer seeking. There was no effect of treatment in either group on the latency to initiate lever press responding.
Acamprosate in dependent subjects (experiment 3)
BECs during vapor exposure averaged 158.9 mg% (±23) in the ethanol-reinforced group and 146.4 mg% (±18) in the sucrose-reinforced group. When mean total ethanol intake was observed over the four conditions, there was no main effect of condition (pre-dependence, 0.80±0.07 g/kg; post-dependence no injection, 0.75±0.06; saline, 0.70±0.07; and acamprosate, 0.73±0.05). However, when analyzed as intake by day (see Table 2) over the 4 days of each condition, while there was again no effect of condition, there was an effect of day [F(3,48)=9.84, p<0.01], which was a result of day 1 (Mondays) being a low intake day relative to Tuesdays through Thursdays. Similarly, analysis of mean total sucrose intake showed no main effect of condition. Also, when analyzed as intake by day over the 4 days of each condition, there was again no effect of condition and an effect of day [F(3,48)=3.52, p<0.05] and a day × condition interaction [F(3,48)=2.65, p<0.05] which resulted from decreased intake again on Mondays, but with the most pronounced difference within the pre-dependence condition. This “Monday” pattern of drinking was not seen in any of the other four experiments including the naltrexone/dependent animals (i.e., not an effect of the dependence protocol) and did occur in all four conditions of the ethanol group (i.e., not an effect of the acamprosate treatment).
Table 2.
From experiments 3 and 4 in ethanol-dependent animals, the daily mean (±SEM) grams per kilogram intake following either saline injection or drug treatment as detailed in “Materials and methods”; statistical analyses described in “Results”
| Group | Treatment | Day |
|||
|---|---|---|---|---|---|
| Mon | Tues | Wed | Thurs | ||
| Experiment 3 | |||||
| Ethanol-reinforced | Saline | 0.59 (0.09) | 0.65 (0.10) | 0.97 (0.07) | 0.78 (0.07) |
| Ethanol-reinforced | Acamprosate (100 mg/kg) | 0.64 (0.06) | 0.83 (0.07) | 0.73 (0.11) | 0.74 (0.07) |
| Sucrose-reinforced | Saline | 0.55 (0.05) | 0.60 (0.06) | 0.66 (0.04) | 0.64 (0.05) |
| Sucrose-reinforced | Acamprosate (100 mg/kg) | 0.59 (0.04) | 0.59 (0.07) | 0.54 (0.06) | 0.60 (0.04) |
| Experiment 4 | |||||
| Ethanol-reinforced | Saline | 0.99 (0.17) | 0.94 (0.19) | 0.91 (0.14) | 0.86 (0.13) |
| Ethanol-reinforced | Naltrexone (0.3 mg/kg) | 0.60 (0.10) | 0.55 (0.07) | 0.58 (0.07) | 0.46 (0.07) |
| Sucrose-reinforced | Saline | 0.57 (0.08) | 0.57 (0.07) | 0.55 (0.08) | 0.54 (0.08) |
| Sucrose-reinforced | Naltrexone (0.3 mg/kg) | 0.51 (0.07) | 0.41 (0.05) | 0.38 (0.04) | 0.43 (0.05) |
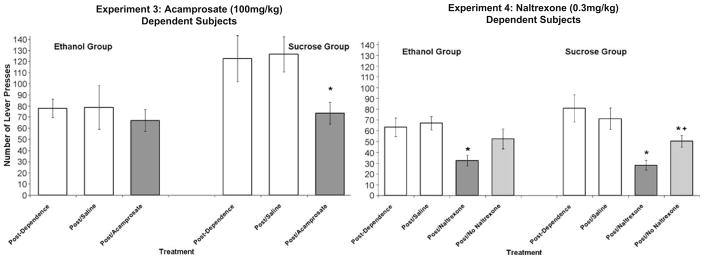
Reinforcer seeking was analyzed following no injection, saline, and acamprosate treatment (Fig. 6, left panel) using the Friday extinction sessions. In the ethanol-reinforced group, there was no effect of condition on total number of ethanol-seeking responses. However, the latency to first response differed by condition [F(2,12)=7.6, p<0.01], reflecting an increase in start latency only in the acamprosate-treated subjects (118±24 s) relative to saline (42±15 s) and no injection (35±8 s). In subjects reinforced with sucrose after ethanol dependence induction, there was no effect of condition on start latency; however, there was a main effect on number of responses [F(2,12)=7.44, p<.01]. Post hoc analysis indicated no difference between no injection compared to saline, but a significant decrease in sucrose seeking that resulted from acamprosate treatment when compared to both no injection and saline.
Fig. 6.
Mean (±SEM) lever-press responses during single, non-reinforced extinction sessions in the ethanol (n=7 both experiments) and sucrose (n=7 experiment 3; n=8 experiment 4) groups as indicated in acamprosate-treated (left panel) or naltrexone-treated (right panel) ethanol-dependent rats. Asterisks indicate a significant difference from the post/saline condition and plus symbol indicates a significant difference from the post/naltrexone condition
Naltrexone in dependent subjects (experiment 4)
BECs during vapor exposure averaged 171.1 mg% (±22) in the ethanol-reinforced group and 189.3 mg% (±26) in the sucrose-reinforced group. Analysis of mean total ethanol intake over the four conditions (pre-dependence, post-dependence no injection, saline, and naltrexone) showed a main effect of condition [F(3,18)=8.8, p<0.001] such that naltrexone treatment decreased intake (to 0.53±0.06 g/kg) relative to all other conditions (pre-dependence, 0.83± 0.06 g/kg; post-dependence no injection, 0.80±0.10; saline, 0.92±0.14), with no differences in intake between the other control conditions. When assessed over each of the 4 days (see Table 2) under each condition, there was again a main effect of condition [F(3,50)=7.2, p<0.01], but not day. In the sucrose-reinforced group, analysis of mean total sucrose intake similarly indicated that naltrexone decreased intake relative to all other conditions with no difference between those conditions [F(3,21)=6.7, p<0.01]. When analyzed as day × condition, there was an effect of condition [F(3,59)= 7.0, p<0.01] and a day × condition interaction [F(9,59)= 3.0, p<0.01], but no effect of day alone. Post hoc analysis showed that the treatment effect of naltrexone did not occur on day 1 (Monday), with decreased sucrose intake on all other days.
Reinforcer seeking was analyzed following no injection, saline, and naltrexone treatments (Fig. 6, right panel). The first three were Friday sessions that immediately followed the treatment indicated, while the final naltrexone/extinction test took place on Monday following 2 days of no injection or reinforcer access (similar to the protocol for experiment 2 in nondependent subjects). In the ethanol group, there was a main effect of condition [F(3,18)=4.0, p<0.05], with post hoc analysis showing that no injection and saline did not differ, and only the first naltrexone treatment condition differed from saline and from no treatment. The second naltrexone “test” with no naltrexone on board showed that ethanol seeking had returned to control levels (saline and no injection). A similar analysis of sucrose seeking also showed a main effect of condition [F(3,21)=14.0, p<0.001]. Saline and no injection did not differ, but both naltrexone treatment conditions differed from saline and from no treatment. There was also a significant difference between the two naltrexone tests, therefore indicating some recovery of sucrose seeking with no naltrexone on board, but not to control levels. Latency to first response was not affected by naltrexone in either the ethanol or sucrose groups.
Discussion
The behavioral paradigm utilizing a low response requirement for intake assessment along with single extinction sessions to assess reinforcer seeking, and the separation of appetitive and consummatory responding within each session, made it possible to determine that each drug treatment had differential effects on the regulation of seeking and drinking behaviors. It is also important to note that we previously have demonstrated that the seeking response (extinction session lever-press behavior) in this model is stable over repeated measurements (Samson et al. 2001) and reflects a prior association with the post-ingestive, pharmacological effects of ethanol (Samson et al. 2004). Therefore, with the inclusion of the sucrose-reinforced control groups, the experiments allowed for detailed comparison between the treatment effects, the identification of the specific behaviors “targeted” by each drug treatment, and the selectivity of treatment effects for ethanol. Overall, naltrexone appeared to be the better pharmacotherapy, and most of the beneficial or “treatment-like” effects of acamprosate were limited to a dose that also caused weight loss and general health issues. This is consistent with most comparison assessments of both drugs in animal and human studies (Morley et al. 2006; Richardson et al. 2008; Stromberg et al. 2001) and a follow-up of the COMBINE project (Combining Medications and Behavioral Interventions) that found that at 1 year post-treatment, naltrexone but not acamprosate was “associated with sustained efficacy beyond discontinuation” of the drugs (Donovan et al. 2008).
To briefly summarize the overall findings, with regard to drinking behavior, both acamprosate and naltrexone had somewhat similar effects in both nondependent and dependent rats in that neither drug was selective for ethanol over sucrose intake. However, in nondependent animals, naltrexone was more efficacious at more doses than acamprosate, and acamprosate’s effects primarily were limited to a dose that also had adverse effects on body weight gain. In dependent animals, naltrexone was again more efficacious since acamprosate did not attenuate either ethanol or sucrose intake at the moderate dose tested. Both pharmacotherapies showed more selectivity when examining a specific reinforcer-seeking response. In nondependent rats, both acamprosate and naltrexone had response-attenuating effects in the ethanol groups, but not the sucrose groups. In fact, acamprosate increased sucrose seeking at the low dose, but unfortunately, the decreases in ethanol seeking were again only noted at the high dose. Of more concern in terms of treatment efficacy, in dependent animals, is that acamprosate had selective effects limited to a decrease in sucrose seeking with no effect on ethanol seeking. Naltrexone, on the other hand, selectively decreased ethanol seeking in nondependent rats with no effects on sucrose seeking and decreased reinforcer seeking in general in ethanol-dependent animals. There was no indication of the development of either tolerance or sensitivity to treatment effects over the 9 or 14-day dosing regimens and no motor impairment as indicated by the lack of effects on latency to respond. The present findings are limited to male Long Evans rats, and other rat strains and females should be examined to assess possible strain or sex-specific treatment effects.
Daily administration of naltrexone dose-dependently decreased ethanol consumption in nondependent rats, while the same treatment decreased sucrose intake, but to a slightly lesser degree at all doses and with a less pronounced dose/response pattern. Naltrexone doses in a similar range (0.15–0.45 mg/kg) previously have been shown to non-selectively decrease “free” home cage intake of sweetened ethanol and the sweetened control solution, with modest selectivity for ethanol responding at the lower dose (0.15 mg/kg) when tested in an operant paradigm in “binge-drinking” rats with baseline intake of approximately 0.8–1.0 g/kg (Ji et al. 2008). While treatment selectivity was not assessed, a 1.0-mg/kg dose of naltrexone decreased cue-induced reinstatement responding for ethanol when tested in rats after a single and multiple ethanol withdrawals after vapor exposure (Ciccocioppo et al. 2003). In the present experiments, when tested 3 days after naltrexone/reinforcer sessions, subjects in the ethanol-reinforced group showed a decreased tendency to seek ethanol, while sucrose-reinforced subjects showed no such effect of the naltrexone/sucrose exposures (Fig. 5). Since subjects had no naltrexone on board at the time of these extinction tests, the decreases in ethanol seeking indicate a decrease in incentive motivation that likely resulted from the reinforcer devaluation caused by the repeated naltrexone/ethanol pairings during the reinforced sessions. It is unlikely that naltrexone produced a general malaise that interfered with subsequent reinforcer seeking, since no decrement in responding was observed in the sucrose-reinforced subjects. Moreover, the use of the sucrose control groups showed that while naltrexone’s effect on the consumption of a reinforcing substance was not specific to ethanol, the decrease in ethanol seeking did not generalize to a highly palatable calorie source, suggesting that the seeking attenuation in nondependent animals may be specific to the psychoactive reinforcing properties of ethanol and possibly related to the phenomenon of craving. This finding is in agreement with the clinical observation that naltrexone seems to be most efficacious in patients that continue to drink some ethanol (Killeen et al. 2004), since some ethanol must be consumed in order to experience that it is less reinforcing with naltrexone on board and then for this experience to subsequently affect ethanol-motivated behavior (Heinälä et al. 2001). In rats, the intake suppression produced by naltrexone over extended drug treatment and following termination of treatment was best accounted for by a learned association of the decreased reinforcing properties of consumed ethanol (Stromberg et al. 1998). The present findings in nondependent rats suggest that naltrexone treatment may be effective for decreasing the motivation to seek ethanol in “binge-drinking” individuals without disrupting motivational processes in general. Moreover, the effects on continued ethanol seeking did not require naltrexone on board, indicating that this reinforcer blocking type of mechanism is perhaps long lasting and again consistent with the follow-up study of patients in the COMBINE Study (Donovan et al. 2008).
In rats, naltrexone has been shown to suppress ethanol intake and ethanol-induced increases in dopamine in the nucleus accumbens during ethanol self-administration (Gonzales and Weiss 1998). Recent evidence extends this finding to show that naltrexone also blocks ethanol-induced increases in tyrosine hydroxylase mRNA levels in the ventral tegmental area (Lee et al. 2005), leading to the conclusion that naltrexone affects the reinforcing properties of ethanol by interfering with ethanol-induced stimulation of the mesolimbic dopaminergic pathway. In humans, naltrexone has been shown to decrease some of the positive reinforcing properties of ethanol on the ascending limb of the blood alcohol curve, such as simulation, vigor, “high”, and positive mood (Ray and Hutchison 2007). Acamprosate has also been shown to affect the dopaminergic system in rats, producing increases in dopamine transporter density and decreases in dopamine D2-like receptor density in the nucleus accumbens, but tolerance develops quickly (within 3 days) to this effect (Cowen et al. 2005). In addition, the combination of naltrexone plus acamprosate treatment showed no evidence of either additive or synergistic effects on ethanol-reinforced responding, suggesting that the two drugs are not working by a common mechanism (Stromberg et al. 2001).
Our previous study using a similar operant model, nondependent rats, and 2 days of acamprosate treatment at the same doses used in this study (Czachowski et al. 2001b) found that all doses decreased ethanol intake relative to saline after 2 days, again supporting the notion of “reinforcer blocking” or that ethanol and acamprosate needed to be experienced together to be effective. In that study, there were no effects on sucrose seeking or intake and no effects of acamprosate on any measure of ethanol seeking at doses that did not cause weight-related side effects. Overall, the short-term study suggested selective effects of acamprosate on ethanol intake, while the present study shows nonselective effects with extended treatment. Also, the low doses of acamprosate were effective at attenuating ethanol drinking early in treatment (first 9 days), but when examined over the whole 14-day treatment in this study, effectiveness was limited to the high dose that also had adverse side effects. We also observed these gastrointestinal problems/weight loss in our previous study after a single injection of 400 mg/kg, and Bowers et al. (2007) reported a similar dose-dependent weight loss over 100- to 500-mg/kg doses, with the high dose reaching significant loss by day 3 and causing one fatality when administered only once daily, with deficits in locomotor activity at 300 and 500 mg/kg.
One rationale for assessing acamprosate in ethanol-dependent subjects was based on findings showing that dependent (LeMagnen et al. 1987) or high-drinking (Boismare et al. 1984) subjects showed greater responsiveness to acamprosate than non-selected nondependent animals (Heyser et al. 1998). Indeed, there was one indication that acamprosate specifically affected ethanol seeking in dependent animals in the present study which was the increase in latency to first response, which was not seen in the sucrose-reinforced subjects and therefore not simple motor impairment. Total responding, however, was affected only in the sucrose-reinforced subjects, suggesting that even at moderate doses, acamprosate may have unwanted side effects. One hypothesis as to the process by which acamprosate decreases ethanol intake is that it inhibits the negative reinforcing properties of ethanol (Littleton 1995; Spanagel and Zieglgansberger 1997); however, the present paradigm tested animals after a withdrawal recovery period, so they were not exposed to the negative reinforcing (i.e., withdrawal-attenuating) properties of ethanol.
In ethanol-dependent rats, a moderate dose of naltrexone decreased ethanol consumption and decreased sucrose intake to a lesser but significant degree. The sustained “devaluation” of ethanol that resulted from multiple naltrexone/ethanol pairings and was evidenced by an attenuation of ethanol seeking in the nondependent rats, however, did not occur following dependence induction (Fig. 6). When tested with naltrexone on board (not previously tested in the nondependent rats), both ethanol and sucrose seeking were significantly decreased. When tested after experience with naltrexone/reinforcer pairings but with no naltrexone on board (similar to the nondependent animals), only sucrose seeking remained attenuated, while ethanol seeking had returned to baseline levels. It should be noted that the effectiveness of naltrexone in nondependent versus dependent animals may reflect differences in the number of naltrexone/ethanol pairings in each experiment which were greater in the nondependent animals (14) versus the dependent animals (4). Alternatively, naltrexone’s effectiveness in the nondependent versus dependent animals may be a result of differences in opiate system function resulting from the repeated dependence/withdrawal cycles. More specifically, recent findings from Walker and Koob (2008) also show that naltrexone produced greater attenuation of ethanol-reinforced responding in nondependent rats as compared to dependent rats and that dysregulation of the κ-opioid system versus the μ-opioid system is primarily involved in dependence-induced ethanol drinking.
Interestingly, the ethanol dependence induction used in the present experiments did not result in an increase in “binge-like” ethanol drinking in the operant chamber (sucrose drinking was also unaffected). It should be noted that animals were tested after a week-long recovery period, so they were not in withdrawal at the time of the operant sessions. Ciccocioppo et al. (2003) similarly observed that repeated ethanol vapor exposure did not augment ethanol cue-induced reinstatement responding and also attributed this to the absence of exposure to ethanol self-administration during the alleviation of withdrawal. Studies that report an increase in intake have used animals tested 6–12 h into withdrawal and are thus assessing the tendency to self-administer ethanol to avoid or decrease withdrawal symptoms (Roberts et al. 1996; Walker and Koob 2007). From a clinical perspective, those approaches would be examining patients with experience of drinking for negative reinforcement, that is, to alleviate ethanol withdrawal, while the present approach more closely models individuals with prolonged abstinence preceding relapse. In terms of “success” of the two pharmacotherapies, it should be noted that treatment-seeking individuals are not modeled by either the binge-drinking or ethanol-dependent animals assessed in the present experiments. Therefore, lack of specificity of the treatments for ethanol reinforcement may be of less concern when choosing a drug treatment. That is, motivated, treatment-seeking individuals may still greatly benefit from a pharmacotherapy that decreases the reinforcing properties of ethanol in order to overcome craving and, in conjunction with other forms of therapy, may be the best approach for controlling the excessive use of alcohol.
Acknowledgments
This work was supported by grant AA013860 to CLC from the National Institute on Alcohol Abuse and Alcoholism. Acamprosate was generously provided by Forest Research Institute, Jersey City, NJ. Dr. Hank Samson provided insight and discussion essential for the development of the behavioral paradigm.
Contributor Information
Cristine L. Czachowski, Email: cczachow@iupui.edu, Center for Alcohol and Addiction Studies, Brown University, Providence, RI 02912, USA. Departments of Psychology and Psychiatry, Institute of Psychiatric Research, Indiana University School of Medicine, 791 Union Drive, Indianapolis, IN 46202, USA
Michael J. DeLory, Center for Alcohol and Addiction Studies, Brown University, Providence, RI 02912, USA
References
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE Study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) Study. Arch Gen Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachteler D, Economidou D, Danysz W, Ciccocioppo R, Spanagel R. The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacology. 2005;30:1104–1110. doi: 10.1038/sj.npp.1300657. [DOI] [PubMed] [Google Scholar]
- Baros AM, Latham PK, Moak DH, Voronin K, Anton RF. What role does measuring medication compliance play in evaluating the efficacy of naltrexone? Alcohol Clin Exp Res. 2007;31:596–603. doi: 10.1111/j.1530-0277.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- Boismare F, Daoust M, Moore N, Saligaut C, Lhuintre JP, Chretien P, Durlach J. A homotaurine derivative reduces the voluntary intake of ethanol by rats: are cerebral GABA receptors involved? Pharmacol Biochem Behav. 1984;21:787–789. doi: 10.1016/s0091-3057(84)80020-9. [DOI] [PubMed] [Google Scholar]
- Bouza C, Angeles M, Muñoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Chou JK, Osborne MP, Gass JT, See RE, Bonci A, Janak PH, Olive MF. Acamprosate attenuates cocaine- and cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2007;195:397–406. doi: 10.1007/s00213-007-0904-y. [DOI] [PubMed] [Google Scholar]
- Chick J, Howlett H, Morgan MY, Ritson B. United Kingdom multicentre acamprosate study (UKMAS): a 6-month prospective study of acamprosate versus placebo in preventing relapse after withdrawal from alcohol. Alcohol Alcohol. 2000;35:176–187. doi: 10.1093/alcalc/35.2.176. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology. 2003;168:208–215. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Adams C, Kraehenbuehl T, Vengeliene V, Lawrence AJ. The acute anti-craving effect of acamprosate in alcohol-preferring rats is associated with modulation of the mesolimbic dopamine system. Addict Biol. 2005;10:233–242. doi: 10.1080/13556210500223132. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Breakpoint determination and ethanol self-administration using a discrete session progressive ratio procedure in the rat. Alcohol Clin Exp Res. 1999;23:1580–1586. [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. The effects of raclopride in the nucleus accumbens on ethanol-seeking and consumption. Alcohol Clin Exp Res. 2001a;25:1431–1440. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Samson HH. The effects of acamprosate on ethanol-seeking and self-administration in the rat. Alcohol Clin Exp Res. 2001b;25:344–350. [PubMed] [Google Scholar]
- Czachowski CL, Santini LA, Legg BH, Samson HH. Separate measures of ethanol seeking and drinking in the rat: effects of remoxipride. Alcohol. 2002;28:39–46. doi: 10.1016/s0741-8329(02)00236-7. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Anton RF, Miller WR, Longabaugh R, Hosking JD, Youngblood M COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence (The COMBINE Study): examination of posttreatment drinking outcomes. J Stud Alcohol Drugs. 2008;69:5–13. doi: 10.15288/jsad.2008.69.5. [DOI] [PubMed] [Google Scholar]
- Ferraro FM, 3rd, Hill KG, Kaczmarek HJ, Coonfield DL, Kiefer SW. Naltrexone modifies the palatability of basic tastes and alcohol in outbred male rats. Alcohol. 2002;27:107–114. doi: 10.1016/s0741-8329(02)00220-3. [DOI] [PubMed] [Google Scholar]
- Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007;1155:172–178. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Heinälä P, Alho H, Kiianmaa K, Lönnqvist J, Kuoppasalmi K, Sinclair JD. Targeted use of naltrexone without prior detoxification in the treatment of alcohol dependence: a factorial double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2001;21:287–292. doi: 10.1097/00004714-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Durbin P, Koob GF. Chronic acamprosate eliminates the alcohol deprivation effect while having limited effects on baseline responding for ethanol in rats. Neuropsychopharmacology. 1998;18:125–133. doi: 10.1016/S0893-133X(97)00130-9. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Moc K, Koob GF. Effects of naltrexone alone and in combination with acamprosate on the alcohol deprivation effect in rats. Neuropsychopharmacology. 2003;28:1463–1471. doi: 10.1038/sj.npp.1300175. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Cox AA, Bratt AM, Camarini R, Iller K, Kelley SP, Mehmert KK, Nannini MA, Olive MF. The discriminative stimulus properties of self-administered ethanol are mediated by GABA(A) and NMDA receptors in rats. Psychopharmacology. 2001;154:13–22. doi: 10.1007/s002130000619. [DOI] [PubMed] [Google Scholar]
- Hölter SM, Landgraf R, Zieglgänsberger W, Spanagel R. Time course of acamprosate action on operant ethanol self-administration after ethanol deprivation. Alcohol Clin Exp Res. 1997;21:862–868. [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen TK, Brady KT, Gold PB, Simpson KN, Faldowski RA, Tyson C, Anton RF. Effectiveness of naltrexone in a community treatment program. Alcohol Clin Exp Res. 2004;28:1710–1717. doi: 10.1097/01.alc.0000145688.30448.2c. [DOI] [PubMed] [Google Scholar]
- Lee YK, Park SW, Kim YK, Kim DJ, Jeong J, Myrick H, Kim YH. Effects of naltrexone on the ethanol-induced changes in the rat central dopaminergic system. Alcohol Alcohol. 2005;40:297–301. doi: 10.1093/alcalc/agh163. [DOI] [PubMed] [Google Scholar]
- LeMagnen J, Tran G, Durlach J, Martin C. Dose-dependent suppression of the high alcohol intake of chronically intoxicated rats by Ca-acetyl homotaurinate. Alcohol. 1987;4:97–102. doi: 10.1016/0741-8329(87)90005-x. [DOI] [PubMed] [Google Scholar]
- Littleton JM. Acamprosate in alcohol dependence: how does it work. Addiction. 1995;90:1179–1188. doi: 10.1046/j.1360-0443.1995.90911793.x. [DOI] [PubMed] [Google Scholar]
- Macenski MJ, Shelton KL. Self-administered ethanol as a discriminative stimulus in rats. Drug Alcohol Depend. 2001;64:243–247. doi: 10.1016/s0376-8716(00)00238-6. [DOI] [PubMed] [Google Scholar]
- McGeary JE, Monti PM, Rohsenow DJ, Tidey J, Swift R, Miranda R., Jr Genetic moderators of naltrexone’s effects on alcohol cue reactivity. Alcohol Clin Exp Res. 2006;30:1288–1296. doi: 10.1111/j.1530-0277.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- Morley KC, Teesson M, Reid SC, Sannibale C, Thomson C, Phung N, Weltman M, Bell JR, Richardson K, Haber PS. Naltrexone versus acamprosate in the treatment of alcohol dependence: a multi-centre, randomized, double-blind, placebo-controlled trial. Addiction. 2006;101:1451–1462. doi: 10.1111/j.1360-0443.2006.01555.x. [DOI] [PubMed] [Google Scholar]
- Naassila M, Legrand E, d’Alche-Biree F, Daoust M. Cyamemazine decreases ethanol intake in rats and convulsions during ethanol withdrawal syndrome in mice. Psychopharmacology. 1998;140:421–428. doi: 10.1007/s002130050785. [DOI] [PubMed] [Google Scholar]
- Nava F, Premi S, Manzato E, Lucchini A. Comparing treatments of alcoholism on craving and biochemical measures of alcohol consumption. J Psychoactive Drugs. 2006;38:211–217. doi: 10.1080/02791072.2006.10399846. [DOI] [PubMed] [Google Scholar]
- NIH. NIH guide. 28. Vol. 25. National Academy Press; Washington, DC: 1996. [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Robin RW, Levenson AL, GreyWolf I, Chance LE, Hodgkinson CA, Romano D, Robinson J, Meandzija B, Stillner V, Wu R, Goldman D. Naltrexone alone and with sertraline for the treatment of alcohol dependence in Alaska natives and non-natives residing in rural settings: a randomized controlled trial. Alcohol Clin Exp Res. 2008;32:1271–1283. doi: 10.1111/j.1530-0277.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paille F, Guelfi JD, Perkins AC, Royer RJ, Steru L, Parot P. Double-blind randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol Alcohol. 1995;30:239–247. [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64:1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Richardson K, Baillie A, Reid S, Morley K, Teesson M, Sannibale C, Weltman M, Haber P. Do acamprosate or naltrexone have an effect on daily drinking by reducing craving for alcohol? Addiction. 2008;103:953–959. doi: 10.1111/j.1360-0443.2008.02215.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology. 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, Slawecki CJ. A new assessment of the ability of oral ethanol to function as a reinforcing stimulus. Alcohol Clin Exp Res. 2000;24:766–773. [PubMed] [Google Scholar]
- Samson HH, Chappell A, Czachowski C, Sharpe A. Measuring ethanol-seeking behavior: the effect of using repeated extinction trials. Alcohol. 2001;24:205–209. doi: 10.1016/s0741-8329(01)00157-4. [DOI] [PubMed] [Google Scholar]
- Samson HH, Cunningham CL, Czachowski CL, Chappell A, Legg B, Shannon E. Devaluation of ethanol reinforcement. Alcohol. 2004;32:203–212. doi: 10.1016/j.alcohol.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Samson HH. Effect of naloxone on appetitive and consummatory phases of ethanol self-administration. Alcohol Clin Exp Res. 2001;25:1006–1011. [PubMed] [Google Scholar]
- Spanagel R, Zieglgansberger W. Anti-craving compounds for ethanol: new pharmacological tools to study addictive processes. Trends Pharmacol Sci. 1997;18:54–59. [PubMed] [Google Scholar]
- Stromberg MF. The effect of baclofen alone and in combination with naltrexone on ethanol consumption in the rat. Pharmacol Biochem Behav. 2004;78:743–750. doi: 10.1016/j.pbb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Volpicelli JR, O’Brien CP. Effects of naltrexone administered repeatedly across 30 or 60 days on ethanol consumption using a limited access procedure in the rat. Alcohol Clin Exp Res. 1998;22:2186–2191. [PubMed] [Google Scholar]
- Stromberg MF, Mackler SA, Volpicelli JR, O’Brien CP. Effect of acamprosate and naltrexone, alone or in combination, on ethanol consumption. Alcohol. 2001;23:109–116. doi: 10.1016/s0741-8329(00)00137-3. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Sengpiel T, Mackler SA, Volpicelli JR, O’Brien CP, Vogel WH. Effect of naltrexone on oral consumption of concurrently available ethanol and cocaine in the rat. Alcohol. 2002a;28:169–179. doi: 10.1016/s0741-8329(02)00280-x. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Rukstalis MR, Mackler SA, Volpicelli JR, O’Brien CP. A comparison of the effects of 6-beta naltrexol and naltrexone on the consumption of ethanol or sucrose using a limited-access procedure in rats. Pharmacol Biochem Behav. 2002b;72:483–490. doi: 10.1016/s0091-3057(02)00721-9. [DOI] [PubMed] [Google Scholar]
- Tempesta E, Janiri L, Bignamini A, Chabac S, Potgieter A. Acamprosate and relapse prevention in the treatment of alcohol dependence: a placebo-controlled study. Alcohol Alcohol. 2000;35:202–209. doi: 10.1093/alcalc/35.2.202. [DOI] [PubMed] [Google Scholar]
- Ulm RR, Volpicelli JR, Volpicelli LA. Opiates and alcohol self-administration in animals. J Clin Psychiatry. 1995;56(Supple 7):5–14. [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:1505–1515. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]