Abstract
Fish constitute an excellent model to understand the mechanistic aspects of metal toxicity vis-à-vis oxidative stress in aquatic ecosystems. Hexavalent chromium (Cr (VI)), due to its redox potential can induce oxidative stress (OS) in fish and impair their health. In the present investigation, we hypothesize that OS plays a key role in chromium induced toxicity in goldfish; leading to the production of reactive oxygen species (ROS) such as O· 2, H2O2, OH·, and subsequent modulation of the activities of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), metallothioneins (MT), glutathione proxidase (GPx), genotoxicity and histopathology. To test this hypothesis, antioxidant enzymes, DNA damage and histopathology assays were performed in liver and kidney tissues of goldfish exposed to different concentrations of Cr (VI) (LC12.5, LC25 and LC50) following 96h static renewal bioassay. The results of this study clearly show that the fish experienced OS as characterized by significant modulation of enzyme activities, induction of DNA damage and microscopic morphological changes in the liver and kidney. In both tissues, CAT activity was decreased whereas SOD activity and hydroperoxide levels were increased. In addition, GPx activity also increased significantly in higher test concentrations, especially in the kidney. MT induction and DNA damage were observed in both tissues in a concentration dependent manner. Microscopic examination of organ morphology indicated degeneration of liver tissue and necrosis of central vein. Necrosis of kidney tubular epithelial cells and tubules was observed at higher Cr (VI) concentrations. Taking together the findings of this study are helpful in organ-specific risk assessment of Cr (VI)-induced oxidative stress, genotoxicity and histopathology in fish.
Keywords: Hexavalent chromium, acute exposure, goldfish, oxidative stress, genotoxicity, histopathology
1. Introduction
Heavy metals constitute a core group of aquatic pollutants due to their bioacuumulative and non-biodegradable properties. Their excessive contamination of aquatic ecosystems has evoked major environmental and health concerns worldwide [1]. Chromium is the sixth most abundant heavy metal in the earth crust [2]. While this metal exhibits various oxidation states, the trivalent and hexavalent states are predominant in the environment [3]. Cr (VI) is a strong oxidizing agent and able to form chromate and dichromate ions in the environment [4]. In the United States, chromate ore is used for the production of thousands of tons of products every year in metallurgical, chemical and refractory industries [5]. Inadequate treatment of effluents from these industries often leads to the large-scale contamination of water resources by chromium [2]. Eventually, the metal exerts a great influence on the survival of fish and other aquatic biota. Several studies on chromium have reported it’s cytotoxic, immunological, hematological, histological and genotoxic effects to fish [6–14].
Fish serve as an excellent model to understand the mechanistic aspects of metal toxicity vis-à-vis oxidative stress in aquatic ecosystems. Heavy metals including chromium, due to their redox potential can induce oxidative stress in fish and impair their health [15, 8]. Fish upon exposure to pollutants elicit the production of reactive oxygen species (ROS) like superoxide anion, hydrogen peroxide and hydroxyl radical [16]. As the ROS levels increase, the biological system develops a first line defense mechanism by modulating the activities of antioxidants such as catalase (CAT), superoxide dismutase (SOD), metallothioneins (MT) and glutathione related enzymes [17, 18].
Genotoxic potential of Cr (VI) has been reported from human and rodent studies [19–21]. The assessment of the genotoxicity of metals in terrestrial and aquatic ecosystems has been a major thrust area of current research and there is growing concern to develop methods for detection of genotoxic effects in aquatic animals. Though, many methods like micronucleus test, chromosomal aberrations and DNA damage assays have been used for assessing genotoxicity of various chemicals in different animals [22–27], the DNA damage (comet assay) protocol is known to be simple, sensitive, more reliable and cost effective, and has been used to investigate the genotoxic potential of toxicants in the environment [28–30]. However, scientific data on the genotoxic potential of Cr (VI) in aquatic animals is scarce.
Microscopic examination of target tissues is an important end-point in the evaluation of toxic potential and risk assessment of chemicals in the environment. Histopathology studies of target organs along with the studies of oxidative stress and DNA damage would give the complete risk assessment and toxic potential of Cr (VI) in aquatic animals [24]. Cr (VI) is one of the known core toxicants for induction of morphological changes such as epithelial hyperplasia, and epithelial lifting [17], degeneration of secondary gill lamellae, hyperplasia of lamellar cells and atrophy of central axis in tissues [13]. Much information is not available on the toxic potential of Cr (VI) to aquatic animals at the genetic and histopathological levels. Therefore, in this research, we have investigated the acute toxicity, and evaluated the biomarkers of Cr (VI)-induced oxidative stress, genotoxicity and histopathology in liver and kidney tissues of goldfish, Carassius auratus. An attempt has been made to unravel the relationships between oxidative stress, DNA damage and histopathology resulting from Cr (VI) exposure.
2. Materials and methods
2. 1. Experimental animals
Carassius auratus, commonly known as goldfish, was selected as an experimental animal model. The fish (average length 7.4±0.54cm and average weight 9.2±0.85 g) were purchased from a commercial store in Jackson, Mississippi, and acclimatized to the laboratory conditions for 15 days. The fish were placed in glass aquaria with tap water (pH 7.2±0.6, temperature 28.2±0.5°C, dissolved oxygen 3.65mg /L, conductivity 222µs/ cm, total suspended solids (TSS) 105.6 mg/L and salinity 0%). No mortality was observed during the acclimatization. The fish were fed with aquarium flake food twice a day and 12hr light and 12hr dark photoperiod was maintained during the acclimatization.
2.2. Chemicals and reagents
Sodium dichromate, ethanol, xylene, 10% formalin, Tris borate electrophoresis buffer, chloroform, formaldehyde, mannitol, ethylene glycol tetra acetic acid (EGTA), dimethyl sulfoxide, methanol, protease inhibitor cocktail, sucrose, 5-5 dithiobis (2-nitrobenzoic acid) (DTNB), reduced glutathione, ethyl di-amine tetra acetic acid (EDTA), 2-Phenoxyethanol and human albumin were purchased from Sigma-Aldrich (St Louis, MO, USA). 4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid (HEPES) buffer, potassium phosphate, Tris-HCl, catalase assay kit, superoxide dismutase assay kit, lipid hydro-peroxides assay kit and glutathione peroxidase assay kits were purchased from Calbiochem (La Jolla, CA, USA). Phosphate buffered saline (PBS) and Comet assay kit were purchased from Trevigen Inc, (Gaithersburg, MD, USA). Heparin, hematoxylin and eosin Y were purchased from Fisher scientific (Suwanee, GA, USA).
2.3. Experimental design
From a previous static renewal bioassay, the 96h-LC50 (lethal concentration inducing 50% mortality of goldfish) was found to be 85.7±0.4 mg/L [31]. Subsequently, the acclimatized fish were randomly separated into adequate number of groups and exposed to different concentrations of Cr (VI) including control, LC12.5, LC25 and LC50 i.e. 0 mg/L, 21.42 mg/L, 42.85 mg/L and 85.7 mg/L, respectively. During exposure, feeding was stopped and photoperiod was maintained with 12h light and 12h dark. The aqueous chemical solutions in the aquaria were renewed for every 24h with the respective concentrations of toxicant. After the 96h exposure, the fish were anesthetized with 0.1% 2-phenoxyethanol and sacrificed for biochemical analysis. The dissected tissues were stored at −80°C for further analysis. Liver and kidney of 3 fish from each treatment (control, LC12.5, LC25 and LC50) were used to analyze each of the studied parameters. Three sample replicates were made from each fish liver and kidney. Hence, a total of nine samples were analyzed for each parameter.
2.4. Catalase (CAT) assay
Dissected tissues were rinsed with phosphate buffer (pH 7.4) to remove red blood cells, and homogenized in cold buffer (1:8 w/v) containing 50 mM potassium phosphate, pH 7.0 and 1 mM ethylene diamine tetra acetic acid (EDTA) per gram of tissue. The supernatant from each tissue was used as the enzyme source and analyzed according to the method described by Johansson and Borg [32] with few modifications. Briefly, three replicates of 20 µl samples were mixed with 100 µl of 100 mM potassium phosphate, pH 7.0 and 30 µl methanol. The reaction was initiated with 20 µl of 35mM hydrogen peroxide and the mixture was incubated on shaker at room temperature for 20 min. The reaction was terminated by adding 30 µl of 10 M potassium hydroxide. Thirty µl of 4-amino-3-hydrazino-5-mercapto-1, 2, 4-triazole (chromogen) was added to the three replicates of each sample, and incubated on a shaker for 10 min at room temperature. After incubation, 10 µl of potassium periodate was added to the mixture and incubated for 5 min at room temperature. Finally, the reaction mixture’s absorbance was recorded at 540nm using 96 plate reader (Multiskan Ascent, Lab systems USA). The principle of this assay is based on the reaction of the CAT with methanol in the presence of an optimal concentration of hydrogen peroxide, and the measurement of formaldehyde produced. One unit of CAT is defined as the amount of CAT that will cause the formation of 1.0nmol formaldehyde per min at 25°C. Reference standard curve was prepared with formaldehyde solution.
2.5. Superoxide dismutase (SOD) assay
The tissues were rinsed with phosphate buffer (pH 7.4) containing 0.16 mg/ml heparin, to remove any red blood cells. The tissues were homogenized (1:8, w/v) in cold 20mM HEPES buffer, pH 7.2, containing 1mM EGTA, 210 mM mannitol, and 70 mM sucrose per gram of tissue. Each tissue homogenate was centrifuged at 1500× g for 5 min at 4°C (Beckman XL-100K, USA). The supernatants were transferred to a 96 well plate, and the activity of SOD was analyzed according to a previously published method [33, 34], with few modifications. Briefly, three replicates of 10 µl of each sample were mixed with 200 µl of radical detector. Radical detector was prepared with 50 µl of tetrazolium salt solution and 19.95 ml of 50 mM Tris-HCl (pH 8.0, containing 0.1 mM diethylene triamine penta acetic acid (DTPA) and 0.1 mM hypoxanthine). The reaction was initiated by adding 20 µl of xanthine oxidase and the reaction mixture was incubated on a shaker for 20 min at room temperature. The xanthine oxidase was prepared with 50 µl of xanthine oxidase solution and 1.95 ml of 50 mM Tris-HCl, pH 8.0. After 20 min of incubation, the reaction mixture’s absorbance was recorded at 450 nm using 96 wells plate reader (Multiskan Ascent, Lab systems USA). The assay principle is based on the utilization of a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical. The standard reference curve was prepared with solution of bovine erythrocyte SOD.
2.6. Lipid peroxidation (LPO) assay
Lipid hydroperoxides in liver and kidney were estimated according to the method described by Mihaljevic [35] with few modifications. Briefly, the tissues were homogenized (1:8, w/v) in cold HPLC-grade water. Five hundred µl of the each tissue homogenate was taken in a glass test tube and equal volume of Calbiochem supplied Extract R saturated methanol was added. The mixture was vortexed for few minutes and 1 ml of cold deoxygenated chloroform was added, and vortexed thoroughly. The mixture was centrifuged at 1500× g for 5 min at 0°C and the bottom chloroform layer was collected. Five hundred µl of the bottom chloroform was mixed with 450 µl of chloroform: methanol (2:1) mixture and 50µl of Calbiochem supplied chromogen (thiocyanate ion). Then the mixture was incubated for 5 min and the absorbance of each sample was recorded at 500 nm wavelength using spectrophotometer (2800 Unico spectrophotometer USA). This method directly measures the lipid hydro-peroxides utilizing redox reactions with ferrous ions. The produced lipid hydro peroxides are highly unstable and react readily with ferrous ions to produce ferric ions that are detected using thiocyanate ion as chromogen. Calbiochem supplied lipid hydro peroxide solution were used as reference standards.
2.7. Glutathione peroxidase (GPx) assay
GPx activity was analyzed according to the method described by Paglia and Valentine [36], with few modifications. Briefly, the tissues were perfused with phosphate buffer saline (PBS), pH 7.4, and containing 0.16 mg/ml heparin, to remove red blood cells. The tissues were homogenized (1:8, w/v) in cold buffer containing 50 mM Tris-HCl, pH 7.5, 5 mM EDTA and 1 mM DTT per gram tissue. The homogenates were centrifuged at 1500× g for 15 min at 4°C and each tissue supernatant was separated (Beckman XL-100K, USA). Three replicates of 20 µl of each sample were mixed with 100 µl of 50 mM Tris-HCl, pH 7.6, containing 5 mM EDTA and 50 µl of co-substrate mixture (Calbiochem supplied NADPH, glutathione, and glutathione reductase). The reaction was initiated by adding 20 µl of cumene hydroperoxide solution. The reactions mixture was vortexed properly, and the absorbance was recorded at 340nm wavelength (Multiskan Ascent, Lab systems USA). The assay indirectly measures the activity of GPx by a coupled reaction with glutathione reductase. One unit of GPx is defined as the amount of enzyme that will cause the oxidation of 1.0 nmol of NADPH to NADP+ per minute at 25°C.
2.8. Metallothionein (MT) quantification
Liver and kidney of goldfish were dissected and MT was quantified as described previously [37]. Briefly, the tissues were homogenized separately (1:8, w/v) in a buffer containing 20 mM Tris-HCl, pH 8.6, 0.01% β-mercaptoethanol, protease inhibitor cocktail and 0.5 M sucrose. The homogenates were centrifuged at 30000× g for 20 min (Beckman XL-100K, USA); and the supernatants were collected and resuspended in 49% ethanol containing 3.7% chloroform. The mixture was centrifuged at 30000× g for 20 min and again resuspended in 49% ethanol containing 3.7% chloroform, and centrifuged at 6000× g for 10 min. The supernatant was collected, acidified cold 87% ethanol was added and the mixture was incubated at −20°C for 1 hr. The mixture was centrifuged at 6000× g for 10 min, the pellet was resuspended in 20 mM Tris-HCl buffer, pH 8.6, containing 87% ethanol and 1% chloroform, and centrifuged at 6000× g for 10 min. The pellet was allowed to dry under a nitrogen gas stream and resuspended in a solution containing 0.16 M NaCl, 0.5N HCl, and 2 mM EDTA. The resuspended 0.3 ml aliquots were mixed with 4.2 ml of 0.43mM DTNB (5-5 dithiobis (2-nitrobenzoic acid)). The DTNB was prepared in 0.2 M phosphate buffer, pH 8.0, supplemented with 2M NaCl. The mixture was centrifuged at 3000× g for 5 min at room temperature and the absorbance of the supernatant was measured at 412 nm using reduced glutathione as reference standard.
2.9. Total Protein quantification
Liver and kidney tissues were dissected and homogenized with phosphate buffer (1:8, w/v). Protein concentration was measured according to the Bradford method [38] using bovine serum albumin as reference standard.
2.10. Comet assay
Comet assay was performed according to the protocol that had been previously described by Singh et al [30] and modified by Patlolla et al [21]. Liver and kidney tissues were dissected, chopped in to large pieces and placed in 1 ml of ice cold phosphate buffer saline (PBS) (with out Ca++ and Mg ++) to remove red blood cells. The tissues were minced with scissors into small pieces. The chopped tissues were placed in 1 ml of PBS containing 20 mM EDTA. The mixture was incubated for 5 min, and the cell suspension was transferred into another tube by avoiding debris. The number of cells in the cell suspension were counted using hemocytometer and pelleted at 4°C. The pellet was suspended in 1 ml of ice cold PBS at 1 × 105 cells/ml. Molten agarose was prepared in a boiling water bath, cooled down to 37°C and mixed with isolated cells in a 1:10 ratio in a eppendorf tube. Seventy-five µl of the mixture of agarose and cells were taken on comet slides. The comet slides were placed in dark for 10 min at 4°C to solidify the gel. After 10 min, the slides were placed in lysis solution containing dimethyl sulfoxide, for 30 min at 4°C. Then the excess solution was removed and the slides were placed in alkaline solution to denature the DNA for 40 min at room temperature. After 40 min, the slides were subjected to electrophoresis in tris borate electrophoresis buffer (TBE) with 1 volt/ cm current between the two electrodes for 10 min. After 10 min of electrophoresis, the slides were fixed with 70% ethanol for 5 min. The slides were stained with syber green and air-dried. Control comet slides were prepared along with the exposed cells comet slides. The whole process was done under yellow light in order to minimize the UV light damage. The processed slides were examined for DNA damage using an epifluorescent microscope (Olympus BX51 TRF, USA). Liver and kidney tissues of 3 fish were analyzed per treatment. In each of 3 fish, a minimum of 75 individual cells per sample were screened, and a total of 225 individual cells (triplicate) were examined per organ. The data were analyzed using a DELL computer equipped with a DNA damage analysis software (Loats Associates Inc., USA).
2.11. Histopathology
Liver and kidney tissues of control and Cr(VI)-treated fish were processed and evaluated according the method described by Kerem et al [40], with few modifications. Dissected tissues were washed in ice cold 0.9% sodium chloride solution, and subsequently fixed in 10% formalin solution for 48 hr. After 48 hr, the tissues were transferred into 70% ethanol. After three days, the organs were placed in cassettes and incubated in 50% alcohol for overnight. Dehydration of the organs was accomplished by soaking in increasing alcohol percentages for 5 hr as follows: 70%, 90%, 95%, 95%, and 100% ethanol, xylene and lastly xylene: paraffin mixture (1:1 ratio). The organs were incubated overnight in xylene: paraffin mixture at 65°C in preparation for embedding (Tissue processor, Triangle biomedical sciences USA). On the next day, the organs were embedded in paraffin and sectioned using an ultra microtome (Olympus CUT 4055E, USA) to obtain sections of 5 µm in size. The sectioned tissues were fixed on the microscope slides and air-dried for 24 hr. For each Cr(VI) concentration, three fish were dissected, and triplicate slides were made; resulting in a total of 9 slides per treatment and per organ. The slides were later stained with hematoxylin and counter stained with eosin. The stained tissues were visualized under microscope and evaluated for changes in morphology of Cr (VI) exposed tissues compared to the control.
Morphologies of liver and kidney tissues were evaluated as described by Kerem et al [40] using Axiovert S-100, inverted light microscope (Carl Zeiss Micro Imaging Inc, Thornwood, NY). The liver morphology was scored as follows: 0= normal, 1 = mild cellular disruption in less than 25% of field area, 2 = moderate cellular disruption and hepato cellular vacuolation greater than 50% of field area, 3 = extensive cell disruption, hepato-cellular vacuolation and condensed nuclei (pycknotic) of hepatocytes in greater than 50% of field area, 4 = extensive cell disruption, hepatocellular vacuolation, pycknotic and occasional central vein injury, and 5= extensive cell disruption, multi central vein necrosis and degenerating of liver in more than 50% of field area. The ‘cell disruption’ refers to the degree of central vein damage, hepato-cellular vacuolation, tissue necrosis and number of pycknotic cells in the liver. Histopathology of kidney was evaluated in terms of tubular injury. Tubular injury was defined as tubular dilation, tubular necrosis, renal tubular separation, disintegration of tubules and glomerular necrosis. Glomerular cells and all kinds of tubules damage were included in the scoring system: 0 = no injury, 1= < 10% of injury, 2 = 10–25% of injury, 3 = 26 to 50 % of injury, 4 = 51–75% of injury, and 5 = > 75% of injury.
2.12. Statistical Analysis
The data was analyzed using SAS 9.1 software and expressed as arithmetic mean ± standard deviation. One way ANOVA and Dunnett test were performed to determine if there were significant differences among and between treatment groups. Significant differences were considered at p < 0.05.
3. Results
3.1. Antioxidant enzymes activities
The activity of catalase (CAT), superoxide dismutase (SOD), glutathione proxidase (GPx), lipid peroxidation (LPO), metallothioneins (MT) and total protein levels were determined in liver and kidney homogenates of control and Cr (VI) exposed fish for 96 h. Further, DNA damage and histopathology of liver and kidney tissues were evaluated.
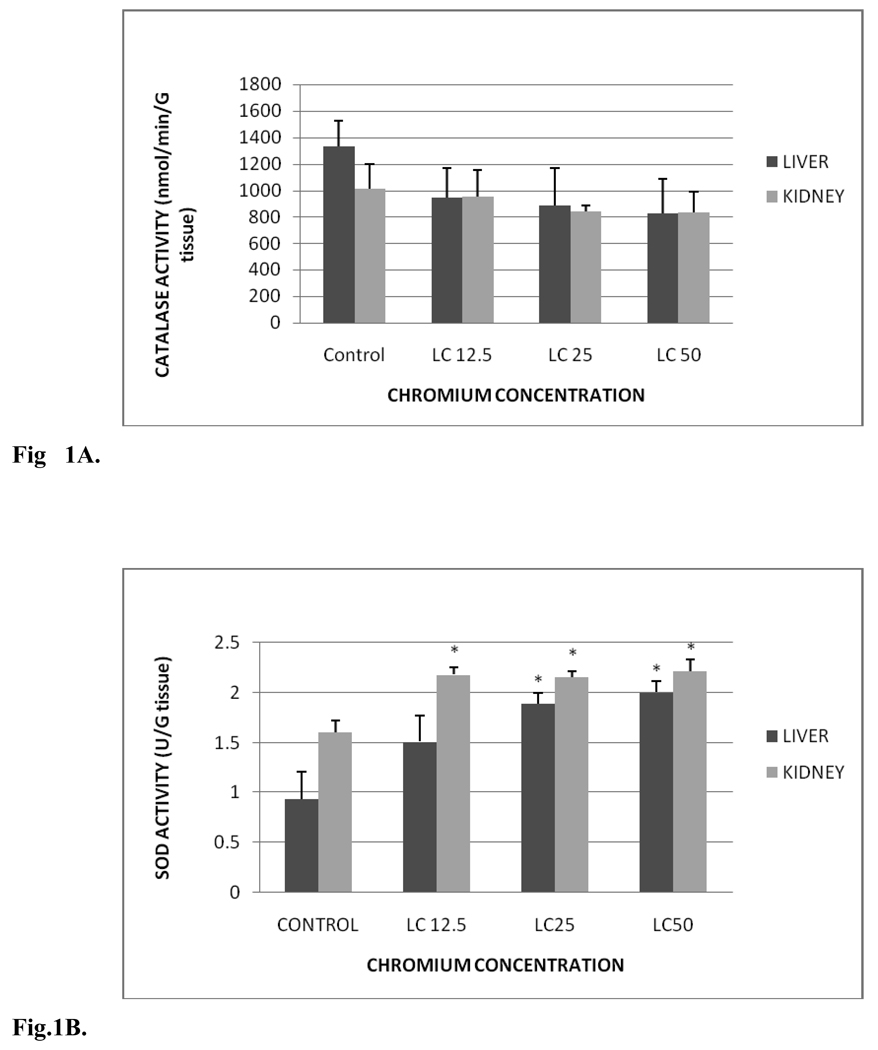
Fig. 1 A summarizes the CAT activity in liver and kidney of control and exposed fish. CAT activity levels in liver were 1,329.03±197.52, 946.71±220.99, 885.01±282.03 and 825.04±262.36 nmol/min/gram tissue for control, LC12.5, LC25 and LC50, respectively. The amounts in kidney were 1012.93±186.18, 950.79±203.80, 839.55±43.35 and 834.2±152.39 nmol/min/gram tissue for control, LC12.5, LC25 and LC50, respectively. No significant differences in CAT activity were observed between control and Cr (VI) treated fish. However, there was slight decrease in the CAT activity of treatment groups compared to the control groups of liver and kidney, and this decrease was concentration-dependent.
Fig. 1.
A. Catalase activity in Carassius auratus liver and kidney exposed to various concentrations of Cr (VI) for 96h. Each point represents a mean value and standard deviation of three replicates.
B. SOD activity in Carassius auratus liver and kidney exposed to various concentrations of Cr (VI) for 96h. Each point represents a mean value and standard deviation of three replicates. *indicates significantly different from the control according to Dunnett’s multiple comparison test.
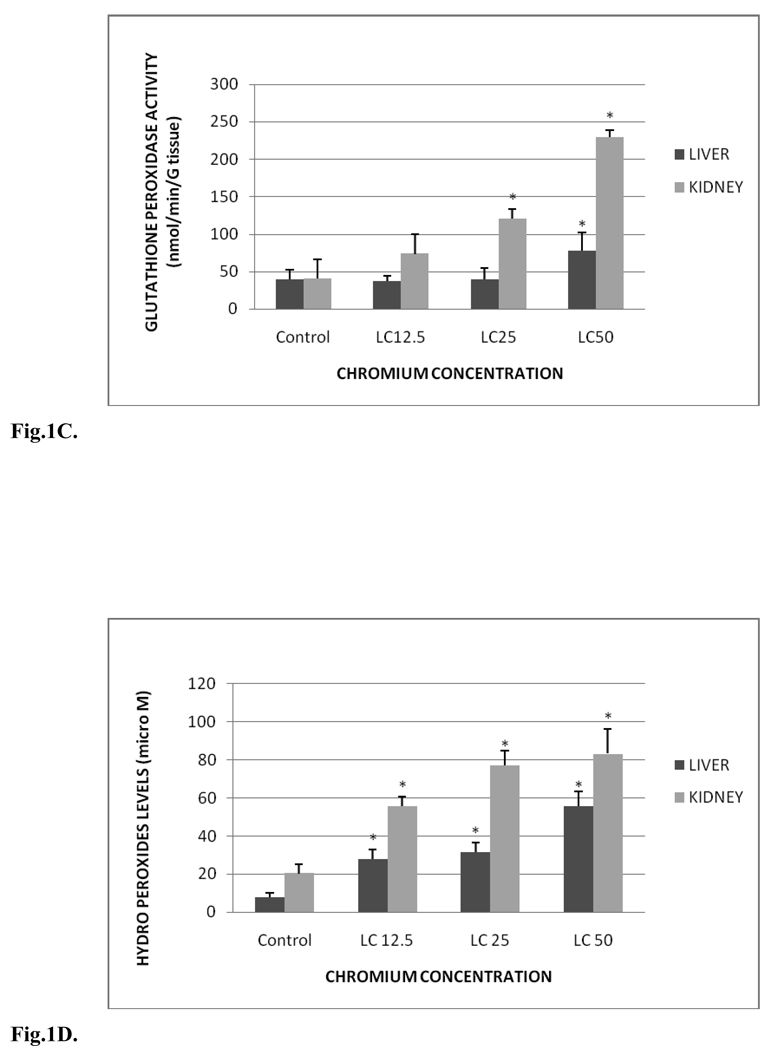
C. Glutathione peroxidase activity in Carassius auratus liver and kidney exposed to various concentrations of Cr (VI) for 96h. Each point represents a mean value and standard deviation of three replicates. *indicates significantly different from the control according to Dunnett’s multiple comparison test.
D. Hydro peroxides levels in Carassius auratus liver and kidney exposed to various concentrations of Cr (VI) for 96h. Each point represents a mean value and standard deviation of three replicates. *indicates significantly different from the control according to Dunnett’s multiple comparison test.
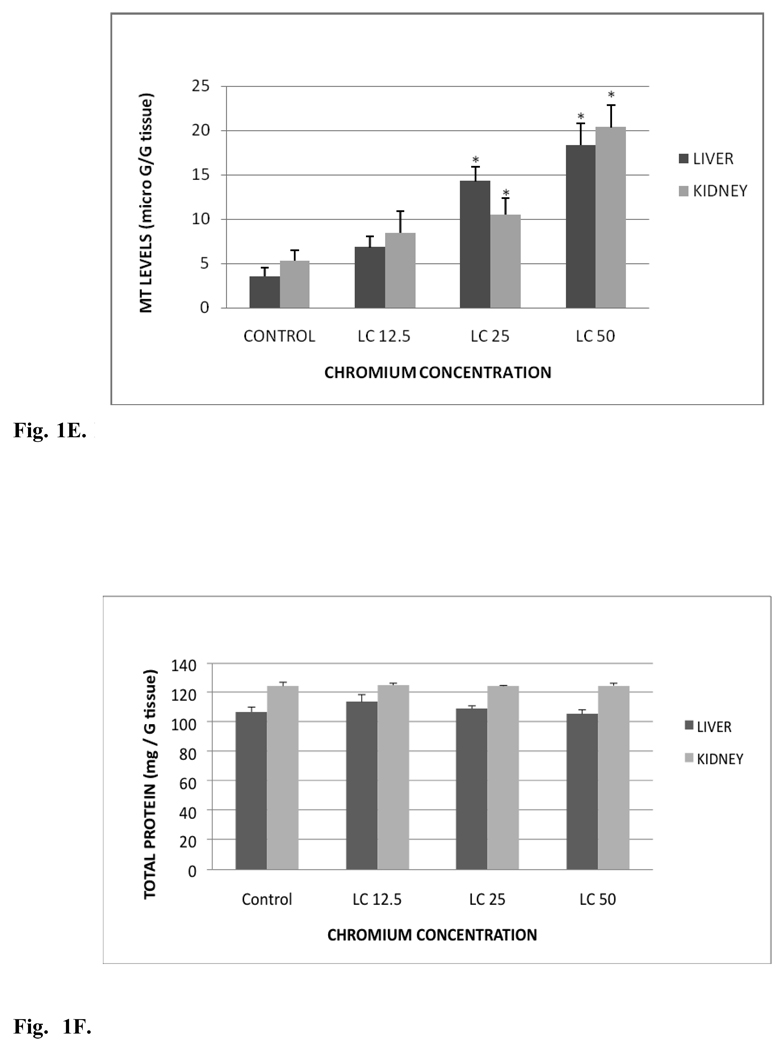
E. MT levels in Carassius auratus liver and kidney exposed to various concentrations of Cr (VI) for 96h. Each point represents a mean value and standard deviation of three replicates. *indicates significantly different from the control according to Dunnett’s multiple comparison test.
F. Total protein levels in Carassius auratus liver and kidney exposed to various concentrations of Cr (VI) for 96h. Each point represents a mean value and standard deviation of three replicates.
The SOD activity in liver and kidney of control and treated groups is presented in Fig.1B. SOD activity levels in liver were 0.93±0.28, 1.51±0.26, 1.89±0.11 and 2.00±0.12 units/gram tissue for control, LC12.5, LC25 and LC50, respectively. The amounts in the kidney tissue were 1.60±0.12, 2.18±0.07, 2.15±0.05 and 2.22±0.12 units/gram tissue for control, LC12.5, LC25 and LC50, respectively. In both the organs a concentration-dependent increase in SOD activity was observed. Significant increases (p < 0.05) in the SOD activity of liver were observed in LC50 and LC25 treatment groups compared to the control. Although increased SOD activity in the liver was demonstrated in fishes under LC12.5 treatment, this increase was insignificant (p > 0.05). On the other hand, the SOD activity in the kidney was significantly increased in all the test concentrations. The SOD activity increase in the liver and kidney was time- and concentration-dependent.
Fig. 1C shows the GPx activity in liver and kidney tissues of control and treated groups. GPx activity levels in liver were 39.30±12.80, 37.15±7.20, 39.53±15.28 and 77.72±24.74 nmol/min/gram tissue for control, LC12.5, LC25 and LC50, respectively. The amounts in kidney were 40.45±26.14, 73.56±26.75, 120.5±13.34 and 229.10±9.63 nmol/min/gram tissue for control, LC12.5, LC25 and LC50, respectively. GPx activities of liver were increased in all the tested concentrations compared to the control group. However, increase in the activity of GPx of liver was significant (p < 0.05) only in the LC50 treatment group compared to the control. In the kidney, the activity was increased significantly (p < 0.05) in both LC25 and LC50 exposed fish groups compared to the control.
3.2. Lipid peroxidation
Lipid hydroperoxide (LHP) levels in liver and kidney tissues of control and treatment groups are presented in Fig. 1D. In the liver, the levels were 7.84±2.14, 27.79±4.87, 31.68±4.9 and 55.55±7.93 µM for control, LC12.5, LC25 and LC50, respectively. The LHP levels in kidney were 20.31±4.84, 55.78±4.7, 77.1±7.92 and 83.25±13.1µM for control, LC12.5, LC25 and LC50, respectively. These levels were significantly (p < 0.05) increased in all treated groups compared to the control fish; showing a concentration-dependent increase of hydro peroxides in both organs.
3.3. Metallothionein content
Metallothionein (MT) profile in liver and kidney tissues of control and exposed fish is presented in Fig. 1E. The MT levels in liver were 3.54±0.98, 6.82±1.20, 14.28±1.58 and 18.32±2.35 µg/g tissue for control, LC12.5, LC25 and LC50, respectively. The MT levels in kidney were 5.32±1.20, 8.42±2.43, 10.53±1.82 and 20.35±2.50 µg/g tissue for control, LC12.5, LC25 and LC50, respectively. MT levels of both organs were significantly increased (p< 0.05) in LC25 and LC50 treatment groups compared to the controls. The increased activity in the LC12.5 treated group was insignificant (p > 0.05). However, the increase in MT levels was concentration-dependent in both the organs
3.4. Total protein content
The proteins levels in liver and kidney tissues of the control and treatment groups are presented in Fig. 1F. Total protein amounts in liver were 107.08±3.29, 113.87±4.95, 109.28±1.95 and 105.68±2.90 mg/g tissue for control, LC12.5, LC25 and LC50, respectively. The total protein levels in kidney were 124.57±2.61, 125.23±1.32, 124.56±0.5 and 124.91±1.62 mg/ g tissue for control, LC12.5, LC25 and LC50, respectively. The proteins levels of liver were elevated in LC12.5, LC25 treatment groups and decreased in LC50 treatment group compared to the control group. In kidney, the protein content was increased in all the treatment groups compared to the control. However, the changes in proteins levels in liver and kidney of treatment group were not significantly different (p> 0.05) from the control fish.
3.5. Genotoxicity
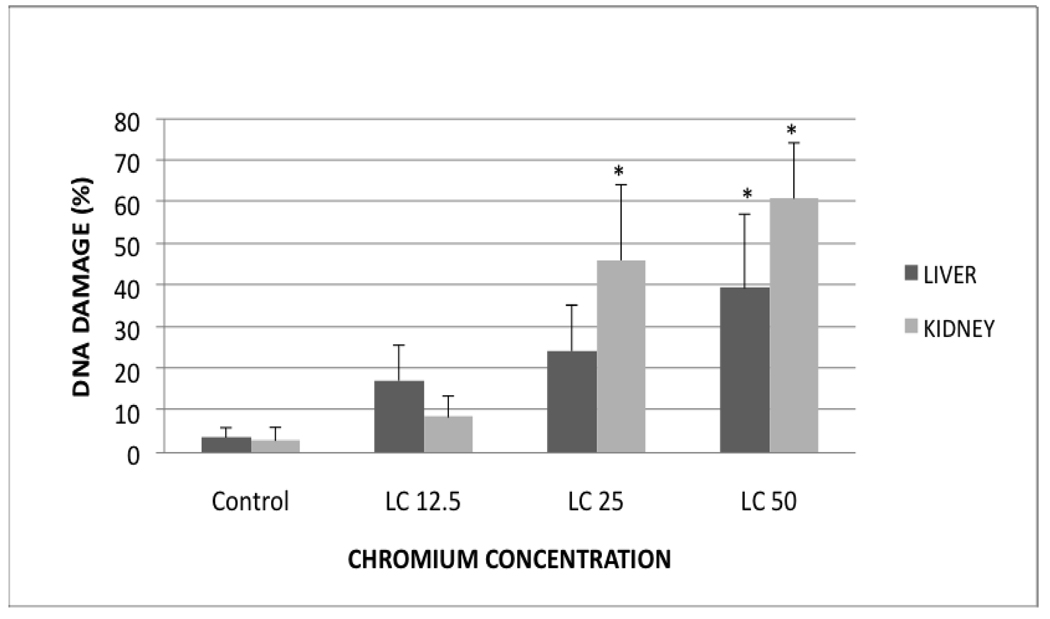
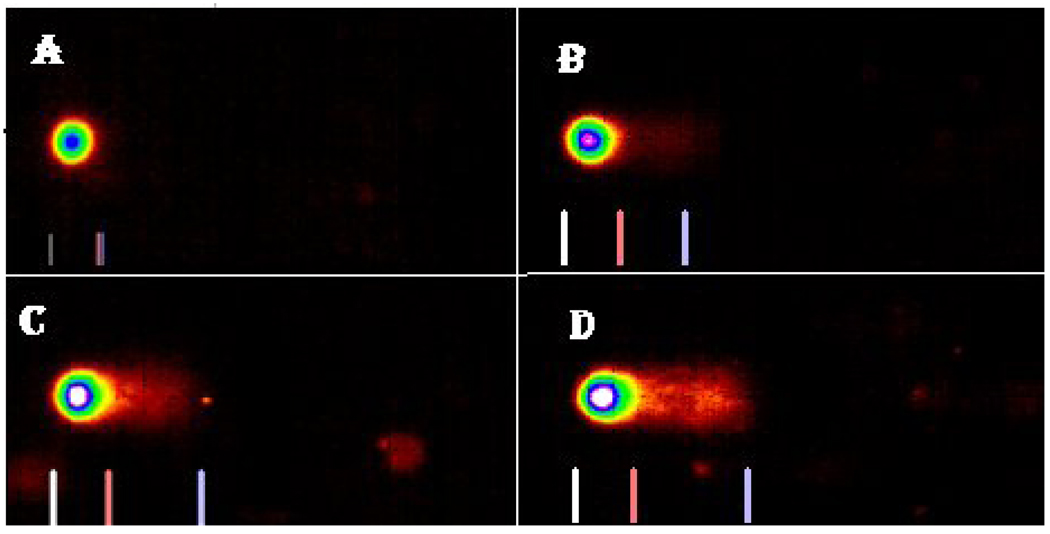
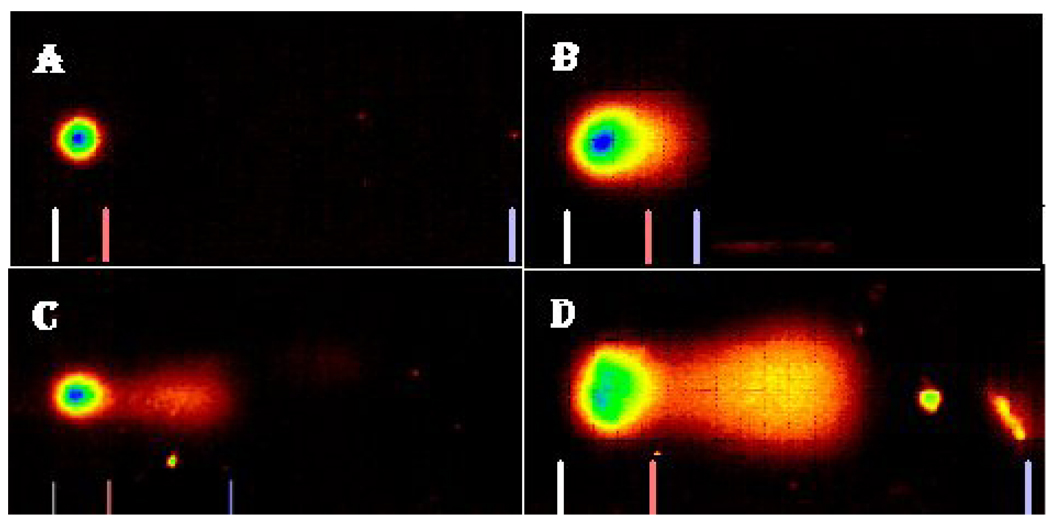
The profiles of DNA damage in liver and kidney of control and Cr (VI) treated fish are presented in Fig. 2. The percentages of DNA damage in liver and kidney cells of all the treatments are presented in Table 1. There was a concentration-dependent increase in percentage of DNA damage with chemical exposure. In the liver, the DNA damage of LC50 treatment group was significantly different from the control group. In the kidney, the DNA damage of LC50 and LC25 were significantly different from the control. Both organs showed a concentration-dependent increase in percentage of DNA damage (Fig. 3 & Fig. 4).
Fig. 2.
Profile of DNA damage in Carassius auratus liver and kidney exposed to various concentrations of Cr (VI) for 96h. Each point represents a mean value and standard deviation of three replicates. *indicates significantly different from the control according to Dunnett’s multiple comparison test.
Table 1.
Percentages of DNA damage in liver and kidney of Carassius auratus exposed to Cr (VI) for 96h. The evaluation was done as described in the Materials and Methods section. Each point represents a mean value and standard deviation of 3 replicates.
| DNA Damage (%) | Control | LC12.5 | LC25 | LC50 |
|---|---|---|---|---|
| Liver | 3.6±2.0 | 17.3±8.0 | 24.4±11.0 | 39.4±17.8* |
| Kidney | 2.9±3.0 | 8.5±5.0 | 46.1±18.0* | 60.9±13.4* |
indicates significantly different from the control according to the Dunnett’s multiple comparison test.
Fig. 3.
Comet assay SYBR images of DNA damage profile in Carassius auratus liver cells exposed to Cr (VI) for 96 h. A= Control, B= LC12.5, C= LC25 and D= LC50. The comet assay was performed as described in the Materials and Methods section.
Fig. 4.
Comet assay SYBR images of DNA damage profile in Carassius auratus kidney cells exposed to Cr (VI) for 96 h. A= Control, B= LC12.5, C= LC25 and D= LC50. The comet assay was performed as described in the Materials and Methods section.
3.6. Histopathology
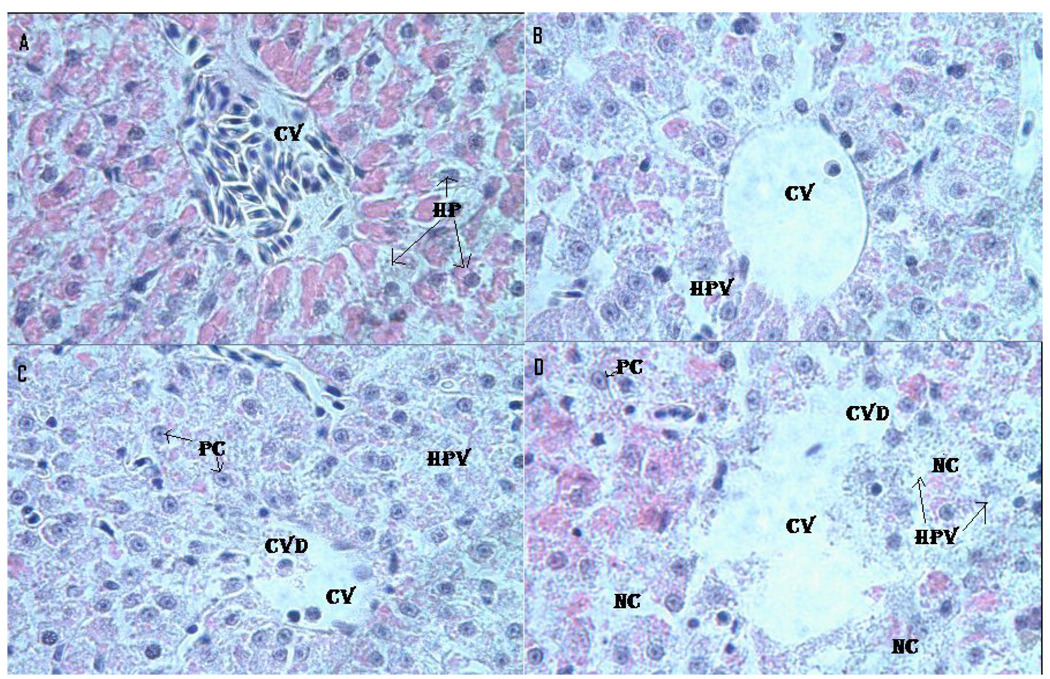
Fig. 5 presents the morphology of the liver of control and Cr (VI)-treated fish. Microscopic examination of the control liver showed a normal structure with compactly arranged hepatocytes. Sinusoids were scattered randomly all over the hepatocytes and the hepatocytes had uniform morphology along with central vein. However, the fish exposed to LC12.5, LC25 and LC50 presented remarkable morphological alterations. Hepatocytes disruption and hepatocellular vacuolation were observed in microscopic examination of LC12.5 exposed fish liver. In addition to the LC12.5 alterations, pycknotic or karyomegaly (condensed nuclei) of hepatocytes and partial disruption of central vein were observed in LC25 exposed fish liver. In addition to the above alterations, degeneration of liver (atrophy) and central vein injury were observed in LC50 exposed fish liver.
Fig. 5.
Histopathological evaluation of Carassius auratus liver exposed to Cr (VI) for 96 h. A= Control (CV= Central vein, HP=Hepatocytes); B= LC12.5 exposed liver (CV= Central vein, HPV= Hepato cellular vacuolation); C= LC25 exposed liver (CV=Central vein, CVD= Central vein damage, HPV= Hepato cellular vacuolation and PC=Pycknotic); and D= LC50 exposed liver (CV=Central vein, CVD= Central vein damage, NC= Necrosis and PC=Pycknotic). H & E Staining 1000 X.
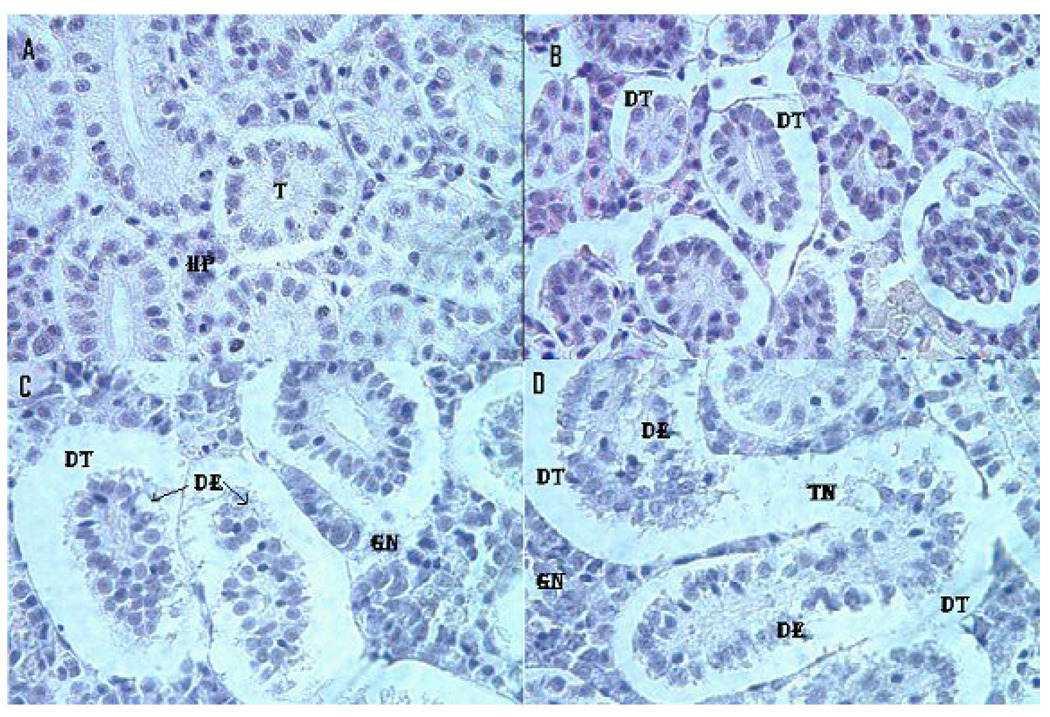
Treated and control kidney morphologies are presented in the Fig. 6. Microscopic examination of the control fish kidney showed uniformly formed functional units called tubules and the interstices of the tubules contain hematopoietic tissue. However, the kidney of LC12.5-, LC25- and LC50-exposed fish showed morphological alterations. The kidney exposed to LC12.5 showed tubular dilation and renal tubular separation, whereas the kidney exposed to LC25 showed tubular necrosis, disintegration of tubules, degeneration of hematopoietic tissue and esonophillic exudates. Further, in the kidney exposed to LC50, progressive dilation of tubules, tubular necrosis, renal tubular separation, degeneration of hematopoietic tissue and tubular lumen were observed. Table 2 represents the statistical data for liver and kidney histopathology.
Fig. 6.
Histopathological evaluation of Carassius auratus kidney exposed to Cr (VI) for 96h. A= Control (T= Tubule, HP= Hematopoietic tissue), B= LC12.5 exposed kidney (DT=Dilated tubule), C= LC25 exposed kidney (DT=Dilated tubule, DE= Degeneration of epithelial cells, GN=Glomerular necrosis) and D= LC25 exposed kidney (DT=Dilated tubule, DE= Degeneration of epithelial cells, TN=Tubular necrosis, GN=Glomerular necrosis). H & E staining 1000 X.
Table 2.
Histopathological evaluation of Carassius auratus liver and kidney exposed to Cr (VI) for 96h. The evaluation was done as described in the Materials and Methods section. Each point represents a mean value and standard deviation of nine values per sample.
| Control | LC12.5 | LC25 | LC50 | |
|---|---|---|---|---|
| Liver damage | 0.00±00 | 0.62±0.42 | 1.65±1.20 | 3.07±1.26 * |
| Kidney damage | 0.00±00 | 1.10±0.80 | 2.20±0.94 | 3.00±1.50* |
indicates significantly different from the control according to the Dunnett’s multiple comparison test.
4. Discussion
In this study, we have investigated the oxidative stress, genotoxicity and histopathology of liver and kidney of Goldfish, Carassius auratus, upon acute exposure to Cr (VI). The results of this study clearly show that the fish experienced oxidative stress by modulating the antioxidant enzyme activities, exhibited DNA damage and microscopic morphological changes in the liver and kidney tissues.
4.1. Oxidative stress
The present study indicates that CAT activity decreased in both liver and kidney of fish exposed to LC12.5, LC25 and LC50 compared to the control. However, this decrease is not significant. Similar results were also reported in earlier studies from which the authors pointed out that the inhibition of CAT activities may be associated with over production of superoxide anion radicals [41, 42]. In another study, Anguilla anguilla L exposed to chromium showed inhibition of CAT activity due to the generation of CAT inhibitors [25]. The inhibition of CAT activity might also be due to the carbonylation of proteins [43, 44] and the change of protein function by lipid peroxidation products [45].
In the present study, SOD activity in liver and kidney of exposed fish was significantly elevated in all the concentrations compared to the controls. It has been reported that elevated levels of ROS induce antioxidant levels and as a part of an adaptive mechanism to oxidative stress SOD activity will be elevated [46]. Similar findings reported earlier are in agreement of these findings [47, 17]. Thus, the increase in SOD activity observed in the current study suggests that Cr (VI) is capable of inducing oxidative stress to the goldfish.
Lipid hydroperoxides are the products of saturated and unsaturated lipids resulting from oxidative injury. Assessment of oxidative damage by measuring the hydro peroxides levels is a novel and direct method compared to the traditional, indirect calorimetric assessment of malonaldehyde (MDA) and 4-hydroxynonenal (4-HNE). The hydroperoxide levels were increased in all test concentrations compared to the controls of liver and kidney. The results of present study clearly suggest that the fish group were subjected to oxidative stress because of the lipid peroxidation in response to ROS generation [16]. On the other hand, less effective antioxidant defense mechanism against the ROS may lead to the increase of lipid peroxidation [25], which can be considered as one of the biomarkers for cell death [48, 49].
GPx enzyme exhibits pivotal role in protection of animals from oxidative damage by reducing lipid hydro peroxides to alcohols. The activity of GPx increased significantly in higher test concentrations in liver and kidney suggesting elevated levels of hydro peroxides in liver and kidney. The results are corroborated with the previous findings [25, 42]. Induction of GPx was more in kidney compared to the liver; which could be due to the higher production of lipid hydro peroxides by superoxide radicals (O2 −) in the kidney. Previously it has been reported that the kidney is the primary organ of Cr (VI) toxicity in fish [24].
Metallothioneins (MT) are low molecular weight metal binding proteins induced as a part of defense mechanism against metals. In the present study, the levels of MT were elevated in a concentration-dependent manner in liver and the kidney of Cr (VI) exposed fish. Induction of MT could be result from direct activation of metal responsive elements (MRE), indirect displacement of other bound metals of MRE by intracellular ligands or activation of antioxidant responsive elements [50, 51]. Roberts et al [17] have reported similar findings of significant MT induction from an investigation of the sub-lethal effects of Cr (VI) to Rainbow trout. Though the total protein content in liver and kidney was altered, the changes in protein levels were insignificant when compared to the control fish. The alterations in the protein levels might be due to the adaptation of the animals to metal stress. Our data supported by previous reports [52] have shown the decrease of total protein content in fish exposed to Cr (VI). Decreased rate of protein synthesis, utilization for energy or secreted mucous proteins could alter the protein levels in animals under metallic stress [12, 53].
4.2. Genotoxicity
The present study clearly showed that the Cr (VI) is genotoxic to the goldfish. DNA damage was observed in liver and kidney cells of all the fish exposed to Cr (VI) in a concentration-dependent manner. The DNA damage at higher test concentrations in the liver and kidney could be due to the elevated levels of hydroproxides in both the tissues compared to their controls, respectively. Induction of ROS under metallic stress could attack the DNA and damage its integrity. Our present study results are corroborated with the previous reports [25]. In another study, medaka fin cell lines exposed to Cr (VI) to examine the genotoxic potentials, have demonstrated DNA double strand breaks and chromosome damage in a concentration-dependent manner [6]. In a particular study, Abbas et al [54] studied the genotoxic potential of Cr (VI) in Orechromis spp. The fish were exposed to sub lethal concentrations (43.7 mg/L) of Cr (VI) for 24 and 96h. The results showed polymorphic bands in long time exposed fish whereas no polymorphic bands in short time exposed fish. Additional studies have reported the genotoxic potential of Cr (VI) in Pimephales promelas, the fathead minnow, based on the induction of micronucleus in erythrocytes [26].
4.3. Histopathology
Microscopic examination of organs to evaluate the toxic effects of contaminants has become a vital tool in detecting early effects on morphology. Also it has been used as a biomarker to evaluate the toxicity of various pollutants [55, 56]. In the present study, the liver of Cr (VI) exposed fish showed distorted hepatocytes with vacuolation. It has been reported that toxicant exposed liver show vacuolation because of the excessive accumulation of fat in the cytoplasm [57]. Pycknotic (karyomegaly) is the state of condensed nuclei present in the hepatocytes; it might be due to the deposition of lipids and glycogen [58]. In the present study, the sections of the liver exposed to higher Cr (VI) concentrations, showed remarkable damage of the central vein was because of the infiltration of neutrophils and lymphocytes. Degeneration of liver tissue and necrosis of central vein could be due to the accumulation of neutrophils and lymphocytes. Similar results have been found from studies of African catfish exposed to fuel oil for 14 days [59]. Lipid peroxidation is the primary source for tubular dilation, and disintegration tubules. Our results are corroborated by the similar findings of kidney pathology in Chinook salmon exposed to aqueous chromium [24]. The generation of ROS as a result of chromium exposure leads to the peroxidation of unsaturated fatty acids in the cell membranes and development of necrosis [60–62]. In our study, degeneration of tubular epithelial cells and tubular necrosis was observed at higher concentrations of Cr (VI) exposure. This may be due to the accumulation of inflammatory cells associated with chromium toxicity [63]. Epithelial degeneration of tubule was earlier reported in fish exposed to higher doses of antibiotics [64, 65].
4.4. Organ specificity
We made an attempt to assess the organ specific toxic effects of Cr (VI) in goldfish liver and kidney for acute exposure. Organ specificity depends on the bioaccumulation of toxicants and defensive mechanism of the particular organ [25]. The organ-specific Cr (VI) toxicity data in fish is scarce.
The results from our studies indicated that CAT activity was altered in both liver and kidney compared to the controls. However, neither organs antioxidant levels showed significant change to the Cr (VI) exposure. On the other hand, the SOD activity was found to be elevated in both organs and the antioxidant levels were significantly higher in kidney compared to the liver. A similar pattern of GPx levels induction was observed in liver and kidney showing organ specific toxicity. These antioxidant profiles showed more resistance to Cr (VI)-induced ROS in liver and less in kidney; it could be due to the differential antioxidant defensive capacity of liver and kidney. Also, the toxicant exposed fish showed adaptability to the metal contaminated environment by inducing MT levels and altering total protein content in the liver and kidney. However, the MT induction was more in kidney compared to the liver. This could be due to the more accumulation of the metal in the kidney.
The differential toxicity of Cr (VI) in liver and kidney was further evidenced with the ROS induced lipid peroxidation, genotoxicity and histopathology. The lipid hydro peroxides levels, degree of DNA damage and microscopic morphological changes were observed more in the kidney compared to the liver. The organ-specific toxic potential of Cr (VI) can be due to the differential sensitivity of liver and kidney cells, which may be due to the differential expression of receptors and cellular components that interact with the metal and metal-produced ROS. Also, it has been reported that oxidative stress interferes with cell cycle check points and alters the process of cell division [66]. Because the kidney is a blood-forming organ, cell division may be more active in this tissue; making it more susceptible to oxidative stress than the liver which is primarily involved in the biotransformation of xenobiotic compounds.
5. Conclusions
The current study demonstrates that the Cr (VI) induced oxidative stress, genotoxicity and histopathological effects in goldfish liver and kidney at all the tested concentrations for 96h exposure. As a result of oxidative stress, the antioxidant enzyme levels were significantly modulated in both organs in a concentration-dependent manner. Cr (VI) induced significant DNA damage in goldfish liver and kidney cells leading to believe that the elevated levels of antioxidants were inadequate to combat the high level of ROS generated.
Also, significant morphological changes were observed in liver and kidney tissues of exposed fish. These changes included distorted hepatocytes with vacuolation, karyomegaly, degeneration of liver tissue and necrosis of central vein in the liver, and tubular dilation and disintegration of tubules in the kidney. However, the induced histopathological effects were significantly higher at higher test concentration; it might be attributed to the higher accumulation of Cr and inflammatory reactions in the tissues as a result of oxidative stress and lipid peroxidation leading to tubular dilation and kidney disintegration. Hence, Cr (VI) specifically altered the morphology and physiology of the kidney. Further, the subtle effects seen at low concentrations suggest that kidney is more sensitive to Cr (VI) toxicity.
Acknowledgements
This research was supported in by the NIH-RCMI Grant No. 2G12RR013459, and in part by a grant from the NOAA-ECSC Grant No. NA06060AR4810164 & Subcontract No. 000953.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vutukuru SS, Prabhath NA, Raghavender M, Yerramilli A. Effect of arsenic and chromium on the serum amino-transferases activity in Indian major carp, Labeo rohita. Int. J. Environ. Res. Public. Health. 2007;4:224–227. doi: 10.3390/ijerph2007030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U. S. Environmental protection agency, Locating and Estimating Air Emissions from Sources of Chromium. Traingle Park. 1984 [Google Scholar]
- 3.Towill LE. Reviews of the Environmental Effects of Pollutants: III, Chromium. 1978:12–17. EPA-600/1-78-023 and ORNL/EIS-80. [Google Scholar]
- 4.National Academy of Sciences, Committee on Biologic Effects of Atmospheric Pollutants, Chromium. Washington D.C: 1974:2–6. ISBN 0-309-02217-7.
- 5.Papp JF. Chromium, Mineral Commodity Profiles 1983. Washington D.C: U.S. Bureau of Mines; 1984. [Google Scholar]
- 6.Goodale BC, Walter R, Pelsue SR, Thompson WD, Wise SS, Winn RN, Mitani H, Wise JP., Sr The cytotoxicity and genotoxicity of hexavalent chromium in medaka (Oryzias latipes) cells. Aquat. Toxicol. 2008;87:60–67. doi: 10.1016/j.aquatox.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Tan F, Wang M, Wang W, Lu Y. Comparative evaluation of the cytotoxicity sensitivity of six fish cell lines to four heavy metals in vitro. Toxicol. In. Vitro. 2008;22:164–170. doi: 10.1016/j.tiv.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Prabakaran M, Binuramesh C, Steinhagen D, Michael RD. Immune response in the tilapia, Oreochromis mossambicus on exposure to tannery effluent. Ecotoxicol. Environ. Saf. 2007;68:372–378. doi: 10.1016/j.ecoenv.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Bozcaarmutlu A, Arinc E. Effect of mercury, cadmium, nickel, chromium and zinc on kinetic properties of NADPH-cytochrome P450 reductase purified from leaping mullet (Liza saliens) Toxicol. In. Vitro. 2007;21:408–416. doi: 10.1016/j.tiv.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Stokes RM, Fromm PO. Effects of chromate on glucose transport by the gut of rainbow trout. Physiol. Zool. 1965;38:202–205. [Google Scholar]
- 11.Sastry KV, Sunita KM. Effect of cadmium and chromium on the intestinal absorption of glucose in the Snakehead Fish, Channa punctatus. Toxicol. Lett. 1982;10:293–296. doi: 10.1016/0378-4274(82)90090-x. [DOI] [PubMed] [Google Scholar]
- 12.Vutukuru SS. Acute effects of hexavalent chromium on survival, oxygen consumption, hematological parameters and some biochemical profiles of the Indian Major Carp, Labeo rohita. Int. J. Environ. Res. Public. Health. 2005;2:456–462. doi: 10.3390/ijerph2005030010. [DOI] [PubMed] [Google Scholar]
- 13.Sesha Srinivas V, Rao BM. Chromium induced alterations in the gill of the freshwater teleost fish, Labeo rohita. Ind. J. Compar. Anim. Physiol. 1998;17:31–33. [Google Scholar]
- 14.Zhu Y, Wang J, Bai Y, Zhang R. Cadmium, chromium, and copper induced polychromatocyte micronuclei in Carp (Cyprinus carpio L.) Bull. Environ. Contamn. Toxicol. 2004;72:78–86. doi: 10.1007/s00128-003-0243-6. [DOI] [PubMed] [Google Scholar]
- 15.Maples NL, Bain LJ. Trivalent chromium alters gene expression in the mummichog (Fundulus heteroclitus) Environ. Tox. Chem. 2004;23:626–631. doi: 10.1897/03-130. [DOI] [PubMed] [Google Scholar]
- 16.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. third ed. Oxford: Clarendon Press; 1999. [Google Scholar]
- 17.Roberts AP, Oris JT. Multiple biomarker response in rainbow trout during exposure to hexavalent chromium. Comp. Biochem. Physiol. C. 2004;138:221–228. doi: 10.1016/j.cca.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Bagnyukova TV, Chahrak OI, Lushchak VI. Coordinated response of goldfish antioxidant defenses to environmental stress. Aqua. Toxicol. 2006;78:325–331. doi: 10.1016/j.aquatox.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Maeng SH, Chung HW, Kim KJ, Lee BM, Shin YC, Kim SJ, Yu IJ. Chromosome aberration and lipid peroxidation in chromium-exposed workers. Biomarkers. 2004;9:418–434. doi: 10.1080/13547500400022200. [DOI] [PubMed] [Google Scholar]
- 20.Wise SS, Holmes AL, Xie H, Thompson WD, Wise JP., Sr Chronic exposure to particulate chromate induces spindle assembly checkpoint bypass in human lung cells. Chem. Res. Toxicol. 2006;19:1492–1498. doi: 10.1021/tx0601410. [DOI] [PubMed] [Google Scholar]
- 21.Patlolla AK, Barnes C, Yedjou C, Velma VR, Tchounwou PB. Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environ. Toxicol. 2008;24:66–73. doi: 10.1002/tox.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavas T, Ergene-Gozukara S. Induction of micronuclei and nuclear abnormalities in Oreochromis niloticus following exposure to petroleum refinery and chromium processing plant effluents. Aqua. Toxicol. 2005;74:264–271. doi: 10.1016/j.aquatox.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Cavas T, Konen S. Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus test and the comet assay. Mutagenesis. 2007;22:263–268. doi: 10.1093/mutage/gem012. [DOI] [PubMed] [Google Scholar]
- 24.Farag AM, May T, Marty GD, Easton M, Harper DD, Little EE, Clevelnad L. The effect of chronic chromium exposure on the health of Chinook salmon (Oncorhynchus tshawytscha) Aqua. Toxicol. 2006;76:246–257. doi: 10.1016/j.aquatox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Iqbal Ahmad, Maria VL, Oliveira M, Pacheco M, Santos MA. Oxidative stress and genotoxic effects in gill and kidney of Anguilla anguilla L. exposed to chromium with or without pre-exposure to β-naphthoflavone. Mutat. Res. 2006;608:16–28. doi: 10.1016/j.mrgentox.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 26.De Lemos CT, Rodel PM, Terra NR, Erdtman B. Evaluation of basal micronucleus frequency and hexavalent chromium effects in fish erythrocytes. Environ. Toxicol. Chem. 2001;20:1320–1324. doi: 10.1897/1551-5028(2001)020<1320:eobmfa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Patlolla AK, Tchounwou PB. Cytogenetic evaluation of arsenic trioxide toxicity in Sprague-Dawley rats. Mutat. Res. 2005;587:126–133. doi: 10.1016/j.mrgentox.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Dhawan A, Bajpayee M, Parmar D. Comet assay: a reliable tool for the assessment of DNA damage in different models. Cell. Biol. Toxicol. 2009;25:5–32. doi: 10.1007/s10565-008-9072-z. [DOI] [PubMed] [Google Scholar]
- 29.Andrade VM, de Freitas TR, da Silva J. Comet assay using mullet (Mugil sp.) and sea catfish (Netuma sp.) erythrocytes for the detection of genotoxic pollutants in aquatic environment. Mutat. Res. 2004;560:57–67. doi: 10.1016/j.mrgentox.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Ateeq B, Abul Farah M, Ahmad W. Detection of DNA damage by alkaline single cell gel electrophoresis in 2,4-dichlorophenoxyacetic- acid- and butachlor-exposed erythrocytes of Clarias batrachus. Ecotoxicol. Environ. Saf. 2005;62:348–354. doi: 10.1016/j.ecoenv.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Velma V, Tchounwou PB. Acute toxicity of sodium dichromate to goldfish, Carassium auratus. Metal. Ions. Biology. Medicine. 2008;10:642–646. [Google Scholar]
- 32.Johansson LH, Borg LAH. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988;174:331–336. doi: 10.1016/0003-2697(88)90554-4. [DOI] [PubMed] [Google Scholar]
- 33.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 34.Marklund SL. In: In Handbook of Methods for Oxygen Radical Research. Greenwald RA, editor. Boca Raton: CRC Press; 1985. pp. 243–247. [Google Scholar]
- 35.Mihaljevi B, Katusin-razem B, razem D. The reevaluation of the ferric thiocyanate assay for lipid hydroperoxides with special considerations of the mechanistic aspects of the response. Free. Radical. Biol and Medicine. 1996;21:53–63. doi: 10.1016/0891-5849(95)02224-4. [DOI] [PubMed] [Google Scholar]
- 36.Paglia E, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Cm. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 37.Viarengo A, Ponzano E, Dondero F, Fabbri R. A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic molluscs. Mar. Environ. Res. 1997;44:69–84. [Google Scholar]
- 38.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 1976;2:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 39.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell. Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 40.Kerem M, Bedirli N, Gurbuz N, Ekinci O, Bedirli A, Akkaya T, Akrak O, Pafiao H. Effects of acute fenthion toxicity on liver and kidney function and histology in rats, Turk. J. Med. Sci. 2007;37:281–288. [Google Scholar]
- 41.Kono Y, Fridovich I. Superoxide radicals inhibit catalase. J. Biol.Chem. 1982;257:5751–5754. [PubMed] [Google Scholar]
- 42.Pandey S, Ahmad I, Pervez S, Hafeez B, Haque R, Raisuddin S. Effect of endosulfan on antioxidants of freshwater fish Channa punctatus Bloch. 1. Protection against lipid peroxidation in liver by copper pre-exposure. Arch. Environ. Contam. Toxicol. 2001;41:345–352. doi: 10.1007/s002440010258. [DOI] [PubMed] [Google Scholar]
- 43.Starke PE, Oliver CN, Stadtman ER. Modification of hepatic proteins in rats exposed to high oxygen concentration. FASEB J. 1987;1:36–39. doi: 10.1096/fasebj.1.1.2886388. [DOI] [PubMed] [Google Scholar]
- 44.Stadtman ER, Oliver CN, Starke-Reed PE. Implication of metalcatalyzed oxidation of enzymes in aging, protein turnover, and oxygen toxicity, Korean. J. Biochem. 1991;23:49–54. [Google Scholar]
- 45.Stadtman ER, Levine RL. Protein oxidation. Ann. N. Y. Acad. Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang XF, Xing ML, Xin Zhu YS, Xu LH. Oral administration of Cr (VI) induced oxidative stress, DNA damage and apoptotic cell death in mice. Toxicol. 2006;228:16–23. doi: 10.1016/j.tox.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Ritola O, Livingstone DR, Peters LD, Lindstrom-Seppa P. Antioxidant processes are affected in juvenile rainbow trout (Oncorhynchus mykiss) exposed to ozone and oxygen-supersaturated water. Aquaculture. 2002;210:1–19. [Google Scholar]
- 48.Dreiem A, Ring A, Fonnum F. Organic solvent-induced cell death in rat cerebellar granule cells: structure dependence of C10 hydrocarbons and relationship to reactive oxygen species formation. Neurotoxicology. 2005;26:321–330. doi: 10.1016/j.neuro.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Sayes CM, Gobin AM, Ausman KD, Mendez J, West JL, Colvin VL. Nano-C60 cytotoxicity is due to lipid peroxidation. Biomaterials. 2005;26:7587–7595. doi: 10.1016/j.biomaterials.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 50.Roesijadi G. Metallothionein and its role in toxic metal regulation. Comp. Biochem. Physiol. C. 1996;113:117–123. [Google Scholar]
- 51.Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 52.Vutukuru SS. Chromium induced alterations in some biochemical profiles of the Indian major carp, Labeo rohita (Hamilton) Bull. Environ. Contam. Toxicol. 2003;70:118–123. doi: 10.1007/s00128-002-0164-9. [DOI] [PubMed] [Google Scholar]
- 53.Nagai M, Ikeda S. Carbohydrate metabolism in Fish-1. Effects of starvation and dietary composition on the blood glucose level and haepatopancreatic glycogen and lipid contents in Cyprinus carpio. Bull. Jap. Soc. Scient. Fish. 1971;37:404–409. [Google Scholar]
- 54.Abbas HH, Ali FK. Study the effect of hexavalent chromium on some biochemical, citotoxicological and histopathological aspects of the Orechromis spp. Fish, Pak. J. Biol. Sci. 2007;10:3973–3982. doi: 10.3923/pjbs.2007.3973.3982. [DOI] [PubMed] [Google Scholar]
- 55.Mela M, Randi MAF, Ventura DF, Carvalho CEV, Pelletier E, Oliveira Ribeiro CA. Effects of dietary methyl mercury on liver and kidney histology in the neotrophical fish Hoplias malabaricus. Ecotox. Environ. Saf. 2007;68:426–435. doi: 10.1016/j.ecoenv.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Adams SM. Biological indicators of aquatic ecosystem stress. Amer. Fish. Soc. 2002;3:104–112. [Google Scholar]
- 57.Bogiswariy S, Jegathambigai R, Marimuthu K. Effect of acute exposure of cadmiunchloride in the morphology of the liver and kidney of mice. Intern. Conf. Environ. Res. Technol. (ICERT) 2008 [Google Scholar]
- 58.Myers MS, Rhodes LD, McCain BB. Pathologic anatomy and patterns of occurrence of hepatic neoplasms, putative preneoplastic lesions and other idiopathic hepatic conditions in English sole (Parophrys vetulus) from Puget Sound, Washington, U. S. A. J. Natl. Cancer Inst. 1987;78:333–363. [PubMed] [Google Scholar]
- 59.Gabriel UU, Ezeri GNO, Amakiri EU. Liver and kidney histopathology: Biomakers of No. 1 fuel toxicosis in African catfish, Clarias gariepinus. J. Animal Veterinary Advances. 2007;6:379–384. [Google Scholar]
- 60.Lloyd DR, Phillips DH, Carmichael PL. Generation of putative intrastrand cross-links and strand breaks in DNA by transition metal ion-mediated oxygen radical attack. Chem. Res. Toxicol. 1997;10:393–400. doi: 10.1021/tx960158q. [DOI] [PubMed] [Google Scholar]
- 61.Lloyd DR, Carmichael PL, Phillips DH. Comparison of the formation of 8-hydroxy-2’-deoxyguanosine and singleand double-strand breaks in DNA mediated by fenton reactions. Chem. Res. Toxicol. 1998;11:420–427. doi: 10.1021/tx970156l. [DOI] [PubMed] [Google Scholar]
- 62.Susa N, Ueno S, Furukawa Y, Sugiyama M. Protective effect of Vitamin E on chromium (VI)-induced cytotoxicity and lipid peroxidation in primary cultures of rat hepatocytes. Arch. Toxicol. 1996;71:20–24. doi: 10.1007/s002040050353. [DOI] [PubMed] [Google Scholar]
- 63.Kurtov ić B, Teskered žić E, Teskered žić Z. Histological comparison of spleen and kidney tissue from farmed and wild European sea bass (Dicentrarchus labrax L.) ACTA ADRIAT. 2008;49:147–154. [Google Scholar]
- 64.Lauren DJ, Wishkovsky A, Grof JM, Hedrick RP, Hinton DE. Toxicity and pharmacokinetics of the antibiotic fumagilin in yearling rainbow trout (Salmo gairdneri) Toxicol. Appl. Pharm. 1989;98:444–453. doi: 10.1016/0041-008x(89)90173-7. [DOI] [PubMed] [Google Scholar]
- 65.Hicks BD, Geraci JR. A histological assesment of damage in rainbow trout, Salmo gairdneri Richardson fed rations containing erythromycin. J. Fish Dis. 1984;7:457–466. [Google Scholar]
- 66.Shackelford RE, Kaufmann WK, Praules RS. Oxidative stress and cell cycle checkpoint function. Free. Radic. Biol. Med. 2000;28:1387–1404. doi: 10.1016/s0891-5849(00)00224-0. [DOI] [PubMed] [Google Scholar]