Abstract
Extracellular nucleotides and their receptor antagonists have therapeutic potential in disorders such as inflammation, brain disorders, and cardiovascular diseases. Pancreatic β cells express several purinergic receptors, and reported nucleotide effects on insulin secretion are contradictory. We studied the effect of P2Y receptors on insulin secretion and cell death in MIN6, mouse pancreatic β cells. Expression of P2Y1 and P2Y6 receptors was revealed by total mRNA analysis using RT-PCR. MIN6 cells were stimulated in the presence of 16.7 mM glucose with or without P2Y1 and P2Y6 agonists, 2-MeSADP and Up3U, respectively. Both the agonists increased insulin secretion with EC50 values of 44.6±7.0 nM and 30.7±12.7 nM respectively. The insulin secretion by P2Y1 and P2Y6 agonists was blocked by their selective antagonists MRS2179 and MRS2578, respectively. Binding of the selective P2Y1 receptor antagonist radioligand [125I]MRS2500 in MIN6 cell membranes was saturable (KD 4.74±0.47 nM), and known P2Y1 ligands competed with high affinities. Inflammation and glucose toxicity leads to pancreatic β cell death in diabetes. Flow cytometric analysis revealed that Up3U but not 2-MeSADP protected MIN6 cells against TNF-α induced apoptosis. Overall, the results demonstrate that selective stimulation of P2Y1 and P2Y6 receptors increases insulin secretion that accompanies intracellular calcium release, suggesting potential application of P2Y receptor ligands in the treatment of diabetes.
Keywords: nucleotides, G protein-coupled receptors, phospholipase C, radioligand binding
1. Introduction
Recent reports have revealed the therapeutic potential of extracellular nucleotides in disorders such as inflammation, brain disorders, and cardiovascular diseases [1-4]. Nucleotides are released in the extracellular fluid by various actions such as cell lysis, exocytosis, efflux, and cellular stress upon changes in osmolarity and mechanical perturbations. The released nucleotides exert their activity by binding to nucleotide receptors. P2Y receptors are a class of GPCRs activated by nucleotides and so far eight different subtypes of P2Y receptors have been identified: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14. These eight subtypes are divided into two classes based on their G-protein coupling properties: P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11 couple to Gq protein and activate phospholipase C (PLC), P2Y11 also activates adenylate cyclase in addition to Gq coupling, whereas P2Y12, P2Y13 and P2Y14 receptors are coupled to Gi protein and inhibit adenylate cyclase [5].
Although P2Y receptors play important roles in different disease conditions, the activity of these receptors varies greatly depending on the cell type, their environment, the assembly of signaling molecules, and interactions with other receptors. The exact role of each of the subtypes of P2Y receptors, their distribution, expression, and biological functions has not yet been fully elucidated. Human, rat, and mouse pancreatic islets are reported to express several purinergic receptors, and reports on the modulation of insulin secretion by extracellular nucleotides are contradictory, with both stimulatory [6-8] and inhibitory [9,10] effects reported. The P2Y1 receptor is among the most widely studied purinergic receptors, and its agonists, such as ADP, ADPβS, and MeSATP, have been proposed as insulin secretagogues [4, 11]. The stable UDP analog UDPβS, a selective P2Y6 agonist, has been shown to increase insulin secretion at 8.3 mM and 16.7 mM glucose concentrations in mouse pancreatic islets [12]. Lee et al. have also reported an increased insulin secretion induced by ATP, α,β-MeATP, and 2-MeATP through stimulation of the P2Y11 receptor [13].
In this study, we have characterized the binding and functional properties of P2Y1 and P2Y6 receptors endogenously expressed in the MIN6 cells, a mouse pancreatic β cell line. P2Y1 receptors are distributed widely among platelets, heart, skeletal muscles, neuronal tissues, and the digestive tract. The distribution pattern for P2Y6 receptors is in the lungs, heart, spleen, aorta and brain. P2Y1 receptors are activated by ADP and its analogs, and P2Y6 receptors are activated by UDP and its analogs [14]. Recent medicinal chemistry studies have identified useful ligand tools for studying these receptors. 2-Methylthioadenosine-5′-diphosphate (2-MeSADP) potently activates P2Y1, P2Y12, and P2Y13 receptors (Figure 1), while the (N)-methanocarba equivalent, MRS2365, is truly a potent and selective agonist of the P2Y1 receptor [15,16]. UDP, a P2Y6 agonist, is readily hydrolyzed by nucleotidases present on the cell surface. The dinucleotide Up3U is a potent agonist at the P2Y6 receptor [17,18], but is more stable than UDP toward enzymatic breakdown. The following EC50 values (μM) were determined for Up3U in PLC activation in transfected astrocytoma cells: P2Y2, 1.31 ± 0.21; P2Y4, 0.87 ± 0.11; P2Y6, 0.27 ± 0.07 [17]. Selective, reversible, and competitive antagonists of the P2Y6 receptor have not yet been reported [19], but there are potent antagonists of the P2Y1 receptor that are useful in receptor characterization. For example, the (N)-methanocarba bisphosphate, MRS2179 (N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate), and a bridged carbocyclic 2-iodo analogue, MRS2500 (2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate), have been used as tracers in radioligand binding experiments [20,21].
Figure 1.
Structures of nucleotide agonists (A) and antagonists (B) used as pharmacological probes of P2Y1 and P2Y6 receptors.
We recently introduced [125I]MRS2500 as a useful radioligand [22], which we utilized in the present study for characterization of the P2Y1 receptor expressed by MIN6 cells. We have also examined the ability of nucleotides to modulate apoptosis induced by tumor necrosis factor (TNF-α). We previously demonstrated that apoptosis induced by TNF-α in transfected astrocytoma cells and in mouse smooth muscle cells could be attenuated by the activation of P2Y6 receptors, but not P2Y1 receptors [23,24]. We have now extended this analysis to MIN6 cells, to conclude that the cytoprotective nature of P2Y6 ligands could be of interest in treating diabetes, potentially by preserving the existing β-cell mass in diabetic patients.
2. Materials and methods
2.1. Materials
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Life Technologies (Rockville, MD). Plastic collagen-coated cellware was purchased from Becton Dickinson (Bedford, MA). Calcium Mobilization Assay Kit was purchased from Molecular Devices (Sunnyvale, CA). 2-MeSADP, propidium iodide (PI) and Hoechst 33342 (HO342) were purchased from Sigma Aldrich (St. Louis, MO). TNF-α was purchased from Invitrogen Corporation (Carlsbad, CA). MRS2179, MRS2279, MRS2500, MRS2578 and MRS2211 were obtained from Tocris Biosciences (Ellisville, MO). All other buffers and reagents were from Gibco, Invitrogen (Carlsbad, CA).
2.2. Cell culture
MIN6, a murine β cell line, was used for the present study. MIN6 cells, established from an insulinoma, were obtained by targeted expression of Simian Virus 40T antigen gene in transgenic mice [25]. Unless otherwise mentioned, MIN6 cells were grown to confluence in Dulbecco’s modified eagle medium (DMEM) with 25 mM of glucose in the presence of 15% fetal bovine serum (FBS) under 5% CO2 and 95% air at 37° C.
2.3 Reverse transcription-polymerase chain reaction (RT-PCR) in MIN6 cells
The total RNA of the MIN6 cells was isolated with RNeasy Mini Kit (Qiagen, Germantown, MD) along with DNase digestion using RNase free DNase (Qiagen). The RNA (1 μg) was reverse transcribed into cDNA and then amplified by PCR with 40 cycles according to manufacturer’s instructions using a Superscript One-Step RT-PCR with a Platinum Taq kit (Invitrogen). In short, first reverse transcription was performed at 50°C for 30 min followed by PCR with gene-specific primers for P2Y1, P2Y6, P2Y13 and β-actin (internal control) [26]. The primers were synthesized by Eurofins MWG Operon (Huntsville, AL) according to the sequences given in Table 1. Contamination of genomic DNA in the total RNAs was examined by substituting Taq-DNA polymerase instead of RT/Platinum® Taq Mix.
Table 1.
Primers for mouse P2Y receptors
| Receptor or protein |
Primer | Fragment Length, bp |
|---|---|---|
| P2Y1 | ||
| Forward | 5′-TGGCGTGGTGTACCCTCTCAAGTC-3′ | 410 |
| Reverse | 5′-ACCGTGCTCGCAAATTCATCGTT -3′ | |
| P2Y6 | ||
| Forward | 5′-CCTGGCACTGGCGGACCTGAT-3′ | 452 |
| Reverse | 5′-GGCGGGCCATGCGACAATAAC-3′ | |
| P2Y13 | ||
| Forward | 5′- ACCGTGGCCCTCTGGGTGTT -3′ | 440 |
| Reverse | 5′- ACCACCTGATGCCACCACAGC -3′ | |
| β-Actin | ||
| Forward | 5′-ATCCATGAAACTACATTCAATTCCAT-3′ | 199 |
| Reverse | 5′-ACCGATCCACACAGAGTACTTGCGC-3′ | |
2.4. Membrane preparation
MIN6 cells were grown to 80% confluence and then harvested for the preparation of membranes. The cells were homogenized, and the nuclear fraction was removed by centrifuging at 100 × g for 5 min. The pellet was resuspended in 50 mM tris(hydroxymethyl) aminomethane (Tris) hydrochloride buffer (pH 7.4). The suspension was homogenized with a Polytron homogenizer (Brinkmann) for 10 s and ultracentrifuged at 20,000 × g for 20 min at 4° C. The resultant pellets were resuspended in Tris buffer (pH 7.4), and the membranes were stored at −80° C until the binding experiments. The protein content of the membranes was determined by BCA protein assay kit (Thermo Scientific, Waltham, MA).
2.5. Radioligand binding assays
A saturation curve of the high affinity radioligand [125I]MRS2500 [22,27] was performed on MIN6 cell membranes to determine its KD value. MIN6 cell membranes were incubated with 0.2-7.2 nM [125I]MRS2500 in assay buffer (50 mM Tris, MgCl2, pH 7.4) in a reaction volume of 200 μl for 60 min at 4° C. Binding reactions were terminated by the addition of 2 ml of ice-cold assay buffer followed by filtration through Whatman GF/B glass-fiber filters under reduced pressure with a MT-24 cell harvester (Brandel), and radioactivity was determined with a 1414 liquid scintillation counter (Wallac, Perkin Elmer Life Sciences, Boston, MA). Binding experiments on P2Y1 receptors were performed as previously described [25]. Specific binding was determined as total binding of [125I]MRS2500 bound minus binding occurring in the presence of 10 μM MRS2500. Maximal binding was calculated as binding per mg of protein present in the membrane. The competition of various known P2Y1 antagonists, MRS2500, MRS2279, and MRS2179, and agonists, 2-MeSADP and ADP, against [125I]MRS2500 binding was determined on MIN6 cell membranes. Radioligand concentrations used in competition binding assays approximated its KD value at the receptor.
2.6. Insulin secretion assay
MIN6 cells were seeded in a 96-well plate at a density of 40,000 cells/well and cultured in DMEM at 37° C. After 48 h, the medium was replaced by Kreb’s ringer bicarbonate buffer (KRBB) containing 3.3 mM glucose and 0.3% albumin, for 1 h. Then, insulin secretion was induced by supplementing KRBB containing 16.7 mM glucose in the presence or absence of P2Y1 and P2Y6 agonists in KRBB solution for 1 h at 37° C. Insulin secretion was measured by ELISA using insulin ELISA kit with mouse insulin standard (Crystal Chem, Inc., Downer’s Grove, IL).
2.7. Determination of inositol phosphate derivatives
The quantity of inositol trisphosphates and their derivatives (IP3) was determined according to the method of Mamedova et al. [19] with appropriate modifications. In short, the MIN6 cells were grown to 80% confluence in 6-well plates in the presence of myo-[3H]-inositol (2 μCi/ml) for 48 h. Cells were then treated for 30 min at 37°C with P2Y ligands or buffer in the presence of 20 mM lithium chloride. The reaction was terminated upon aspiration of the medium and addition of cold formic acid (20 mM). After 30 min, supernatants were neutralized with ammonium hydroxide, and applied to Bio-Rad AG1-X8 anion exchange columns. The columns were then washed with water followed by a 60 mM sodium formate solution containing 5 mM sodium tetraborate. Total IP3 was eluted with 1 M ammonium formate containing 0.1 M formic acid, and radioactivity was measured using a liquid scintillation counter.
2.8. Calcium mobilization assay
Cells were grown overnight in 100 μl of medium in 96-well flat-bottom plates at 37°C at 5% CO2 until they reached ~80% confluency at a density of 40,000 cells/well. After 48 h, the DMEM medium was replaced by calcium-4 dye (Molecular Devices) and incubated for 1 h at 37° C. The calcium-4 assay kit was used as directed with no washing of cells. Cells were loaded with 40 μl of dye in each well and incubated for 1 h at room temperature. The compound plate was prepared with dilutions of various compounds in Hank’s Balanced Buffer at pH 7.2. The change in calcium was measured by addition of P2Y1 and P2Y6 agonists to the dye and the change in calcium was measured by change in intracellular fluorescence. Samples were run in duplicate with a FLIPRTETRA (Molecular Devices) at room temperature. Cell fluorescence (excitation = 485 nm; emission = 525 nm) was monitored following exposure to a compound. Increases in intracellular calcium are reported as the maximum fluorescence value after exposure minus the basal fluorescence value before exposure.
2.9. Induction and detection of apoptosis
Viable, necrotic and apoptotic cells can be differentiated by using specific DNA binding dyes [28, 29]. To evaluate this, cells were exposed to DNA binding dyes PI and HO342. HO342 freely crosses the plasma membrane to enter cells with intact membranes as well as cells with damaged membranes and stains DNA blue, whereas PI, a highly polar dye which is impermeable to cells with preserved membranes, stains DNA red. Apoptosis was induced in MIN6 cells using TNF-α as described previously [30]. In brief, MIN6 cells were grown on coverslips for 48 h, and after 48 h the cells were grown in a DMEM medium supplemented with 5% FBS. MIN6 cells were pretreated with a 10 μM concentration of either 2-MeSADP or Up3U for 30 min and the experiment was continued in the presence of agonists. After pretreatment, apoptosis was induced by treating MIN6 cells with 50 ng/ml of TNF-α for 16 h. Use of PBS alone served as a negative control. Cells were stained with both PI (10 μg/ml) and HO342 (20 μg/ml) for 10 min and then mounted on a sterile glass slide for examination by fluorescence microscopy (Axio Imager D1, Carl Zeiss, Germany) with excitation at 365 nm. While the intact cells are stained blue, apoptotic cells were identified by the presence of condensed or fragmented nuclei stained blue, pink, or red, depending on the stage of the apoptotic process.
For flow cytometry experiments, 24 h after the induction of apoptosis, the cells grown in 6-well plates were trypsinized and stained by PI (l0 μg/ml; Sigma, St. Louis, MO) for 30 min [31]. PI-stained cells were analyzed using a Becton Dickinson FACS Calibur. Cells within a distinct sub-G1 peak were considered apoptotic, because they showed DNA condensation and fragmentation, which resulted in reduced PI staining [32].
2.10. Statistical analysis
All the experiments were performed in duplicate or triplicate assays and were carried out at least three times. Results are presented as mean ± s.e.m., n represents the number of individual experiments performed. Pharmacological parameters were analyzed with the GraphPAD Prism software (version 4.0, GraphPAD Prism, San Diego, CA). P values less than 0.05 (P<0.05) were considered to be statistically significant. Statistical significance between the results was analyzed by ANOVA followed by the Tukey-Kramer multiple comparison test.
3. Results
3.1. Demonstration of the expression of P2Y receptors in MIN6 cells
We used the published sequences of mouse P2Y1, P2Y6 and P2Y13 receptors for the synthesis of primers for the above receptors [26] (Table 1). RT-PCR analysis of MIN6 cell total RNA revealed the expression of P2Y1, P2Y6 and P2Y13 receptors (Figure 2). The PCR products were of the expected size and were further confirmed by sequencing.
Figure 2.
RT-PCR analysis of the mRNAs of three P2Y receptor subtypes in MIN6 cells.
3.2. High affinity binding of [125I]MRS2500 to P2Y1 receptor in MIN6 cells
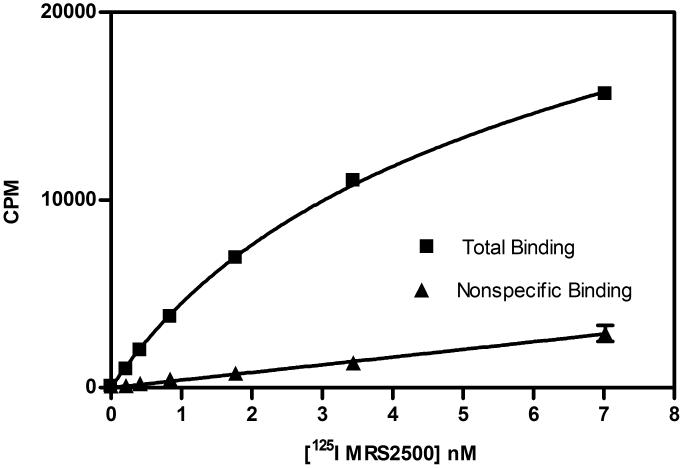
MRS2500 is a selective antagonist of nanomolar affinity at the P2Y1 receptor [19,20]. [125I]MRS2500 [22,27] was used for radioligand binding assays to characterize the expression of the P2Y1 receptor in MIN6 cells membranes (Figure 3). Optimal conditions for radioligand binding experiments were determined in preliminary experiments. Saturation binding experiments were performed to determine the affinity of [125I]MRS2500 for the mouse P2Y1 receptor expressed in MIN6 membranes. Saturation binding isotherms exhibited one-site binding with a KD of 4.74±0.47 nM and an average of Bmax of 307±22 fmol receptor per mg protein (n=3) from three experiments each performed on a single membrane preparation.
Figure 3.
(A) Saturation curve and (B) Scatchard analysis for [125I]MRS2500 binding in MIN6 cell membranes. The figures are from a representative experiment, and the KD value was 4.74±0.47 nM. Data represent mean±s.e.m, n=3.
3.3. Pharmacology of P2Y receptors in MIN6 cells
The capacity of the several agonists and antagonists of the P2Y1 receptor to compete with [125I]MRS2500 for binding in MIN6 cell membranes was determined. Agonists known to bind to the P2Y1 receptor inhibited binding of [125I]MRS2500 in a concentration dependent manner. The potency observed was in the order of 2-MeSADP>ADP (Table 2). This order was in agreement with the predicted potencies at the P2Y1 receptor in previous studies [21,33,34].
Table 2.
Binding affinities of various P2Y1 receptor ligands against [125I]MRS2500 in MIN6 cell membranes. Ki values were calculated as an mean ± s.e.m. of three individual experiments
| Compound | Effect on P2Y1 receptors | Ki, nM |
|---|---|---|
| MRS2500 | Antagonist | 2.92 ± 0.21 |
| MRS2279 | Antagonist | 6.86 ± 0.12 |
| MRS2179 | Antagonist | 49.8 ± 0.23 |
| 2-MeSADP | Agonist | 50.2 ± 0.22 |
| ADP | Agonist | 334.9 ± 3.9 |
P2Y1 receptor antagonists were also investigated for their capacity to compete with [125I]MRS2500 for binding to the P2Y1 receptor. The nucleotide antagonists MRS2179, MRS2279 and MRS2500 inhibited [125I]MRS2500 binding with Ki values in accordance with the KB values determined for these same antagonists for inhibition of P2Y1 receptor-promoted second messenger signaling (Table 2) [35,36,37].
3.4. Effect of P2Y agonists on glucose stimulated insulin secretion from MIN6 cells
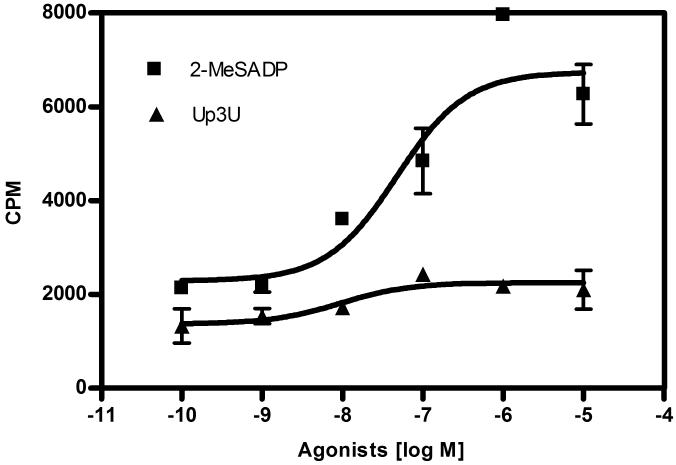
MIN6 cells were stimulated by 16.7 mM glucose in the absence or presence of nucleotide agonists of P2Y receptors, and insulin release was measured by ELISA (Figure 4). 2-MeSADP, MRS2365 and Up3U produced a concentration-dependent increase in insulin secretion at high glucose levels (16.7 mM) when compared with 16.7 mM glucose alone as a control. The EC50 values were 44.6±7 nM, 25.8±5.6 nM and 30.7±12.7 nM (n=3), respectively for 2-MeSADP, MRS2365 and Up3U.
Figure 4.
(A) The effect of P2Y1 and P2Y6 agonists, 2-MeSADP and Up3U respectively, on insulin secretion was studied in the presence of 16.7 mM glucose. EC50 (nM) values obtained were: 2-MeSADP (EC50 = 44.6±7.0), MRS2365 (EC50 = 25.8±5.6), and Up3U (EC50 = 30.7±12.7). (B) Effect of muscarinic receptor agonist (oxotremorine-M) and P2Y1 receptor agonists (ADP and 2-MeSADP) on insulin secretion in the presence of 16.7 mM glucose. (C) Effect of P2Y1 antagonist, MRS2179 (30 μM), and P2Y13 antagonist, MRS2211 (30 μM), on insulin secretion induced by 2-MeSADP and the effect of P2Y6 antagonist MRS2578 (10 μM) on Up3U induced insulin secretion. (D) Glucose-dependent increase in insulin secretion observed in response to 2-MeSADP (10 μM) and Up3U (10 μM) was evaluated under increasing concentrations of glucose in MIN6 cells. Insulin secretion by both 2-MeSADP and Up3U was significantly increased in the presence of higher glucose concentrations compared with 5 mM glucose concentration. Data represent mean±s.e.m, n=3. Analysis of results was performed by ANOVA, followed by Tukey-Kramer multiple comparison test. *P<0.05 when compared to control; aP<0.05 when compared to 10 μM 2-MeSADP; bP<0.05 when compared to 10 μM Up3U.
The P2Y1/P2Y12/P2Y13 agonists ADP and 2-MeSADP at 10 μM stimulated insulin release, although not to the same extent as the muscarinic agonist oxotremorine-M (300 μM) (Figure 4B). In concentration response studies, the levels of insulin secretion induced by 10 μM ADP and 100 nM 2-MeSADP were found to be maximal (Figure 4B).
Since 2-MeSADP is also an agonist of P2Y13 receptors, we tried to determine if the amplification of insulin secretion by 2-MeSADP is a combined action of P2Y1, P2Y12, and P2Y13 receptors or otherwise. Insulin secretion by 2-MeSADP was studied in the presence of MRS2179, a specific P2Y1 antagonist, and MRS2211, a specific P2Y13 antagonist. 10 μM of MRS2179 significantly decreased the insulin secretion by 2-MeSADP, by blocking the P2Y1 receptors in MIN6 cells. The increase in insulin release induced by 10 μM of 2-MeSADP was not antagonized by MRS2211 (Figure 4C). The inability of MRS2211 (1 – 30 μM) to either antagonize or enhance the effects of 2-MeSADP in insulin release (16.7 mM glucose) was also demonstrated at 10 and 100 nM 2-MeSADP. MRS2211 (30 μM) also had no effect on the baseline insulin release in these cells (data not shown). This demonstrates that the increased insulin secretion through 2-MeSADP could be mediated through the P2Y1 receptor. Also, the P2Y6 receptor antagonist MRS2578 (10 μM) significantly blocked the insulin secretagogue effect of P2Y6 agonist Up3U at 10 μM. This confirmed that the increased insulin secretion by Up3U was mediated through P2Y6 receptors present in MIN6 cells.
A more detailed study on the effects of P2Y ligands at varying glucose concentrations (5 - 35 mM) clearly demonstrated the glucose dependency of the P2Y receptor-induced insulin secretion (Figure 4D). Both P2Y1 agonist 2-MeSADP (10 μM) and P2Y6 agonist Up3U (10 μM) could increase insulin secretion with increasing glucose concentrations. A significant (P<0.05) increase in insulin concentration was observed at higher glucose concentration (10-35 mM) when compared to low glucose concentration (5 mM). A 4-fold increase by 2-MeSADP and a 2-fold increase by Up3U in insulin secretion was observed at 35 mM glucose when compared with glucose alone as controls in MIN6 cells. In contrast, at a low glucose concentration (3.3 mM) Up3U did not show any significant effect on insulin secretion, while 2-MeSADP produced a significant increase (P<0.05) in insulin secretion (data not shown). This demonstrated that the P2Y ligands act as glucose-dependent insulin secretagogues.
3.5. Effect of P2Y ligands on intracellular calcium and IP3 in MIN6 cells
P2Y1 and P2Y6 receptors act through Gq proteins, and stimulation of these receptors results in activation of PLC. PLC activation leads to increased intracellular levels of IP3 and diacyl glycerol (DAG) [21,38]. The actions of these second messengers include increased release of intracellular calcium from endoplasmic reticulum by IP3 and activation of protein kinase C (PKC) by DAG. Our earlier reports have confirmed the increased concentrations of intracellular calcium and IP3 through activation of P2Y1 and P2Y6 receptors in pancreatic islets and other cells. With this background, we tried to establish the mechanism of insulin secretion by P2Y receptors in MIN6 cells.
The functional responses of P2Y1 and P2Y6 receptors to the stimulation by their respective ligands 2-MeSADP and Up3U were determined in MIN6 cells (Figure 5A). 2-MeSADP and Up3U induced an increased accumulation of intracellular IP3 in MIN6 cells when compared to control MIN6 cells. Although the intensity of the IP3 accumulation was low in response to Up3U when compared to 2-MeSADP, nevertheless both ligands exhibited high potency with EC50 values of 11.7±1.2 and 29±7.3 nM, respectively. The IP3 assay confirmed the expression of functionally active P2Y1 and P2Y6 receptors in mouse pancreatic islets.
Figure 5.
The functional response in MIN6 cells to P2Y1 and P2Y6 receptor agonists, 2-MeSADP and Up3U, respectively. Activation of PLC by Gq-coupled P2Y1 and P2Y6 receptors lead to significant accumulation of intracellular IP3 (A) and intracellular calcium (B) in MIN6 cells. EC50 (nM) values for accumulation of intracellular IP3 were: 2-MeSADP (EC50 = 11.7±1.2) and Up3U (EC50 = 29±7.3). Data represent mean±s.e.m, n=3.
Subconfluent MIN6 cells were incubated in a fluorescent calcium-4 dye and then treated with 2-MeSADP and Up3U. The change in intracellular calcium was determined through change in fluorescence intensities. Both 2-MeSADP and Up3U exhibited a significant concentration-dependent increase in intracellular calcium levels when compared to the control MIN6 cells. The EC50 was calculated as 3.4±0.6 nM and 3.2±0.7 nM for 2-MeSADP and Up3U, respectively (Figure 5B).
3.6. Nuclei and chromatin staining of MIN6 cells
In order to elucidate the role of P2Y receptors in the preservation of β cell mass, which may help in the attenuation of diabetes in both type 1 and type 2 diabetes, we evaluated apoptosis in TNF-α-treated MIN6 cells. Nuclei and chromatin staining of MIN6 cells was performed using the fluorophores HO342 and PI. In untreated control cells, HO342 fluorescence was homogenously distributed throughout the cell nuclei, and no chromatin condensation or nuclear fragmentation was found (Figure 6A). This staining pattern was observed in approximately 90% of the control cells in each field-of-view. In contrast, the nuclei of MIN6 cells exposed to 50 ng/ml of TNF-α exhibited condensed or fragmented chromatin as evidenced in Figure 6B, which is a characteristic of apoptosis. About 30-40% of nuclei in each field-of-view also showed double staining with HO342 and PI, an indicator of late stage apoptosis. In the presence of 10 μM of Up3U, TNF-α treated MIN6 cells showed a clear reduction in chromatin condensation as evidenced by decreased double staining of nuclei. This type of staining pattern was evident in only ~10% of the cells, and more than 80% of the cells were homogeneously stained only with HO342 (Figure 6C). In the presence of 2-MeSADP, TNF-α treated MIN6 cells showed no significant cytoprotection (Figure 6D). These results show that the activation of P2Y6 receptors may lead to prevention of cell death in TNF-α-induced apoptosis in pancreatic islet cells.
Figure 6.
MIN6 cells were pretreated with either 2-MeSADP or Up3U and apoptosis was induced by treatment with TNF-α (50 ng/ml). After 24 h, cells were stained with HO342 and PI. Fluorescence micrographs of: (A) control cells; (B) MIN6 cells treated with TNF-α showing apoptosis; (C) MIN6 cells treated with Up3U + TNF-α showing significant cytoprotection; (D) MIN6 cells treated with 2-MeSADP + TNF-α with no apparent changes when compared to (B). Bars shown indicate 50 μm.
3.6 Cytoprotective effect of the P2Y6 agonist Up3U in MIN6 cells
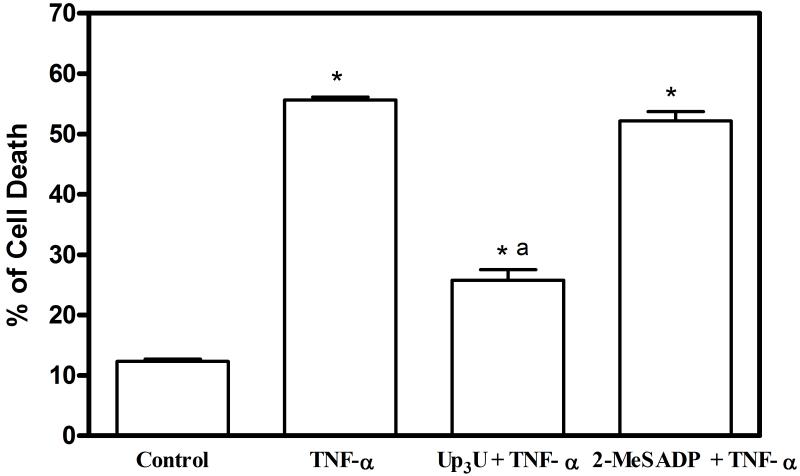
The efficiency of the selective P2Y agonists in preventing cell death induced by TNF-α in MIN6 cells was further evaluated using flow cytometry (Figure 7). Flow cytometric analysis of PI-stained apoptotic nuclei resulted in a broad peak of hypodiploid particles, clearly separated from the sharp diploid DNA peak of normal cells. The baseline rate of apoptosis in untreated MIN6 cells was 10.2%. In MIN6 cells exposed to 50 ng/ml of TNF-α, the percentage of dead cells observed for a sub-G1 peak was 54%. MIN6 cells pretreated with Up3U and TNF-α showed a significant reduction in cell death (25%) when compared to cells exposed to TNF-α alone. Though the cell death was reduced in 2-MeSADP-pretreated cells exposed to TNF-α (43%), no significant reduction was observed when compared to cells exposed to TNF-α only. A significant reduction of cell death in TNF-α exposed MIN6 cells pre-treated with Up3U clearly indicated that the stimulation of P2Y6 receptors resulted in cytoprotection of MIN6 cells.
Figure 7.

Cytoprotective effect of Up3U against TNF-α induced cell death was determined using flow cytometry. MIN6 cells were cultured in the absence or presence of TNF-α (50 ng/ml) either alone or in the presence of Up3U or 2-MeSADP for 24 h. The percentage of cells displaying sub-G1 quantities of DNA was determined using a FACS Calibur. Representative FACS histograms are shown with horizontal bars indicating the gate setting (M1) used to detect sub-G1 (apoptotic) cells. (B) Data represent mean±s.e.m, n=3. Analysis of results was performed by ANOVA, followed by Tukey-Kramer multiple comparison test. *P<0.05 when compared to control; aP<0.05 when compared to TNF-α.
4. Discussion
In this study, we demonstrated that the activation of P2Y1 and P2Y6 receptors by their selective ligands lead to increased insulin secretion in mouse pancreatic β cells. This is in seeming contradiction to our previous work, in which we have reported a decreased insulin secretion through stimulation of P2Y receptors at high glucose concentrations in the mouse pancreatic β-TC6 cell line [33]. Stimulation of insulin secretion by nucleotides is more consistent with earlier reports. Therefore, the MIN6 cell line might be more representative of native pancreatic cells than β-TC6 cells.
In addition to their biochemical role in the storage and transfer of energy, nucleotides also function as signaling molecules. This signaling role of nucleotides, which may be due to both intra- and extracellular actions, is being explored as a drug target for various disorders [38]. The role of P2Y receptors, which are activated by these extracellular nucleotides, is well documented in pancreatic β cells [11,38-40]. The activation of P2Y receptors by specific agonists results in enhanced insulin secretion, which could be of therapeutic value in the treatment of diabetes.
In the present study, we analyzed the effects of activation of P2Y1 and P2Y6 receptors in MIN6 cells through selective agonists. Both P2Y1 and P2Y6 receptor agonists, 2-MeSADP and Up3U, respectively, potentiated insulin secretion in MIN6 cells with high efficacy. Although both ligands showed high EC50 values, the amount of insulin secretion was much higher through stimulation by 2-MeSADP when compared to that of Up3U. As both P2Y1 and P2Y6 act through Gq-coupled receptors, this difference in stimulation may be ascribed to differences in the receptor expression or in interaction with other receptors. Also, insulin secretion in the presence of specific P2Y1 and P2Y13 receptor antagonists demonstrated that the amplification of insulin secretion by 2-MeSADP was through the P2Y1 receptor. MRS2365, a specific P2Y1 agonist, was also used to show that the insulin secreting effect of 2-MeSADP was mediated through P2Y1 receptors and not through either P2Y12 or P2Y13 receptors. Similarly, the blocking of the insulin secreting effect of Up3U by P2Y6-specific antagonist MRS2578 also highlighted the involvement of P2Y6 receptors in insulin secretion.
Apart from the elevated insulin secretion in the present study, the ability of these P2Y receptors to secrete insulin in the presence of increased glucose was also apparent from previous studies [41,42]. Both 2-MeSADP and Up3U showed a significant increase in insulin secretion at higher glucose concentration (35 mM) in comparison to low glucose concentration (5 mM). This further emphasizes the potential therapeutic value of these P2Y receptor agonists. Since these ligands boost insulin secretion at higher glucose concentrations, the development of hypoglycemia would be abated in diabetic patients. Though there is a significant increase in insulin secretion at low glucose concentration (3.3 mM, data not shown) by 2-MeSADP, we strongly believe that the 4-fold increase in glucose concentration at higher glucose concentrations (35 mM) is due to the response of 2-MeSADP to the shift in glucose from lower to higher concentrations. Hence on the basis of our results, we propose that these P2Y agonists act as insulin secretagogues in a glucose-dependent manner.
Though the effect of P2Y receptors on insulin secretion is well documented, the exact mechanism of action of these P2Y receptors on pancreatic islets is not yet fully determined. Specifically in this study, G protein-coupled P2Y receptors mediate intracellular signal transduction pathways in pancreatic β cells. The activation of islet GPCRs results in multiple pathways of β-cell signaling, involving alterations in intracellular levels of cAMP, IP3, and calcium ions, as well as changes in protein phosphorylation and protein acylation [39,43-44]. This leads to modulation of other signaling pathways such as kinases, some but not all of which are documented and which can potentiate insulin secretion. Our study also shows significantly increased levels of IP3 and calcium ions in MIN6 cells in response to P2Y1 and P2Y6 agonists. This increase in second messengers could account for the increased insulin secretion through P2Y receptor signalling pathways. However, additional studies will be required to elucidate the exact mechanism of action of these P2Y receptors.
Type 1 and type 2 diabetes are characterized by β cell failure, and apoptosis is the main form of β cell death in both types of diabetes. In type 1 diabetes, autoimmune responses can lead to β cell failure and death. Pancreatic β cell death in type 2 diabetes is influenced by a number of factors, such as glucose toxicity, free radicals, and intra-islet inflammatory mediators [45]. A recent report by Donath et al. [46] states that islet inflammation caused by activation of interleukin-1β is a major causative factor for β cell death in type 2 diabetes. Though increased insulin secretion by insulin secretagogues may decrease circulating glucose and thus decrease β cell death caused by glucose toxicity [47], it is also essential to preserve the existing β-cell mass. In this context, we made an approach to study the cytoprotective effect of P2Y receptor ligands on TNF-α induced apoptosis in MIN6 cells. We found that the P2Y6 receptor agonist Up3U could significantly reduce TNF-α induced cell death, and the P2Y1 receptor agonist 2-MeSADP did not display a significant effect on cell death. This could be due to the cytoprotective effect of P2Y6 receptor, as described in our earlier reports on astrocytoma cells and skeletal muscle cells [48,49]. Though the mechanism of action is not yet fully understood, we could interpret that the stimulation of P2Y6 receptor leads to cell survival either by activation of isozymes of protein kinase C (PKC) or through activation of NF-κB and ERK [21,23,48-50]. This cytoprotective effect exhibited by the P2Y6 agonist Up3U resulted in the preservation of β-cell mass. This potential of P2Y6 ligands to protect β cells as well as to stimulate insulin secretion could be beneficial therapeutically.
In conclusion, the results demonstrate that the activation of P2Y1 and P2Y6 receptors by their selective agonists leads to increased insulin secretion through G protein signaling. Also, P2Y6 receptor activation by Up3U resulted in the prevention β cell death induced by TNF-α. Decreasing β cell mass is widely recognized as an inhibiting factor in the long term treatment of diabetic patients. Hence, this ability of the P2Y6 receptor agonists to increase insulin secretion as well as preserve β cell mass could be an important consideration for its therapeutic application. Further analysis of the effects of extracellular nucleotides on pancreatic β cells will be needed to elucidate the mechanism and development of a P2Y receptor agonist as a drug for diabetes.
Acknowledgments
This work was supported in part by the Intramural Research Program of NIDDK, National Institutes of Health, Bethesda, MD, USA. We thank Dr. Takami Oka and Dr. Masahiro Ohtani (Dept. of Pharmacology, Osaka Dental University, Osaka, Japan) and Prof. T.K. Harden (Univ. of North Carolina School of Medicine, Chapel Hill, NC) for helpful discussions.
Abbreviations
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- KRBB
Kreb’s ringer bicarbonate buffer
- MRS2179
N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate
- MRS2211
pyridoxal-5′-phosphate-6-azo-(2-chloro-5-nitrophenyl)-2,4-disulfonic acid
- MRS2279
2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate
- MRS2365
(N)-methanocarba-2′-deoxy-2-methylthio-adenosine-5′-diphosphate
- MRS2500
2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate
- 2-MeSADP
2-methylthioadenosine 5′-diphosphate
- Tris
tris(hydroxymethyl) aminomethane
- Up3U
P1,P4-di(uridine 5′-)triphosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Burnstock G, Williams M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J Pharmacol Exp Ther. 2000;295:862–9. [PubMed] [Google Scholar]
- [2].Burnstock G. Physiological and pathological roles of purines: an update. Drug Dev Res. 1998;28:195–206. [Google Scholar]
- [3].Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–92. [PubMed] [Google Scholar]
- [4].Abbracchio MP, Saffrey MJ, Hopker V, Burnstock G. Modulation of astroglial cell proliferation by analogues of adenosine and ATP in primary cultures of rat striatum. Neuroscience. 1994;59:67–76. doi: 10.1016/0306-4522(94)90099-x. [DOI] [PubMed] [Google Scholar]
- [5].Van Kolen K, Slegers H. Integration of P2Y receptor-activated signal transduction pathways in G protein-dependent signalling networks. Purinergic Signal. 2006;2:451–69. doi: 10.1007/s11302-006-9008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Verspohl EJ, Johannwille B, Waheed A, Neye H. Effect of purinergic agonists and antagonists on insulin secretion from INS-1 cells (insulinoma cell line) and rat pancreatic islets. Can J Physiol Pharmacol. 2002;80:562–68. doi: 10.1139/y02-079. [DOI] [PubMed] [Google Scholar]
- [7].Bertrand G, Chapal J, Puech R, Loubatieres-Mariani MM. Adenosine-5′-O-(2-thiodiphosphate) is a potent agonist at P2 purinoceptors mediating insulin secretion from perfused rat pancreas. Br J Pharmacol. 1991;102:627–30. doi: 10.1111/j.1476-5381.1991.tb12223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fernandez-Alvarez J, Hillaire-Buys D, Loubatieres-Mariani MM, Gomis R, Petit P. P2 receptor agonists stimulate insulin release from human pancreatic islets. Pancreas. 2001;22:69–71. doi: 10.1097/00006676-200101000-00012. [DOI] [PubMed] [Google Scholar]
- [9].Hauge-Evans AC, Squires PE, Belin VD, Roderigo-Milne H, Ramracheya RD, Persaud SJ, et al. Role of adenine nucleotides in insulin secretion from MIN6 pseudoislets. Mol Cell Endocrinol. 2002;191:167–76. doi: 10.1016/s0303-7207(02)00051-5. [DOI] [PubMed] [Google Scholar]
- [10].Poulsen CR, Bokvist K, Olsen HL, Hoy M, Capito K, Gilon P, et al. Multiple sites of purinergic control of insulin secretion in mouse pancreatic β-cells. Diabetes. 1999;48:2171–81. doi: 10.2337/diabetes.48.11.2171. [DOI] [PubMed] [Google Scholar]
- [11].Farret A, Vignaud M, Dietz S, Vignon J, Petit P, Gross R. P2Y purinergic potentiation of glucose-induced insulin secretion and pancreatic β-cell metabolism. Diabetes. 2004;53:S63–6. doi: 10.2337/diabetes.53.suppl_3.s63. [DOI] [PubMed] [Google Scholar]
- [12].Parandeh F, Abaraviciene SM, Amisten S, Erlinge D, Salehi A. Uridine diphosphate (UDP) stimulates insulin secretion by activation of P2Y6 receptors. Biochem Biophys Res Commun. 2008;370:499–503. doi: 10.1016/j.bbrc.2008.03.119. [DOI] [PubMed] [Google Scholar]
- [13].Lee DH, Park KS, Kim DR, Lee JW, Kong ID. Dual effect of ATP on glucose-induced insulin secretion in HIT-T15 cells. Pancreas. 2008;37:302–8. doi: 10.1097/MPA.0b013e318168daaa. [DOI] [PubMed] [Google Scholar]
- [14].von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–32. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- [15].Bourdon DM, Mahanty SK, Jacobson KA, Boyer JL, Harden TK. (N)-methanocarba-2MeSADP (MRS2365) is a subtype-specific agonist that induces rapid desensitization of the P2Y1 receptor of human platelets. J Thromb Haemost. 2006;4:861–8. doi: 10.1111/j.1538-7836.2006.01866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analog. J Pharmacol Exp Ther. 2004;311:1038–43. doi: 10.1124/jpet.104.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ko H, Carter RL, Cosyn L, Petrelli R, de Castro S, Besada P, et al. Synthesis and potency of novel uracil nucleotides and derivatives as P2Y2 and P2Y6 receptor agonists. Bioorg Med Chem. 2008;16:6319–32. doi: 10.1016/j.bmc.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pendergast W, Yerxa BR, Douglass JG, Shaver SR, Dougherty RW, Redick CC, Sims IF, Janet L, Rideout JL. Synthesis and P2Y receptor activity of a series of uridine dinucleoside 5′-polyphosphates. Bioorg Med Chem Lett. 2001;11:157–160. doi: 10.1016/s0960-894x(00)00612-0. [DOI] [PubMed] [Google Scholar]
- [19].Mamedova LK, Joshi BV, Gao ZG, von Kügelgen I, Jacobson KA. Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol. 2004;67:1763–70. doi: 10.1016/j.bcp.2004.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hechler B, Nonne C, Roh EJ, Cattaneo M, Cazenave JP, Lanza F, et al. MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. J Pharmacol Exp Ther. 2006;316:556–63. doi: 10.1124/jpet.105.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Houston D, Ohno M, Nicholas RA, Jacobson KA, Harden TK. [32P]2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate [32P]MRS2500), a novel radioligand for quantification of native P2Y1 receptors. Br J Pharmacol. 2006;147:459–67. doi: 10.1038/sj.bjp.0706453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ohlmann P, de Castro S, Gachet C, Jacobson KA, Harden TK. Quantification of recombinant and platelet P2Y1 receptors utilizing a [125I]-labeled high affinity antagonist 2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate [125I]MRS2500) in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim SG, Gao ZG, Soltysiak KA, Chang TS, Brodie C, Jacobson KA. P2Y6 nucleotide receptor activates PKC to protect 1321N1 astrocytoma cells against tumor necrosis factor-induced apoptosis. Cell Mol Neurobiol. 2003;23:401–18. doi: 10.1023/A:1023696806609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim SG, Soltysiak KA, Gao ZG, Chang TS, Chung E, Jacobson KA. Tumor necrosis factor alpha-induced apoptosis in astrocytes is prevented by the activation of P2Y6, but not P2Y4 nucleotide receptors. Biochem Pharmacol. 2003;65:923–31. doi: 10.1016/s0006-2952(02)01614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, et al. Establishment of a pancreatic β cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–32. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- [26].Vial C, Evans RJ. P2X1 receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol Pharmacol. 2002;62:1438–45. doi: 10.1124/mol.62.6.1438. [DOI] [PubMed] [Google Scholar]
- [27].Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji XD, et al. Quantitation of the P2Y1 receptor with a high affinity radiolabeled antagonist. Mol Pharmacol. 2002;62:1249–57. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yasuda J, Nishioka W, Sakudo A, Yama S, Setoguchi R, Saeki K, et al. Suppressor mechanism of serum thymic factor on tumor necrosis factor-α-induced apoptosis in the mouse pancreatic β-cell line. Biophys Biochem Res Comm. 2003;311:501–5. doi: 10.1016/j.bbrc.2003.10.025. [DOI] [PubMed] [Google Scholar]
- [29].Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protocols. 2006;1:1458–61. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- [30].Choi SE, Choi KM, Yoon IH, Shin JY, Kim JS, Park WY, et al. IL-6 protects pancreatic islet β cells from pro-inflammatory cytokines-induced cell death and functional impairment in vitro and in vivo. Transpl Immunol. 2004;13:43–53. doi: 10.1016/j.trim.2004.04.001. [DOI] [PubMed] [Google Scholar]
- [31].Hoorens A, Van de Casteele M, Klöppel G, Pipeleers D. Glucose promotes survival of rat pancreatic β cells by activating synthesis of proteins which suppress a constitutive apoptotic program. J Clin Invest. 1996;98:1568–74. doi: 10.1172/JCI118950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kimoto K, Suzuki K, Kizaki T, Hitomi Y, Ishida H, Katsuta H. Gliclazide protects pancreatic β-cells from damage by hydrogen peroxide. Biochem Biophys Res Comm. 2003;303:112–19. doi: 10.1016/s0006-291x(03)00310-3. [DOI] [PubMed] [Google Scholar]
- [33].Ohtani M, Suzuki J-I, Jacobson KA, Oka T. Evidence for the possible involvement of the P2Y6 receptor in Ca2+ mobilization and insulin secretion in mouse pancreatic islets. Purinergic Signal. 2008;4:365–75. doi: 10.1007/s11302-008-9122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jacobson KA, Ivanov AA, de Castro S, Harden TK, Ko H. Development of selective agonists and antagonists of P2Y receptors. Purinergic Signal. 2009;5:75–89. doi: 10.1007/s11302-008-9106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kim HS, Ohno M, Xu B, Kim HO, Choi Y, Ji XD, et al. 2-Substitution of adenine nucleotide analogues containing a bicyclo[3.1.0]hexane ring system locked in a northern conformation: enhanced potency as P2Y1 receptor antagonists. J Med Chem. 2003;46:4974–87. doi: 10.1021/jm030127+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Boyer JL, Adams M, Ravi RG, Jacobson KA, Harden TK. 2-Chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine- 3′,5′-bisphosphate is a selective high affinity P2Y1 receptor antagonist. Br J Pharmacol. 2002;135:2004–10. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Boyer JL, Mohanram A, Camaioni E, Jacobson KA, Harden TK. Competitive and selective antagonism of P2Y1 receptors by N6-methyl-2′-deoxyadenosine-3-,5-bisphosphate. Br J Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Petit P, Lajoix AD, Gross R. P2 purinergic signalling in the pancreatic β-cell: control of insulin secretion and pharmacology. Eur J Pharm Sci. 2009;37:67–75. doi: 10.1016/j.ejps.2009.01.007. [DOI] [PubMed] [Google Scholar]
- [39].Winzell MS, Ahrén B. G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacol Ther. 2007;116:437–48. doi: 10.1016/j.pharmthera.2007.08.002. [DOI] [PubMed] [Google Scholar]
- [40].Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8:369–85. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- [41].Petit P, Hillaire-Buys D, Manteghetti M, Debrus S, Chapal J, Loubatières-Mariani MM. Evidence for two different types of P2 receptors stimulating insulin secretion from pancreatic B cell. Br J Pharmacol. 1998;125:1368–74. doi: 10.1038/sj.bjp.0702214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Farret A, Filhol R, Linck N, Manteghetti M, Vignon J, Gross R, Petit P. P2Y receptor mediated modulation of insulin release by a novel generation of 2-substituted-5′-O-(1-boranotriphosphate)-adenosine analogues. Pharm Res. 2006;23:2665–71. doi: 10.1007/s11095-006-9112-4. [DOI] [PubMed] [Google Scholar]
- [43].Hutchinson DS, Summers RJ, Bengtsson T. Regulation of AMP-activated protein kinase activity by G-protein coupled receptors: potential utility in treatment of diabetes and heart disease. Pharmacol Ther. 2008;119:291–310. doi: 10.1016/j.pharmthera.2008.05.008. [DOI] [PubMed] [Google Scholar]
- [44].Chevassus H, Roig A, Belloc C, Lajoix AD, Broca C, Manteghetti M, et al. P2Y receptor activation enhances insulin release from pancreatic beta-cells by triggering the cyclic AMP/protein kinase A pathway. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:464–9. doi: 10.1007/s00210-002-0620-4. [DOI] [PubMed] [Google Scholar]
- [45].Zhou Y-P, Teng D, Dralyuk F, Ostrega D, Roe MW, Philipson L, et al. Apoptosis in insulin-secreting cells: Evidence for the role of intracellular Ca2+ stores and arachidonic acid metabolism. J Clin Invest. 1998;101:1623–32. doi: 10.1172/JCI1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care. 2008;31:S161–4. doi: 10.2337/dc08-s243. [DOI] [PubMed] [Google Scholar]
- [47].Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A. Thioredoxin-interacting protein: A critical link between glucose toxicity and β-cell apoptosis. Diabetes. 2008;57:938–44. doi: 10.2337/db07-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mamedova LK, Joshi BV, Gao ZG, von Kügelgen I, Jacobson KA. Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol. 2004;67:1763–70. doi: 10.1016/j.bcp.2004.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mamedova LK, Wang R, Besada P, Liang BT, Jacobson KA. Attenuation of apoptosis in vitro and ischemia/reperfusion injury in vivo in mouse skeletal muscle by P2Y6 receptor activation. Pharmacol Res. 2008;58:232–9. doi: 10.1016/j.phrs.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Korcok J, Raimundo LN, Du X, Sims SM, Dixon SJ. P2Y6 nucleotide receptors activate NF-kappaB and increase survival of osteoclasts. J Biol Chem. 2005;280:16909–15. doi: 10.1074/jbc.M410764200. [DOI] [PubMed] [Google Scholar]