Abstract
The balance between GABA-mediated inhibitory and glutamate-mediated excitatory synaptic transmission represents a fundamental mechanism for controlling nervous system function, and modulators that can alter this balance may participate in the pathophysiology of neuropsychiatric disorders. Pregnenolone sulfate (PS) is a neuroactive steroid that can modulate the activity of ionotropic glutamate and GABAA receptors either positively or negatively, depending upon the particular receptor subtype, and modulates synaptic transmission in a variety of experimental systems. To evaluate the modulatory effect of PS in vivo, we infused PS into rat striatum for 20 min via a microdialysis probe while monitoring local extracellular dopamine (DA) levels. The results demonstrate that PS at low nanomolar concentrations significantly increases extracellular DA levels. The PS -induced increase in extracellular DA is antagonized by the NMDA receptor antagonist, D-AP5, but not by the σ receptor antagonist, BD 1063. The results demonstrate that exogenous PS, at nanomolar concentrations, is able to increase DA overflow in the striatum through an NMDA receptor mediated pathway.
Introduction
Neuroactive steroids exhibit rapid non-genomic modulatory actions on receptors and ion channels located in the neuronal membrane, altering excitatory and inhibitory synaptic transmission. PS, a negatively charged sulfated steroid, has been detected in both human and rat brain, and enzymes for conversion of cholesterol to pregnenolone (P450scc) and sulfation of pregnenolone to PS (pregnenolone sulfotransferase) are present in neural tissue (Tsutsui, 2006). The activity of pregnenolone sulfotransferase in rat C6 glioma cells has been shown to be regulated by AMPA receptors (Kohjitani et al., 2008).
PS exhibits direct modulatory activity at NMDA receptors (NMDARs) (Wu et al., 1991; Malayev et al., 2002). It potentiates spontaneously occurring EPSCs in hippocampal cell cultures (Park-Chung et al., 1997) and exacerbates NMDAR-mediated increases in intracellular Ca2+ and excitotoxicity (Weaver et al., 2000). PS also modulates activity of other neurotransmitter receptors and ion channels, including AMPA, kainate, (Wu et al., 1991; Yaghoubi et al., 1998) and GABAA receptors (Majewska et al., 1988), as well as certain voltage-gated calcium channels (Spence et al., 1991; Bukusoglu and Sarlak, 1996; Hige et al., 2006).
PS modulates neurotransmitter release in multiple systems (Maurice, 2004; Gibbs et al., 2006) and enhances long term potentiation in hippocampal slices (Chen et al., 2007; Sabeti et al., 2007). A PS-like retrograde modulatory factor released by depolarization of CA1 neurons has been reported to play a role in plasticity of immature hippocampal synapses (Mameli et al., 2005). The ability of PS to modulate neurotransmission in multiple receptor systems and by multiple mechanisms suggests that PS may play a role as an endogenous modulator of neurotransmission.
We chose to investigate the effect of PS on striatal dopamine (DA) release using in vivo using microdialysis to determine whether PS might act as a modulator of nigrostriatal DA release. The striatum is the major target of DA neurons in the brain and its activity is critical for the regulation of voluntary movement. Parkinson’s disease results from the specific, progressive degeneration of striatal DA neurons. The striatum is also involved in procedural learning, and changes in striatal activity are implicated in neuropsychiatric disorders including depression and drug addiction. Elucidation of the mechanisms of action of endogenous neurosteroids in this nucleus could lead to approaches for therapeutic intervention in pathologies related to both mood and movement.
Methods
Subjects
Male Sprague-Dawley rats (225-300 g) from Charles River Laboratories (Wilmington, MA) were initially housed in shoe-box cages (2 rats/cage) and were provided with food and water ad libitum. The cages were kept in a temperature-controlled room with a 12-hour light/dark cycle. All experiments were performed during the light cycle. All procedures were carried out under a protocol approved by the Boston University School of Medicine Institutional Animal Care and Use Committee.
Materials
Steroids were purchased from Steraloids (Newport, RI) and prepared as 500× stock solutions in DMSO. All other chemicals were purchased from Sigma-Aldrich Co. (St. Louis, MO).
Stereotaxic surgery
Prior to surgery, animals were anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine and placed in a stereotaxic apparatus (David Kopf Instruments; Tujunga, CA). A rostro-caudal incision was made to expose the dorsal surface of the skull, and a hole was drilled in the skull above the striatum with a stereotaxic drill [+ 0.5 mm A/P and + 2.9 mm M/L relative to bregma, (Paxinos and Watson, 1997)]. Two other holes were drilled in the skull and screws were secured. A dialysis-guide cannula (CMA Microdialysis; Acton, MA) was lowered 3 mm ventrally into the striatum and then was secured to the skull with dental cement. All rats were housed in individual cages following surgery.
In vivo microdialysis
No less than two days after surgery, rats were anesthetized briefly with isoflurane to facilitate removal of the dummy probe from the guide cannula and insertion of a microdialysis probe (CMA 12, dialysis membrane length of 2 mm of polycarbonate, with a molecular weight cut-off of 20 kD) that extended 2 mm below the end of the guide cannula into the striatum. Animals were placed in the experimental cage for at least 12 hours prior to the start of the experiment, while artificial cerebrospinal fluid (aCSF: 145 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 0.2 mM ascorbate, 5.0 mM glucose, pH 7.4) was pumped through the dialysis probe at a rate of 0.2 μl/minute. Forty-five minutes before collection of the first sample, the flow rate was increased to 2 μl/minute. Samples were collected at 20 min intervals. Perfusion tubing had a volume of approximately 2 μl from syringe to probe, and 2 μl from probe to collector, resulting in a perfusion delay of approximately 1 min from syringe to probe and 1 min from probe to collector. As these times are short relative to the 20 min collection interval, no correction was made for the perfusion delay. aCSF was perfused through the microdialysis probe during the collection of all other samples. Samples were stored at -80° C until analysis. After a minimum of 6 baseline samples had been collected, the perfusate was switched via a three-way valve (Rheodyne LLC) from aCSF alone to aCSF plus one of the following: PS (1, 5, 10, 25, 50 nM, or 100 μM), d-(-)-2-amino-5-phosphonopentanoic acid (D-AP5; 100 μM), PS + D-AP5, progesterone (10 nM or 100 μM), pregnenolone (10 nM, 50 nM, or 100 μM), pregnanolone (10 nM, 50 nM, or 100 μM), pregnenolone hemisuccinate (PHS; 5, 50, 300 nM), (+)-N-allylnormetazocine (SKF 10,047; 100 μM), PS (50 nM) + D-AP5 (100 μM), 1(-)[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine (BD 1063; 30 μM), PS (50 nM) + BD1063. After collection of one sample (20 min), the perfusate was switched back to aCSF. For inhibition studies, a two-treatment protocol was employed in which a second drug solution was perfused through the probe for 20 min during collection of sample 13. For these studies a balanced crossover experimental design was used in which the order of treatment with drug and drug + inhibitor was reversed from subject to subject to eliminate potential treatment order bias. DMSO was added to solutions where necessary, such that all perfusing solutions contained the same final concentration of DMSO (0.2%).
Samples were analyzed using high-pressure liquid chromatography with electrochemical detection (HPLC-EC) (ESA; Chelmsford, MA). The mobile phase consisted of 50 mM Na2HPO4, 20 mM citric acid, 1.5 mM heptanesulfonic acid, 0.1 mM EDTA, and 10% methanol at pH 4.5. DA was separated on a 15-cm reverse phase C-18 column (Alltech; Deerfield, IL) and oxidized at a potential of 150 mV. DA concentrations per sample were normalized to an external standard curve and plotted as percentage change from baseline. Using this system, the detection limit for DA is approximately100 pM.
Histology
Upon completion of the microdialysis experiments, animals were given an overdose of sodium pentobarbital (100 mg/kg, i.p.) and were perfused intracardially with 0.9% saline followed by 10% formalin. The brains were removed and coronal sections (75 μm) were taken at the level of the striatum using a Vibratome (Technical Products International; St. Louis, MO). The sections were stained with Cresyl violet. Cannulae placement was determined by an individual who was unaware of prior treatments. Examination of Cresyl violet-stained brain sections revealed some gliosis immediately surrounding the probe secondary to probe placement, but without indication of toxicity, as the amount of gliosis and the number of healthy cells did not differ among treatment groups.
Statistical analysis
DA content of samples was normalized to percent of baseline and analyzed with a one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. Results are presented as the percentage change in extracellular DA levels.
Results
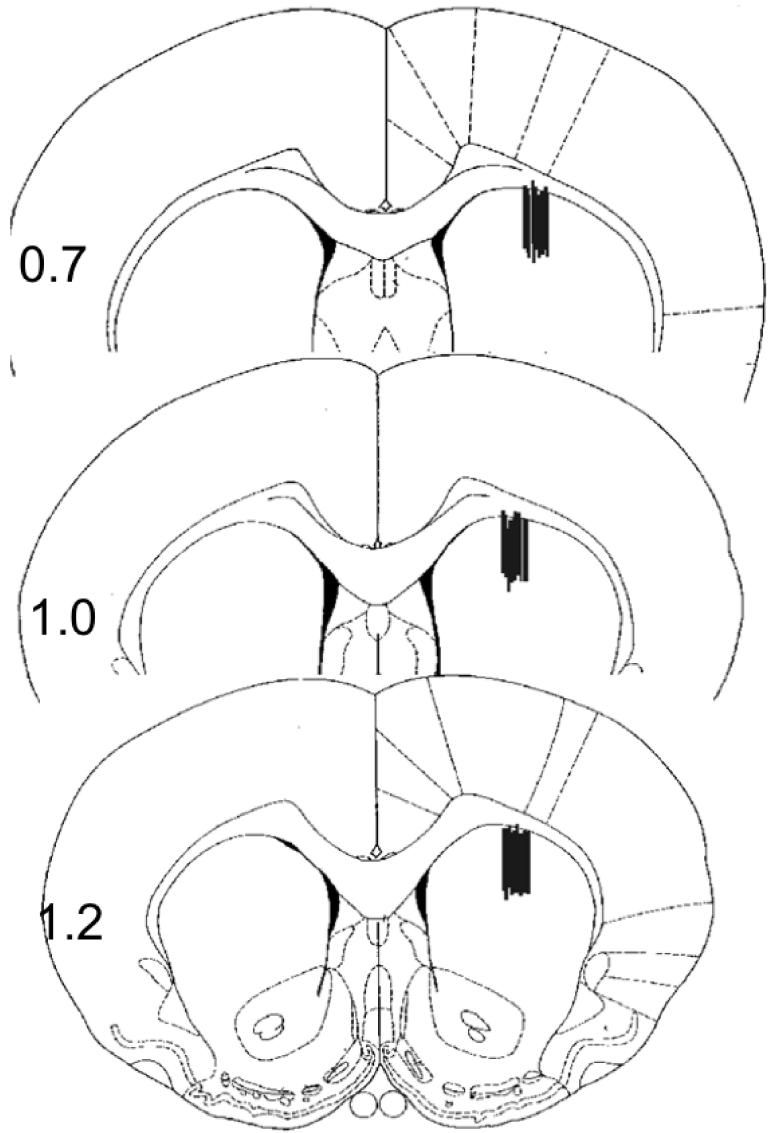
To evaluate whether PS is able to modulate release of DA from synaptic terminals derived from neurons in the substantia nigra, we measured levels of extracellular DA in the striatum using in vivo microdialysis. Microdialysis probes were placed within the dorsal striatum (subsequently verified by histologic examination; Fig. 1) as described previously (Sadri-Vakili et al., 2003).
Figure 1.
Histological verification of microdialysis probe placement in the striatum. Lines indicate the placement of the microdialysis probes in the striatum, and numbers indicate distance from bregma (mm) according to the atlas of Paxinos and Watson (Paxinos and Watson, 1997).
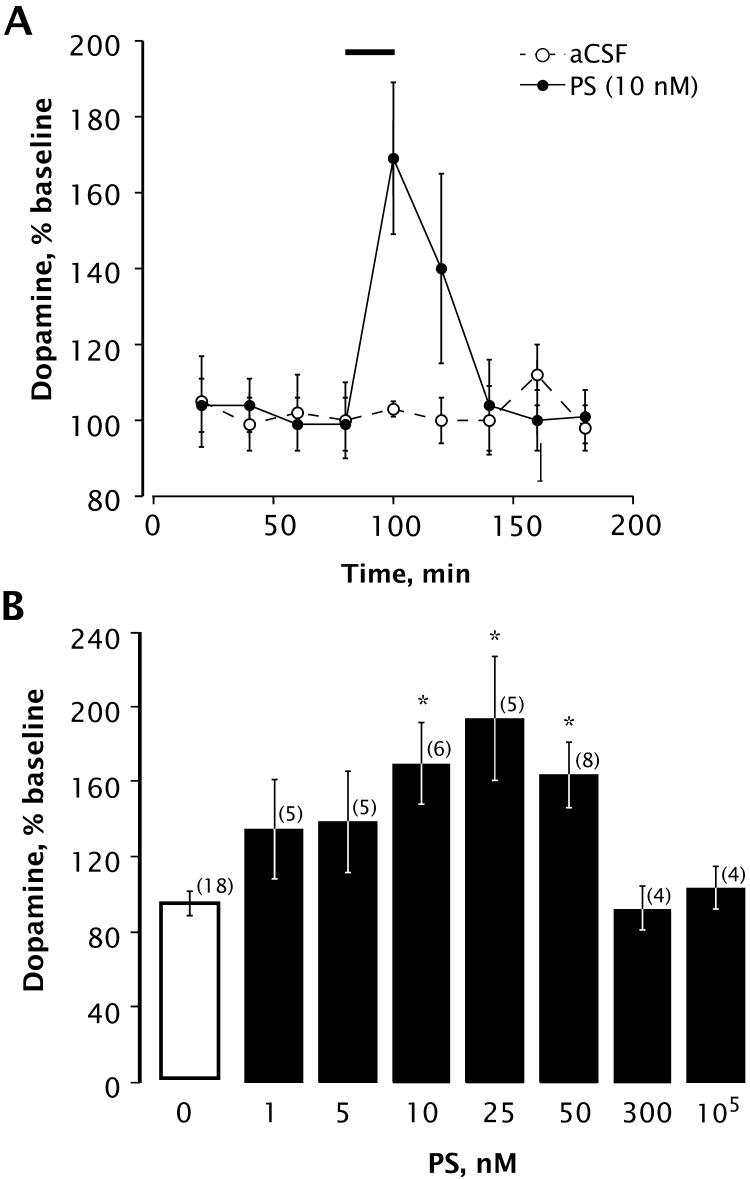
After collection of baseline samples, the perfusate was switched to an aCSF solution containing PS (1, 5, 10, 25, 50, 300 nM, or 100 μM) for 20 minutes. DA content is reported as percent of baseline, with baseline defined as the average of all aCSF samples collected prior to treatments. The average level of DA in baseline samples was 2.0 ± 0.9 nM (n = 8). As shown in Fig. 2A, perfusion of 10 nM PS through the microdialysis probe for 20 min increased the level of extracellular DA in striatum. We observed a significant rise in extracellular DA during perfusion with 10, 25, or 50 nM PS, but DA levels were unaffected by higher concentrations of 300 nM or 10 μM PS (Fig. 2B).
Figure 2.
PS induces an increase in extracellular DA in the rat striatum. A, time course showing DA levels with or without infusion of PS (10 nM) through the dialysis probe (horizontal bar indicates period of PS infusion). Analysis by mixed factors ANOVA with repeated measures over time revealed a significant effect of time [F(8,88) = 4.593; p < 0.0001] and a significant time × treatment interaction [F(8,88) = 4.074; p < 0.0004]. Number of animals is 8 for aCSF group, 6 for PS group. B, concentration dependence of PS-induced increase in striatal extracellular DA. Bars indicate mean ± SEM DA content of the sample collected during 20-min perfusion with the indicated concentration of PS, expressed as percentage of DA content of baseline samples. Statistical analysis revealed a significant main effect of treatment [F(7,69) = 6.789; p < 0.0001]. * Denotes significant differences from baseline values (Bonferroni, p < 0.003). Number of animals is given in parentheses.
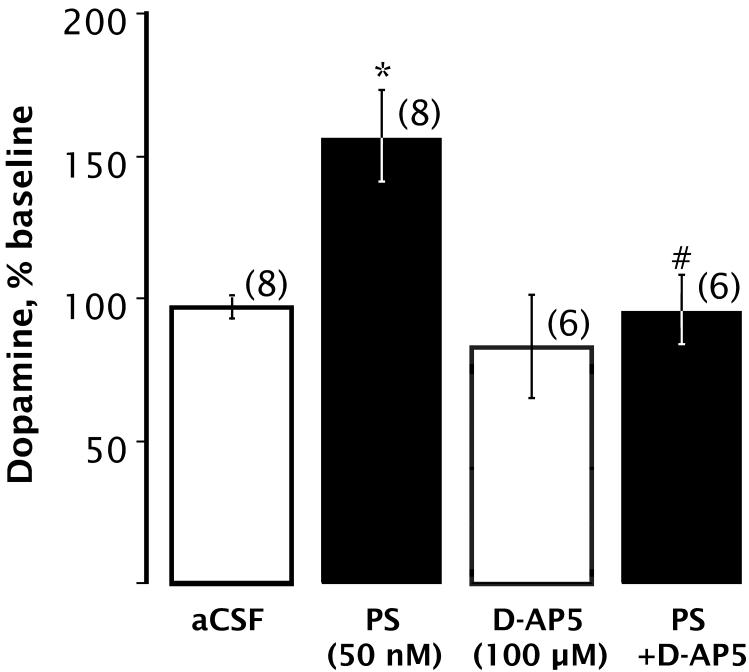
To examine the role of NMDARs in the PS-induced increase in DA overflow, we perfused the specific NMDAR antagonist D-AP5 (100 μM) through the dialysis probe for 20 minutes either alone or together with 50 nM PS. As shown in Fig. 3, D-AP5 blocked the PS-induced increase in extracellular DA, indicating that NMDARs play a role in this action of PS.
Figure 3.
The PS-induced DA increase is inhibited by D-AP5. Bars indicate mean DA content of dialysate collected while perfusing PS and/or D-AP5 through the dialysis probe for 20 min, expressed as percentage of baseline. There was a significant main effect on extracellular DA levels [F(3,25) = 9.948; p < 0.0002]. * Denotes significant differences from baseline values (Bonferroni, p < 0.05). # Denotes significant difference from 50 nM PS (Bonferroni, p < 0.002). Number of animals per group is given in parentheses.
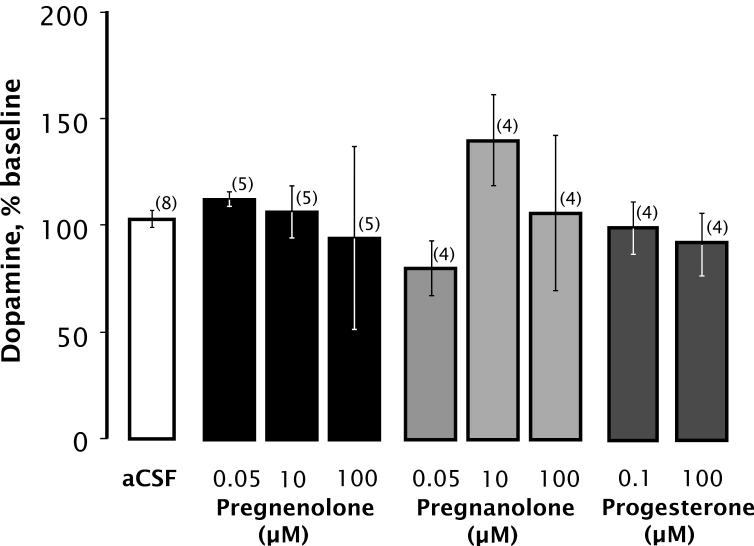
To evaluate the specificity of PS action, we examined the effects of three metabolically related steroids, pregnenolone, pregnanolone, and progesterone. None of these steroids had any significant effect on DA overflow when perfused through the microdialysis probe (Fig. 4).
Figure 4.
PS metabolites do not increase extracellular DA. Statistical analysis revealed no significant differences in DA recovery compared with control (F(8,33) = 1.075; p < 0.404). Data are mean ± SEM DA content of dialysate collected during 20 min perfusion with the indicated compound, expressed as percentage of baseline. Number of animals per group is given in parentheses.
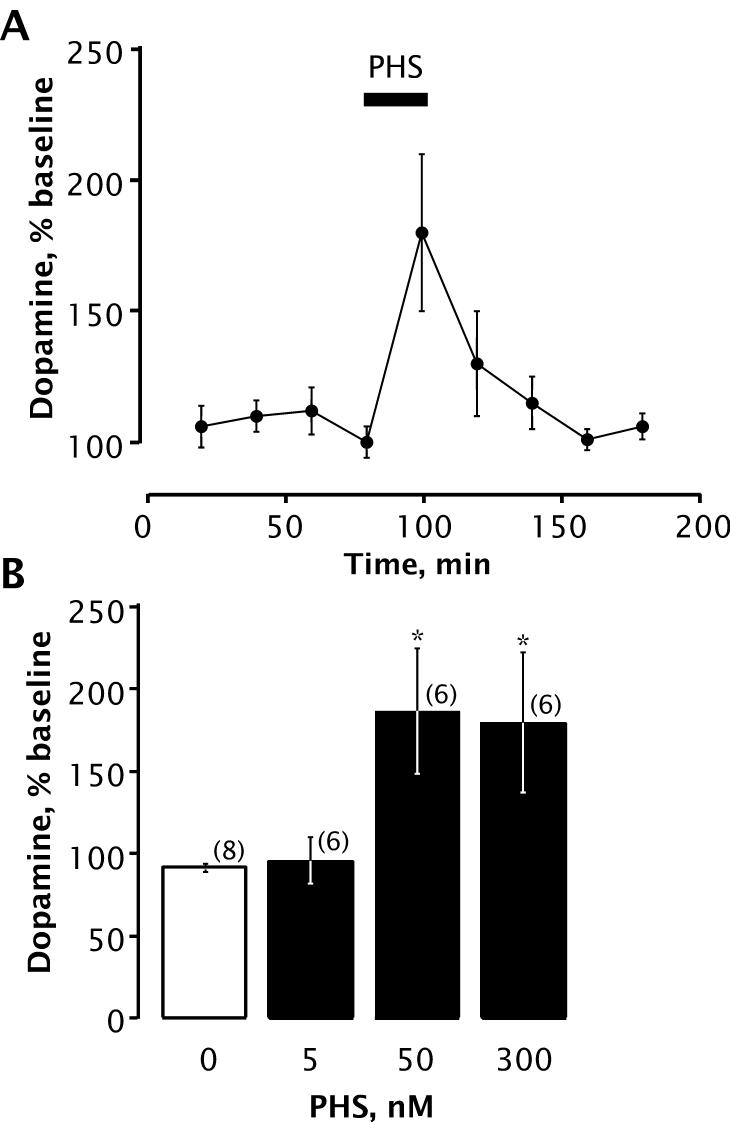
We also assessed the effect of a synthetic steroid mimic of PS bearing a negative charge at C-3: pregnenolone hemisuccinate (PHS), an analog of PS that similarly enhances NMDAR activity (Weaver et al., 2000). Like PS, PHS (50 nM or 300 nM) significantly increased striatal DA levels when perfused through the microdialysis probe (Fig. 5).
Figure 5.
PHS increases extracellular DA. A, time course showing effect of PHS (50 nM) on DA levels. Number of animals is 6 per group. Horizontal bar indicates period of PHS infusion. B, concentration dependence of PHS induced increase in extracellular DA. PHS perfusion resulted in a significant increase in dopamine recovery [F(4, 25) = 3.369; p < 0.025]. * Denotes significant differences from baseline values (Bonferroni, p < 0.05). Data are mean ± SEM DA content of dialysate collected during infusion of PHS (20 min), presented as percentages of baseline values.
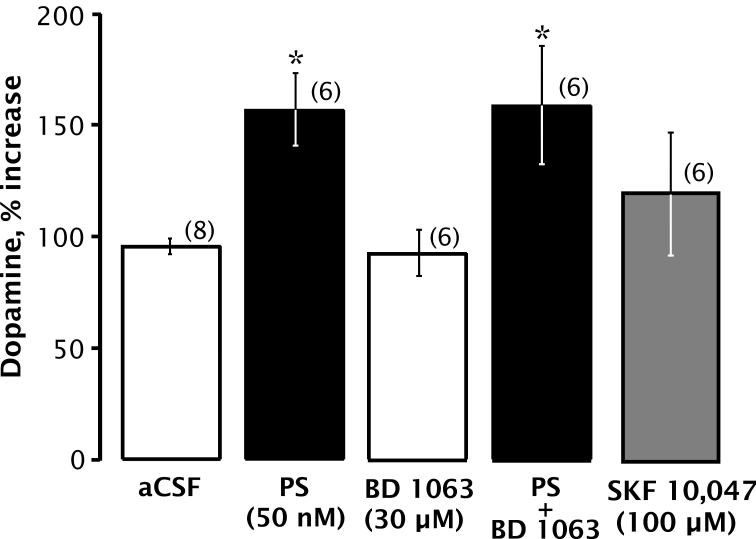
To test for involvement of σ receptors in PS-induced DA release, we investigated the effects of BD 1063, a specific σ-receptor antagonist (Matsumoto et al., 1995). BD 1063 (30 μM) neither reproduced nor blocked PS-induced DA release (Fig. 6), arguing that PS is not acting as a σ agonist or antagonist to increase extracellular DA. Similarly, the σ agonist SKF 10,047 failed to alter extracellular DA levels.
Figure 6.
DA overflow is unaffected by σ receptor ligands. PS and/or BD 1063 or SKF 10,047 was perfused through the dialysis probe. Bars indicate mean ± SEM DA content of the sample collected while perfusing 50 nM PS and/or 30 μM BD 1063 or 100 μM SKF 10,047 through the dialysis probe for 20 min, expressed as percentage of baseline. Statistical analysis revealed a significant main effect of drug treatment [F(4,28) = 3.934, p < 0.011]. Post-hoc analyses revealed a significant increase in DA levels following PS and PS + BD 1063, indicating that BD 1063 does not block the PS-induced increase in DA overflow. SKF 10,047 administration into the striatum through the dialysis probe did not significantly affect extracellular DA levels compared with control. * Denotes significant differences from baseline values (Bonferroni, p < 0.03). Number of animals is 6-8 per group.
Discussion
PS modulates NMDAR function in neuronal cultures (Wu et al., 1991; Weaver et al., 2000) and in heterologous expression systems (Malayev et al., 2002; Horak et al., 2006), and NMDAR activation increases extracellular DA in striatum in vivo (David et al., 2005). PS has been reported to increase DA levels in nucleus accumbens in vivo (Barrot et al., 1999), although in that study PS was injected intracerebroventricularly, so the effective concentration and site of action of PS was uncertain. Here, we examine the effect of introducing low nanomolar concentrations of PS into striatum by reverse in vivo microdialysis.
The tissue content of PS in the rat striatum has been reported to be 17 ng/g (Wang et al., 1997), which if evenly distributed would amount to a concentration of 43 nM, but recent studies have called the methodology of this determination into question (Gibbs et al., 2006), with revised estimates of tissue content corresponding to 2.7 nM in frontal cortex and 4.4 nM in cerebellum of postmortem human brain, and less than 1 nM in rodent brain (Liere et al., 2004). The concentration of free PS in the extracellular space is unknown, but our observation that as little as 10 nM PS administered by reverse microdialysis increases DA overflow in the striatum sets an upper limit to the extracellular concentration of PS. That is, endogenous free PS in the vicinity of the probe must be less than 10 nM, or else there could not be net efflux of PS out of the probe. 10 nM is likely an upper limit for the minimally effective concentration of PS, because with reverse microdialysis the concentration of drug reaching the surrounding tissue is typically 10-50% of that in the probe (Morrison et al., 1991).
PS can be converted by brain sulfatases to pregnenolone, which is inactive at GABAA (Majewska et al., 1988) and NMDA (Weaver et al., 2000) receptors, but can be metabolized to progesterone, which is moderately effective as a positive modulator of the GABAA receptor (Wu et al., 1990). Progesterone can be reduced to yield pregnanolone isomers. In the human, both the 3α,5β (pregnanolone) and 3α,5α (allopregnanolone) isomers are formed, whereas in the rat the 3α,5α isomer is favored. These two pregnanolone isomers are similarly potent and efficacious GABAA receptor positive modulators (Berezhnoy et al., 2007). Perfusion of pregnenolone or progesterone failed to increase DA overflow, arguing against the hypothesis is DA release is induced by pregnenolone metabolites. As a more stringent test of whether GABA receptor modulation could be responsible for DA release, we infused pregnanolone, a potent, high-efficacy GABAA receptor positive modulator. No significant increase in DA overflow was detected.
The PS-induced increase in DA overflow is smaller than that elicited by perfusion of 1 mM NMDA (Sadri-Vakili et al., 2003), but is blocked by D-AP5, indicating an involvement of NMDARs. PS allosterically enhances activation of NMDARs containing NR2A or NR2B subunits (Malayev et al., 2002), suggesting that PS could increase DA overflow by enhancing endogenous glutamate-induced activation of striatal NMDARs. Supporting this hypothesis is the observation that striatal DA overflow is also increased by PHS, a synthetic analog of PS that also enhances NMDAR activation. In contrast, the uncharged steroids pregnenolone, pregnanolone, and progesterone, which are inactive at NMDARs, failed to increase striatal DA levels. Notably, the increase in extracellular DA is lost at PS concentrations of 300 nM or more, which could reflect a lower potency inhibitory effect of PS on DA release. At micromolar concentrations, PS produces net inhibition of NMDARs containing NR2C or NR2D subunits (Malayev et al., 2002), and rapid application studies indicate that an inhibitory action of PS is also present with NMDARs containing the NR2A and NR2B subunits, but is mostly masked by the potentiating action of PS (Horak et al., 2006), arguing that multiple PS modulatory sites with opposing actions are associated with NMDARs. The decline in DA release at higher concentrations could be due to an action of PS at inhibitory sites associated with NMDARs, or it could reflect an indirect inhibitory effect mediated by another receptor type (e.g. kainate, GABAA, or σ receptors).
A difficulty with this hypothesis is that PS stimulates striatal DA release at much lower concentrations than expected from studies of PS modulation of NMDA-induced currents in neuronal cultures or heterologous expression systems, which typically requires PS concentrations ≥1 μM (Wu et al., 1991; Park-Chung et al., 1997; Weaver et al., 2000; Malayev et al., 2002). In contrast, studies of PS modulation of 3H-ifenprodil binding to neuronal membranes (Johansson et al., 2005) or NR1/NR2B receptors expressed in CHO cells (Johansson et al., 2008) reveal that PS at nM concentrations enhances ifenprodil binding, suggesting that there is a higher-affinity mode of interaction between PS and NMDARs. Like PS enhancement of DA overflow, PS enhancement of 3H-ifenprodil binding exhibits a bell-shaped dose-response curve, with loss of modulation at higher concentrations (Johansson et al., 2005; Johansson et al., 2008). It is unclear whether this high potency modulatory effect of PS is mediated through the same binding site(s) that are responsible for modulation of NMDA-induced currents or via an additional site of interaction. The details of the mechanism whereby high-affinity interactions of PS with NMDARs couple to enhancement of DA release remain to be elucidated.
The question of whether presynaptic NMDARs play a role in regulating release of DA from nigrostriatal dopaminergic terminals is controversial. Local application of NMDA increases extracellular DA levels in the striatum, and lesioning of DA neurons in the substantia nigra depletes NMDARs in striatum (David et al., 2005). In contrast, immunolabeling studies have detected only postsynaptic NMDARs in rat striatum, indicating that presynaptic NMDARs, if present, must be at much lower density than on the postsynaptic membrane (Bernard and Bolam, 1998; Gracy et al., 1999). Nevertheless, a role for presynaptic NMDARs in regulating DA release is supported by the observation that NMDA stimulates release of 3H-DA from striatal synaptosomes (Krebs et al., 1991; Pittaluga et al., 2001). We have found, using a mixed population of synaptosomes and synaptoneurosomes, that glutamate or NMDA stimulates the release of newly-accumulated 3H-DA via an NMDAR-dependent mechanism. Moreover, 25 nM PS stimulates the release of 3H-DA by striatal synaptosomes/synaptoneurosomes, but does not affect 3H-DA uptake. Our finding that D-AP5 blocks the PS-induced increase in striatal DA overflow is in agreement with the observation that D-AP5 blocks the PS-induced stimulation of 3H-DA release by striatal synaptosomes/synaptoneurosomes. (Whittaker et al., 2008). It therefore seems likely that the PS-induced increase in striatal DA overflow reflects an action of PS on presynaptic NMDARs located on nigrostriatal dopaminergic terminals.
Another possibility is that PS could act through σ receptors (Gibbs and Farb, 2000; Maurice, 2004), perhaps in concert with NMDARs, to release DA. σ receptors regulate neurotransmitter release in several experimental systems, including NMDA-stimulated release of preloaded 3H-DA from striatal slices (Gonzalez-Alvear and Werling, 1995). PS at nM concentrations inhibits NMDA-induced 3H-norepinephrine release from rat hippocampal slices (Monnet et al., 1995) and reduces IPSC frequency in hippocampal cultures (Mtchedlishvili and Kapur, 2003). These effects are blocked by the σ receptor antagonist BD-1063, as is PS enhancement of paired-pulse facilitation in hippocampal slices (Schiess et al., 2006). Similarly, the σ receptor antagonist NE-100 blocks the ability of PS to attenuate the amnestic effects of MK-801 in vivo (Zou et al., 2000).
PS-induced stimulation of striatal DA release was not affected by co-microdialysis of the σ-antagonist BD 1063, while the σ-agonist SKF 10,047 did not increase extracellular DA levels. Similarly, progesterone, which is also a ligand for σ receptors (Maurice, 2004), failed to alter DA overflow. Given the lack of activity of σ receptor agonists and antagonists, a role for σ receptors in PS regulation of striatal DA release seems unlikely.
The results described in this report demonstrate that nM concentrations of PS influence striatal extracellular DA levels in vivo, supporting the hypothesis that PS could function as an endogenous modulator of neurotransmission. The data show that this action of PS involves NMDARs and is independent of σ receptor activity. The physiological consequences of PS modulation of striatal DA release would depend on which synapses are affected. If PS acts generally to enhance DA release, then it would be expected to enhance the action of DA in regulating the balance between the direct and indirect pathways, which tends to suppress the indirect pathway and favor the direct pathway, thereby enhancing motor activity. Changes in DA levels may also alter mood, motivation, and reward pathways, raising the possibility that changes in modulation of excitatory transmission by endogenous PS could play a role in the etiology of movement and psychiatric disorders. Conversely, compounds that affect PS levels or that mimic or inhibit PS modulation may offer a novel therapeutic approach for diseases involving underactivation or overactivation of DA receptors.
Acknowledgments
This work was supported by NIMH grant R01MH049469, NIDA grant R01DA013724, and NIGMS grant T32GM008541
Abbreviations
- PS
pregnenolone sulfate
- PHS
pregnenolone hemisuccinate
- DA
dopamine
- aCSF
artificial cerebrospinal fluid
- NMDAR
NMDA receptor
References
- Barrot M, Vallee M, Gingras MA, Le Moal M, Mayo W, Piazza PV. The neurosteroid pregnenolone sulphate increases dopamine release and the dopaminergic response to morphine in the rat nucleus accumbens. Eur J Neurosci. 1999;11:3757–3760. doi: 10.1046/j.1460-9568.1999.00816.x. [DOI] [PubMed] [Google Scholar]
- Berezhnoy D, Gravielle M, Farb D. Pharmacology of the GABAA Receptor. In: Silbey D, Kuhar M, Hanin I, Skolnick P, editors. Handbook of Contemporary Neuropharmacology. John Wiley & Sons/Wiley-Interscience; Hoboken, NJ: 2007. pp. 465–568. [Google Scholar]
- Bernard V, Bolam JP. Subcellular and subsynaptic distribution of the NR1 subunit of the NMDA receptor in the neostriatum and globus pallidus of the rat: co-localization at synapses with the GluR2/3 subunit of the AMPA receptor. Eur J Neurosci. 1998;10:3721–3736. doi: 10.1046/j.1460-9568.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- Bukusoglu C, Sarlak F. Pregnenolone sulfate increases intracellular Ca2+ levels in a pituitary cell line. Eur J Pharmacol. 1996;298:79–85. doi: 10.1016/0014-2999(95)00772-5. [DOI] [PubMed] [Google Scholar]
- Chen L, Miyamoto Y, Furuya K, Mori N, Sokabe M. PREGS induces LTP in the hippocampal dentate gyrus of adult rats via the tyrosine phosphorylation of NR2B coupled to ERK/CREB signaling. J Neurophysiol. 2007;98:1538–1548. doi: 10.1152/jn.01151.2006. [DOI] [PubMed] [Google Scholar]
- David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of “intact” animals. Brain Res Brain Res Rev. 2005;50:336–360. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Gibbs TT, Farb DH. Dueling enigmas: neurosteroids and sigma receptors in the limelight. Sci STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.60.pe1. [DOI] [PubMed] [Google Scholar]
- Gibbs TT, Russek SJ, Farb DH. Sulfated steroids as endogenous neuromodulators. Pharmacol Biochem Behav. 2006;84:555–567. doi: 10.1016/j.pbb.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alvear GM, Werling LL. σ1 Receptors in rat striatum regulate NMDA-stimulated [3H]dopamine release via a presynaptic mechanism. Eur J Pharmacol. 1995;294:713–719. doi: 10.1016/0014-2999(95)00617-6. [DOI] [PubMed] [Google Scholar]
- Gracy KN, Clarke CL, Meyers MB, Pickel VM. N-methyl-D-aspartate receptor 1 in the caudate-putamen nucleus: ultrastructural localization and co-expression with sorcin, a 22,000 mol. wt calcium binding protein. Neuroscience. 1999;90:107–117. doi: 10.1016/s0306-4522(98)00440-0. [DOI] [PubMed] [Google Scholar]
- Hige T, Fujiyoshi Y, Takahashi T. Neurosteroid pregnenolone sulfate enhances glutamatergic synaptic transmission by facilitating presynaptic calcium currents at the calyx of Held of immature rats. Eur J Neurosci. 2006;24:1955–1966. doi: 10.1111/j.1460-9568.2006.05080.x. [DOI] [PubMed] [Google Scholar]
- Horak M, Vlcek K, Chodounska H, Vyklicky L., Jr. Subtype-dependence of N-methyl-D-aspartate receptor modulation by pregnenolone sulfate. Neuroscience. 2006;137:93–102. doi: 10.1016/j.neuroscience.2005.08.058. [DOI] [PubMed] [Google Scholar]
- Johansson T, Frandberg PA, Nyberg F, Le Greves P. Low concentrations of neuroactive steroids alter kinetics of [3H]ifenprodil binding to the NMDA receptor in rat frontal cortex. Br J Pharmacol. 2005;146:894–902. doi: 10.1038/sj.bjp.0706397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson T, Frandberg PA, Nyberg F, Le Greves P. Molecular mechanisms for nanomolar concentrations of neurosteroids at NR1/NR2B receptors. J Pharmacol Exp Ther. 2008;324:759–768. doi: 10.1124/jpet.107.130518. [DOI] [PubMed] [Google Scholar]
- Kohjitani A, Fuda H, Hanyu O, Strott CA. Regulation of SULT2B1a (pregnenolone sulfotransferase) expression in rat C6 glioma cells: relevance of AMPA receptor-mediated NO signaling. Neurosci Lett. 2008;430:75–80. doi: 10.1016/j.neulet.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Krebs MO, Desce JM, Kemel ML, Gauchy C, Godeheu G, Cheramy A, Glowinski J. Glutamatergic control of dopamine release in the rat striatum: evidence for presynaptic N-methyl-D-aspartate receptors on dopaminergic nerve terminals. J Neurochem. 1991;56:81–85. doi: 10.1111/j.1471-4159.1991.tb02565.x. [DOI] [PubMed] [Google Scholar]
- Liere P, Pianos A, Eychenne B, Cambourg A, Liu S, Griffiths W, Schumacher M, Sjovall J, Baulieu EE. Novel lipoidal derivatives of pregnenolone and dehydroepiandrosterone and absence of their sulfated counterparts in rodent brain. J Lipid Res. 2004;45:2287–2302. doi: 10.1194/jlr.M400244-JLR200. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Mienville J-M, Vicini S. Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neurosci Lett. 1988;90:279–284. doi: 10.1016/0304-3940(88)90202-9. [DOI] [PubMed] [Google Scholar]
- Malayev AA, Gibbs TT, Farb DH. Inhibition of the NMDA Response by pregnenolone sulfate reveals subtype selective modulation of NMDA receptors by sulfated steroids. British Journal of Pharmacology. 2002;135:901–909. doi: 10.1038/sj.bjp.0704543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Carta M, Partridge LD, Valenzuela CF. Neurosteroid-induced plasticity of immature synapses via retrograde modulation of presynaptic NMDA receptors. J Neurosci. 2005;25:2285–2294. doi: 10.1523/JNEUROSCI.3877-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. Characterization of two novel σ receptor ligands: antidystonic effects in rats suggest σ receptor antagonism. Eur J Pharmacol. 1995;280:301–310. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- Maurice T. Neurosteroids and sigma1 receptors, biochemical and behavioral relevance. Pharmacopsychiatry. 2004;37(Suppl 3):S171–S182. doi: 10.1055/s-2004-832675. [DOI] [PubMed] [Google Scholar]
- Monnet FP, Mahe V, Robel P, Baulieu EE. Neurosteroids, via σ receptors, modulate the [3H]norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc Natl Acad Sci USA. 1995;92:3774–3778. doi: 10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison P, Bungay P, Hsiao J, Mefford I, Dykstra K, Dedrick R. Quantitative microdialysis. In: Robinson T, Justice J, editors. Microdialysis in the Neurosciences. Elsevier; Amsterdam: 1991. pp. 47–79. [Google Scholar]
- Mtchedlishvili Z, Kapur J. A presynaptic action of the neurosteroid pregnenolone sulfate on GABAergic synaptic transmission. Mol Pharmacol. 2003;64:857–864. doi: 10.1124/mol.64.4.857. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Wu F-S, Purdy RH, Malayev AA, Gibbs TT, Farb DH. Distinct sites for inverse modulation of NMDA receptors by sulfated steroids. Mol Pharmacol. 1997;52:1113–1123. doi: 10.1124/mol.52.6.1113. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1997. [DOI] [PubMed] [Google Scholar]
- Pittaluga A, Pattarini R, Feligioni M, Raiteri M. N-methyl-D-aspartate receptors mediating hippocampal noradrenaline and striatal dopamine release display differential sensitivity to quinolinic acid, the HIV-1 envelope protein gp120, external pH and protein kinase C inhibition. J Neurochem. 2001;76:139–148. doi: 10.1046/j.1471-4159.2001.00057.x. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Nelson TE, Purdy RH, Gruol DL. Steroid pregnenolone sulfate enhances NMDA-receptor-independent long-term potentiation at hippocampal CA1 synapses: Role for L-type calcium channels and sigma-receptors. Hippocampus. 2007;17:349–369. doi: 10.1002/hipo.20273. [DOI] [PubMed] [Google Scholar]
- Sadri-Vakili G, Johnson DW, Janis GC, Gibbs TT, Pierce RC, Farb DH. Inhibition of NMDA-induced striatal dopamine release and behavioral activation by the neuroactive steroid 3a-hydroxy-5b-pregnan-20-one hemisuccinate. J Neurochem. 2003;86:92–101. doi: 10.1046/j.1471-4159.2003.01814.x. [DOI] [PubMed] [Google Scholar]
- Schiess AR, Scullin CS, Partridge LD. Neurosteroid-induced enhancement of short-term facilitation involves a component downstream from presynaptic calcium in hippocampal slices. J Physiol. 2006;576:833–847. doi: 10.1113/jphysiol.2006.118505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence KT, Plata-Salaman CR, ffrench-Mullen JMH. The neurosteroids pregnenolone and pregnenolone-sulfate but not progesterone, block Ca2+ currents in acutely isolated hippocampal CA1 neurons. Life Sci. 1991;49:PL235–PL239. doi: 10.1016/0024-3205(91)90649-v. [DOI] [PubMed] [Google Scholar]
- Tsutsui K. Biosynthesis, mode of action and functional significance of neurosteroids in the developing Purkinje cell. J Steroid Biochem Mol Biol. 2006;102:187–194. doi: 10.1016/j.jsbmb.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Wang MD, Wahlstrom G, Backstrom T. The regional brain distribution of the neurosteroids pregnenolone and pregnenolone sulfate following intravenous infusion. J Steroid Biochem Mol Biol. 1997;62:299–306. doi: 10.1016/s0960-0760(97)00041-1. [DOI] [PubMed] [Google Scholar]
- Whittaker MT, Gibbs TT, Farb DH. Pregnenolone sulfate induces NMDA receptor dependent release of dopamine from synaptic terminals in the striatum. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05627.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CE, Jr., Land MB, Purdy RH, Richards KG, Gibbs TT, Farb DH. Geometry and charge determine pharmacological effects of steroids on N-methyl-D-aspartate receptor induced Ca2+ accumulation and cell death. J Pharmacol Exp Ther. 2000;293:747–754. [PubMed] [Google Scholar]
- Wu F-S, Gibbs TT, Farb DH. Inverse modulation of γ-aminobutyric acid- and glycine-induced currents by progesterone. Mol Pharmacol. 1990;37:597–602. [PubMed] [Google Scholar]
- Wu F-S, Gibbs TT, Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the NMDA receptor. Mol Pharmacol. 1991;40:333–336. [PubMed] [Google Scholar]
- Yaghoubi N, Malayev A, Russek SJ, Gibbs TT, Farb DH. Neurosteroid modulation of recombinant ionotropic glutamate receptors. Brain Res. 1998;803:153–160. doi: 10.1016/s0006-8993(98)00644-1. [DOI] [PubMed] [Google Scholar]
- Zou LB, Yamada K, Sasa M, Nakata Y, Nabeshima T. Effects of σ1 receptor agonist SA4503 and neuroactive steroids on performance in a radial arm maze task in rats. Neuropharmacology. 2000;39:1617–1627. doi: 10.1016/s0028-3908(99)00228-2. [DOI] [PubMed] [Google Scholar]