Abstract
Background
High levels of insulin and lipids following a meal are recognized risk factors for atherosclerosis. Monitoring such risk factors in the general population is hampered by the inconvenience of venipuncture blood collection, particularly for both premeal and postmeal analyses. This study examined insulin and triglyceride levels in dried blood spots (DBSs) collected after different breakfast meal challenges to assess the potential of this method for risk assessment.
Methods
Glucose levels were measured using a glucose meter, and insulin and triglycerides were determined in DBS samples collected from 19 healthy volunteers before and at four time points up to 2.5 h after consuming each of five typical breakfast meals varying in nutritional composition.
Results
At 2 h, glucose was within normal postprandial values (<140 mg/dl) for all meals; significantly lower glucose was seen after meal 2 (the lowest carbohydrate content) compared to the other meals. Insulin returned to normal fasting levels (<15 μIU/ml) in significantly more subjects (90%) after meal 2 and significantly fewer subjects (31%) after meal 4 (highest carbohydrate content) than the other meals. Triglycerides were elevated to a similar extent in all subjects, with no significant differences between meals; levels were still rising at 2.5 h.
Conclusions
Subjects were able to collect blood spots with minimum disruption to their normal daily activities. Relative ease of collection, analyte stability in dried blood, and the close correlation with serum levels that we have previously demonstrated makes DBS a convenient and simple tool for assessing the individual impact of different diets on postprandial dysmetabolism.
Keywords: blood spot, cardiometabolic risk, insulin, postprandial, postprandial dysmetabolism, triglycerides
Introduction
The metabolic conditions that predispose individuals to the development of atherosclerosis and coronary heart disease (CHD) may be largely a postprandial phenomenon described as “postprandial dysmetabolism.”1 O'Keefe and colleagues2 have reviewed dietary strategies that lower risk of cardiovascular disease by reducing postprandial glucose (PPG), lipids, and inflammation.
In societies such as the United States that predominantly have a positive energy balance, such that caloric intake exceeds calorie expenditure, obesity is prevalent, and the incidence of metabolic syndrome, diabetes, and cardiovascular disease is rising. Poor dietary habits result in much of an individual's time being spent in a nonfasting state.3 Visceral obesity has been shown to increase levels of atherogenic lipid particles after a meal challenge.4
There is increasing evidence that postprandial levels of insulin and triglycerides are a valuable clinical tool for determining risk for developing cardiovascular disease and diabetes. In people with diabetes, postprandial insulin levels are higher than in people without diabetes. However, when monitored over time, declining post-prandial insulin levels in patients with type 2 diabetes are a powerful indicator of deteriorating blood sugar control, because they are a measure of the decline in beta-cell responsiveness as the disease progresses.5,6 In subjects with no known diabetes, high postprandial insulin levels have been found to be an independent risk factor for CHD.7,8 Triglyceride levels measured 2–4 h after a meal are also highly predictive of cardiovascular events, especially in women.9,10
Cardiometabolic risk markers are routinely measured in fasting blood samples in order to provide meaningful levels of cholesterol, insulin, and triglycerides that are not affected by recent food consumption. However, standardized protocols have not yet been established for the assessment of nonfasting triglycerides and insulin.3 Clinical studies reporting postprandial parameters have used either a variety of types of meal or an oral glucose or fat load that does not represent what might be consumed by an individual in their choice of a typical meal. It has therefore been difficult to determine appropriate reference ranges for postprandial levels of these markers and cutoffs that may define increased risk.
Another important factor is the inconvenience of collecting blood samples at various time points after eating, as this requires staying at a blood collection center or repeatedly returning to have blood collected. The majority of clinical meal studies involve intravenous catheterization for collection of multiple samples after a meal challenge. These studies typically can only assess a subject after a single meal and require the subject to remain in the clinic for several hours during sample collection. This artificial clinical environment and type of meal served are not what would be happening during that subject's normal day, particularly with respect to physical activity or the types of foods they routinely consume. Capillary (finger stick) blood spot collection on filter paper by an individual in the home or work environment, on the other hand, affords a simple and convenient way to collect blood samples. Such methods of blood collection can be employed in larger-scale epidemiological studies looking at postprandial dysmetabolism and its longer- term effects on cardiovascular disease and diabetes in people in their normal living situation after eating typical meals.
To appreciate the simplicity and convenience of using dried blood spots (DBSs) to study dysmetabolism, we investigated postprandial levels of insulin and triglycerides in DBSs up to 2.5 h after five different breakfast meals in 19 healthy volunteers. The aim was to determine whether different meals would elicit differing responses and to identify a suitable protocol for detecting postprandial dysmetabolism for routine cardiometabolic risk assessment.
Methods
The procedures followed were in accordance with the current revision of the Helsinki Declaration, and all subjects gave their informed consent. Nineteen healthy volunteers, 13 women and 6 men, were recruited from ZRT Laboratory staff after being informed of the contents of all five meals and confirming that they would be able to eat all of them. None of the volunteers was receiving statin medications.
Volunteers were asked to fast by consuming nothing except water from the previous evening until arriving for the study. Length of fasting time was recorded on arrival; fasting time varied between 10 and 14 h over the course of the study. On each test day, volunteers provided a fasting blood spot sample and then ate one of five test meals (see Table 1 for meal composition). Blood spot samples were then collected 30 min, 1 h, 2 h, and 2.5 h after finishing the meal during the volunteers' normal workday activities. At least one week elapsed between each test meal. Following finger stick with Unistik lancets (Fisher Scientific), blood drops were collected on filter paper blood spot collection cards (Whatman 901). Immediately prior to collecting blood spots, the first drop of blood was used to test for glucose using a glucose meter (One Touch Ultra Mini), and the subsequent drops were applied to the filter paper. Blood spots on the collection cards were allowed to dry at room temperature for one day, and the samples were then stored individually in Ziploc bags with desiccant at -70 °C until assays were run.
Table 1.
Test Meal Descriptions and Nutritional Composition
| Meal # | Meal contents | Nutritional composition | Calories (kcal) |
|---|---|---|---|
| 1 | 2 glazed donuts (Krispy Kreme, total 104 g), 1 fruit smoothie (Dannon, 207 ml) | Carbohydrate: 79 g (sugars 53 g) = 69% (46%) | 580 |
| Fat: 26.5 g = 23% | |||
| Protein: 9 g = 8% | |||
| Fiber: 2 g | |||
| 2 | 2 boiled eggs, 2 sausages (total 35 g), 250 ml 2% milk | Carbohydrate: 13.3 g (sugars 11 g) = 23% (19%) | 590 |
| Fat: 25.6 g = 45% | |||
| Protein: 18.6 g = 32% | |||
| Fiber: 0 g | |||
| 3 | 1 bagel (120 g) with 1 28 g packet cream cheese, 1 hard-boiled egg, 250 ml 2% milk | Carbohydrate: 61.4 g (sugars 16.3 g) = 63% (17%) | 522 |
| Fat: 13.8 g = 14% | |||
| Protein: 23 g = 23% | |||
| Fiber: 2 g | |||
| 4 | 3 pancakes (116 g) with 30 ml syrup, tea with 9 ml cream (half and half), 1 3.5 g sugar packet | Carbohydrate: 78 g (sugars 35 g) = 83% (37%) | 425 |
| Fat: 10 g = 11% | |||
| Protein: 6 g = 6% | |||
| Fiber: 1 g | |||
| 5 | 1 packet (50 g) instant oatmeal, 28 g almonds, 1 medium Granny Smith apple (150 g), 125 ml skim milk | Carbohydrate: 67 g (sugars 30.5 g) = 67% (31%) | 426 |
| Fat: 16.5 g = 17% | |||
| Protein: 16.4 g = 16% | |||
| Fiber: 11 g |
Insulin and triglyceride levels were measured in extracted DBS as previously described,11 where correlation with simultaneously collected venous serum samples for both analytes was demonstrated (r = 0.93 and 0.91, respectively). All assays were run on the same day after administration of all test meals and within a maximum of six weeks after sample collection. Quality control samples were included with each assay. Intra- and inter- assay coefficients of variation of the assay are <10% for triglycerides and <15% for insulin over the range of values measured in this study.
All volunteers were assessed for body mass index (BMI) and criteria for metabolic syndrome.12 Five of the 19 volunteers met the National Cholesterol Education Panel's criteria for metabolic syndrome (three or more of the following: waist circumference >40 in. in men or 35 in. in women, fasting triglycerides >150 mg/dl, high-density lipoprotein cholesterol <40 mg/dl for men or <50 mg/dl for women, blood pressure >130/85, and fasting plasma glucose >110 mg/dl). Clinical diabetes was an exclusion criterion when enrolling volunteers as study subjects. However, during the course of the study, fasting blood glucose levels in four subjects were observed to be just within the range currently defined by the American Diabetes Association as “prediabetic,” i.e., between 100 and 125 mg/dl; fasting blood glucose levels, averaged over the study test days for each of these four subjects, were 100, 104, 104, and 105 mg/dl.
For purposes of statistical analysis, both insulin and triglyceride levels were transformed into normal/abnormal and optimal/nonoptimal classifications. Insulin levels were classified as normal if they were ≤15 μIU/ml. Levels ≤8 μIU/ml were considered optimal. Triglyceride levels were considered normal if they were ≤150 mg/dl and were considered optimal if they were ≤100 mg/dl. All glucose levels were in the normal 2 h postprandial range (<140 mg/dl) and thus could not be transformed into a dichotomous variable. A mixed model logistic regression was used to analyze the dichotomous insulin and triglyceride data.
Results
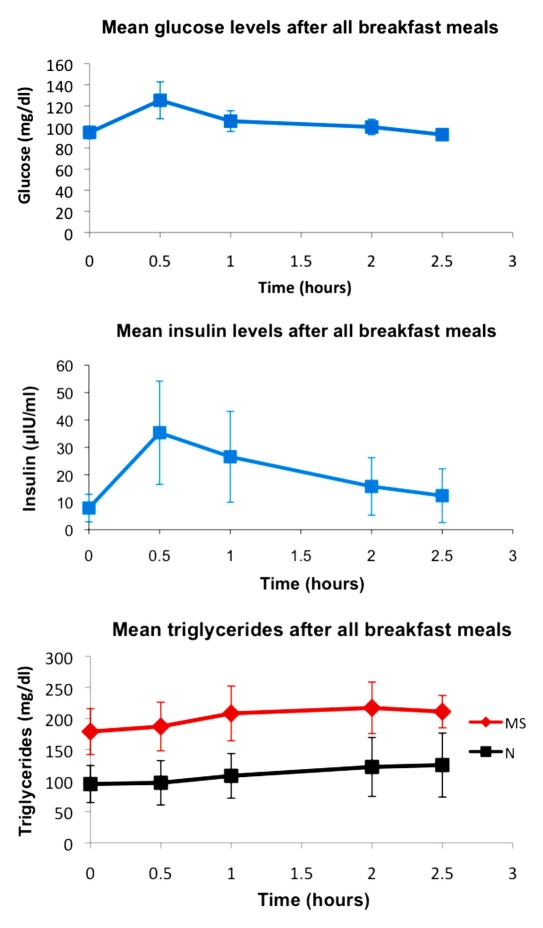
Glucose, insulin, and triglyceride levels averaged over all meals for all volunteers at each time point are shown in Figure 1. Glucose and insulin peaked at the 30 min postprandial time point and then declined. Triglycerides showed a more gradual increase and tended to be still rising at the 2.5 h time point. Because of overall higher triglyceride levels in the subjects who met the criteria for metabolic syndrome, the triglycerides graph shows metabolic syndrome and nonmetabolic syndrome subjects separately.
Figure 1.
Glucose, insulin, and triglyceride levels (including all subjects, mean value ± standard deviation) for all meals at each time point. Subjects with metabolic syndrome are shown separately from subjects without metabolic syndrome for triglycerides, because triglyceride levels were substantially higher overall in metabolic syndrome subjects. Glucose and insulin levels both peaked at the 30 min time point, while triglycerides showed a gradual increase continuing through the 2.5 h time point. At 2 h, glucose was comparable to baseline levels. MS, metabolic syndrome; N, subjects without metabolic syndrome.
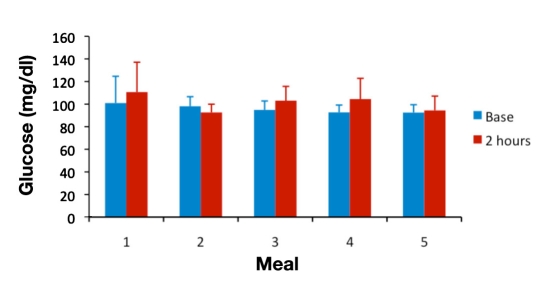
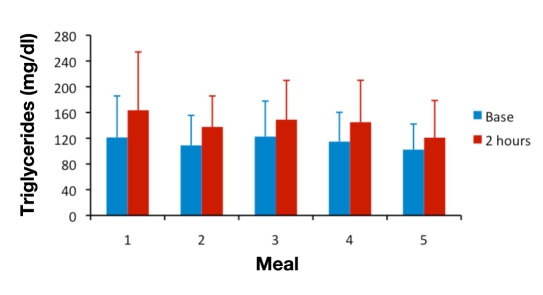
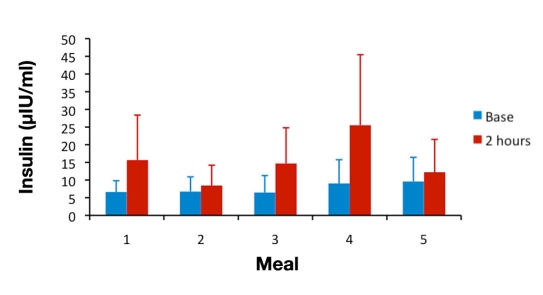
For analyses of between-meal differences, the 2 h time point was chosen because published studies looking at postprandial hyperinsulinemia5,7,8,13 have used this time point, at which glucose and insulin levels are expected to have returned to normal values in healthy subjects. Plasma glucose concentrations normally peak within 1 h after a meal and return to normal values within 2–3 h.14 Population studies of postprandial hypertriglyceridemia have found it to be most predictive of cardiovascular events when measured 2 to 4 h after eating.10 Figures 2–4 show mean glucose, insulin, and triglyceride levels for all subjects at baseline and at the 2 h time point for meals 1–5.
Figure 2.
Mean ± standard deviation glucose levels at baseline and at 2 h postprandial for meals 1 to 5.
Figure 4.
Mean ± standard deviation DBS triglyceride levels at baseline and at 2 h postprandial for meals 1 to 5.
For statistical analysis of the between-meal differences in postprandial insulin levels, a mixed logistic regression model for insulin was tested for both the normal/abnormal classification and the optimal/nonoptimal classification. The model was identical for both outcome measures. Participants' gender and BMI were included in the model to control for their influence, as was each participant's baseline (preprandial) insulin (classified as normal/abnormal or optimal/nonoptimal using the same criteria as those described earlier) for each meal. Gender, baseline insulin, and meal type were all treated as categorical variables, and participants were treated as a repeated variable.
The model for normal insulin resulted in a good fit. Neither BMI (p > .28) nor baseline normal insulin were statistically significant (p > .32), but gender and meals both displayed trends toward significance (gender p < .08; meal p < .08). The model for optimal insulin also resulted in a good fit. Gender was not significant (p > .36), but baseline optimal insulin tended toward significance (p < .11). Both meals (p < .03) and BMI (p < .02) were significant.
A Wilcoxon paired sign test was then used to compare participants' normal/abnormal and optimal/nonoptimal insulin classifications for any given meal to every other meal (using only participants who had data for both meals in a given pairing), for a total of 10 comparisons. Table 2 displays the proportion of participants who had normal insulin levels for a given meal, and the table note indicates which meal comparisons were significant. Meal 2 produced the highest proportion of normal insulin classifications, and meal 4 produced the lowest proportion of normal insulin classifications.
Table 2.
Number and Percentage of Subjects with Normal Insulin (≤15 μIU/ml) for Each Meal at 2 h Postprandiala
| Meal number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Normal/total n | 13/16 | 17/19 | 12/18 | 5/16 | 10/14 |
| % normal | 81% | 90% | 67% | 31% | 71% |
Analysis of between-meal comparisons showed that meal 4 had a lower percentage of people with normal levels and meal 2 produced a tendency for having a higher percentage of people with normal levels. Significantly different meal/meal comparisons were 1 to 4 (Z = 2.45, p < .014, 14 persons), 2 to 4 (Z = 3.00, p < .003, 16 persons), 3 to 4 (Z = 2.00, p < .046, 15 persons), 5 to 4 (Z = 2.00, p < .046, 13 persons), and 3 to 2 (Z = 2.24, p < .025, 18 persons). Number of persons indicates the number of subjects for whom data were available and who ate both meals in the given pairing; subjects who did not eat both meals were excluded from the analysis for that pairing. All other meal/meal comparisons were nonsignificant.
Table 3 provides the same information using the optimal/nonoptimal classification. Again, meal 2 produced the highest proportion of optimal insulin classifications, and meal 4 produced the lowest proportion of optimal meal classifications.
Table 3.
Number and Percentage of Subjects with Optimal Insulin Levels (≤8 μIU/ml) for Each Meal at 2 h Postprandiala
| Meal number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Optimal/total (n) | 3/16 | 12/19 | 7/18 | 3/16 | 7/14 |
| % optimal | 19% | 63% | 39% | 19% | 50% |
Analysis of between-meal comparisons showed that meal 4 had a lower percentage of people with optimal levels and meal 2 had a higher percentage of people with optimal levels. Significantly different (p < .10) meal/meal comparisons were 2 to 4 (Z = 2.65, p < .008, 16 persons), 3 to 4 (Z = 1.73, p < .083, 15 persons), 5 to 4 (Z = 1.73, p < .083, 13 persons), 1 to 2 (Z = 3.00, p < .003, 16 persons), 3 to 2 (Z = 1.67, p < .096, 18 persons), 5 to 2 (Z = 1.73, p < .083, 14 persons), and 1 to 5 (Z = 2.00, p < .046, 12 persons). Number of persons indicates the number of subjects for whom data were available and who ate both meals in the given pairing; subjects who did not eat both meals were excluded from the analysis for that pairing. All other meal/meal comparisons were nonsignificant.
Mixed logistic regression models identical to those for insulin were used to examine normal/abnormal and optimal/ nonoptimal triglyceride classifications, substituting the appropriate baseline triglyceride classification into the models. For the normal classification, the model was a reasonable fit. Neither gender (p > .53) nor meals (p > .43) nor BMI (p > .25) were significant. Baseline normal/ abnormal triglycerides were a significant predictor (p < .04). For the optimal classification, the model was a very good fit. Gender was not significant (p > .59). Both baseline optimal/nonoptimal triglycerides (p < .04) and BMI (p < .05) were significant, and meals tended toward significance (p < .09).
As for insulin, Wilcoxon paired sign tests were used to compare triglycerides for all meals to one another. Proportions of normal and optimal triglyceride levels are presented in Tables 4 and 5. Unlike insulin, no meal-to-meal comparisons of triglycerides were statistically significant. It should be noted that at 2 h postprandial, far fewer triglyceride levels were normal or optimal than was the case for insulin at 2 h.
Table 4.
Number and Percentage of Subjects with Normal Triglycerides (≤150 mg/dl) by Meal at 2 h Postprandiala
| Meal number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Normal/total (n) | 8/16 | 10/19 | 8/18 | 8/16 | 9/14 |
| % normal | 50% | 53% | 44% | 50% | 64% |
None of the meal/meal comparisons was statistically significant.
Table 5.
Number and Percentage of Subjects with Optimal Triglycerides (≤100 mg/dl) by Meal at 2 h Postprandiala
| Meal number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Optimal/total (n) | 4/16 | 5/19 | 6/18 | 5/16 | 6/14 |
| % optimal | 25% | 26% | 33% | 31% | 43% |
None of the meal/meal comparisons was statistically significant.
The mixed linear regression model for glucose resulted in a good fit. Neither BMI (p > .71), baseline glucose (p > .77), nor gender were statistically significant (p > .72), but meal type was significant (p < .008).
A Wilcoxon paired sign test was then used to compare participants' glucose levels for any given meal to every other meal (using only participants who had data for both meals in a given pairing), for a total of 10 comparisons. Because of the day-to-day variability in baseline glucose levels within individuals, accommodation was done by subtracting baseline glucose levels from glucose 2 h levels and using that difference score as the unit of analysis. Table 6 displays the participants' means and standard deviations for the change in glucose from baseline to 2 h for each meal and the results of the meal-to-meal comparisons. Meal 2 produced a slight decrease in glucose levels overall at 2 h relative to baseline and differed significantly from the other four meals. Meals 1, 3, 4, and 5 did not differ significantly from one another.
Table 6.
Change in Glucose between Baseline and 2 h: Differences between Mealsa
| Meal number | Number of subjects | Baseline to 2 h difference score for glucose | |||
|---|---|---|---|---|---|
| Mean | Standard deviation | Minimum | Maximum | ||
| 1 | 19 | 8.7 | 15.2 | −21.0 | 42.0 |
| 2 | 19 | −5.5 | 5.9 | −16.0 | 5.0 |
| 3 | 19 | 9.8 | 17.8 | −12.0 | 51.0 |
| 4 | 17 | 11.8 | 19.6 | −12.0 | 60.0 |
| 5 | 15 | 1.7 | 12.6 | −21.0 | 30.0 |
Analysis of between-meal comparisons showed that meal 2 produced the only significant change in glucose at 2 h relative to baseline and resulted in lower mean glucose at 2 h compared to baseline. Significantly different (p < .10) meal/meal comparisons were 2 to 1 (Z = 2.80, p < .005, 19 persons), 2 to 3 (Z = 2.72, p < .007, 19 persons), 2 to 4 (Z = 2.84, p < .005, 17 persons), 2 to 5 (Z = 2.62, p < .024, 15 persons), and 4 to 5 (Z = 1.85, p < .064, 15 persons). Number of persons indicates the number of subjects for whom data were available and who ate both meals in the given pairing; subjects who did not eat both meals were excluded from the analysis for that pairing. The five remaining meal/meal comparisons were all nonsignificant, with p > .15.
When subjects with (5 subjects) or without (14 subjects) metabolic syndrome were analyzed separately, similar between-meal differences were seen for insulin, and for triglycerides, the main difference between the two groups was generally higher levels throughout in subjects with metabolic syndrome. However, because of the small number of subjects, there was limited statistical power in drawing any separate conclusions for this group.
Discussion
As the body of evidence for the impact of diet and lifestyle on long-term risk of cardiovascular disease and diabetes grows, efficient ways of studying risk markers that can help to provide strategies for managing these health problems are urgently needed. With the identification of postprandial dysmetabolism as an important risk factor, it is becoming apparent that traditional fasting blood draws may no longer be appropriate for total risk assessment.
Collection of postprandial samples requires that subjects have eaten a meal within a specific time of sample collection, and studies investigating the phenomenon may require multiple samples at a range of postprandial times. The inherent complications in attending clinics for a blood draw mean that such studies could not be carried out under the conditions of a normal day. However, blood spot collection allows convenient, simple sample collection at home or at work while subjects continue their daily activities. This opens up the significant possibility of studying postprandial dysmetabolism, either under prescribed dietary conditions or as a means of studying people who are allowed to consume their regular diets, so that long-term risks can be assessed.
Postprandial glucose measurement has long been used as a determinant of glycemic control in the medical management of diabetes; it is easily monitored using a glucose meter and requires no blood draw. Postprandial glucose provides a reasonable assessment of postprandial hyperglycemia; however, there is limited clinical value in monitoring PPG to assess risk of diabetic complications or cardiovascular disease.14 In this study, blood glucose levels were determined using a standard glucose meter because of its practicality. All the volunteers achieved PPG levels <140 mg/dl by 2 h postprandial, which is considered to be within the normal range; therefore, at least in this study, PPG would not have given an abnormal result that might indicate the presence of postprandial dysmetabolism. As shown in Figure 2, between-meal differences were not large. As expected, however, the meal with the lowest carbohydrate content (meal 2) did produce significantly lower PPG than the other meals in our study (see Table 6).
Postprandial insulin tends to mirror PPG, but clinically, postprandial insulin levels have been found to predict CHD irrespective of PPG levels. For example, in the Paris Protective Study15 investigating risk factors in a large male population, 2 h postload insulin level was a major independent predictor of CHD death, whereas impaired glucose tolerance was not. Similarly, the Helsinki Policemen Study16 showed that the predictive value of high plasma insulin for CHD was also independent of other risk factors, including blood glucose levels; 1 and 2 h postprandial levels were better associated with CHD events than fasting insulin.
In this study, postprandial insulin levels at the 2 h time point revealed significant between-meal differences, which appeared to be related to carbohydrate content of the mixed meals. As seen in Figure 3, meal 2 (with the lowest carbohydrate content) produced the lowest postprandial insulin, while meal 4 (with the highest carbohydrate content) produced the highest postprandial insulin. The statistical analysis, which compared each meal to all other meals for every individual who had consumed both meals in a pairing, found significantly more normal or optimal insulin levels at 2 h postprandial after meal 2 and significantly fewer normal or optimal levels after meal 4 (see Tables 2 and 3). Protein and fat content of the meals (see Table 1) had minimal effects in the amounts realistically found in meals that people eat, as has been noted in another meal study looking at typical food consumption rather than artificially high glucose or fat loads.17
Figure 3.
Mean ± standard deviation DBS insulin levels at baseline and at 2 h postprandial for meals 1 to 5.
In light of these results, larger-scale studies may determine that a simple blood spot collection 2 h after eating a typical breakfast meal may constitute a convenient test to help identify cardiometabolic risk in individuals whose fasting insulin levels are normal. Protocols could be established to determine suitable cutoffs, such as the “normal” or “optimal” classifications that revealed between-meal differences in this study. Such individuals who have abnormal levels at 2 h may be encouraged to modify their diets to mitigate risk of postprandial dysmetabolism and potential for developing cardiovascular disease or diabetes.
Triglyceride levels at 2 h did not differ significantly between meals, as shown in Figure 4 and Tables 4 and 5; however, other studies in the literature have found that triglyceride levels peak around 4 h postprandial, and in our subjects, the triglyceride levels were still rising at the 2.5 h time point. In this study, carried out in healthy volunteers during their regular working day, we noted the potential for low compliance when attempting to study subjects for longer than 3 h after a breakfast meal without further consumption of beverages or snacks. In a larger study looking at a 4 h sample collection, subjects should be closely monitored to ensure that they do not consume anything during this time.
Postprandial triglycerides have been found to be an independent risk factor of cardiovascular disease in larger population studies and were most useful when levels were determined around 4 h postprandial.10 Larger-scale studies taking advantage of the convenience of blood spot collection may determine whether a routine blood spot triglycerides test at 4 h after a typical breakfast may help identify individuals at risk.
This study, although small, demonstrated the use of a DBS test to assess the presence of postprandial dysmetabolism in ambulant subjects after a variety of breakfast meal types. As in the general population, a proportion of our subjects (5/19) met the criteria for metabolic syndrome. While the overall conclusions regarding the postprandial responses to different meals were similar for metabolic syndrome and nonmetabolic syndrome subjects, it would be interesting to conduct a larger study of dietary influences on postprandial metabolism in metabolic syndrome. The convenience and practicality of DBS collection paves the way for the possibility of large-scale studies, in a variety of populations, of the impact of dietary habits on risk of metabolic syndrome, diabetes, and cardiovascular disease, which would not be practical using traditional venipuncture blood collection.
Conclusions
This study demonstrates the convenience and simplicity of using DBSs to assess markers of postprandial dysmetabolism in an ambulant group of subjects after consuming a variety of breakfast meals. The results of this small study demonstrated significant differences in postprandial insulin responses after a variety of typical breakfast meals in people without diabetes, suggesting a higher risk of postprandial dysmetabolism after consumption of mixed meals with high overall carbohydrate content. Larger-scale or population studies of postprandial dysmetabolism using the convenience of blood spot collection could result in the development of a routine test that can be carried out at home to help monitor risk. This methodology would also facilitate the study of the effects of dietary or therapeutic regimens implemented to treat dysmetabolism in people at risk.
Acknowledgments
The authors thank Danielle Moore of ZRT Laboratory for her diligent work in organizing the meals and volunteers and for helping with blood spot collection, Myra Turman and Wendy Norris of ZRT Laboratory for conducting the laboratory assays, and William Gregory, Ph.D., of the Helfgott Research Institute, Portland, OR, for his expert statistical analyses.
Abbreviations
- BMI
body mass index
- CHD
coronary heart disease
- DBS
dried blood spot
- PPG
postprandial glucose
References
- 1.O'Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007;100(5):899–904. doi: 10.1016/j.amjcard.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 2.O'Keefe JH, Gheewala NM, O'Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008;51(3):249–255. doi: 10.1016/j.jacc.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Kolovou GD, Anagnostopoulou KK, Daskalopoulou SS, Mikhailidis DP, Cokkinos DV. Clinical relevance of postprandial lipaemia. Curr Med Chem. 2005;12(17):1931–1945. doi: 10.2174/0929867054546609. [DOI] [PubMed] [Google Scholar]
- 4.Van Hees AM, Saris WH, Dallinga-Thie GM, Hul GB, Martinez JA, Oppert JM, Stich V, Astrup A, Arner P, Sørensen TI, Blaak EE. Fasting and postprandial remnant-like particle cholesterol concentrations in obese participants are associated with plasma triglycerides, insulin resistance, and body fat distribution. J Nutr. 2008;138(12):2399–2405. doi: 10.3945/jn.108.094516. [DOI] [PubMed] [Google Scholar]
- 5.Shim WS, Kim SK, Kim HJ, Kang ES, Ahn CW, Lim SK, Lee HC, Cha BS. Decrement of postprandial insulin secretion determines the progressive nature of type-2 diabetes. Eur J Endocrinol. 2006;155(4):615–622. doi: 10.1530/eje.1.02249. [DOI] [PubMed] [Google Scholar]
- 6.Albarrak AI, Luzio SD, Chassin LJ, Playle RA, Owens DR, Hovorka R. Associations of glucose control with insulin sensitivity and pancreatic beta-cell responsiveness in newly presenting type 2 diabetes. J Clin Endocrinol Metab. 2002;87(1):198–203. doi: 10.1210/jcem.87.1.8152. [DOI] [PubMed] [Google Scholar]
- 7.Karabulut A, Iltumur K, Toprak N, Tuzcu AK, Kara IH, Kaplan A, Aksu Y. Insulin response to oral glucose loading and coronary artery disease in nondiabetics. Int Heart J. 2005;46(5):761–770. doi: 10.1536/ihj.46.761. [DOI] [PubMed] [Google Scholar]
- 8.Baltali M, Korkmaz ME, Kiziltan HT, Muderris IH, Ozin B, Anarat R. Association between postprandial hyperinsulinemia and coronary artery disease among non-diabetic women: a case control study. Int J Cardiol. 2003;88(2-3):215–221. doi: 10.1016/s0167-5273(02)00399-6. [DOI] [PubMed] [Google Scholar]
- 9.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298(3):299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 10.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 11.Kapur S, Kapur S, Zava D. Cardiometabolic risk factors assessed by a finger stick dried blood spot method. J Diabetes Sci Technol. 2008;2(2):236–241. doi: 10.1177/193229680800200210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C American Heart Association, National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro-Filho FF, Faria AN, Kohlmann NE, Zanella MT, Ferreira SR. Two-hour insulin determination improves the ability of abdominal fat measurement to identify risk for the metabolic syndrome. Diabetes Care. 2003;26(6):1725–1730. doi: 10.2337/diacare.26.6.1725. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Postprandial blood glucose. Diabetes Care. 2001;24(4):775–778. doi: 10.2337/diacare.24.4.775. [DOI] [PubMed] [Google Scholar]
- 15.Fontbonne AM, Eschwège EM. Insulin and cardiovascular disease. Paris Prospective Study. Diabetes Care. 1991;14(6):461–469. doi: 10.2337/diacare.14.6.461. [DOI] [PubMed] [Google Scholar]
- 16.Pyörälä K, Savolainen E, Kaukola S, Haapakoski J. Plasma insulin as coronary heart disease risk factor: relationship to other risk factors and predictive value during 9 1/2-year follow-up of the Helsinki Policemen Study population. Acta Med Scand Suppl. 1985;701:38–52. doi: 10.1111/j.0954-6820.1985.tb08888.x. [DOI] [PubMed] [Google Scholar]
- 17.Wolever TM, Yang M, Zeng XY, Atkinson F, Brand-Miller JC. Food glycemic index, as given in glycemic index tables, is a significant determinant of glycemic responses elicited by composite breakfast meals. Am J Clin Nutr. 2006;83(6):1306–1312. doi: 10.1093/ajcn/83.6.1306. [DOI] [PubMed] [Google Scholar]