Abstract
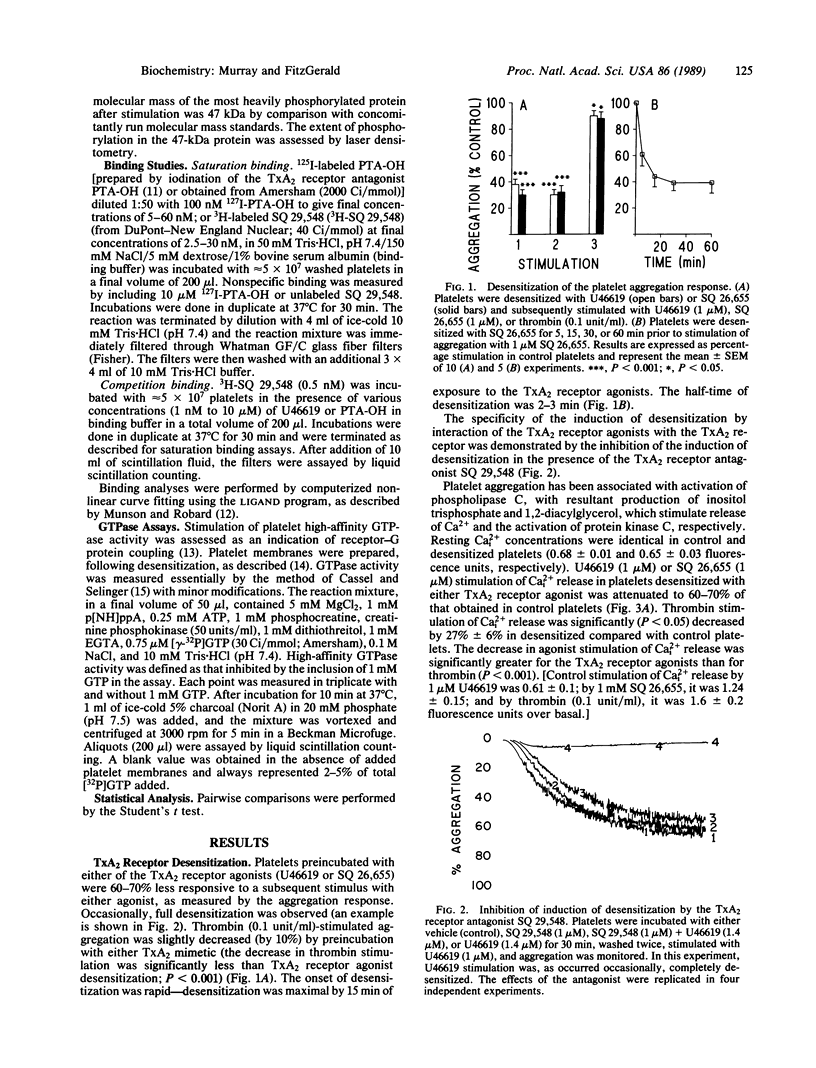
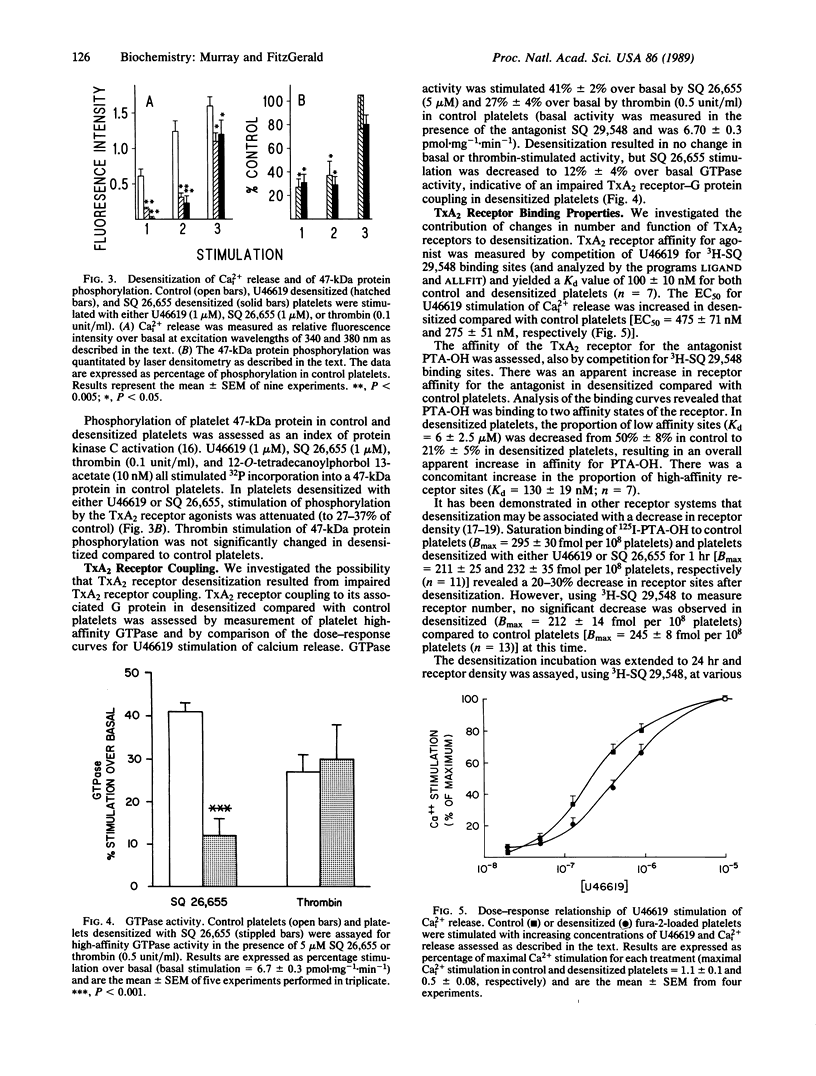
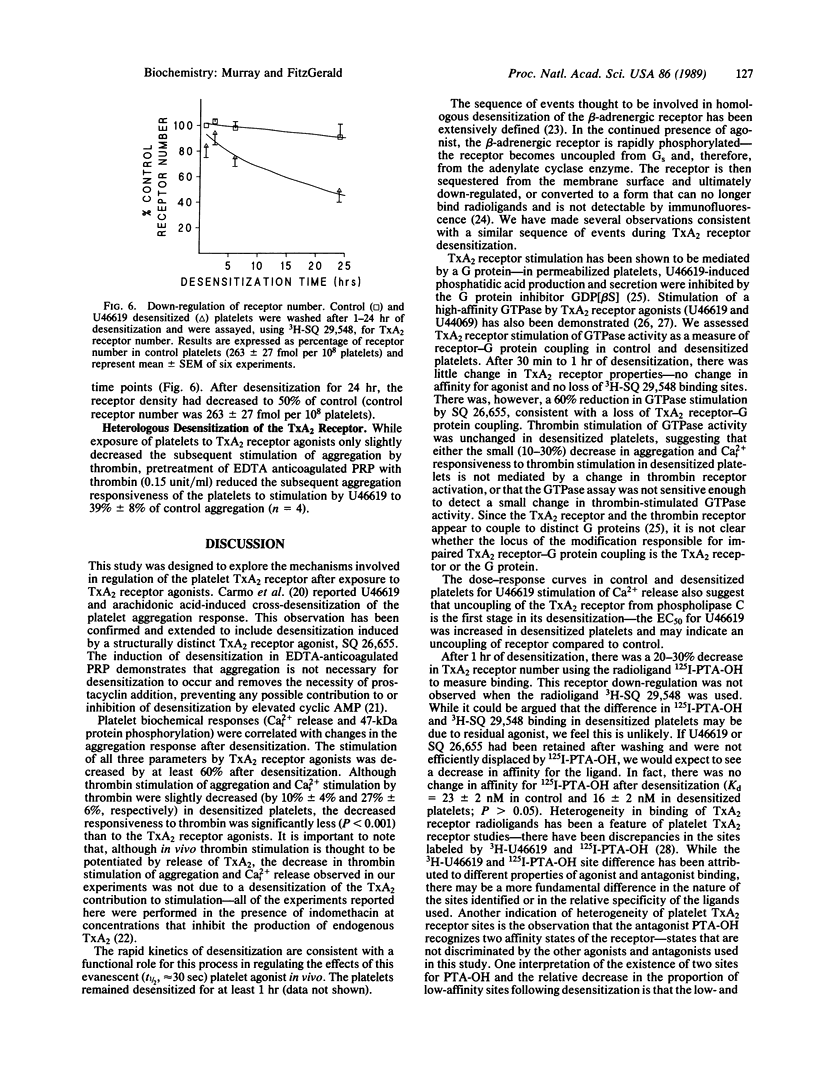
Thromboxane A2 (TxA2) is a potent platelet agonist that serves as an amplifying signal after exposure of platelets to other stimulants, such as thrombin, in vitro. Exposure of platelets to the TxA2 receptor agonists U46619 and SQ 26,655 (1.4 microM) resulted in a 60-90% decrease in subsequent TxA2 receptor-stimulated aggregation, calcium release, and protein kinase C activation. The desensitization was rapid, with a half-time of 2-3 min. The sequence of events involved in TxA2 receptor desensitization involves initial uncoupling of the receptor from a guanine nucleotide binding (G) protein followed by eventual receptor down-regulation. Consistent with this hypothesis were (i) a 60-70% decrease in SQ 26,655-stimulated platelet GTPase activity, (ii) a shift to the right of the dose-response curve for U46619-stimulated release of calcium [EC50, 275 +/- 51 nM (control)] vs. 475 +/- 71 nM (desensitized); P less than 0.01], and (iii) a delayed loss of receptor sites. In summary, exposure of platelets to TxA2 receptor agonists results in rapid desensitization of the biochemical and functional responses to interaction with its receptor in human platelets. The kinetics of these events are consistent with the hypothesis that this icosanoid functions in the regulation as well as amplification of platelet activation in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avdonin P. V., Svitina-Ulitina I. V., Leytin V. L., Tkachuk V. A. Interaction of stable prostaglandin endoperoxide analogs U46619 and U44069 with human platelet membranes: coupling of receptors with high-affinity GTPase and adenylate cyclase. Thromb Res. 1985 Oct 1;40(1):101–112. doi: 10.1016/0049-3848(85)90354-8. [DOI] [PubMed] [Google Scholar]
- Brass L. F., Shaller C. C., Belmonte E. J. Inositol 1,4,5-triphosphate-induced granule secretion in platelets. Evidence that the activation of phospholipase C mediated by platelet thromboxane receptors involves a guanine nucleotide binding protein-dependent mechanism distinct from that of thrombin. J Clin Invest. 1987 Apr;79(4):1269–1275. doi: 10.1172/JCI112947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo L. G., Hatmi M., Rotilio D., Vargaftig B. B. Platelet desensitization induced by arachidonic acid is not due to cyclo-oxygenase inactivation and involves the endoperoxide receptor. Br J Pharmacol. 1985 Aug;85(4):849–859. doi: 10.1111/j.1476-5381.1985.tb11084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Activation of turkey erythrocyte adenylate cyclase and blocking of the catecholamine-stimulated GTPase by guanosine 5'-(gamma-thio) triphosphate. Biochem Biophys Res Commun. 1977 Aug 8;77(3):868–873. doi: 10.1016/s0006-291x(77)80058-2. [DOI] [PubMed] [Google Scholar]
- Coleman R. A., Humphrey P. P., Kennedy I., Levy G. P., Lumley P. Comparison of the actions of U-46619, a prostaglandin H2-analogue, with those of prostaglandin H2 and thromboxane A2 on some isolated smooth muscle preparations. Br J Pharmacol. 1981 Jul;73(3):773–778. doi: 10.1111/j.1476-5381.1981.tb16814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald G. A., Oates J. A., Hawiger J., Maas R. L., Roberts L. J., 2nd, Lawson J. A., Brash A. R. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest. 1983 Mar;71(3):676–688. doi: 10.1172/JCI110814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Halushka P. V., MacDermot J., Knapp D. R., Eller T., Saussy D. L., Jr, Mais D., Blair I. A., Dollery C. T. A novel approach for the study of thromboxane A2 and prostaglandin H2 receptors using an 125I-labeled ligand. Biochem Pharmacol. 1985 Apr 15;34(8):1165–1170. doi: 10.1016/0006-2952(85)90490-3. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden T. K. Agonist-induced desensitization of the beta-adrenergic receptor-linked adenylate cyclase. Pharmacol Rev. 1983 Mar;35(1):5–32. [PubMed] [Google Scholar]
- Harris D. N., Greenberg R., Phillips M. B., Michel I. M., Goldenberg H. J., Haslanger M. F., Steinbacher T. E. Effects of SQ 27,427, a thromboxane A2 receptor antagonist, in the human platelet and isolated smooth muscle. Eur J Pharmacol. 1984 Aug 3;103(1-2):9–18. doi: 10.1016/0014-2999(84)90183-3. [DOI] [PubMed] [Google Scholar]
- Hatmi M., Rotilio D., Haye B., Antonicelli F., Joseph D., Vargaftig B. B. Modulation by cyclic AMP of arachidonic acid-induced platelet desensitization. Eur J Pharmacol. 1986 Dec 16;132(2-3):219–228. doi: 10.1016/0014-2999(86)90608-4. [DOI] [PubMed] [Google Scholar]
- Homburger V., Lucas M., Cantau B., Barabe J., Penit J., Bockaert J. Further evidence that desensitization of beta-adrenergic-sensitive adenylate cyclase proceeds in two steps. Modification of the coupling and loss of beta-adrenergic receptors. J Biol Chem. 1980 Nov 10;255(21):10436–10444. [PubMed] [Google Scholar]
- Houslay M. D., Bojanic D., Wilson A. Platelet activating factor and U44069 stimulate a GTPase activity in human platelets which is distinct from the guanine nucleotide regulatory proteins, Ns and Ni. Biochem J. 1986 Mar 15;234(3):737–740. doi: 10.1042/bj2340737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambayashi J., Morimoto K., Hakata N., Kobayashi T., Kosaki G. Involvement of calmodulin in platelet reaction. Thromb Res. 1981 Jun 1;22(5-6):553–558. doi: 10.1016/0049-3848(81)90053-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liel N., Mais D. E., Halushka P. V. Binding of a thromboxane A2/prostaglandin H2 agonist [3H]U46619 to washed human platelets. Prostaglandins. 1987 Jun;33(6):789–797. doi: 10.1016/0090-6980(87)90107-9. [DOI] [PubMed] [Google Scholar]
- Lyons R. M., Stanford N., Majerus P. W. Thrombin-induced protein phosphorylation in human platelets. J Clin Invest. 1975 Oct;56(4):924–936. doi: 10.1172/JCI108172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Ardlie N. G., Packham M. A. Preparation of suspensions of washed platelets from humans. Br J Haematol. 1972 Feb;22(2):193–204. doi: 10.1111/j.1365-2141.1972.tb08800.x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Reilly I. A., Doran J. B., Smith B., FitzGerald G. A. Increased thromboxane biosynthesis in a human preparation of platelet activation: biochemical and functional consequences of selective inhibition of thromboxane synthase. Circulation. 1986 Jun;73(6):1300–1309. doi: 10.1161/01.cir.73.6.1300. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Differential activation of platelet phospholipases by thrombin and ionophore A23187. J Biol Chem. 1981 May 10;256(9):4153–4155. [PubMed] [Google Scholar]
- Saussy D. L., Jr, Mais D. E., Burch R. M., Halushka P. V. Identification of a putative thromboxane A2/prostaglandin H2 receptor in human platelet membranes. J Biol Chem. 1986 Mar 5;261(7):3025–3029. [PubMed] [Google Scholar]
- Scarpace P. J., Abrass I. B. Desensitization of adenylate cyclase and down regulation of beta adrenergic receptors after in vivo administration of beta agonist. J Pharmacol Exp Ther. 1982 Nov;223(2):327–331. [PubMed] [Google Scholar]
- Shima S., Komoriyama K., Hirai M., Kouyama H. Blockade of heterologous desensitization of prostate adenylate cyclase without blockade of homologous down regulation of receptors or loss of GTP regulation of agonist binding. J Biol Chem. 1983 Feb 25;258(4):2083–2086. [PubMed] [Google Scholar]
- Vachon L., Costa T., Herz A. Opioid receptor desensitization in NG 108-15 cells. Differential effects of a full and a partial agonist on the opioid-dependent GTPase. Biochem Pharmacol. 1987 Sep 15;36(18):2889–2897. doi: 10.1016/0006-2952(87)90199-7. [DOI] [PubMed] [Google Scholar]
- Watson S. P., Ruggiero M., Abrahams S. L., Lapetina E. G. Inositol 1,4,5-trisphosphate induces aggregation and release of 5-hydroxytryptamine from saponin-permeabilized human platelets. J Biol Chem. 1986 Apr 25;261(12):5368–5372. [PubMed] [Google Scholar]
- Zemcik B. A., Strader C. D. Fluorescent localization of the beta-adrenergic receptor on DDT-1 cells. Down-regulation by adrenergic agonists. Biochem J. 1988 Apr 15;251(2):333–339. doi: 10.1042/bj2510333. [DOI] [PMC free article] [PubMed] [Google Scholar]



