Abstract
Background
It has been shown previously that the suppression of slow-wave sleep (SWS) markedly reduced insulin sensitivity and led to an impairment of glucose tolerance. We hypothesized that a decreased amount of SWS is a feature peculiar to subjects with type 2 diabetes.
Method
A retrospective case-control study analyzed polysomnographic recordings and covariate data of 22 type 2 diabetic and 22 nondiabetic subjects [n = 44; 8 women, 36 men, aged 57.5 ± 5.5 years, body mass index (BMI) 33.8 ± 5.9 kg/m2, apnea–hypopnea index (AHI) 29.6 ± 22.2 episodes/hr] matched individually for sex, race, age, BMI, and severity of sleep-related breathing disorders (SRBD). We assessed differences in sleep architecture between the study group and the control group. Primary end points included the percentage of total sleep time spent in each sleep stage.
Results
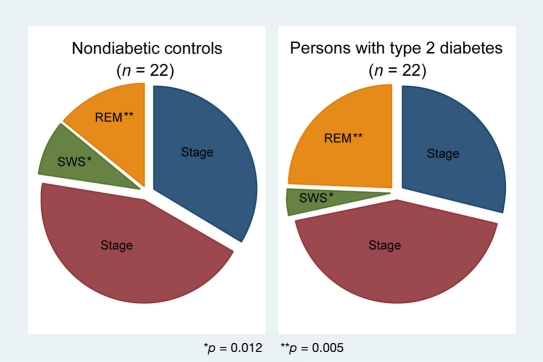
Despite similar age and severity of SRBD, subjects with type 2 diabetes demonstrated a significantly decreased amount of SWS (3.9 ± 5.95% vs 8.4 ± 4.57%; p = 0.012), increased percentage time in rapid eye movement sleep (24.1 ± 12.14% vs 13.8 ± 6.96%; p = 0.005), and higher arousal index (44.3 ± 19.53/hr vs 35.7 ± 12.67/hr; p = 0.037) compared to nondiabetic controls. After adjustment for sex, BMI, AHI, and smoking, age and presence of type 2 diabetes were independent predictors of the decreased SWS percentage (p = 0.001). Variables in this model accounted for 34% of the variance in the SWS percentage in our cohort.
Conclusions
Results demonstrated distinct differences in sleep architecture in our cohort with decreased amounts of SWS in type 2 diabetes. These findings suggest that polysomnographic recognition of altered sleep architecture may be partially implicated in the early detection of persons with type 2 diabetes.
Keywords: polysomnography, sleep architecture, sleep-related breathing disorders, slow-wave sleep, type 2 diabetes
Introduction
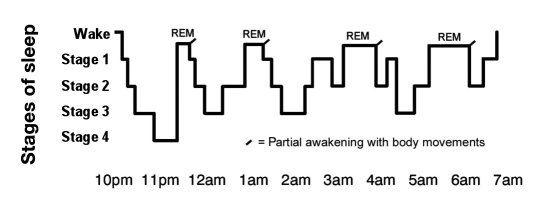
Sleep is a dynamic state with its own distinctive stages that cycle throughout the night. The succession of cycles, their component stages, and the duration of each stage and cycle compose a person's sleep architecture (Figure 1).1 Sleep architecture includes macro- and microarchitectural features that characterize sleep integrity and continuity, global sleep-stage structure, and presumed underlying physiologic mechanisms, e.g., changes in cholinergic–adrenergic brain mechanisms. American Academy of Sleep Medicine (AASM) sleep scoring criteria2 divide sleep into rapid eye movement (REM) sleep and three stages of nonrapid eye movement (NREM) sleep based on electroencephalographic (EEG), electromyographic, and electrooculographic measures. Stage 1 is characterized by slight slowing of the EEG, whereas stage 2 is characterized by high amplitude K complexes and spindles (low amplitude clusters). Slow, high amplitude delta waves characterize stages 3 and 4. Eye movements and loss of muscle tone, in conjunction with a stage 1 EEG, characterize REM sleep.3 As of 2008, the AASM has discontinued the use of stage 4 of NREM sleep according to the Rechtschaffen and Kales standard of 1968,3 and previous stages 3 and 4 are now combined as stage N3. This stage is also termed slow-wave sleep (SWS) or deep sleep and is thought to be the most restorative of all sleep stages.
Figure 1.
Sleep stages during one night.1
Overnight polysomnography (PSG) is a diagnostic test used to evaluate qualitative and quantitative abnormalities of sleep and wakefulness. Physiological data obtained during the sleep study complement medical history, physical examination, and study questionnaires, thereby providing important additional insight into the causes of some clinical conditions.
It has been shown previously that sleep is a major modulator of endocrine function, particularly of pituitary-dependent hormonal release.4,5 In 2000, Van Cauter and colleagues4 reported that age-related changes in SWS in healthy men were associated with specific alterations in hormonal systems essential for metabolic regulation. Findings by Tasali and colleagues5 demonstrated a clear role for SWS in the maintenance of normal glucose homeostasis. The authors showed that, in young healthy adults, all-night selective suppression of SWS, without any change in total sleep time, resulted in marked decreases in insulin sensitivity without an adequate compensatory increase in insulin release, leading to reduced glucose tolerance and increased diabetes risk.5
To date, there is a lack of case-control studies investigating disturbances in sleep architecture in persons with type 2 diabetes. We hypothesized that the diminished amount of SWS is a feature peculiar to subjects with type 2 diabetes. We investigated whether differences in sleep architecture exist between persons with type 2 diabetes and nondiabetic controls and explored the relative effects of selected covariates on sleep architecture measures.
Subjects and Methods
Study Design and Setting
This was a retrospective single-center, case-control study conducted in 2008 in the sleep laboratory at PJ Safarik University, Kosice, Slovakia. The study was approved by the local ethics committee and was performed in accordance with the principles of the Declaration of Helsinki on human experimentation as revised in 2000 and in compliance with good clinical practice. Written informed consent was obtained from all subjects before participating in the study.
Study Population
All participants were middle-aged and older patients with suspected sleep-related breathing disorders (SRBD) referred to our sleep laboratory for sleep evaluation. Inclusion criteria included male and female adults with or without type 2 diabetes mellitus, with an ability and willingness to undergo a laboratory-based overnight PSG. Type 2 diabetes was defined as a history of type 2 diabetes or as the current use of oral hypoglycemic agents or insulin. Exclusion criteria were a history of type 1 diabetes, night-shift work, previous sleep deprivation, consumption of alcohol more than 30 grams daily, and history of a coexisting disorder likely to disturb the quality of sleep other than sleep disordered breathing. A total of 22 Caucasian type 2 diabetic subjects and 22 nondiabetic controls well matched individually for sex, race, age, body mass index (BMI), and severity of SRBD were enrolled in this study.
Methods
All study participants completed a sleep questionnaire with the Epworth Sleepiness Scale (ESS) to assess daytime sleepiness,6 fatigue, sleep complaints, and history of nocturnal motor/sensory leg symptoms. Additional information obtained included demographics, medical history, and use of medications, nicotine, and alcohol. Alcohol consumption was estimated through a questionnaire of self-reported drinking habits.
All subjects underwent a laboratory-based overnight polysomnography. Target bedtimes and wake times were determined for a sleep duration of about 8 hours. Most sleep studies were performed between 10 pm and 6 am. In some cases, individual times were adjusted according to each participant's availability and/or preferred sleep and wake timing to ensure that scheduled timing was close to the schedule that he/she would adopt spontaneously. Healthdyne-computerized PSG systems (Alice 3 diagnostic sleep system; Respironics Inc., Murrysville, PA) were used for overnight polysomno-graphic recordings. Polysomnography involved recording of electroencephalograms, bilateral electrooculograms, submental electromyograms, electrocardiogram, oronasal airflow, snoring, respiratory effort, thoracoabdominal and leg movements, body posture, and arterial oxyhemoglobin saturation obtained by finger oximetry. Each polysomnographic recording was scored visually in 30-second epochs according to standard criteria.2,3 The sleep period was defined as the interval separating sleep onset from morning awakening. Total sleep time was calculated as the total sleep period minus the time spent awake during the sleep period. Sleep efficiency was defined as the night sleep duration expressed as a percentage of the total recording time. The total duration of each stage was expressed in minutes, as well as a percentage of the total sleep period. SWS was defined as the sum of stages 3 and 4. Respiratory events and arousals were scored according to established criteria.7,8 An apnea was defined as a drop in the peak oronasal thermal sensor excursion by ≥90% of baseline, lasting 10 seconds or longer. Hypopneas were identified if nasal pressure signal excursions dropped by ≥50% of baseline for at least 10 seconds and there was a ≥3% desaturation from pre-event baseline or the event was associated with arousal. The number of apneas and hypopneas per hour of sleep was calculated to obtain the apnea–hypopnea index (AHI). The oxygen desaturation index (ODI) was calculated for each subject as the total number of oxygen desaturations ≥3% below the baseline level per hour of sleep. The total arousal index (ArI) was defined as the total number of arousals in sleep, divided by the total sleep time. The primary end points of the study were percentages of total sleep time spent in stage 1, in stage 2, in stages 3 and 4 (SWS), and in REM sleep. Secondary end points included AHI, ODI, and ArI.
Statistical Analyses
Descriptive statistics were produced for all variables. The Shapiro–Wilk test was applied to test for a normal distribution. Continuous variables with normal distribution are presented as means ± standard deviation and compared with use of a Student's t test. Continuous variables with nonnormal distributions are presented as medians and interquartile ranges (25 –75th percentile) and compared with use of the Wilcoxon matched-pairs signed-ranks test. We used the Fisher exact probability test to examine patterns between categorical variables. The Pearson product- moment correlation coefficient, Spearman's rank correlation coefficient, and multiple regression analyses were used to examine relationships among the variables of interest (sleep and respiratory data, anthropometric measures, and confounders). The sample size required to detect a minimum detectable significant difference in SWS as a function of type 2 diabetes with an α of 0.05 and β of 20% (hence, the power of 80%) was 21 subjects per each subgroup. Findings were considered to be statistically significant at the 5% level. All statistical calculations were performed using Stata statistical software release 11.0 (StataCorp LP, College Station, TX).
Results
Demographic Characteristics
A total of 44 overnight polysomnographic recordings of 22 type 2 diabetic subjects and 22 nondiabetic controls were available for final analysis. Table 1 shows characteristics of study participants with comparisons between the study group (subjects with type 2 diabetes) and the control group (nondiabetic controls). Of all the 44 participants, 8 (18.2%) were women and 36 (81.8%) were men. Participants had a mean age of 57.5 ± 5.5 years (range 46 to 68) and a BMI of 33.8 ± 5.9 kg/m2 (range 26 to 57). In this cohort, 9 subjects (20.5%) were current smokers of cigarettes (17 ± 11 cigarettes/day). According to self-reported drinking habits questionnaire responses, 29 (65.9%) of the study participants denied drinking any alcohol at all, 8 (18.2%) were occasional drinkers (<14 grams of alcohol per week), and 7 (15.9%) were light to moderate drinkers (14–196 grams of alcohol per week). There were no significant differences in the number of smokers and in drinking habits between the study group and the control group.
Table 1.
Clinical Characteristics of Study Participants (n = 44) with Comparisons between Study Group and Control Groupa
| Persons with type 2 diabetes (n = 22) | Nondiabetic controls (n = 22) | p value | |
|---|---|---|---|
| Age, years | 58.4 ± 5.4 | 56.6 ± 5.6 | 0.115 |
| BMI, kg/m2 | 34.9 ± 6.9 | 32.6 ± 4.6 | 0.092 |
| Male, % (n) | 81.8 (18) | 81.8 (18) | NS |
| Current smokers, % (n) | 22.7 (5) | 18.2 (4) | NS |
| ESS score | 9.2 ± 4.8 | 8.7 ± 4.0 | 0.768 |
| Sleep architecture | |||
| Total sleep time, minutes | 358.1 ± 116.7 | 363.1 ± 95.1 | 0.880 |
| Sleep efficiency, % | 81.1 (70.9–95.2) | 76.6 (60.1–80.2) | 0.205 |
| Stage 1 sleep, % | 28.4 ± 14.6 | 32.9 ± 17.3 | 0.282 |
| Stage 2 sleep, % | 42.4 ± 16.2 | 43.2 ± 15.7 | 0.870 |
| SWS, % | 2.0 (0.2–4.3) | 8.0 (6.1–10.1) | 0.012 |
| REM sleep, % | 24.1 ± 12.14 | 13.8 ± 6.96 | 0.005 |
| Polysomnography results | |||
| AHI, episodes/hour | 27.2 (9.8–43) | 27.9 (8.9–49.6) | 0.685 |
| REM-AHI, episodes/hour | 17.3 (4.7–37.1) | 17.8 (7.1–42.8) | 0.961 |
| NREM-AHI, episodes/hour | 25.2 (10.5–43) | 29.1 (6.5–50.3) | 0.408 |
| OAI, episodes/hour | 6.1 (2.2–16.4) | 6.4 (2.4–13.9) | 0.783 |
| CAI, episodes/hour | 1.8 (0.6–4.7) | 2.1 (0.8–8.5) | 0.077 |
| MAI, episodes/hour | 0.7 (0.2–3.6) | 2.7 (0.5–5.0) | 0.217 |
| Hypopnea index, episodes/hour | 11.3 (4.6–14.5) | 6.6 (4.4–15.1) | 0.548 |
| MeanSatO2, % | 90 (85–92) | 91 (89–91) | 0.221 |
| MinSatO2, % | 83.5 (79–87) | 84.5 (79–86) | 0.406 |
| ODI, episodes/hour | 50.8 ± 26.8 | 41.3 ± 22.2 | 0.067 |
| Arousal index, episodes/hour | 44.3 ± 19.5 | 35.7 ± 12.7 | 0.037 |
Continuous variables with normal distribution are presented as means ± standard deviation if not otherwise stated and compared with use of a Student's t test. Continuous variables with nonnormal distributions are presented as medians [interquartile range (25 –75th percentile)] and compared with use of the Wilcoxon matched-pairs signed-ranks test. The significance of the association between two categorical variables was examined using the Fisher exact probability test. CAI, central apnea index; MAI, mixed apnea index; MeanSatO2, mean oxygen saturation; MinSatO2, minimum oxygen saturation; NS, not significant; OAI, obstructive apnea index.
Polysomnographic Findings
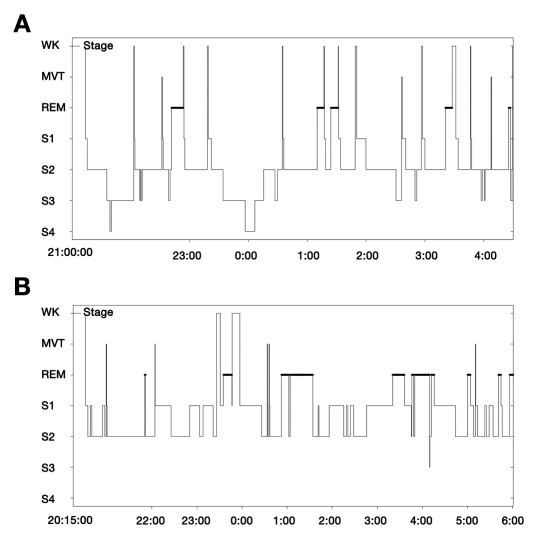
Measurements of daytime sleepiness with the ESS revealed an average score of 9.0 ± 4.4 in our cohort. The average duration of PSG was 507.3 ± 65.8 minutes, with a mean sleep efficiency of 74.3 ± 18.4%. The median AHI in the entire sample was 27.2 events per hour (interquartile range, 9.7 to 46) with a predominance of obstructive events. Polysomnography results with nighttime respiratory outcomes and sleep architecture measures are shown in Table 1. Subjects with type 2 diabetes did not differ from nondiabetic controls in total sleep time, sleep efficiency, excessive daytime sleepiness, and severity of SRBD and in oxygen saturation parameters. Despite similar age and severity of SRBD, participants with type 2 diabetes demonstrated a significantly decreased percentage time in SWS (p = 0.012; Figure 2), an increased percentage time in REM sleep (p = 0.005; Figure 2), and a higher ArI (p = 0.037) compared to their individually matched nondiabetic controls. Figure 3 shows hypnograms of two female study participants with distinct differences in sleep architecture.
Figure 2.
Sleep architecture among study participants (n = 44) with comparisons between the two subgroups.
Figure 3.
Hypnograms of two female study participants with distinct differences in sleep architecture. (A) Hypnogram of a 46-year-old nondiabetic woman with a BMI of 35.3 kg/m2 and an AHI of 3.3 episodes/hour. The sleep study demonstrated normal sleep onset—6.6% of stage 1, 64.4% of stage 2, 20.3% of SWS, and 8.3% of REM sleep. Total sleep time was normal (424 minutes), and sleep efficiency was normal (97.7%). (B) Hypnogram of a 48-year-old woman with type 2 diabetes, a BMI of 38.6 kg/m2, and an AHI of 1.6 episodes/hour. The sleep study demonstrated normal sleep onset—36.5% of stage 1, 43.8% of stage 2, no SWS present, and 19.1% of REM sleep. Total sleep time was normal (551.5 minutes), and sleep efficiency was normal (97.4%). WK, wake; MVT, movement; S1, stage 1 of NREM sleep; S2, stage 2 of NREM sleep; S3, stage 3 of NREM sleep; S4, stage 4 of NREM sleep.
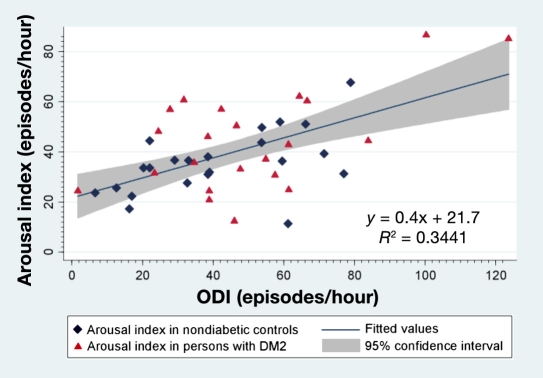
The ArI was correlated positively with ODI in all study participants (r = 0.59; p < 0.001). Figure 4 shows a two-way linear prediction plot of ArI. The prediction for ArI was calculated from a linear regression of ArI on ODI.
Figure 4.
A two-way linear prediction plot of the arousal index. The prediction for the arousal index was calculated from a linear regression of the arousal index on ODI. Blue diamonds in the plot represent the arousal index in nondiabetic study participants. Red triangles represent the arousal index in participants with type 2 diabetes. The thick blue line represents the line of best fit for the arousal index. The shaded gray area represents the 95% confidence interval around the best fit line. DM2, type 2 diabetes mellitus.
Multivariable analyses were conducted to determine whether unadjusted associations persisted after controlling for potential confounders. In a multivariate linear regression model, age and type 2 diabetes were independent predictors of the decreased amount of SWS, even after adjustment for sex, BMI, AHI, and smoking (p = 0.001). Variables in this model accounted for 34% of the variance in the percentage time spent in SWS. After adjustment for sex, age, AHI, and smoking, BMI and type 2 diabetes were independent predictors of the REM sleep percentage (p = 0.014) and altogether explained 23% of the variability in the percentage of total sleep time spent in REM sleep in our cohort.
We explored the relative effects of each of the selected covariates on sleep architecture measures and calculated coefficients of partial determination (partial R2 values) that measure the marginal contribution of one explanatory variable when all others are already included in the model. Table 2 shows partial R2 values for each variable in each model. Age explained the highest proportion of the variance for each NREM sleep stage variable, including nearly 12% of the variance in SWS percentage. Smoking explained 18% of the variance in stage 1 and stage 2 percentages. Type 2 diabetes explained 14% of the variance in SWS percentage and 16.9% of the variance in REM sleep percentage. In general, the AHI explained 0 to 2.6% of the variance in each model for each sleep stage. In contrast, the AHI explained 21% of the variance in the ArI. The ArI was significantly predicted by sex (p = 0.046) and AHI (p = 0.003) and nearly significantly by the presence of type 2 diabetes (p = 0.057).
Table 2.
Percentage Variability (Partial R2) Explained by Each Covariata
| Covariate | % stage 1 | % stage 2 | % stages 3+4 | % stage REM | Arousal index |
|---|---|---|---|---|---|
| Age | 0.1398 | 0.1443 | 0.1191 | 0.0437 | 0.0093 |
| Sex | 0.0080 | 0.0014 | 0.0703 | 0.0021 | 0.1037 |
| BMI | 0.0127 | 0.0786 | 0.0343 | 0.0979 | 0.0001 |
| Smoking | 0.1811 | 0.1888 | 0.0676 | 0.0105 | 0.0065 |
| Type 2 diabetes | 0.0552 | 0.0052 | 0.1408 | 0.1689 | 0.0944 |
| AHI | 0.0169 | 0.0261 | 0.0042 | 0.0000 | 0.2101 |
Values in bold text indicate significant findings.
Discussion
The primary finding of this retrospective case-control study was a significantly decreased amount of SWS in subjects with type 2 diabetes compared to nondiabetic controls. We identified independent predictors of diminished amounts of SWS in our cohort. After adjustment for sex, BMI, AHI, and smoking, the age and presence of type 2 diabetes were independent predictors of the decreased percentage time in SWS and altogether explained 34% of the variability in SWS percentage in the entire sample.
The observation of the decreased amount of SWS in diabetic subjects is partially consistent with previous findings from the Sleep Heart Health Study,9 which indicated differences between diabetic and nondiabetic participants in the unadjusted proportion of sleep time in stages 3 and 4 (SWS). Diabetic participants (n = 470) spent more time in stage 1 and stage 2 and less time in SWS (p < 0.001) compared to persons without diabetes (n = 4402). However, when the investigators adjusted means for age, sex, BMI, race, and neck circumference, multivariable regression analyses eliminated differences for NREM sleep stages.9
In addition to diminished amounts of SWS in type 2 diabetes, our results demonstrated a significantly increased percentage time in REM sleep in type 2 diabetic subjects compared to nondiabetic controls. Using the squared partial correlation, BMI and type 2 diabetes explained the highest proportion of variance for the REM sleep percentage in our cohort. This finding is opposite to previous findings from the Sleep Heart Health Study9 where diabetic participants (n = 470) spent significantly less time in REM sleep than persons without diabetes (n = 4402). The difference in adjusted means of the REM sleep percentage (19.0% among diabetic subjects vs 20.1% among nondiabetic subjects; p < 0.001), while statistically significant due to the large sample size, was not clinically meaningful.9 It is likely that REM sleep has multiple functions.10 Post-training increases in REM sleep intensity during the night following cognitive procedural/implicit task acquisition implicate REM sleep mechanisms in further off-line memory processing and provide a biological marker of learning potential.11
Despite similar age and severity of SRBD, we observed significantly higher ArI in persons with type 2 diabetes compared to nondiabetic controls. During the sleep studies, there were no hypoglycemic episodes reported either by the patient or by the technician. The ArI was significantly predicted by sex and AHI and nearly significantly by the presence of type 2 diabetes in our cohort. There was no significant interaction between age and ArI and between BMI and ArI in our small cohort of mostly middle-aged patients. This observation is consistent with previous findings from the Sleep Heart Health Study9 of higher ArI in individuals with a history of diabetes mellitus compared to those without diabetes. Our results also partially confirm findings by Redline and associates,12 who reported that the ArI was significantly predicted by sex and AHI. The authors showed that the ArI varied substantially with the respiratory disturbance index independent of the associations of other demographic or health variables, suggesting that the ArI may be the most sensitive measure of the sleep-disrupting effects of SRBD.12 Although we did not observe an increased occurrence of central respiratory events among diabetic subjects in our cohort, it is possible that some sleep disturbances may result from diabetes through the deleterious effects of diabetes on the central control of respiration.9
Other factors may influence sleep architecture, such as a fall in core body temperature,13–15 cigarette smoking,16 or weight loss.17 In our small cohort, current smokers spent more time in stage 2 and less time in stage 1 compared to nonsmokers. Several studies have documented the direct effect of macronutrient intake on sleep. Phillips and colleagues18 showed a significantly less SWS percentage in young healthy male subjects after consuming a high-carbohydrate/low-fat diet (10% protein, 10% fat, and 80% carbohydrate) than after consuming a normal balanced diet or a low-carbohydrate/high-fat diet (10% protein, 77% fat, and 13% carbohydrate). Both high-carbohydrate/ low-fat and low-carbohydrate/high-fat isocaloric diets, especially the former, were associated with significantly more REM sleep than a normal balanced diet.18 In our study, patients were asked to follow a normal balanced diet on the day of the sleep study. Substantial evidence indicates that the structure of sleep changes with age.19 Table 3 shows changes in the quality of sleep across the life span20—the latency of sleep onset is lengthened, awakenings are frequent, naps are more common, and sleep is subjectively and objectively lighter. REM sleep remains fairly constant across the adult life span and decreases as people age. Stage 1 of NREM sleep increases with age, whereas the amount of deep SWS is reduced considerably and may be nonexistent for some older people.
Table 3.
Quality of Sleep across the Life Spana
| Sleep in early adulthood (18–30 years) | Sleep in early middle age (30–45 years) | Sleep in later middle age (45–60 years) | Sleep in old age (60+ years) | |||||
|---|---|---|---|---|---|---|---|---|
| Sex | Males | Females | Males | Females | Males | Females | Males | Females |
| Total sleep time | 450–480 min | 399–436 min | 394–448 min | 340–440 min | 396–466 min | 286–460 minc | 349–461 minc | |
| Sleep efficiency | 91–99% | 94–98% | 85–99% | 90–99% | 88–96% | 86–100% | 57–97% | 73–96% |
| Awakeningsb | 0–6 | 0–2 | 1–7 | 0–5 | 4–7 | 3–7 | 4–11d | 2–7d |
| Stage 1 | 2–6% | 3–11% | 2–8% | 4–12% | 3–7% | 6–14% | 4–12% | |
| Stage 2 | 41–51% | 46–58% | 45–66% | 45–63% | 52–72% | 51–65% | 38–72% | 44–64% |
| SWS | 6–26% | 11–25% | 2–18% | 4–21% | 0–12% | 5–17% | 0–3% | 0–18% |
| Stage REM | 22–34% | 21–29% | 19–27% | 21–31% | 17–25% | 19–25% | 11–27% | 15–25% |
Adapted from Margaret H. White, 2000.20
Greater than 2 minutes in duration.
Plus an afternoon nap (1 hour usually); cannot keep sleep consolidated at night
Ages 70–79 years: 1–10 in males and 3–14 in females.
Obstructive sleep apnea is substantially more common in men than in women, with male:female ratios ranging between 2:1 and 10:1, depending on the study design.21,22 Participants in our study were subjects with suspected SRBD referred to our sleep laboratory for sleep evaluation. The unequal numbers of men and women in our cohort are likely to be related to an increased prevalence of sleep apnea in men compared with women. Possible implications of the mostly male cohort may include sex differences in sleep physiology, including those in sleep architecture. An increasing body of evidence suggests that women have greater amounts of SWS than men,12,23,24 as well as a tendency for longer sleep latencies and fewer awakenings, despite no differences in age,23,24 AHI, or oxygen saturation.24 Redline and colleagues12 also demonstrated that men, not women, showed evidence of poorer sleep with aging, suggesting important sex differences in sleep physiology. We did not aim to examine sex differences in sleep physiology. Therefore, subjects in both study and control groups were well matched individually for sex, race, age, BMI, and severity of SRBD.
A main limitation of our study was the lack of spectral analysis in assessing the depth or intensity of the SWS. Another limitation was that we did not have anthropometric variables such as neck circumference, waist-to-hip ratio, sagittal–abdominal diameter, or percentage of body fat on all subjects. Inclusion of these markers may have en abled us to more accurately assess relative effects of obesity and central adiposity on sleep architecture measures. Our limited sample size precluded the use of more complex multivariate modeling. Another potential limitation was the self-reported nature of alcohol consumption. Our study was restricted to individuals who reported drinking less than 30 grams of alcohol per day. Because of the possibility of underreporting, we did not assess whether lesser quantities of alcohol could have affected the study findings.
The decreased SWS profile is not a disease-specific measure. Despite the limitations, our study indicated distinct differences in sleep architecture in persons with type 2 diabetes compared to nondiabetic controls.
A number of studies have consistently demonstrated that insulin sensitivity can be altered by several well-known pathological processes, such as obesity25 or sleep apnea.26,27 Future questions that remain to be answered include elucidating the role of SWS in the development and progression of insulin resistance that presents a fundamental aspect of the etiopathophysiology of type 2 diabetes.
Conclusions
To our knowledge, this was the first case-control study that assessed differences in sleep architecture between type 2 diabetic and nondiabetic subjects with SRBD matched individually for sex, race, age, BMI, and severity of SRBD and that explored the relative effects of selected covariates on sleep architecture measures. Our study demonstrated that persons with type 2 diabetes had significantly decreased amounts of SWS compared to nondiabetic controls independently of age, obesity, and severity of sleep apnea. Findings suggest that polysomnographic recognition of altered sleep architecture may be partially implicated in the early detection of persons with type 2 diabetes, thus offering a possibility to intervene. Understanding the complex interactions among sleep, nocturnal hormonal changes affecting the adipo–insulin axis, insulin sensitivity, and glucose regulation, as well as future research into the effects of restored/stimulated SWS on the prevention and treatment of type 2 diabetes, may have important implications in an innovative 21st-century approach to type 2 diabetes management.
Acknowledgment
Parts of this study were presented at the Eighth Annual Diabetes Technology Meeting, November 13–15, 2008 in Bethesda, Maryland.
Abbreviations
- AASM
American Academy of Sleep Medicine
- AHI
apnea–hypopnea index
- ArI
total arousal index
- BMI
body mass index
- EEG
electroencephalogram
- ESS
Epworth Sleepiness Scale
- NREM
nonrapid eye movement
- ODI
oxygen desaturation index
- PSG
polysomnography
- REM
rapid eye movement
- SRBD
sleep-related breathing disorders
- SWS
slow-wave sleep
References
- 1.Dement W, Kleitman N. The relation of eye movements during sleep to dream activity: an objective method for the study of dreaming. J Exp Psychol. 1957;53(5):339–346. doi: 10.1037/h0048189. [DOI] [PubMed] [Google Scholar]
- 2.Iber C, Ancoli-Israel S, Chesson A. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specification. 1st. Westchester, IL: American Academy of Sleep Medicine; 2007. Quan SF for the American Academy of Sleep Medicine. [Google Scholar]
- 3.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: BIS/BRI University of California; 1968. [Google Scholar]
- 4.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284(7):861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 5.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 7.The Report of an American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 8.A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. EEG arousals: scoring rules and examples. Sleep. 1992;15(2):173–184. [PubMed] [Google Scholar]
- 9.Resnick HE, Redline S, Shahar E, Gilpin A, Newman A, Walter R, Ewy GA, Howard BV, Punjabi NM Sleep Heart Health Study. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26(3):702–709. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 10.Rechtschaffen A. Current perspectives on the function of sleep. Perspect Biol Med. 1998;41(3):359–390. doi: 10.1353/pbm.1998.0051. [DOI] [PubMed] [Google Scholar]
- 11.Smith CT, Nixon MR, Nader RS. Posttraining increases in REM sleep intensity implicate REM sleep in memory processing and provide a biological marker of learning potential. Learn Mem. 2004;11(6):714–719. doi: 10.1101/lm.74904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164(4):406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 13.Berger RJ, Palca JW, Walker JM, Phillips NH. Correlation between body temperatures, metabolic rate and slow wave sleep in humans. Neurosci Lett. 1988;86(2):230–234. doi: 10.1016/0304-3940(88)90576-9. [DOI] [PubMed] [Google Scholar]
- 14.McGinty D, Szymusiak R. Keeping cool: a hypothesis about the mechanisms and functions of slow-wave sleep. Trends Neurosci. 1990;13(12):480–487. doi: 10.1016/0166-2236(90)90081-k. [DOI] [PubMed] [Google Scholar]
- 15.Wehr TM. A brain-warming function for REM sleep. Neurosci Biobehav Rev. 1992;16(3):379–397. doi: 10.1016/s0149-7634(05)80208-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Samet J, Caffo B, Punjabi NM. Cigarette smoking and nocturnal sleep architecture. Am J Epidemiol. 2006;164(6):529–537. doi: 10.1093/aje/kwj231. [DOI] [PubMed] [Google Scholar]
- 17.Willi SM, Oexmann MJ, Wright NM, Collop NA, Key LL., Jr The effects of a high-protein, low-fat, ketogenic diet on adolescents with morbid obesity: body composition, blood chemistries, and sleep abnormalities. Pediatrics. 1998;101(1 Pt 1):61–67. doi: 10.1542/peds.101.1.61. [DOI] [PubMed] [Google Scholar]
- 18.Phillips F, Chen CN, Crisp AH, Koval J, McGuinness B, Kalucy RS, Kalucy EC, Lacey JH. Isocaloric diet changes and electroencephalographic sleep. Lancet. 1975;2(7938):723–725. doi: 10.1016/s0140-6736(75)90718-7. [DOI] [PubMed] [Google Scholar]
- 19.Kales JC, Carvell M, Kales A. Sleep disorders. In: Cassel CK, Riesenberg DE, Sorensen LB, Walsh JR, editors. Geriatric medicine. 2nd. New York: Springer-Verlag; 1990. [Google Scholar]
- 20.Sleep across the lifespan. Fullerton: Department of Psychology, California State University; California State University, Fullerton [Internet] c2008 [updated 2000 Jan 25; cited 2009 May 31]. Available from: http://psych.fullerton.edu/mwhite/473pdf/473%20 Sleep%20Across%20Lifespan.pdf. [Google Scholar]
- 21.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 22.Dancey DR, Hanly PJ, Soong C, Lee B, Shepard J, Jr, Hoffstein V. Gender differences in sleep apnea: the role of neck circumference. Chest. 2003;123(5):1544–1550. doi: 10.1378/chest.123.5.1544. [DOI] [PubMed] [Google Scholar]
- 23.Rediehs MH, Reis JS, Creason NS. Sleep in old age: focus on gender differences. Sleep. 1990;13(5):410–424. [PubMed] [Google Scholar]
- 24.Valencia-Flores M, Bliwise DL, Guilleminault C, Rhoads NP, Clerk A. Gender differences in sleep architecture in sleep apnoea syndrome. J Sleep Res. 1992;1(1):51–53. doi: 10.1111/j.1365-2869.1992.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 25.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4):473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165(5):670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 27.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165(5):677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]