Abstract
Background
For microvascular outcomes, there is compelling historical and contemporary evidence for intensive blood glucose reduction in patients with either type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM). There is also strong evidence to support macrovascular benefit with intensive blood glucose reduction in T1DM. Similar evidence remains elusive for T2DM. Because cardiovascular outcome trials utilizing conventional algorithms to attain intensive blood glucose reduction have not demonstrated superiority to less aggressive blood glucose reduction (Action to Control Cardiovascular Risk in Diabetes; Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation; and Veterans Affairs Diabetes Trial), it should be considered that the means by which the blood glucose is reduced may be as important as the actual blood glucose.
Methods
By identifying quantitative differences between antidiabetic agents on carbohydrate exposure (CE), hepatic glucose uptake (HGU), hepatic gluconeogenesis (GNG), insulin resistance (IR), peripheral glucose uptake (PGU), and peripheral insulin exposure (PIE), we created a pharmacokinetic/pharmacodynamic model to characterize the effect of the agents on the glucose supply and insulin demand dynamic. Glucose supply was defined as the cumulative percentage decrease in CE, increase in HGU, decrease in GNG, and decrease in IR, while insulin demand was defined as the cumulative percentage increase in PIE and PGU. With the glucose supply and insulin demand effects of each antidiabetic agent summated, the glucose supply (numerator) was divided by the insulin demand (denominator) to create a value representative of the glucose supply and insulin demand dynamic (SD ratio).
Results
Alpha-glucosidase inhibitors (1.25), metformin (2.20), and thiazolidinediones (TZDs; 1.25–1.32) demonstrate a greater effect on glucose supply (SD ratio >1), while secretagogues (0.69–0.81), basal insulins (0.77–0.79), and bolus insulins (0.62–0.67) demonstrate a greater effect on insulin demand (SD ratio <1).
Conclusion
Alpha-glucosidase inhibitors, metformin, and TZDs demonstrate a greater effect on glucose supply, while secretagogues, basal insulin, and bolus insulin demonstrate a greater effect on insulin demand. Because T2DM cardiovascular outcome trials have not demonstrated macrovascular benefit with more aggressive blood glucose reduction when using conventional algorithms that predominantly focus on insulin demand, it would appear logical to consider a model that incorporates both the extent of blood glucose lowering (hemoglobin A1c) and the means by which the blood glucose was reduced (SD ratio) when considering macrovascular outcomes.
Keywords: cardiovascular outcomes, glucose, insulin, pharmacodynamics, pharmacokinetics
Background
Historically, diabetes management has been guided by the positive microvascular outcomes demonstrated in the Diabetes Control and Complications Trial (DCCT)1 and United Kingdom Prospective Diabetes Study (UKPDS).2 In the DCCT, comparing intensive (hemoglobin A1c [HbA1c] = 7.0%) and conventional (HbA1c = 9.0%) management of patients with type 1 diabetes mellitus (T1DM), intensive therapy reduced all diabetes-specific complications evaluated (retinopathy, nephropathy, and neuropathy) by as much as 76%. Similarly, the UKPDS compared intensive (HbA1c = 7.0%) and conventional (HbA1c = 7.9%) management in patients with type 2 diabetes mellitus (T2DM); again, intensive therapy was observed to significantly reduce risk for microvascular outcomes. In 2005, long-term follow-up data from the DCCT demonstrated that, after a mean of 17 years, intensive treatment significantly reduced the risk of nonfatal myocardial infarction, stroke, or death from cardiovascular disease by 57%.3 In 2008, the UKPDS 10-year post-trial follow-up study revealed that, despite the loss of glycemic differences between intensive and conventional cohorts, there was continued microvascular benefit and an emergent reduction in myocardial infarction and all-cause mortality.4
With respect to microvascular outcomes, there appears to be compelling historical and contemporary evidence for intensive blood glucose reduction in patients with both T1DM and T2DM. There is also strong evidence to support a beneficial impact on macrovascular outcomes with intensive blood glucose reduction in patients with T1DM. Similar evidence remains elusive in T2DM.5 The nonsignificant reductions in macrovascular outcomes (myocardial infarction, stroke, heart failure, angina) observed in the UKPDS intervention trial and the more recent Action to Control Cardiovascular Risk in Diabetes (ACCORD),6 Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE),7 and Veterans Affairs Diabetes Trial (VADT)8 have collectively created uncertainty toward the extent of blood glucose reduction and also the optimal therapeutic choice to attain the reduction.
In this situation, where long-term, randomized controlled trials have resulted in counterintuitive outcomes, it is imperative that the methodology of therapeutic intervention be rigorously evaluated. The common theme of the aforementioned historical and contemporary cardiovascular outcome trials has been a focus on the intensive reduction of the primary biomarker HbA1c in a manner consistent with the consensus algorithm issued by the American Diabetes Association and European Association for the Study of Diabetes. Predominantly, this algorithm advocated the initial use of metformin with subsequent addition and intensification of sulfonylurea and/or insulin therapies. Inherent to this pharmacotherapeutic approach, as well as those utilized prior to the guidelines, is an imbalance toward increased insulin exposure and increased peripheral glucose disposal. While this approach is effective at reducing HbA1c, it must be considered that both excessive insulin exposure and excessive peripheral glucose disposal have potentially deleterious effects on the vasculature.9–12 Moreover, a potential explanation for the observed neutral or negative cardiovascular outcomes despite more intensive blood glucose reduction when using therapies that principally impact peripheral insulin exposure (PIE) and peripheral glucose disposal is that the means by which the blood glucose is reduced may be as important as the actual blood glucose. Therefore, we developed a pharmacokinetic/pharmacodynamic model to characterize the effect of conventional antidiabetic therapies on the glucose supply [carbohydrate intake and intestinal absorption (carbohydrate exposure [CE]), hepatic glucose uptake (HGU), hepatic gluconeogenesis (GNG), and insulin resistance (IR)] and insulin demand [PIE and peripheral glucose uptake (PGU)] dynamic in order to identify agents and/or pharmacotherapeutic strategies that may have benefits extending beyond the reduction of HbA1c.
Methods
Therapeutic targets of the glucose supply (CE, 1 + 2; HGU, 3; GNG, 4; IR, 5) and insulin demand (PGU, 6; PIE, 7) model are presented in Figure 1. To identify quantitative differences between antidiabetic agents on CE, HGU, GNG, IR, PGU, and PIE, multidatabase searches (Cochrane Central Register of Controlled Trials and Cochrane Register of Systematic Reviews, Embase, OVID Healthstar, OVID Journals, and PubMed) were conducted, cross-referencing title and keywords for all selected antidiabetic therapies and their respective targets.
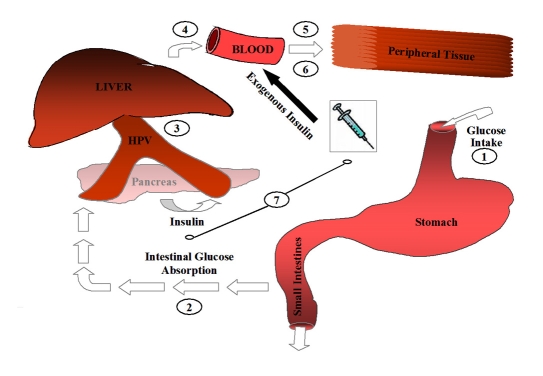
Figure 1.
Glucose supply and insulin demand model. CE, 1+2; HGU, 3;GNG, 4; IR, 5; PGU, 6; PIE, 7. HPV, hepatic portal vein.
Alpha-glucosidase, biguanide, and thiazolidinedione (TZD) studies with long-term, pre–post design at maximal therapeutic doses were identified to simulate chronic administration. To maintain consistency with cardiovascular trials and accommodate known influences of hyperglycemia and hyperinsulinemia on the respective targets,13–16 studies including patients with HbA1c in the range of 6–8% and body mass index (BMI) ≥30 kg/m2 were preferentially selected. In the event multiple studies were available to identify the effect of an agent on a therapeutic target, the mean percent change was used. Conversely, if there was evidence that an agent would elicit a response on a given target but no mathematical representation of the difference was provided, conservative estimates consistent with the scale of other agents and the degree of glucose regulation were instituted to best represent the expected effect. Individual agents were then characterized for the 24 h percent change from baseline for CE, HGU, GNG, IR, PGU, and PIE. Carbohydrate exposure was determined as the combined effect of caloric intake and intestinal carbohydrate absorption. Hepatic glucose uptake was defined as the reported value obtained immediately following oral glucose loading. Because GNG is known to be enhanced in the fasting state and persistent throughout the prandial phase,17,18 effect was determined during the fasting state and considered equivalent throughout the prandial phase. For studies evaluating fasting glucose and insulin concentrations, an index of IR was determined by homeostasis model assessment of insulin resistance (HOMAIR) using the following formula: Insulin (mU/liter) × Glucose (mmol)/22.5.19 To account for known differences in secretory and uptake dynamics during the fasting and prandial phases, studies identifying the impact of therapies during the fasting state (or simulated hyperinsulinemic euglycemic clamp) and prandial phase (or oral glucose load insulin clamp) were specifically identified for changes in PGU (glucose infusion rate) and PIE (insulin concentrations) according to Equation (1):
| (1) |
For sulfonylurea and insulin-based therapies, insulin concentration time profiles were obtained and super-imposed on the baseline 24 h insulin concentration time profile of T2DM patients (Figure 2) to calculate the increase in PIE (trapezoidal rule).20 Calculated increases in incremental and cumulative insulin exposure were correlated to known insulin dose-response effects on HGU, GNG, and PGU (Figure 3),18,21 according to the equation y = mx + b. Twenty-four-hour increases in HGU, PGU, and PIE and decreases in GNG were compared to baseline values and percent change calculated.
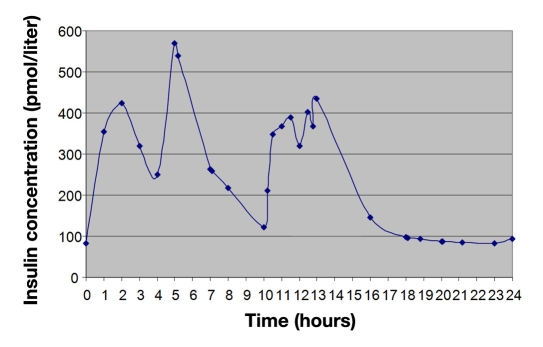
Figure 2.
Standard insulin concentration time profile (T2DM).
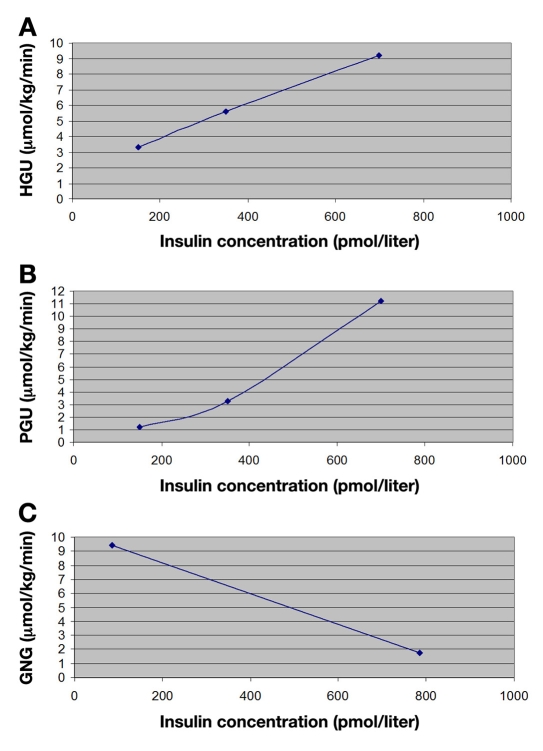
Figure 3.
(A) HGU insulin-dose response relationship. (B) PGU insulin-dose response relationship. (C) GNG insulin-dose response relationship.
With alpha-glucosidase, biguanide, TZD, secretagogue, and insulin therapies characterized for their respective impacts on CE, HGU, GNG, IR, PIE, and PGU, identification of their effect on the glucose supply (decrease in CE, increase in HGU, decrease in GNG, decrease in IR) and insulin demand (increase in PIE, increase in PGU) dynamic was assessed according to Equation (2):
| (2) |
Results
Alpha-Glucosidase Inhibitors (Acarbose and Miglitol)
The alpha-glucosidase inhibitors (1) have no significant effect on total caloric intake,22 (2) delay and decrease carbohydrate absorption,23–27 (3) have not been directly evaluated for HGU, (4) have negligible effect on hepatic glucose output,28,29 (5) reduce IR,22,30–33 (6) have variable effects on PGU,22,28,30,34–38 and (7) reduce plasma insulin concentrations.22,39–45 Studies meeting the review criteria for the target effects of the alpha-glucosidase inhibitors on the respective targets are summarized here. Estimates for the effect of alpha-glucosidase inhibitors on the respective targets are presented in Table 1.
Table 1.
Glucose Supply : Insulin Demand Ratio for Antidiabetic Therapies at Maximal Therapeutic Dose
| Antidiabetic agent | CE | HGU | GNG | IR | PIE | PGU | Therapeutic dose | SD ratioa |
|---|---|---|---|---|---|---|---|---|
| Miglitol | 0.30 | 0.15 | 0.05 | 0.15 | 0.05 | 0.25 | 300 mg | 1.25 |
| Acarbose | 0.30 | 0.15 | 0.05 | 0.15 | 0.05 | 0.25 | 300 mg | 1.25 |
| Metformin | 0.15 | 0.40 | 0.35 | 0.38 | -0.10 | 0.14 | 2000 mg | 2.20 |
| Acetohexamide | 0.00 | 0.14 | 0.07 | 0.00 | 0.21 | 0.36 | 1500 mg | 0.77 |
| Chlorpropamide | 0.00 | 0.14 | 0.07 | 0.00 | 0.21 | 0.36 | 500 mg | 0.77 |
| Tolazamide | 0.00 | 0.14 | 0.07 | 0.00 | 0.21 | 0.36 | 1000 mg | 0.77 |
| Tolbutamide | 0.00 | 0.14 | 0.07 | 0.00 | 0.21 | 0.36 | 2000 mg | 0.77 |
| Glimepiride | 0.00 | 0.18 | 0.08 | 0.00 | 0.24 | 0.39 | 8 mg | 0.77 |
| Glipizide | 0.00 | 0.18 | 0.08 | 0.00 | 0.24 | 0.39 | 10 mg | 0.77 |
| Glyburide | 0.00 | 0.14 | 0.07 | 0.00 | 0.21 | 0.36 | 10 mg | 0.77 |
| Nateglinide | 0.00 | 0.21 | 0.11 | 0.00 | 0.34 | 0.60 | 360 mg | 0.69 |
| Repaglinide | 0.00 | 0.16 | 0.07 | 0.00 | 0.20 | 0.31 | 12 mg | 0.81 |
| Pioglitazone | 0.00 | 0.40 | 0.21 | 0.35 | -0.10 | 0.59 | 45 mg | 1.32 |
| Rosiglitazone | 0.00 | 0.40 | 0.23 | 0.39 | -0.10 | 0.70 | 8 mg | 1.27 |
| Troglitazone | 0.00 | 0.40 | 0.22 | 0.35 | -0.10 | 0.67 | 600 mg | 1.25 |
| Insulin aspart | 0.00 | 0.23 | 0.14 | 0.00 | 0.42 | 0.80 | 0.5 U/kg | 0.62 |
| Insulin lispro | 0.00 | 0.23 | 0.14 | 0.00 | 0.42 | 0.80 | 0.5 U/kg | 0.62 |
| Insulin regular | 0.00 | 0.21 | 0.11 | 0.00 | 0.33 | 0.64 | 0.5 U/kg | 0.67 |
| Insulin isophane | 0.00 | 0.23 | 0.10 | 0.00 | 0.28 | 0.40 | 0.5 U/kg | 0.79 |
| Insulin aspart protamine | 0.00 | 0.23 | 0.10 | 0.00 | 0.28 | 0.40 | 0.5 U/kg | 0.79 |
| Insulin lispro protamine | 0.00 | 0.23 | 0.10 | 0.00 | 0.28 | 0.40 | 0.5 U/kg | 0.79 |
| Insulin lente | 0.00 | 0.23 | 0.10 | 0.00 | 0.28 | 0.40 | 0.5 U/kg | 0.79 |
| Insulin ultralente | 0.00 | 0.17 | 0.08 | 0.00 | 0.24 | 0.38 | 0.5 U/kg | 0.77 |
| Insulin glargine | 0.00 | 0.24 | 0.10 | 0.00 | 0.30 | 0.42 | 0.5 U/kg | 0.78 |
Estimates of effect for oral medications on CE, HGU, GNG, IR, PIE, and PGU were calculated for maximal therapeutic dose and linearly extrapolated for decreasing doses. Insulin, having no maximal therapeutic dose, was linearly extrapolated for increasing or decreasing dose. All combination effects on CE, HGU, GNG, IR, PIE, and PGU were considered additive.
Caloric Intake and Intestinal Carbohydrate Absorption
Meneilly and associates evaluated the effects of acarbose on total caloric intake by means of 3-day food recall and dietician interview.22 Acarbose was administered at an initial dose of 50–100 mg three times daily. At the conclusion of 52 weeks of acarbose therapy, there was no significant change in proportion of calories as carbohydrate (-0.7 ± 0.8%), fat (0.9 ± 0.8%), or protein (-0.5 ± 0.5%), nor was there a significant change in total caloric intake (90 ± 50 kcal). Radziuk and coworkers evaluated the effect of 0, 50, and 100 mg of acarbose on the absorption of the glucose moiety of sucrose in overnight-fasted subjects receiving labeled 100 g oral sucrose load ([1-14C]glucose) and simultaneous intravenous infusion of [3-3H]glucose.24 Acarbose increased malabsorption in a dose-dependent manner; at 50 mg, there was a modest effect (6%), whereas at 100 mg, it was approximately 30%, and at the highest 150 mg dose, it was approximately 66%. These findings are supported by Sobajima et al., where carbohydrate malabsorption, measured by hydrogen excretion following 2-month acarbose administration (50–100 mg three times daily) was estimated to be 31.6% of baseline.25
Hepatic Glucose Uptake and Hepatic Gluconeogenesis
No studies directly evaluate the impact of alpha-glucosidase inhibitors on HGU. However, evidence does suggest acarbose delays carbohydrate absorption26,27 and increases glucagon-like peptide-1 secretion.46,47 Therefore, it would be anticipated that alpha-glucosidase inhibitors would exhibit modest effects on retaining carbohydrate in the splanchnic area. Likewise, there is limited data regarding the impact of alpha-glucosidase inhibitors on hepatic GNG. Schnack and associates evaluated the effect of long-term miglitol therapy on hepatic glucose output in poorly controlled T2DM patients (HbA1c = 9.9%). After eight weeks of therapy (300 mg/day), miglitol had no significant effect on hepatic glucose output versus placebo (0.37 ± 0.15 versus 0.35 ± 0.17 mg/kg-1/min-1) under euglycemic clamp conditions.28 Sels and coworkers evaluated the effects of miglitol on fasting plasma glucose (FPG) in T2DM patients. Finding similar results, 200 mg of miglitol at bedtime for 1 week was not associated with a change in hepatic glucose production.29
Insulin Resistance
In the study by Meneilly and colleagues, IR was assessed at baseline and after 12 months of acarbose (HOMAIR). Insulin resistance was significantly improved following acarbose treatment (6.1 ± 0.5 versus 5.0 ± 0.5).22 At the same acarbose dose, Calle-Pascual and associates observed reductions in FPG and fasting plasma insulin (FPI) and a slightly greater reduction in IR (∼27%), as calculated by HOMAIR, after 16 weeks of therapy.30 Concurrent with these results, Delgado et al. observed an approximate 15% reduction in IR after 16 weeks of therapy at a lower therapeutic dose of acarbose (100 mg daily).33 Contradicting the findings of the previous authors, Hanefeld and colleagues as well as Fischer and associates found no significant alterations in IR.38,41
Peripheral Glucose Uptake
Kinoshita and group evaluated the effect of acarbose 300 mg daily on glucose utilization rate (M value) (mg/kg-1/min-1) under euglycemic hyperinsulinemic conditions.37,48 After allowing the HbA1c to fall to ≤8%, baseline clamp study was performed, with follow-up study at 6 months. At the conclusion of therapy, glucose utilization rate was increased (8.00 ± 1.96 versus 9.94 ± 2.35 mg/kg-1/min-1). At the same daily dose for 16 weeks, Fischer et al. observed a nonsignificant increase in glucose disposal rate during euglycemic hyper-insulinemic clamp (3.2 versus 2.3 mg/kg-1/min-1).38 In the study by Meneilly and associates, glucose infusion rate during the final 20 min of the 2 h hyperglycemic clamp (5.4 mM above basal) was assessed at baseline and after 12 months of therapy. Glucose infusion rate increased significantly after acarbose therapy (1.68 ± 0.19 versus 2.69 ± 0.19 mg/kg-1/min-1).22 Despite this evidence, multiple studies under similar experimental conditions do not confirm the observed increases in peripheral glucose disposal after sustained alpha-glucosidase therapy.28,35,36,49
Peripheral Insulin Exposure
Numerous studies have identified a reduced postprandial insulin response following acarbose administration to T2DM patients.42–45 Meneilly and associates as well as Hanefeld and coworkers have evaluated the combined fasting and postprandial effects of long-term acarbose administration.22,41 Meneilly and associates assessed fasting and postprandial insulin secretion at baseline and 12 months of acarbose therapy (100 mg three times daily), observing significant decreases in both increments (-13 ± 4 and -271 ± 159 pmol/liter, respectively).22 Hanefeld and coworkers evaluated the effect of acarbose therapy (100 mg three times daily) on the 24 h insulin concentration time profile. After 16 weeks of therapy, acarbose was not found to change the 24 h area under the curve of insulin from baseline.41
Biguanides (Metformin)
Metformin has been shown to (1) reduce caloric intake,50–52 (2) have variable effects on intestinal carbohydrate absorption,53–70 (3) increase HGU,71 (4) diminish hepatic GNG,72,73 (5) reduce IR,71,72,74–77 (6) increase PGU,74,78 and (7) reduce insulin exposure.71,72,76,78 Studies meeting review criteria for the target effects of metformin are presented here. Estimates for the effect of metformin on the respective targets are presented in Table 1.
Caloric Intake and Intestinal Carbohydrate Absorption
Anorexia is occasionally reported following the introduction of metformin therapy to T2DM patients.51 Lee and Morley evaluated the effect of metformin on caloric intake in patients with T2DM. Patients were randomly given placebo, 850, or 1700 mg of metformin for three days and subsequently evaluated for caloric intake during three consecutive 10 min intake periods. Caloric intake was reduced during each eating interval in a dose-dependent manner. Total caloric intake during the 30 min period was reduced 30% and 50% at 850 and 1700 mg, respectively.52 Despite the substantial reductions in caloric intake observed at the respective doses, it should be considered that the impact is thought to be sustained with only extremely high doses (>2 g/kg-1/day-1).60,79
Animal and human studies to determine the impact of biguanides on intestinal carbohydrate absorption have yielded conflicting results.53–59 Bailey reviewed the effects of metformin on intestinal glucose handling (absorption and metabolism) in animal and human models.60 In vitro animal studies have demonstrated metformin to cause a concentration-dependent decrease in glucose transport at concentrations in the millimolar range.61–64 In vivo, Wilcock and Bailey observed net glucose transfer in the serosal fluid was reduced 12% in mice at a dosage of 50 mg/kg (slightly greater than the maximum 3 g dose).65 In a preparation of brush border vesicles isolated from rabbit intestine (5 mM metformin), Kessler and colleagues observed a nominal decrease in glucose uptake.66 In clinical studies of noninsulin-dependent diabetes mellitus patients, there is evidence to suggest that biguanides may delay the rate, but not the extent of glucose absorption.58,67 During a 75 g oral glucose load challenge with labeled [1-14C] glucose, Jackson et al. observed the absorption of glucose to be slightly delayed but ultimately unaltered over the 3 h study period.67
Metformin has also been noted to increase intestinal glucose utilization.68,69 Pénicaud et al. administered 350 mg/kg-1/day-1 to obese fa/fa rats for eight days, observing an increased glucose utilization by 39% in the jejunum.68 During intravenous glucose tolerance test, Bailey and colleagues administered metformin 250 mg/kg-1 to normal rats, observing an increased glucose utilization by 30–60% in mucosa from different regions of the intestine.69 Despite substantial increases in intestinal glucose utilization induced by metformin, it must be considered that evidence suggests an increased lactate exposure in the hepatic portal vein.70 The increased exposure to lactate may yield increased glucose–lactate cycling between the splanchnic tissues and diminish the impact of intestinal metabolism on overall glycemia.60
Hepatic Glucose Uptake
Iozzo et al. evaluated the impact of metformin (2000 mg daily) and rosiglitazone (8 mg daily) therapy on HGU.71 Positron-emission tomography (PET) studies in combination with [18F]2-fluoro-2-deoxyglucose ([18F]FDG) and the insulin clamp technique48 were performed before treatment and at 26 weeks to assess HGU. At 90 min of the 150 min normoglycemic hyperinsulinemic period, patients were intravenously administered [18F]FDG, and consecutive scans of the liver were obtained at 20 min. Although baseline HGU was not presented, metformin and rosiglitazone similarly and significantly increased HGU (placebo-subtracted value = +0.008 ± 0.004 and +0.007 ± 0.004 μmol/kg-1/min-1, respectively). Despite the failure of this study to define a specific increase versus baseline in HGU following an oral glucose load, the relationship identified between metformin and TZD would infer a similar impact.
Hepatic Gluconeogenesis
Stumvoll and associates evaluated the metabolic effects of metformin in T2DM patients receiving metformin 2550 mg daily.72 Prior to and at the conclusion of the 16-week treatment period, patients were fasted and assessed for the rate of plasma lactate to plasma glucose conversion (GNG). Metformin was found to reduce the rate of conversion by 37% (7.3 ± 0.7 versus 4.6 ± 0.6 μmol/kg-1/min-1). Hundal and coworkers also evaluated the mechanism by which metformin reduces glucose production in patients with T2DM.73 To address known methodological limitations used in previous studies assessing GNG, two independent and complimentary methods (nuclear magnetic resonance spectroscopy and 2H2O method) were employed to assess the impact of metformin therapy (2550 mg daily). Supporting the findings of Stumvoll and associates, the rate of hepatic GNG was reduced 36% as evaluated by the nuclear magnetic resonance method (0.59 ± 0.03 versus 0.18 ± 0.03 mmol/m-2/min-1) and 33% by the 2H2O method (0.42 ± 0.04 versus 0.28 ± 0.03 mmol/m-2/min-1) after three months of treatment.
Insulin Resistance
In a meta-analysis of randomized controlled trials in people at risk for T2DM, metformin reduced calculated IR (HOMAIR) by 22.6%. In studies of patients with T2DM and maximal therapeutic doses of metformin,71,72,74,75 calculated IR was reduced 38–44%.
Peripheral Glucose Uptake
In the aforementioned analysis by Stumvoll et al., it was noted that the rate of plasma glucose turnover (hepatic glucose output and systemic glucose disposal) was reduced with metformin from 2.8 ± 0.2 to 2.0 ± 0.2 mg/kg-1/min-1.72 Importantly, the reduction in plasma glucose turnover was attributed to the reduction in hepatic glucose output; systemic glucose disposal did not change.72 Corroborating evidence that metformin does not substantially increase PGU, Tiikkainen et al. and Inzucchi and associates observed nominal increases with long-term administration of metformin at therapeutic doses. Tiikkainen and colleagues clamped patients at 144 mg/dl before and after 16 weeks of metformin 2000 mg daily. The glucose rate of disappearance remained unchanged (0.09 ± 0.01 versus 0.10 ± 0.01 mg/kg-1/min-1).74 Inzucchi and associates clamped patients at 100 mg/dl before and after 12 weeks of metformin 2000 mg daily. During the euglycemic hyperinsulinemic clamp period, glucose infusion rate was increased 13% (240 versus 272 mg/m-2/min-1).78
Peripheral Insulin Exposure
Metformin was consistently found to reduce FPI concentrations (range: 10–30%). In the aforementioned studies by Iozzo and group and Stumvoll and colleagues, FPI was reduced ∼30% (63 ± 12 to 43.0 ± 5.0 pmol/liter) and 17% (12 ± 5 to 10 ± μU/ml), respectively.71,72 Tiikkainen and associates observed an ∼30% reduction in FPI (13 versus 9 mU/liter), and Sharma et al. found an ∼10% reduction (76.0 ± 54.5 to 69.0 ± 45.0 pmol/liter) following administration of metformin 2000 mg daily for 16 weeks.74,75 Evaluating both the fasting and mealtime effects of metformin, Inzucchi and colleagues found mean fasting and postprandial plasma insulin concentrations to be slightly, but not significantly, reduced with metformin 2000 mg daily for 12 weeks.78
Thiazolidinediones (Pioglitazone, Rosiglitazone, and Troglitazone)
The TZD agents (1) have no significant effect on total caloric intake,80–82 (2) have no evidence for diminished intestinal absorption, (3) increase HGU,71,83,84 (4) diminish hepatic GNG,17,78,85 (5) reduce IR,86–89 (6) increase PGU,74,78,83 and (7) reduce insulin exposure.17,89–91 Studies meeting review criteria for the target effects of the TZDs are presented here. Estimates for the effect of TZDs on the respective targets are presented in Table 1.
Caloric Intake and Intestinal Carbohydrate Absorption
The effect of TZDs on caloric intake has been evaluated in T2DM patients treated with pioglitazone and rosiglitazone. Smith and associates estimated subjective measures of hunger (visual analog scale) and satiety in patients treated with pioglitazone 45 mg/day.80 At the conclusion of 24 weeks, pioglitazone demonstrated no effect on hunger and satiety. Strowig and Raskin assessed caloric intake via food records in patients administered rosiglitazone 4 mg twice daily.81 At the conclusion of 32 weeks, mean caloric intake did not differ between treatment groups (rosiglitazone 2066.4 ± 589.2 and 1994.9 ± 726.5 calories/day for baseline and week 32, respectively). The effect of troglitazone on caloric intake in patients with diabetes has not been directly evaluated. However, in healthy volunteers, Cominancini and coworkers evaluated the effects of troglitazone 400 mg daily.82 Troglitazone was not associated with changes in carbohydrate or total caloric intake after 2 weeks of therapy.
Hepatic Glucose Uptake
Bajaj and colleagues and Kawamori and associates have evaluated the effect of pioglitazone on HGU.83,84 Kawamori and associates administered pioglitazone 30 mg daily to patients treated with either diet alone or sulfonylurea therapy. Following 12 weeks of therapy, the rate of splanchnic glucose uptake increased from 28.5 ± 19.4% to 59.4 ± 27.1% (p = .010).84 Bajaj et al. administered pioglitazone 45 mg once daily after a 48 h medication washout period. At 16 weeks, splanchnic glucose uptake increased from 33.0 ± 2.8% to 46.2 ± 5.1%.83 As previously mentioned, Iozzo and coworkers evaluated the effects of rosiglitazone on HGU, utilizing the insulin clamp technique and PET studies.71 After 26 weeks, rosiglitazone 4 mg twice daily significantly increased HGU versus placebo (+0.007 μmol/min-1/kg-1). Since the study did not present baseline data to allow for percent change calculation, rosiglitazone was considered to have similar characteristics to pioglitazone for HGU. Troglitazone has not been directly evaluated for impact on HGU and was considered comparable to pioglitazone and rosiglitazone.
Hepatic Gluconeogenesis
Gastaldelli et al. evaluated the fasting and mixed-meal effects of pioglitazone and rosiglitazone on hepatic GNG.17,85 Pioglitazone 45 mg daily for 16 weeks reduced fasting endogenous glucose production (13.1 ± 0.3 versus 12.0 ± 0.6 7 μmol/min-1/kg-1) and GNG contribution (73.1 ± 2.4% versus 64.4 ± 3.1%). During the mixed meal, endogenous glucose production was again reduced (6.5 ± 0.7 versus 5.4 ± 0.7 μmol/min-1/kg-1) as was the contribution of GNG to the total rate of appearance (45.6 ± 1.7% versus 41.3 ± 2.6%).17 In the second study, rosiglitazone 8 mg daily for 12 weeks reduced fasting endogenous glucose production (18.6 ± 0.9 versus 16.3 ± 0.6 μmol/min-1/kg-1) and GNG contribution (67 ± 4% versus 59 ± 3%).85 The direct effect of troglitazone on hepatic GNG has not been evaluated. However, Inzucchi and associates evaluated the effect of troglitazone on endogenous glucose production and found no significant difference after administration of troglitazone 400 mg daily for 12 weeks.78
Insulin Resistance
Langenfield et al. evaluated the effect of pioglitazone on IR as determined by HOMAIR.86 Pioglitazone at a dose of 45 mg daily for 24 weeks in T2DM patients resulted in a decrease in IR from 6.15 ± 4.05 to 3.85 ± 1.92. Comparative analyses have identified similar effects of pioglitazone and rosiglitazone on IR. In a 12-week trial of pioglitazone 45 mg daily and rosiglitazone 4 mg twice daily, Goldberg and colleagues reported a reduction from 8.2 ± 0.3 to 5.4 ± 0.2 and 7.8 ± 0.4 to 4.8 ± 0.2, respectively.87 Under the same experimental design, Deeg and colleagues observed similar reductions in IR for pioglitazone and rosiglitazone (8.3 versus 5.4 and 7.9 versus 4.7, respectively).88 Yatagai et al. evaluated the effects of troglitazone 400 mg daily on IR (HOMAIR). After 12 weeks, IR was reduced from 5.7 ± 0.7 to 4.5 ± 0.8.89
Peripheral Glucose Uptake
Pioglitazone, rosiglitazone, and troglitazone have been shown to increase basal and incremental PGU. Bajaj et al. observed the glucose infusion rate to be significantly greater during euglycemic insulin clamp (5.6 mmol/liter) after treatment with pioglitazone 45 mg daily for 16 weeks (6.9 ± 0.5 versus 5.0 ± 0.5 mg/kg-1/min-1).83 Glucose infusion rate was also significantly increased during the 180–420 min period of the 75 g oral glucose load-insulin clamp (5.3 ± 0.5 versus 2.9 ± 0.5 mg/kg-1/min-1). Tiikkainen and associates demonstrated that rosiglitazone 4 mg twice daily for 16 weeks increased glucose disposal rate (0.10 ± 0.02 versus 0.17 mg/kg-1/min-1) with glycemic maintenance at ∼8 mmol/liter.74 Inzucchi and coworkers found administration of troglitazone 400 mg daily for 12 weeks significantly increased glucose disposal rate (172 versus 265 mg/m-2/min-1) during the final hour of hyper-insulinemic–euglycemic clamp study (5.6 mmol/liter).78
Peripheral Insulin Exposure
Gastaldelli and colleagues evaluated the effect of pioglitazone 45 mg daily for 16 weeks on the metabolic and hormonal response to a mixed meal in T2DM patients.17 Fasting plasma insulin and plasma insulin during the mixed meal challenge (0–6 h) were similarly reduced versus baseline (88 versus 81 pmol/liter and 268 versus 248 pmol/liter, respectively). Miyazaki and associates evaluated the dose-response effect of 7.5–45 mg of pioglitazone on fasting insulin secretion after 26 weeks. Fasting plasma insulin concentrations were similarly reduced (15–25%) at the respective pioglitazone doses.90
Miyazaki and DeFronzo have reported that rosiglitazone demonstrates similar effects to pioglitazone on insulin secretion.91 After three months of therapy with rosiglitazone 8 mg daily, FPI was reduced (18 ± 1 versus 13 ± 1 μU/ml) without change in the mean insulin concentration (37 ± 4 versus 36 ± 4 μU/ml) during a 2 h oral glucose tolerance test (OGTT). Pioglitazone similarly reduced FPI (15 ± 1 versus 13 ± 2 μU/ml) and also demonstrated no change in mean insulin concentration during a 2 h OGTT.
Yatagai and coworkers evaluated the effects of troglitazone 400 mg daily on FPI concentration in T2DM patients.89 After 12 weeks of therapy, FPI concentration was found to be slightly reduced (14.3 ± 2.1 to 12.9 ± 2.6 μU/ml). Similarly, Inzucchi and colleagues evaluated the effects of troglitazone 400 mg daily for 12 weeks.78 At the conclusion of the study, fasting and postprandial plasma insulin concentrations were reported to be slightly, but not significantly, reduced.
Secretagogues and Exogenous Insulin
Secretagogues and exogenous insulin (1) have variable effects on caloric intake,92–104 (2) have no evidence for diminished intestinal carbohydrate absorption, (3) increase HGU,21 (4) diminish GNG,18,21 (5) have variable effects on IR,38,103,105–112 (6) increase PGU,21 and (7) increase PIE.113–122
Caloric Intake and Intestinal Carbohydrate Absorption
It has been hypothesized that increased plasma insulin concentrations increase appetite and cause undesirable weight gain.92–95 The UKPDS and other studies in T2DM patients have demonstrated that initiation of insulin is often accompanied by duration- and intensity-dependent weight gain (5–10%).96–100 The potential cause of increased weight gain has been attributed to increased caloric intake secondary to hyperinsulinemia or hypoglycemic fear and also a reduction in the basal metabolic rate.97,101,102 However, it must be considered that weight gain is not a universal finding and that modest reductions in daily caloric intake have been observed.103,104 Moreover, insulin therapy is commonly, but not unequivocally, associated with increased caloric intake and subsequent weight gain.
Standard and Insulin Concentration Time Profiles
Gannon and Nuttall identified the 24 h insulin secretion profile in patients with T2DM prior to initiating dietary control measures (Figure 2). On average, patients were aged 63 years (range 51–82), with a 4-year duration of diabetes (range 1–15), BMI of 31 kg/m2 (range 27–36), and a total glycosylated hemoglobin of 9.6% (range 8.6–11.2).20
Hepatic Glucose Uptake, Hepatic Gluconeogenesis, and Peripheral Glucose Uptake
Basu and associates evaluated the insulin dose-response curves for stimulation of splanchnic (hepatic) glucose uptake, suppression of endogenous glucose production, and PGU.21 Patients were fed a standard 10 cal/kg meal (55% carbohydrate, 30% fat, 15% protein) and stabilized overnight at a glucose level of ∼5 mmol/liter (90 mg/dl). On the subsequent morning, insulin was infused at variable rates from 0 to 180 min (∼0.5 mU/kg-1/min-1), 181 to 300 min (∼1.0 mU/kg-1/min-1), and 301 to 420 min (∼2.0 mU/kg-1/min-1). The insulin dose-response relationship for splanchnic glucose uptake and PGU during the final 30 min of the low- (∼150 pmol/liter), medium- (∼350 pmol/liter), and high- (∼700 pmol/liter) dose insulin infusions are presented in Figure 3. To most accurately quantify the hepatic contribution to glucose supply, the insulin dose-response relationship to hepatic GNG was utilized in place of total endogenous glucose production. Gastaldelli and coworkers evaluated the effect of physiological hyperinsulinemia on GNG in T2DM.18 Under euglycemic clamp conditions, total rates of glucose appearance were calculated from a previously established two-compartmental model.123 Endogenous glucose output was subsequently calculated as the difference between the rate of glucose appearance and the exogenous glucose rate. Percent contribution of GNG to the plasma glucose was calculated as the ratio of C5:2H2O enrichments. Under basal conditions, mean plasma insulin concentration was 12.2 ± 1.2 μU/ml (∼85 pmol/liter) and increased to 113 ± 6 μU/ml (∼780 pmol/liter) during euglycemic hyperinsulinemic clamp. Endogenous glucose output reduced from 15.2 ± 0.4 to 7.1 ± 0.9 and plasma C5:2H2O ratio declined from 0.60 ± 0.02 to 0.25 ± 0.02. The insulin dose-response relationship for suppression of hepatic GNG is presented in Figure 3.
Insulin and Sulfonylurea Concentration Time Profiles
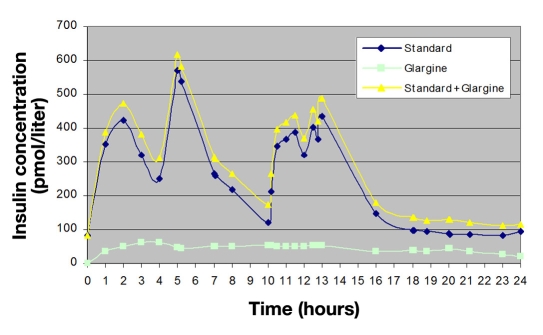
Twenty-four-hour insulin concentration time curves were obtained for sulfonylurea, meglitinide, and exogenously administered insulin products.113–122 Due to a lack of available evidence characterizing the 24 h insulin concentration profile of first generation sulfonylurea agents, comparable dose relationships were drawn with the profile for glyburide. Twenty-four -hour steady state insulin concentration time curves were superimposed on the baseline secretion profile of the standard T2DM patient. As an example, the concentration time profile of insulin glargine at a dose of 0.5 U/kg is presented in Figure 4. Using the trapezoidal rule, glargine increased PIE 30% versus baseline (5765 versus 7495 pmol/h-1/liter-1, respectively). Applying the superimposed 24 h insulin concentration time curve to the insulin dose-response relationships for HGU, GNG, and PGU, glargine was observed to increase HGU and PGU (24% and 42%, respectively), while decreasing GNG by 10%. Hepatic glucose uptake, GNG, PGU, and PIE values for the remaining exogenously administered insulin products and sulfonylurea agents are presented in Table 1.
Figure 4.
Standard + insulin glargine concentration time profile (T2DM).
Insulin Resistance
In 1993, Hotamisligil and colleagues identified the relationship between inflammation and metabolic conditions, such as obesity and IR, by demonstrating adipocyte expression of the proinflammatory cytokine tumor necrosis factor-α (TNF-α) and that expression in the adipocytes of obese animals is markedly increased.124 Further efforts in the area of obesity have identified obesity to be a state of chronic inflammation, as indicated by increased plasma concentrations of C-reactive protein, interleukin-6 (IL-6), and plasminogen activator inhibitor-1 (PAI-1).125–127 Dandona et al. have characterized the anti-inflammatory effect of insulin (reduction of reactive oxygen species generation by mononuclear cells, nicotinamide adenine dinucleotide phosphate oxidase suppression, reduced intranuclear NF-κB, suppressed plasma intercellular adhesion molecule-1 and monocyte chemotactic protein-1, reduced intranuclear Egr-1, monocyte chemotactic protein-1, and PAI-1) as well as the link between IR, obesity, and diabetes.128–130 Crook et al. and Pickup et al. first proposed T2DM to be a chronic inflammatory condition characterized by increased concentrations of acute phase reactants (sialic acid, IL-6).131,132 Indeed, several studies have confirmed the presence of inflammatory mediators predicts T2DM.133–139 It has been noted that the increased concentration of proinflammatory cytokines (i.e., TNF-α, IL-6) associated with obesity and T2DM may interfere with insulin action by suppressing signal transduction. Therefore, the anti-inflammatory effects of insulin may be blunted, which, in turn, may promote inflammation.130
The extensive characterization of obesity and T2DM as inflammatory conditions with blunted anti-inflammatory (and possibly proinflammatory) effects of insulin creates inconsistency when characterizing insulin's effect on IR. It has been argued that, by increasing weight gain, insulin therapy would exacerbate IR.112 So too, there is conflicting evidence that insulin and sulfonylurea agents have no significant effect, or alternatively a beneficial effect, on IR as assessed by HOMAIR.38,103,105–111 Contradictory evidence in combination with known pathophysiologic evidence would indicate a net neutral effect of insulin on IR.
Discussion
The administration of therapies that increase exposure to insulin and increase peripheral glucose disposal may not be without consequence. It has been extensively documented that hyperinsulinemia, hyperglycemia, IR, and inflammation are central to the development of atherosclerosis.9–12,130,140,141 Hence, it may not be altogether unsurprising that there is difficulty in drawing direct relationships between blood glucose reduction and macrovascular outcomes when the strategies employed to rigorously reduce blood glucose do so by preferentially increasing the burden of insulin exposure and peripheral glucose disposal.
After reviewing the available literature for the impact on the respective targets, alpha-glucosidase inhibitors (1.25), TZDs (1.27–1.35), and metformin (2.20) were associated with the highest ratios, while insulin (0.62–0.79) and secretagogues (0.69–0.81) were associated with the lowest ratios. Keeping with the hypothesis that preferentially increasing insulin exposure and peripheral glucose disposal versus more selectively modifying CE, HGU, GNG, and IR may be detrimental, our glucose supply and insulin demand characterization is consistent with evaluations of oral antidiabetic therapies on cardiovascular outcomes wherein alpha-glucosidase inhibitors, metformin, and TZDs appear to be associated with cardiovascular benefit, and sulfonylureas appear to be associated with possible negative effects.111,142–153
To test the hypothesis that the means by which the blood glucose is reduced is as important as the actual blood glucose, it is necessary to construct a longitudinal model that incorporates macrovascular outcomes, the extent of blood glucose reduction (HbA1c), and the manner by which it was reduced (glucose supply:insulin demand [SD] ratio). This hypothesis should be tested in a longitudinal dataset that includes validated macro-vascular outcomes, continuous laboratory follow-up, and an accurate representation of the antidiabetic agents and doses throughout the assessment period. Intuitively, the optimal dataset would be from the recent long-term cardiovascular outcome trials (ACCORD, ADVANCE, VADT); however, it must be considered that our model is heavily dependent on accurate quantification of medication exposure that would be assumed without detailed adherence documentation or pharmacy claims history. Alternatively, the construction of a retrospective dataset from an electronic data source may serve to quantify medication exposure more accurately but would also be complicated by small patient yield due to complexities of validating macrovascular outcomes and identifying patients with continuous laboratory and medication follow-up.
Model Assumptions and Limitations
The construction of the glucose supply and insulin demand model required multiple assumptions. The 24 h effect profile for all antidiabetic therapies has not been comprehensively assessed. This imposed limitations on our ability to more accurately quantify hepatic GNG throughout the prandial phase. With respect to insulin's effect on hepatic GNG, there was insufficient evidence to determine the nonlinear dose-response relationship. Therefore, because hepatic glucose production is very sensitive to suppression by low concentrations of insulin,21,74,154 it is possible that we may have underestimated insulin's impact on GNG inhibition.
Second, the standard 24 h insulin concentration time profile was identified in patients who were not subjected to dietary control measures, were relatively early in the disease course, and had a large HbA1c range. Despite evidence that decreased insulin secretion over time is not inevitable in the course of diabetes,155 it is generally accepted that T2DM patients have a progressive loss of insulin secretion over time.156–161 Therefore, it may be necessary to account for progressive decreases in insulin secretion over time in subsequent analyses. Concordantly, it is also possible that there may be variable effects with differing degrees of insulinemia and glycemia.
Third, insulin concentrations were considered additive on the standard 24-insulin concentration time profile. Under euglycemic conditions in normal subjects, it has been suggested that endogenous insulin secretion may be diminished with exogenous insulin administration.162–164 The effect of exogenous insulin to suppress endogenous insulin secretion would suggest an overestimation of the effect on HGU, hepatic GNG, PIE, and PGU.
Lastly, estimates of effect for oral medications on CE, HGU, GNG, IR, PIE, and PGU were calculated for maximal therapeutic dose and linearly extrapolated for decreasing doses, while insulin, having no maximal therapeutic dose, was linearly extrapolated for increasing or decreasing dose. It is possible that the effects of therapies on the respective targets are not linear or completely synergistic. It is also likely that the respective targets may have differing impacts on disease progression and require multiple coefficients to optimize the model.
Conclusions
Alpha-glucosidase inhibitors, metformin, and TZDs demonstrate a greater effect on glucose supply (CE, HGU, GNG, IR), while secretagogues, basal insulin, and bolus insulin demonstrate a greater effect on insulin demand (PIE, PGU). Because T2DM cardiovascular outcome trials have not demonstrated a macrovascular benefit with more aggressive blood glucose reduction when using conventional algorithms that predominantly focus on insulin demand (secretagogues and basal and bolus insulins), it would appear logical to consider a model that incorporates both the extent of blood glucose lowering (HbA1c) and the means by which the blood glucose was reduced (SD ratio) when considering macrovascular outcomes.
Acknowledgment
To Steve Feuerstein for data extraction and formatting.
Abbreviations
- [18F]FDG
[18F]2-fluoro-2-deoxyglucose
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- ADVANCE
Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation
- BMI
body mass index
- CE
carbohydrate exposure
- DCCT
Diabetes Control and Complications Trial
- FPG
fasting plasma glucose
- FPI
fasting plasma insulin
- GNG
gluconeogenesis
- SD
glucose supply:insulin demand ratio
- HbA1c
hemoglobin A1c
- HGU
hepatic glucose uptake
- HOMAIR
homeostasis model assessment of insulin resistance
- IL-6
interleukin-6
- IR
insulin resistance
- OGTT
oral glucose tolerance test
- PAI-1
plasminogen activator inhibitor-1
- PET
positron-emission tomography
- PGU
peripheral glucose uptake
- PIE
peripheral insulin exposure
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- TNF-α
tumor necrosis factor-α
- TZD
thiazolidinedione
- UKPDS
United Kingdom Prospective Diabetes Study
- VADT
Veterans Affairs Diabetes Trial
References
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 3.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 5.Nathan DM. Glycemic management of type 2 diabetes: how tight is right and how to get there. Arch Intern Med. 2008;168(19):2064–2066. doi: 10.1001/archinte.168.19.2064. [DOI] [PubMed] [Google Scholar]
- 6.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 8.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA. Impaired glucose tolerance: do pharmacological therapies correct the underlying metabolic disturbance? Br J Diabetes Vasc Dis. 2003;3(1):S24–S40. [Google Scholar]
- 10.Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105(3):311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279(41):42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 12.Du X, Matsumura T, Edelstein D, Rossetti L, Zsengellér Z, Szabó C, Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112(7):1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Ferrannini E, Hendler R, Wahren J, Felig P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci U S A. 1978;75(10):5173–5177. doi: 10.1073/pnas.75.10.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toschi E, Camastra S, Sironi AM, Masoni A, Gastaldelli A, Mari A, Ferrannini E, Natali A. Effect of acute hyperglycemia on insulin secretion in humans. Diabetes. 2002;51(Suppl 1):S130–S133. doi: 10.2337/diabetes.51.2007.s130. [DOI] [PubMed] [Google Scholar]
- 15.Elahi D, Nagulesparan M, Hershcopf RJ, Muller DC, Tobin JD, Blix PM, Rubenstein AH, Unger RH, Andres R. Feedback inhibition of insulin secretion by insulrelation to the hyperinsulinemia of obesity. N Engl J Med. 1982;306(20):1196–1202. doi: 10.1056/NEJM198205203062002. [DOI] [PubMed] [Google Scholar]
- 16.Erdmann J, Mayr M, Oppel U, Sypchenko O, Wagenpfeil S, Schusdziarra V. Weight-dependent differential contribution of insulin secretion and clearance to hyperinsulinemia of obesity. Regul Pept. 2009;152(1-3):1–7. doi: 10.1016/j.regpep.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Gastaldelli A, Casolaro A, Pettiti M, Nannipieri M, Ciociaro D, Frascerra S, Buzzigoli E, Baldi S, Mari A, Ferrannini E. Effect of pioglitazone on the metabolic and hormonal response to a mixed meal in type II diabetes. Clin Pharmacol Ther. 2007;81(2):205–212. doi: 10.1038/sj.clpt.6100034. [DOI] [PubMed] [Google Scholar]
- 18.Gastaldelli A, Toschi E, Pettiti M, Frascerra S, Quiñones-Galvan A, Sironi AM, Natali A, Ferrannini E. Effect of physiological hyper-insulinemia on gluconeogenesis in nondiabetic subjects and in type 2 diabetic patients. Diabetes. 2001;50(8):1807–1812. doi: 10.2337/diabetes.50.8.1807. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Gannon MC, Nuttall FQ. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes. 2004;53(9):2375–2382. doi: 10.2337/diabetes.53.9.2375. [DOI] [PubMed] [Google Scholar]
- 21.Basu R, Basu A, Johnson CM, Schwenk WF, Rizza RA. Insulin dose-response curves for stimulation of splanchnic glucose uptake and suppression of endogenous glucose production differ in nondiabetic humans and are abnormal in people with type 2 diabetes. Diabetes. 2004;53(8):2042–2050. doi: 10.2337/diabetes.53.8.2042. [DOI] [PubMed] [Google Scholar]
- 22.Meneilly GS, Ryan EA, Radziuk J, Lau DC, Yale JF, Morais J, Chiasson JL, Rabasa-Lhoret R, Maheux P, Tessier D, Wolever T, Josse RG, Elahi D. Effect of acarbose on insulin sensitivity in elderly patients with diabetes. Diabetes Care. 2000;23(8):1162–1167. doi: 10.2337/diacare.23.8.1162. [DOI] [PubMed] [Google Scholar]
- 23.Laube H. Acarbose: an update of its therapeutic use in diabetes treatment. Clin Drug Invest. 2002;22(3):141–156. [Google Scholar]
- 24.Radziuk J, Kemmer F, Morishima T, Berchtold P, Vranic M. The effects of an alpha-glucoside hydrolase inhibitor on glycemia and the absorption of sucrose in man determined using a tracer method. Diabetes. 1984;33(3):207–213. doi: 10.2337/diab.33.3.207. [DOI] [PubMed] [Google Scholar]
- 25.Sobajima H, Mori M, Niwa T, Muramatsu M, Sugimoto Y, Kato K, Naruse S, Kondo T, Hayakawa T. Carbohydrate malabsorption following acarbose administration. Diabet Med. 1998;15(5):393–397. doi: 10.1002/(SICI)1096-9136(199805)15:5<393::AID-DIA597>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 26.Coniff RF, Shapiro JA, Robbins D, Kleinfield R, Seaton TB, Beisswenger P, McGill JB. Reduction of glycosylated hemoglobin and postprandial hyperglycemia by acarbose in patients with NIDDM. A placebo-controlled dose-comparison study. Diabetes Care. 1995;18(6):817–824. doi: 10.2337/diacare.18.6.817. [DOI] [PubMed] [Google Scholar]
- 27.Hanefeld M, Fischer S, Schulze J, Spengler M, Wargenau M, Schollberg K, Fücker K. Therapeutic potentials of acarbose as first-line drug in NIDDM insufficiently treated with dietalone. Diabetes Care. 1991;14(8):732–737. doi: 10.2337/diacare.14.8.732. [DOI] [PubMed] [Google Scholar]
- 28.Schnack C, Prager RJ, Winkler J, Klauser RM, Schneider BG, Schernthaner G. Effects of 8-wk alpha-glucosidase inhibition on metabolic control, C-peptide secretion, hepatic glucose output, and peripheral insulin sensitivity in poorly controlled type II diabetic patients. Diabetes Care. 1989;12(8):537–543. doi: 10.2337/diacare.12.8.537. [DOI] [PubMed] [Google Scholar]
- 29.Sels JP, Kingma PJ, Wolffenbuttel BH, Menheere PP, Branolte JH, Nieuwenhuijzen Kruseman AC. Effect of miglitol (BAY m-1099) on fasting blood glucose in type 2 diabetes mellitus. Neth J Med. 1994;44(6):198–201. [PubMed] [Google Scholar]
- 30.Calle-Pascual A, Garcia-Honduvilla J, Martin-Alvarez PJ, Calle JR, Maranes JP. Influence of 16-week monotherapy with acarbose on cardiovascular risk factors in obese subjects with non-insulin-dependent diabetes mellitus: a controlled, double-blind comparison study with placebo. Diabetes Metab. 1996;22(3):201–202. [PubMed] [Google Scholar]
- 31.Chiasson JL, Josse RG, Leiter LA, Mihic M, Nathan DM, Palmason C, Cohen RM, Wolever TM. The effect of acarbose on insulin sensitivity in subjects with impaired glucose tolerance. Diabetes Care. 1996;19(11):1190–1193. doi: 10.2337/diacare.19.11.1190. [DOI] [PubMed] [Google Scholar]
- 32.Shinozaki K, Suzuki M, Ikebuchi M, Hirose J, Hara Y, Harano Y. Improvement of insulin sensitivity and dyslipidemia with a new alpha-glucosidase inhibitor, voglibose, in nondiabetic hyperinsulinemic subjects. Metabolism. 1996;45(6):731–737. doi: 10.1016/s0026-0495(96)90139-0. [DOI] [PubMed] [Google Scholar]
- 33.Delgado H, Lehmann T, Bobbioni-Harsch E, Ybarra J, Golay A. Acarbose improves indirectly both insulin resistance and secretion in obese type 2 diabetic patients. Diabetes Metab. 2002;28(3):195–200. [PubMed] [Google Scholar]
- 34.Laube H, Linn T, Heyen P. The effect of acarbose on insulin sensitivity and proinsulin in overweight subjects with impaired glucose tolerance. Exp Clin Endocrinol Diabetes. 1998;106(3):231–233. doi: 10.1055/s-0029-1211981. [DOI] [PubMed] [Google Scholar]
- 35.Reaven GM, Lardinois CK, Greenfield MS, Schwartz HC, Vreman HJ. Effect of acarbose on carbohydrate and lipid metabolism in NIDDM patients poorly controlled by sulfonylureas. Diabetes Care. 1990;13(Suppl 3):32–36. doi: 10.2337/diacare.13.3.32. [DOI] [PubMed] [Google Scholar]
- 36.Jenney A, Proietto J, O'Dea K, Nankervis A, Traianedes K, D'Embden H. Low-dose acarbose improves glycemic control in NIDDM patients without changes in insulin sensitivity. Diabetes Care. 1993;16(2):499–502. doi: 10.2337/diacare.16.2.499. [DOI] [PubMed] [Google Scholar]
- 37.Kinoshita T, Maeda H, Urata S, Hirao K. Effect of acarbose versus sulfonylurea therapy on insulin sensitivity: an insulin clamp study. Curr Ther Res. 1999;61(2):97–104. [Google Scholar]
- 38.Fischer S, Patzak A, Rietzsch H, Schwanebeck U, Köhler C, Wildbrett J, Fuecker K, Temelkova-Kurktschiev T, Hanefeld M. Influence of treatment with acarbose or glibenclamide on insulin sensitivity in type 2 diabetic patients. Diabetes Obes Metab. 2003;5(1):38–44. doi: 10.1046/j.1463-1326.2003.00239.x. [DOI] [PubMed] [Google Scholar]
- 39.Hillebrand I, Boehme K, Graefe KH, Wehling K. The effect of new alpha-glucosidase inhibitors (BAY m 1099 and BAY o 1248) on meal-stimulated increases in glucose and insulin levels in man. Klin Wochenschr. 1986;64(8):393–396. doi: 10.1007/BF01728191. [DOI] [PubMed] [Google Scholar]
- 40.Hillebrand I, Boehme K, Frank G, Fink H, Berchtold P. The effects of the alpha-glucosidase inhibitor BAY g 5421 (Acarbose) on meal-stimulated elevations of circulating glucose, insulin, and triglyceride levels in man. Res Exp Med (Berl) 1979;175(1):81–86. doi: 10.1007/BF01851236. [DOI] [PubMed] [Google Scholar]
- 41.Hanefeld M, Haffner SM, Menschikowski M, Koehler C, Temelkova-Kurktschiev T, Wildbrett J, Fischer S. Different effects of acarbose and glibenclamide on proinsulin and insulin profiles in people with type 2 diabetes. Diabetes Res Clin Pract. 2002;55(3):221–227. doi: 10.1016/s0168-8227(01)00347-3. [DOI] [PubMed] [Google Scholar]
- 42.Uttenthal LO, Ukponmwan OO, Wood SM, Ghiglione M, Ghatei MA, Trayner IM, Bloom SR. Long-term effects of intestinal alpha-glucosidase inhibition on postprandial glucose, pancreatic and gut hormone responses and fasting serum lipids in diabetics on sulphonylureas. Diabet Med. 1986;3(2):155–160. doi: 10.1111/j.1464-5491.1986.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 43.Hoffmann J, Spengler M. Efficacy of 24-week monotherapy with acarbose, glibenclamide, or placebo in NIDDM patients. The Essen Study. Diabetes Care. 1994;17(6):561–566. doi: 10.2337/diacare.17.6.561. [DOI] [PubMed] [Google Scholar]
- 44.Inoue I, Takahashi K, Noji S, Awata T, Negishi K, Katayama S. Acarbose controls postprandial hyperproinsulinemia in non-insulin dependent diabetes mellitus. Diabetes Res Clin Pract. 1997;36(3):143–151. doi: 10.1016/s0168-8227(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 45.Rosak C, Haupt E, Walter T, Werner J. The effect of combination treatment with acarbose and glibenclamide on postprandial glucose and insulin profiles: additive blood glucose lowering effect and decreased hypoglycaemia. Diabetes Nutr Metab. 2002;15(3):143–151. [PubMed] [Google Scholar]
- 46.Seifarth C, Bergmann J, Holst JJ, Ritzel R, Schmiegel W, Nauck MA. Prolonged and enhanced secretion of glucagon-like peptide 1 (7-36 amide) after oral sucrose due to alpha-glucosidase inhibition (acarbose) in type 2 diabetic patients. Diabet Med. 1998;15(6):485–491. doi: 10.1002/(SICI)1096-9136(199806)15:6<485::AID-DIA610>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 47.Gutzwiller JP. Glucagon like peptide-1 is a physiologic regulator of food intake in humans. Gastroenterology. 1997;112:A1153. [Google Scholar]
- 48.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 49.Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (U.K Prospective Diabetes Study 44) Diabetes Care. 1999;22(6):960–964. doi: 10.2337/diacare.22.6.960. [DOI] [PubMed] [Google Scholar]
- 50.Paolisso G, Amato L, Eccellente R, Gambardella A, Tagliamonte MR, Varricchio G, Carella C, Giugliano D, D'Onofrio F. Effect of metformin on food intake in obese subjects. Eur J Clin Invest. 1998;28(6):441–446. doi: 10.1046/j.1365-2362.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- 51.Hermann LS. Metforma review of its pharmacological properties and therapeutic use. Diabete Metab. 1979;5(3):233–245. [PubMed] [Google Scholar]
- 52.Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res. 1998;6(1):47–53. doi: 10.1002/j.1550-8528.1998.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 53.Caspary WF. Biguanides and intestinal absorptive function. Acta Hepatogastroenterol (Stuttg) 1977;24(6):473–480. [PubMed] [Google Scholar]
- 54.Czyzyk A, Tawecki J, Sadowski J, Ponikowska I, Szczepanik Z. Effect of biguanides on intestinal absorption of glucose. Diabetes. 1968;17(8):492–498. doi: 10.2337/diab.17.8.492. [DOI] [PubMed] [Google Scholar]
- 55.Berger W, Kunzli H. Effect of dimethylbiguanide on insulin, glucose and lactic acid contents observed in portal vein blood and peripheral venous blood in the course of intraduodenal glucose tolerance tests. Diabetologia. 1970;6:37. [Google Scholar]
- 56.Gyr M, Berger W, Fridrich R, Denes A, Stadler GA. Der Einfluss von Dimethylbiguanid auf die Magenentleerung und die orale glucosetoleranz. Schw Med Wschr. 1971;101:1876–1879. [PubMed] [Google Scholar]
- 57.Adnitt P, Frayn KN. Effect of metformin on intestinal absorption and intravenous glucose tolerance in man. J Pharmacol. 1971;2:202–204. [Google Scholar]
- 58.Fossati P, Fontaine P, Beuscart R, Romon M, Bourdelle-Hego MF, LePoutre-Vaast D. Escape of non insulin dependent diabetes (NIDD) to the oral hypoglycemic agents control. Rev Fr Endocrinol Clin. 1985;26:105–116. [Google Scholar]
- 59.Cuber JC, Bosshard A, Vidal H, Vega F, Wiernsperger N, Rapin JR. Metabolic and drug distribution studies do not support direct inhibitory effects of metformin on intestinal glucose absorption. Diabete Metab. 1994;20(6):532–539. [PubMed] [Google Scholar]
- 60.Bailey CJ. Metformin and intestinal glucose handling. Diabetes Metab Rev. 1995;11(Suppl 1):S23–S32. doi: 10.1002/dmr.5610110505. [DOI] [PubMed] [Google Scholar]
- 61.Caspary WF, Creutzfeldt W. Analysis of the inhibitory effect of biguanides on glucose absorption: inhibition of active sugar transport. Diabetologia. 1971;7(5):379–385. doi: 10.1007/BF01219474. [DOI] [PubMed] [Google Scholar]
- 62.Lorch E. Inhibition of intestinal absorption and improvement of oral glucose tolerance by biguanides in the normal and in the streptozotocin-diabetic rat. Diabetologia. 1971;7(3):195–203. doi: 10.1007/BF01212553. [DOI] [PubMed] [Google Scholar]
- 63.Coupar IM, McColl I. Glucose absorption from the rat jejunum during acute exposure to metformin and phenformin. J Pharm Pharmacol. 1974;26(12):997–998. doi: 10.1111/j.2042-7158.1974.tb09228.x. [DOI] [PubMed] [Google Scholar]
- 64.Wilcock C, Bailey CJ. Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica. 1994;24(1):49–57. doi: 10.3109/00498259409043220. [DOI] [PubMed] [Google Scholar]
- 65.Wilcock C, Bailey CJ. Reconsideration of inhibitory effect of metformin on intestinal glucose absorption. J Pharm Pharmacol. 1991;43(2):120–121. doi: 10.1111/j.2042-7158.1991.tb06645.x. [DOI] [PubMed] [Google Scholar]
- 66.Kessler M, Meier W, Storelli C, Semenza G. The biguanide inhibition of D-glucose transport in membrane vesicles from small intestine brush borders. Biochim Biophys Acta. 1975;413(3):444–452. doi: 10.1016/0005-2736(75)90127-3. [DOI] [PubMed] [Google Scholar]
- 67.Jackson RA, Hawa MI, Jaspan JB, Sim BM, Disilvio L, Featherbe D, Kurtz AB. Mechanism of metformin action in non-insulin-dependent diabetes. Diabetes. 1987;36(5):632–640. doi: 10.2337/diab.36.5.632. [DOI] [PubMed] [Google Scholar]
- 68.Pénicaud L, Hitier Y, Ferré P, Girard J. Hypoglycaemic effect of metformin in genetically obese (fa/fa) rats results from an increased utilization of blood glucose by intestine. Biochem J. 1989;262(3):881–885. doi: 10.1042/bj2620881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bailey CJ, Mynett KJ, Page T. Importance of the intestine as a site of metformin-stimulated glucose utilization. Br J Pharmacol. 1994;112(2):671–675. doi: 10.1111/j.1476-5381.1994.tb13128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bailey CJ, Wilcock C, Day C. Effect of metformin on glucose metabolism in the splanchnic bed. Br J Pharmacol. 1992;105(4):1009–1013. doi: 10.1111/j.1476-5381.1992.tb09093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iozzo P, Hallsten K, Oikonen V, Virtanen KA, Parkkola R, Kemppainen J, Solin O, Lonnqvist F, Ferrannini E, Knuuti J, Nuutila P. Effects of metformin and rosiglitazone monotherapy on insulin-mediated hepatic glucose uptake and their relation to visceral fat in type 2 diabetes. Diabetes Care. 2003;26(7):2069–2074. doi: 10.2337/diacare.26.7.2069. [DOI] [PubMed] [Google Scholar]
- 72.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333(9):550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 73.Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49(12):2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tiikkainen M, Häkkinen AM, Korsheninnikova E, Nyman T, Mäkimattila S, Yki-Järvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53(8):2169–2176. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- 75.Sharma PK, Bhansali A, Sialy R, Malhotra S, Pandhi P. Effects of pioglitazone and metformin on plasma adiponectin in newly detected type 2 diabetes mellitus. Clin Endocrinol (Oxf) 2006;65(6):722–728. doi: 10.1111/j.1365-2265.2006.02658.x. [DOI] [PubMed] [Google Scholar]
- 76.Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol. 2001;37(5):1344–1350. doi: 10.1016/s0735-1097(01)01129-9. [DOI] [PubMed] [Google Scholar]
- 77.Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149–157. doi: 10.1016/j.amjmed.2007.09.016. e2. [DOI] [PubMed] [Google Scholar]
- 78.Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, Shulman GI. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338(13):867–872. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- 79.Bailey CJ, Flatt PR, Ewan C. Anorectic effect of metformin in lean and genetically obese hyperglycaemic (ob/ob) mice. Arch Int Pharmacodyn Ther. 1986;282(2):233–239. [PubMed] [Google Scholar]
- 80.Smith SR, De Jonge L, Volaufova J, Li Y, Xie H, Bray GA. Effect of pioglitazone on body composition and energy expenditure: a randomized controlled trial. Metabolism. 2005;54(1):24–32. doi: 10.1016/j.metabol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 81.Strowig SM, Raskin P. The effect of rosiglitazone on overweight subjects with type 1 diabetes. Diabetes Care. 2005;28(7):1562–1567. doi: 10.2337/diacare.28.7.1562. [DOI] [PubMed] [Google Scholar]
- 82.Cominacini L, Young MM, Capriati A, Garbin U, Fratta Pasini A, Campagnola M, Davoli A, Rigoni A, Contessi GB, Lo Cascio V. Troglitazone increases the resistance of low density lipoprotein to oxidation in healthy volunteers. Diabetologia. 1997;40(10):1211–1218. doi: 10.1007/s001250050809. [DOI] [PubMed] [Google Scholar]
- 83.Bajaj M, Suraamornkul S, Pratipanawatr T, Hardies LJ, Pratipanawatr W, Glass L, Cersosimo E, Miyazaki Y, DeFronzo RA. Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes. 2003;52(6):1364–1370. doi: 10.2337/diabetes.52.6.1364. [DOI] [PubMed] [Google Scholar]
- 84.Kawamori R, Matsuhisa M, Kinoshita J, Mochizuki K, Niwa M, Arisaka T, Ikeda M, Kubota M, Wada M, Kanda T, Ikebuchi M, Tohdo R, Yamasaki Y AD-4833 Clamp-OGL Study Group. Pioglitazone enhances splanchnic glucose uptake as well as peripheral glucose uptake in non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1998;41(1):35–43. doi: 10.1016/s0168-8227(98)00056-4. [DOI] [PubMed] [Google Scholar]
- 85.Gastaldelli A, Miyazaki Y, Pettiti M, Santini E, Ciociaro D, Defronzo RA, Ferrannini E. The effect of rosiglitazone on the liver: decreased gluconeogenesis in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91(3):806–812. doi: 10.1210/jc.2005-1159. [DOI] [PubMed] [Google Scholar]
- 86.Langenfeld MR, Forst T, Hohberg C, Kann P, Lübben G, Konrad T, Füllert SD, Sachara C, Pfützner A. Pioglitazone decreases carotid intima-media thickness independently of glycemic control in patients with type 2 diabetes mellitus: results from a controlled randomized study. Circulation. 2005;111(19):2525–2531. doi: 10.1161/01.CIR.0000165072.01672.21. [DOI] [PubMed] [Google Scholar]
- 87.Goldberg RB, Kendall DM, Deeg MA, Buse JB, Zagar AJ, Pinaire JA, Tan MH, Khan MA, Perez AT, Jacober SJ GLAI Study Investigators. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28(7):1547–1554. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- 88.Deeg MA, Buse JB, Goldberg RB, Kendall DM, Zagar AJ, Jacober SJ, Khan MA, Perez AT, Tan MH GLAI Study Investigators. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2007;30(10):2458–2464. doi: 10.2337/dc06-1903. [DOI] [PubMed] [Google Scholar]
- 89.Yatagai T, Nakamura T, Nagasaka S, Kusaka I, Ishikawa SE, Yoshitaka A, Ishibashi S. Decrease in serum C-reactive protein levels by troglitazone is associated with pretreatment insulin resistance, but independent of its effect on glycemia, in type 2 diabetic subjects. Diabetes Res Clin Pract. 2004;63(1):19–26. doi: 10.1016/j.diabres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 90.Miyazaki Y, Matsuda M, DeFronzo RA. Dose-response effect of pioglitazone on insulin sensitivity and insulin secretion in type 2 diabetes. Diabetes Care. 2002;25(3):517–523. doi: 10.2337/diacare.25.3.517. [DOI] [PubMed] [Google Scholar]
- 91.Miyazaki Y, DeFronzo RA. Rosiglitazone and pioglitazone similarly improve insulin sensitivity and secretion, glucose tolerance and adipocytokines in type 2 diabetic patients. Diabetes Obes Metab. 2008;10(12):1204–1211. doi: 10.1111/j.1463-1326.2008.00880.x. [DOI] [PubMed] [Google Scholar]
- 92.Grossman SP. The role of glucose, insulin and glucagon in the regulation of food intake and body weight. Neurosci Biobehav Rev. 1986;10(3):295–315. doi: 10.1016/0149-7634(86)90015-1. [DOI] [PubMed] [Google Scholar]
- 93.Grossman MI, Stein IF., Jr Vagotomy and the hunger-producing action of insulin in man. J Appl Physiol. 1948;1(4):263–269. doi: 10.1152/jappl.1948.1.4.263. [DOI] [PubMed] [Google Scholar]
- 94.Thompson DA, Campbell RG. Hunger in humans induced by 2-deoxy-D-glucose: glucoprivic control of taste preference and food intake. Science. 1977;198(4321):1065–1068. doi: 10.1126/science.929188. [DOI] [PubMed] [Google Scholar]
- 95.Westphal SA, Palumbo PJ. Weight gain and management concerns in patients on insulin therapy. Insulin. 2007;2:31–36. [Google Scholar]
- 96.Henry RR, Gumbiner B, Ditzler T, Wallace P, Lyon R, Glauber HS. Intensive conventional insulin therapy for type II diabetes. Metabolic effects during a 6-mo outpatient trial. Diabetes Care. 1993;16(1):21–31. doi: 10.2337/diacare.16.1.21. [DOI] [PubMed] [Google Scholar]
- 97.Mäkimattila S, Nikkilä K, Yki-Järvinen H. Causes of weight gain during insulin therapy with and without metformin in patients with type II diabetes mellitus. Diabetologia. 1999;42(4):406–412. doi: 10.1007/s001250051172. [DOI] [PubMed] [Google Scholar]
- 98.Lindström T, Eriksson P, Olsson AG, Arnqvist HJ. Long-term improvement of glycemic control by insulin treatment in NIDDM patients with secondary failure. Diabetes Care. 1994;17(7):719–721. doi: 10.2337/diacare.17.7.719. [DOI] [PubMed] [Google Scholar]
- 99.Yki-Järvinen H, Ryysy L, Kauppila M, Kujansuu E, Lahti J, Marjanen T, Niskanen L, Rajala S, Salo S, Seppälä P, Tulokas T, Viikari J, Taskinen MR. Effect of obesity on the response to insulin therapy in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1997;82(12):4037–4043. doi: 10.1210/jcem.82.12.4460. [DOI] [PubMed] [Google Scholar]
- 100.Mudaliar S, Edelman SV. Insulin therapy in type 2 diabetes. Endocrinol Metab Clin North Am. 2001;30(4):935–982. doi: 10.1016/s0889-8529(05)70222-x. [DOI] [PubMed] [Google Scholar]
- 101.Rodin J, Wack J, Ferrannini E, DeFronzo RA. Effect of insulin and glucose on feeding behavior. Metabolism. 1985;34(9):826–831. doi: 10.1016/0026-0495(85)90106-4. [DOI] [PubMed] [Google Scholar]
- 102.Heller S. Weight gain during insulin therapy in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2004;65(Suppl 1):S23–S27. doi: 10.1016/j.diabres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 103.Andrews WJ, Vasquez B, Nagulesparan M, Klimes I, Foley J, Unger R, Reaven GM. Insulin therapy in obese, non-insulin-dependent diabetes induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes. 1984;33(7):634–642. doi: 10.2337/diab.33.7.634. [DOI] [PubMed] [Google Scholar]
- 104.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 105.Inukai K, Watanabe M, Nakashima Y, Sawa T, Takata N, Tanaka M, Kashiwabara H, Yokota K, Suzuki M, Kurihara S, Awata T, Katayama S. Efficacy of glimepiride in Japanese type 2 diabetic subjects. Diabetes Res Clin Pract. 2005;68(3):250–257. doi: 10.1016/j.diabres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 106.Koshiba K, Nomura M, Nakaya Y, Ito S. Efficacy of glimepiride on insulin resistance, adipocytokines, and atherosclerosis. J Med Invest. 2006;53(1-2):87–94. doi: 10.2152/jmi.53.87. [DOI] [PubMed] [Google Scholar]
- 107.Scarlett JA, Gray RS, Griffin J, Olefsky JM, Kolterman OG. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982;5(4):353–363. doi: 10.2337/diacare.5.4.353. [DOI] [PubMed] [Google Scholar]
- 108.Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34(3):222–234. doi: 10.2337/diab.34.3.222. [DOI] [PubMed] [Google Scholar]
- 109.Dorkhan M, Frid A, Groop L. Differences in effects of insulin glargine or pioglitazone added to oral anti-diabetic therapy in patients with type 2 diabetes: what to add–insulin glargine or pioglitazone? Diabetes Res Clin Pract. 2008;82(3):340–345. doi: 10.1016/j.diabres.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 110.Li J, Tian H, Li Q, Wang N, Wu T, Liu Y, Ni Z, Yu H, Liang J, Luo R, Li Y, Huang L. Improvement of insulin sensitivity and beta-cell function by nateglinide and repaglinide in type 2 diabetic patients - a randomized controlled double-blind and double-dummy multicentre clinical trial. Diabetes Obes Metab. 2007;9(4):558–565. doi: 10.1111/j.1463-1326.2006.00638.x. [DOI] [PubMed] [Google Scholar]
- 111.Uwaifo GI, Ratner RE. Differential effects of oral hypoglycemic agents on glucose control and cardiovascular risk. Am J Cardiol. 2007;99(4A):51B–67B. doi: 10.1016/j.amjcard.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 112.Genuth S. Insulin use in NIDDM. Diabetes Care. 1990;13(12):1240–1264. doi: 10.2337/diacare.13.12.1240. [DOI] [PubMed] [Google Scholar]
- 113.Groop L, Wåhlin-Boll E, Groop PH, Tötterman KJ, Melander A, Tolppanen EM, Fyhrqvist F. Pharmacokinetics and metabolic effects of glibenclamide and glipizide in type 2 diabetics. Eur J Clin Pharmacol. 1985;28(6):697–704. doi: 10.1007/BF00607919. [DOI] [PubMed] [Google Scholar]
- 114.Strange P, Schwartz SL, Graf RJ, Polvino W, Weston I, Marbury TC, Huang WC, Goldberg RB. Pharmacokinetics, pharmacodynamics, and dose-response relationship of repaglinide in type 2 diabetes. Diabetes Technol Ther. 1999;1(3):247–256. doi: 10.1089/152091599317143. [DOI] [PubMed] [Google Scholar]
- 115.McLeod JF. Clinical pharmacokinetics of nateglinide: a rapidly-absorbed, short-acting insulinotropic agent. Clin Pharmacokinet. 2004;43(2):97–120. doi: 10.2165/00003088-200443020-00003. [DOI] [PubMed] [Google Scholar]
- 116.Plank J, Wutte A, Brunner G, Siebenhofer A, Semlitsch B, Sommer R, Hirschberger S, Pieber TR. A direct comparison of insulin aspart and insulin lispro in patients with type 1 diabetes. Diabetes Care. 2002;25(11):2053–2057. doi: 10.2337/diacare.25.11.2053. [DOI] [PubMed] [Google Scholar]
- 117.Mudaliar SR, Lindberg FA, Joyce M, Beerdsen P, Strange P, Lin A, Henry RR. Insulin aspart (B28 asp-insulin): a fast-acting analog of human insulabsorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care. 1999;22(9):1501–1506. doi: 10.2337/diacare.22.9.1501. [DOI] [PubMed] [Google Scholar]
- 118.Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23(5):644–649. doi: 10.2337/diacare.23.5.644. [DOI] [PubMed] [Google Scholar]
- 119.Heine RJ, Bilo HJ, Fonk T, van der Veen EA, van der Meer J. Absorption kinetics and action profiles of mixtures of short- and intermediate-acting insulins. Diabetologia. 1984;27(6):558–562. doi: 10.1007/BF00276967. [DOI] [PubMed] [Google Scholar]
- 120.Lepore M, Pampanelli S, Fanelli C, Porcellati F, Bartocci L, Di Vincenzo A, Cordoni C, Costa E, Brunetti P, Bolli GB. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49(12):2142–2148. doi: 10.2337/diabetes.49.12.2142. [DOI] [PubMed] [Google Scholar]
- 121.Rosskamp R, Wernicke-Panten K, Draeger E. Clinical profile of the novel sulphonylurea glimepiride. Diabetes Res Clin Pract. 1996;(31 Suppl):S33–S42. doi: 10.1016/0168-8227(96)01228-4. [DOI] [PubMed] [Google Scholar]
- 122.Kabadi MU, Kabadi UM. Effects of glimepiride on insulin secretion and sensitivity in patients with recently diagnosed type 2 diabetes mellitus. Clin Ther. 2004;26(1):63–69. doi: 10.1016/s0149-2918(04)90006-9. [DOI] [PubMed] [Google Scholar]
- 123.Natali A, Toschi E, Camastra S, Gastaldelli A, Groop L, Ferrannini E. Determinants of postabsorptive endogenous glucose output in non-diabetic subjects. European Group for the Study of Insulin Resistance (EGIR) Diabetologia. 2000;43(10):1266–1272. doi: 10.1007/s001250051522. [DOI] [PubMed] [Google Scholar]
- 124.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 125.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 126.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82(12):4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 127.Lundgren CH, Brown SL, Nordt TK, Sobel BE, Fujii S. Elaboration of type-1 plasminogen activator inhibitor from adipocytes. A potential pathogenetic link between obesity and cardiovascular disease. Circulation. 1996;93(1):106–110. doi: 10.1161/01.cir.93.1.106. [DOI] [PubMed] [Google Scholar]
- 128.Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86(7):3257–3265. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 129.Aljada A, Ghanim H, Mohanty P, Kapur N, Dandona P. Insulin inhibits the pro-inflammatory transcription factor early growth response gene-1 (Egr)-1 expression in mononuclear cells (MNC) and reduces plasma tissue factor (TF) and plasminogen activator inhibitor-1 (PAI-1) concentrations. J Clin Endocrinol Metab. 2002;87(3):1419–1422. doi: 10.1210/jcem.87.3.8462. [DOI] [PubMed] [Google Scholar]
- 130.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 131.Crook MA, Tutt P, Pickup JC. Elevated serum sialic acid concentration in NIDDM and its relationship to blood pressure and retinopathy. Diabetes Care. 1993;16(1):57–60. doi: 10.2337/diacare.16.1.57. [DOI] [PubMed] [Google Scholar]
- 132.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40(11):1286–1292. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 133.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353(9165):1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 134.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G Atherosclerosis Risk in Communities Study. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52(7):1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 135.Duncan BB, Schmidt MI, Chambless LE, Folsom AR, Carpenter M, Heiss G. Fibrinogen, other putative markers of inflammation, and weight gain in middle-aged adults–the ARIC study. Atherosclerosis Risk in Communities. Obes Res. 2000;8(4):279–286. doi: 10.1038/oby.2000.33. [DOI] [PubMed] [Google Scholar]
- 136.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 137.Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, Tracy RP. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50(10):2384–2389. doi: 10.2337/diabetes.50.10.2384. [DOI] [PubMed] [Google Scholar]
- 138.Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean ME, Haffner SM. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care. 2002;25(11):2016–2021. doi: 10.2337/diacare.25.11.2016. [DOI] [PubMed] [Google Scholar]
- 139.Pradhan AD, Cook NR, Buring JE, Manson JE, Ridker PM. C-reactive protein is independently associated with fasting insulin in nondiabetic women. Arterioscler Thromb Vasc Biol. 2003;23(4):650–655. doi: 10.1161/01.ATV.0000065636.15310.9C. [DOI] [PubMed] [Google Scholar]
- 140.Dandona P, Aljada A, Chaudhuri A, Bandyopadhyay A. The potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis-related complications in type 2 diabetes. J Clin Endocrinol Metab. 2003;88(6):2422–2429. doi: 10.1210/jc.2003-030178. [DOI] [PubMed] [Google Scholar]
- 141.Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110(12):1564–1571. doi: 10.1161/01.CIR.0000142055.53122.FA. [DOI] [PubMed] [Google Scholar]
- 142.Hanefeld M, Chiasson JL, Koehler C, Henkel E, Schaper F, Temelkova-Kurktschiev T. Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke. 2004;35(5):1073–1078. doi: 10.1161/01.STR.0000125864.01546.f2. [DOI] [PubMed] [Google Scholar]
- 143.Hanefeld M, Cagatay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J. 2004;25(1):10–16. doi: 10.1016/s0195-668x(03)00468-8. [DOI] [PubMed] [Google Scholar]
- 144.Hanefeld M. Treatment of impaired glucose tolerance with acarbose and its effect on intima-media thickness: a substudy of the STOP-NIDDM trial (study to prevent non-insulin-dependent diabetes mellitus) Endocr Pract. 2006;12(Suppl 1):56–59. doi: 10.4158/EP.12.S1.56. [DOI] [PubMed] [Google Scholar]
- 145.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290(4):486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 146.Zeymer U, Schwarzmaier-D'assie A, Petzinna D, Chiasson JL STOP-NIDDM Trial Research Group. Effect of acarbose treatment on the risk of silent myocardial infarctions in patients with impaired glucose tolerance: results of the randomised STOP-NIDDM trial electrocardiography substudy. Eur J Cardiovasc Prev Rehabil. 2004;11(5):412–415. doi: 10.1097/01.hjr.0000140712.71649.5a. [DOI] [PubMed] [Google Scholar]
- 147.Chiasson JL. Acarbose for the prevention of diabetes, hypertension, and cardiovascular disease in subjects with impaired glucose tolerance: the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM) Trial. Endocr Pract. 2006;12(Suppl 1):25–30. doi: 10.4158/EP.12.S1.25. [DOI] [PubMed] [Google Scholar]
- 148.DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med. 1995;333(9):541–549. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- 149.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352(9131):854–865. [PubMed] [Google Scholar]
- 150.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, MassiBenedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J, PROactive investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 151.Meinert CL, Knatterud GL, Prout TE, Klimt CR. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes. 1970;19(Suppl):789–830. [PubMed] [Google Scholar]
- 152.Huupponen R. Adverse cardiovascular effects of sulphonylurea drugs. Clinical significance. Med Toxicol. 1987;2(3):190–209. doi: 10.1007/BF03259864. [DOI] [PubMed] [Google Scholar]
- 153.Pogatsa G, Koltai MZ, Jermendy G, Simon J, Aranyi Z, BallagiPordany G. The effect of sulphonylurea therapy on the outcome of coronary heart diseases in diabetic patients. Acta Med Hung. 1992;49(1-2):39–51. [PubMed] [Google Scholar]
- 154.Yki-Järvinen H, Young AA, Lamkin C, Foley JE. Kinetics of glucose disposal in whole body and across the forearm in man. J Clin Invest. 1987;79(6):1713–1719. doi: 10.1172/JCI113011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zangeneh F, Arora PS, Dyck PJ, Bekris L, Lernmark A, Achenbach SJ, Oberg AL, Rizza RA. Effects of duration of type 2 diabetes mellitus on insulin secretion. Endocr Pract. 2006;12(4):388–393. doi: 10.4158/EP.12.4.388. [DOI] [PubMed] [Google Scholar]
- 156.Levy J, Atkinson AB, Bell PM, McCance DR, Hadden DR. Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow-up of the Belfast Diet Study. Diabet Med. 1998;15(4):290–296. doi: 10.1002/(SICI)1096-9136(199804)15:4<290::AID-DIA570>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 157.UK Prospective Diabetes Study Group. UK prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes. 1995;44(11):1249–1258. [PubMed] [Google Scholar]
- 158.Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH. Sequential changes in serum insulin concentration during develop- ment of non-insulin-dependent diabetes. Lancet. 1989;1(8651):1356–1359. doi: 10.1016/s0140-6736(89)92804-3. [DOI] [PubMed] [Google Scholar]
- 159.Borg H, Gottsäter A, Fernlund P, Sundkvist G. A 12-year prospective study of the relationship between islet antibodies and beta-cell function at and after the diagnosis in patients with adult-onset diabetes. Diabetes. 2002;51(6):1754–1762. doi: 10.2337/diabetes.51.6.1754. [DOI] [PubMed] [Google Scholar]
- 160.Niskanen L, Karjalainen J, Siitonen O, Uusitupa M. Metabolic evolution of type 2 diabetes: a 10-year follow-up from the time of diagnosis. J Intern Med. 1994;236(3):263–270. doi: 10.1111/j.1365-2796.1994.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 161.Mauvais-Jarvis F, Sobngwi E, Porcher R, Riveline JP, Kevorkian JP, Vaisse C, Charpentier G, Guillausseau PJ, Vexiau P, Gautier JF. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origclinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes. 2004;53(3):645–653. doi: 10.2337/diabetes.53.3.645. [DOI] [PubMed] [Google Scholar]
- 162.Liljenquist JE, Horwitz DL, Jennings AS, Chiasson JL, Keller U, Rubenstein AH. Inhibition of insulin secretion by exogenous insulin in normal man as demonstrated by C-peptide assay. Diabetes. 1978;27(5):563–570. doi: 10.2337/diab.27.5.563. [DOI] [PubMed] [Google Scholar]
- 163.Argoud GM, Schade DS, Eaton RP. Insulin suppresses its own secretion in vivo. Diabetes. 1987;36(8):959–962. doi: 10.2337/diab.36.8.959. [DOI] [PubMed] [Google Scholar]
- 164.Fruehwald-Schultes B, Kern W, Born J, Fehm HL, Peters A. Comparison of the inhibitory effect of insulin and hypoglycemia on insulin secretion in humans. Metabolism. 2000;49(7):950–953. doi: 10.1053/meta.2000.6757. [DOI] [PubMed] [Google Scholar]