Abstract
Gc variants of vitamin D binding protein differ in their affinity for vitamin D metabolites that modulate antimycobacterial immunity. We conducted studies to determine whether Gc genotype associates with susceptibility to tuberculosis.
123 adult tuberculosis patients and 140 controls of Gujarati Asian ethnic origin in the United Kingdom, 130 adult tuberculosis patients and 78 controls in Brazil, and 281 children with tuberculosis and 182 controls in South Africa were recruited to case-control studies. Gc genotypes were determined and their frequency was compared between cases vs. controls. Serum 25-hydroxyvitamin D (25[OH]D) concentrations were obtained retrospectively for 139 Gujarati Asians, and case-control analysis was stratified by vitamin D status. Interferon-γ release assays were also performed on 36 Gujarati tuberculosis contacts.
The Gc2/2 genotype was strongly associated with susceptibility to active tuberculosis in Gujarati Asians, compared with Gc1/1 genotype (OR 2.81, 95% CI 1.19 to 6.66, P=0.009). This association was preserved if serum 25(OH)D was <20 nmol/l (P=0.01), but not if serum 25(OH)D was ≥20 nmol/l (P=0.36). Carriage of the Gc2 allele associated with increased PPD-stimulated Interferon-γ release in Gujarati Asian tuberculosis contacts (P = 0.02). No association between Gc genotype and susceptibility to tuberculosis was observed in other ethnic groups studied.
Introduction
Tuberculosis (TB) is a leading global cause of death. Vitamin D deficiency associates with susceptibility to active TB in numerous settings [1] and vitamin D supplementation enhances antimycobacterial immunity [2]. Vitamin D is synthesized in the skin during exposure to ultra-violet light and is metabolized by the liver to form 25-hydroxyvitamin D (25(OH)D), the major circulating vitamin D metabolite and accepted measure of vitamin D status. 25(OH)D then undergoes a further hydroxylation step to form 1,25-dihydroxyvitamin D (1,25(OH)2D), the immunomodulatory metabolite which enhances antimycobacterial activity by pleiotropic mechanisms including the induction of antimicrobial peptides with antituberculous activity [3, 4] and the suppression of matrix metalloproteinase enzymes implicated in degradation of pulmonary extracellular matrix [5].
Vitamin D metabolites in the circulation are bound to vitamin D binding protein (DBP), a highly expressed multifunctional 58 kDa serum glycoprotein encoded on chromosome 4. The DBP locus is among the most polymorphic known [6]. Two common polymorphisms at codons 416 (GAT→GAG, Asp→Glu) and 420 (ACG→AAG, Thr→Lys) of exon 11 of the DBP gene (defined by the presence of restriction endonuclease sites for HaeIII and StyI, respectively) give rise to the three major electrophoretic variants of DBP, termed group-specific component 1 fast (Gc1F), Gc1 slow (Gc1S) and Gc2. These variants differ in their functional characteristics: the Gc1F and Gc1S variants have been reported to have greater affinity for 25(OH)D than the Gc2 variant [7], potentially leading to more efficient delivery of 25(OH)D to the target tissues, while the Gc2 variant is associated with decreased circulating concentrations of 25(OH)D, 1,25(OH)2D and DBP [8, 9]. We therefore reasoned that possession of the Gc2 variant of DBP might associate with susceptibility to TB, and conducted case control studies in three different settings to test this hypothesis. We also conducted functional studies to determine whether antigen-stimulated release of interferon-gamma (IFN-γ) from whole blood of healthy TB contacts varied according to Gc genotype.
Methods
Populations studied
Case-control study participants were recruited at 3 sites (Table 1). One hundred and twenty-three adult TB patients and 140 healthy adult TB contacts, all of Gujarati ethnic origin, were recruited at Northwick Park Hospital, London, UK from 1993 to 2004; 281 children with TB (210 Xhosa, 71 of Cape Coloured ethnic origin) and 182 healthy child TB contacts (163 Xhosa, 19 Cape Coloured ethnic origin) were recruited at Red Cross Children’s Hospital, Cape Town, South Africa, from 2000 to 2003; and 130 adult TB patients (55 white, 44 mixed, 31 black) and 78 healthy adult controls (49 white, 18 mixed and 11 black) were recruited in Instituto de Pesquisa Clínica Evandro Chagas (IPEC) at Fiocruz and in Municipal Health Centres, Rio de Janeiro, Brazil, from 2004 to 2007. Diagnosis of TB was established on the basis of smear positive for acid-fast bacilli and/or culture positive for M. tuberculosis in all adult cases and in 33% of paediatric cases; for remaining paediatric cases, diagnosis was based on WHO criteria for diagnosis of TB in children [10] with 42% classified as having probable TB, and 25% classified as having possible TB. Patients with known HIV infection or taking immunosuppressant drugs were excluded. Adult TB contacts were recruited at participating TB clinics and health centres in London and Rio de Janeiro on the basis of a history of household contact with a TB patient, absence of symptoms of active TB and genetic unrelatedness to any patient recruited as a TB case in the study. Child TB contacts were recruited by locating children living three houses adjacent to a household in which a childhood TB case was identified. Where possible, it was ensured that controls were unrelated to TB cases. All adult participants gave informed consent for inclusion, as did parents of child participants. Permission to conduct the case-control study was obtained from research ethics committees in the UK (Harrow REC ref. 1646), South Africa (University of Cape Town REC ref. 013/2000) and Brazil (IPEC REC ref. 0008.0.009.000-04 and Rio de Janeiro Municipal Health Centre REC ref. S/CRH/DRH/DIC3).
Table 1.
Study populations
| City, Country | Ethnic group | Cases | Controls |
|---|---|---|---|
| London, UK | Gujarati Asian | Adults (n=123) | Adults (n=140) |
| Cape Town, South Africa | Xhosa | Children (n=210) | Children (n=163) |
| Cape Coloured | Children (n=71) | Children (n=19) | |
| Rio de Janeiro, Brazil | White | Adults (n=55) | Adults (n=49) |
| Mixed | Adults (n=44) | Adults (n=18) | |
| Black | Adults (n=31) | Adults (n=11) |
Functional studies were also conducted in a group of 36 healthy TB contacts recruited to a clinical trial of vitamin D supplementation at Northwick Park Hospital (n=26) and Newham Chest Clinic (n=8), London, UK from 2002 to 2005. Characteristics of the whole study population have been reported elsewhere [11]. The sub-group of 36 participants whose results are presented here was selected on the basis of self-assigned Gujarati ethnicity and availability of a result of a whole blood IFN-γ release assay (IGRA) performed prior to randomisation. The study was approved by the research ethics committees of North East London and Harrow (REC refs. P/02/146 and EC 2759, respectively) with written, informed consent to take part in the study obtained from all participants.
Genotyping
Genomic DNA was extracted from peripheral blood samples using the DNA Midi kit (Qiagen, Lewes). A 483-bp fragment of exon 11 of the Gc gene was amplified by polymerase chain reaction (PCR). Reaction mix included 2 μl DNA at 50 μl/ml, 11.1 μl H2O, 2 μl 10× PCR buffer, 0.4 μl 25 mM MgCl2, 0.4 μl 10 mM deoxynucleotide triphosphates (dNTP), 0.1 μl HotStar Taq polymerase (Qiagen) and 2 μl of 20 μM primers. Primer sequences were 5′-AAATAATGAGCAAATGAAAGAAGAC-3′ (forward) and 5′-CAATAACAGCAAAGAAATGAGTAGA-3′ (reverse). Cycling conditions were 95°C for 15 minutes, followed by 35 cycles at 94°C for 45 seconds, 51°C for 45 seconds, 72°C for 45 seconds, then a final 7 minutes at 72°C. PCR products were digested separately with restriction enzymes HaeIII (for 4h at 37°C) and Sty I (overnight at 37°C) (New England Biolabs, Mississauga, Ontario). Digestion with HaeIII produces 297- and 186-bp fragments in the presence of the h allele and digestion with StyI produces 305- and 178-bp fragments in the presence of the s allele. Digested products were visualised on 2% agarose gels stained with ethidium bromide. The presence of restriction sites was assigned by the lower case (h for HaeIII, s for StyI), and absence was assigned by the upper case (H for HaeIII and S for StyI). Gc genotype was assigned as per Table 2.
Table 2.
Deduction of DBP genotype from HaeIII and StyI genotypes
| HaeIII/StyI Genotype |
Potential HaeIII/StyI haplotypes |
Deduced HaeIII/StyI haplotypes |
Corresponding DBP genotype |
|---|---|---|---|
| HH SS | HS/HS | HS/HS | Gc1F/ Gc1F |
| HH Ss | HS/Hs | HS/Hs | Gc1F/Gc2 |
| HH ss | Hs/Hs | Hs/Hs | Gc2/Gc2 |
| Hh SS | HS/hS | HS/hS | Gc1F/Gc1S |
| Hh Ss | Hs/hS or HS/hs* | Hs/hS | Gc2/Gc1S |
| hh SS | hS/hS | hS/hS | Gc1S/Gc1S |
| hh Ss | hS/hs* | Gc1s/Not deduced | Not assigned |
frequency of the hs haplotype is extremely low due to linkage disequilibrium between loci; therefore subjects heterozygous at both loci were assumed to carry Hs/hS haplotypes, and subjects with potential haplotype hs were not assigned a DBP genotype.
Determination of 25(OH)D concentrations
Serum 25(OH)D concentrations of Gujarati participants recruited in London were determined by radio-immunoassay (DiaSorin, Stillwater, MN) in a clinical biochemistry laboratory that participates in the international Vitamin D external quality assessment program (http://www.deqas.org/). For participants with active TB, serum 25(OH)D concentrations were determined at diagnosis. Vitamin D deficiency was defined as serum 25(OH)D < 20 nmol/l.[12]
Interferon-gamma Release Assay (IGRA)
The IGRA used in this study has been described elsewhere [13]. Triplicate samples of venous blood diluted 1:10 with RPMI-1640 (Life Technologies, Paisley, UK) were cultured with 1000 U/ml PPD (Statens Serum Institute, Denmark), 2.5 μg/ml recombinant ESAT-6 or 5 μg/ml CFP-10 (Lionex, Braunschweig, Germany) at 37°C in 5% CO2. Supernatants were aspirated at 96 hours for determination of IFN-γ concentration by ELISA.
Statistical analysis
Due to similar functional characteristics [7] Gc1F and Gc1S allele carriers were combined to produce a total of 3 genotypes: Gc1/1, Gc2/1 and Gc2/2. Contingency tables were analyzed using chi-square tests, unless more than 20% of cells in a table had an expected frequency of <5, when Fisher’s exact tests were employed. Linkage disequilibrium was evaluated by calculating estimating predicted haplotypes frequencies based on random assortment of HaeIII and StyI alleles, and comparing these with observed frequencies by a chi square test; D′ was calculated using Lewontin’s equation [14]. Mean ages were compared between groups using unpaired t-tests and one-way ANOVA, and median serum 25(OH)D concentrations were compared between different groups using Kruskal-Wallis tests. Chi square tests were performed to test for association between genotype and susceptibility to TB, and binary logistic regression analysis was conducted to adjust odds ratios (OR) for age and sex. Data were analyzed using SPSS (version 12.0.1, 2003) and GraphPad Prism (version 4.03, 2005) software packages.
Results
Participant characteristics: case-control study
The characteristics of case-control study participants are presented in Table 3. Gujarati Asian cases and controls did not differ with respect to age or sex distribution. Males were over-represented in cases vs. controls among participants recruited in Cape Town (Xhosa: 55.2% vs. 41.1% male, P = 0.007; Cape Coloured: 47.9% vs. 21.1% male, P = 0.04) and Rio (black: 61.3% vs. 27.3% male, P = 0.01; mixed: 63.6% vs. 38.9% male, P = 0.02). The mean age of cases was lower than that of controls among Xhosa participants in Cape Town (5.2 years vs. 6.1 years, P=0.002) and higher than that of controls among white participants in Rio (38.7 years vs. 29.6 years, P < 0.001).
Table 3.
Characteristics of TB cases and controls
| Ethnic group, City | Cases | Controls | P | |
|---|---|---|---|---|
| Gujarati, London | Mean age, yr, (s.d.) Male sex, n (%) |
43.8 (16.2) 47 (38.2) |
41.9 (13.3) 67 (47.9) |
0.3 0.12 |
| Xhosa, Cape Town | Mean age, yr, (s.d.) Male sex, n (%) |
5.2 (3.9) 116 (55.2) |
6.1 (3.6) 67 (41.1) |
0.002 0.007 |
| Cape Coloured, Cape Town | Mean age, yr, (s.d.) Male sex, n (%) |
4.4 (3.9) 34 (47.9) |
5.5 (4.1) 4 (21.1) |
0.16 0.04 |
| White, Rio | Mean age, yr, (s.d.) Male sex, n (%) |
38.7 (13.8) 37 (67.3) |
29.6 (10.3) 16 (32.7) |
<0.001 0.2 |
| Mixed, Rio | Mean age, yr, (s.d.) Male sex, n (%) |
36.6 (11.9) 28 (63.6) |
34.9 (13.6) 7 (38.9) |
0.63 0.02 |
| Black, Rio | Mean age, yr, (s.d.) Male sex, n (%) |
38.0 (13.7) 19 (61.3) |
33.6 (14.8) 3 (27.3) |
0.37 0.01 |
DBP allele frequency varies between ethnic groups
DBP allele frequency varied significantly between different ethnic groups (Table 4, P<0.0001). Frequency of the Gc2 allele was highest among Gujarati participants in London and white participants in Rio (35.9% and 33.2% respectively) and lowest among black participants in Rio and Xhosa participants in Cape Town (11.9% and 4.6% respectively). A similar ethnic distribution was observed for the 1S allele (47.9% in London Gujaratis, 41.8% in Rio whites, 19% in Rio blacks and 7.9% in Cape Town Xhosa). The opposite ethnic distribution was observed for the 1F allele, which was most common among Xhosa participants in Cape Town and black participants in Rio (87.5% and 69.0% respectively), and least common among white participants in Rio and Gujarati participants in London (25.0% and 16.2% respectively). Intermediate frequencies of all three alleles were found among ethnically admixed participants recruited in Rio and Cape Town. Both loci were in Hardy-Weinberg equilibrium in all populations studied. The loci were in linkage disequilibrium in Gujaratis (D′=0.52, P < 0.0001), in white participants in Rio (D′=0.36, P < 0.0001) and in ethnically admixed populations in Rio (D′=0.15, P < 0.0001) and Cape Town (D′=0.06, P=0.0002), but not in Xhosa participants in Cape Town (P=0.08) or black participants in Rio (P=0.10).
Table 4.
Frequency of DBP alleles by ethnic group
| Ethnic group, City | DBP allele, n (%) | P | ||
|---|---|---|---|---|
| Gc2 | Gc1F | Gc1S | ||
| Gujarati, London | 189 (35.9) | 85 (16.2) | 252 (47.9) | <0.0001 |
| Xhosa, Cape Town | 34 (4.6) | 653 (87.5) | 59 (7.9) | |
| Cape Coloured, Cape Town | 24 (13.3) | 108 (60.0) | 48 (26.7) | |
| White, Rio | 69 (33.2) | 52 (25.0) | 87 (41.8) | |
| Mixed, Rio* | 26 (21.3) | 52 (42.6) | 45 (36.3) | |
| Black, Rio | 10 (11.9) | 58 (69.0) | 16 (19.0) | |
Allele not assigned for one individual (haplotype hs)
Carriage of the Gc2 allele associates with susceptibility to active TB in Gujarati Asians
Given that the allele frequency varied by ethnic group, case-control analysis of DBP variant frequency was stratified by ethnic group. Among Gujarati Asian participants, the Gc genotype was associated with active TB (Table 5; P=0.04). The unadjusted OR for Gc2/2 compared with Gc1/1 was 2.81 (95% CI 1.19 to 6.66, P=0.009), while the unadjusted OR for Gc2/1 compared with Gc1/1 was 1.69 (95% CI 0.96 to 2.96, P=0.052). The unadjusted OR for genotypes Gc2/1 and Gc2/2 combined vs. Gc1/1 was 1.89 (95% CI 1.11 to 3.22, P=0.012). These associations were unaltered by adjustment for age and sex (adjusted OR for Gc2/2 vs. Gc1/1 was 2.83, 95% CI 1.27 to 6.31, P=0.01; adjusted OR for Gc2/1 vs. Gc1/1 was 1.73, 95% CI 1.01 to 2.96, P = 0.045; adjusted OR for Gc2/1 and Gc2/2 combined vs. Gc1/1 was 1.94, 95% CI 1.17 to 3.23, P=0.01). No statistically significant variation in frequency of DBP genotype was observed between cases and controls among any of the populations studied in Cape Town or Rio, either before or after adjustment for age and sex.
Table 5.
Frequency of DBP genotypes: TB cases vs. controls
| Ethnic group, City | Cases / Controls | DBP genotype, n (%) | P | ||
|---|---|---|---|---|---|
| Gc2/2 | Gc2/1 | Gc1/1 | |||
| Gujarati, London | Cases (n=123) Controls (n=140) |
22 (17.9) 13 (9.3) |
60 (48.7) 59 (42.1) |
41 (33.3) 68 (48.5) |
0.04 |
| Xhosa, Cape Town | Cases (n=210) Controls (n=163) |
0 (0) 0 (0) |
20 (9.5) 14 (8.6) |
190 (90.5) 149 (91.4) |
0.86 |
| Cape Coloured, Cape Town |
Cases (n=71) Controls (n=19) |
3 (4.2) 0 (0) |
14 (19.7) 4 (21.1) |
54 (76.1) 15 (78.9) |
<0.99 |
| White, Rio | Cases (n=55) Controls (n=49) |
8 (14.5) 5 (10.2) |
20 (36.4) 23 (46.9) |
27 (49.1) 21 (42.9) |
0.56 |
| Mixed, Rio | Cases (n=44)* Controls (n=18) |
2 (4.5) 2 (11.1) |
12 (27.3) 6 (33.3) |
29 (65.9) 10 (55.6) |
0.64 |
| Black, Rio | Cases (n=31) Controls (n=11) |
1 (3.2) 0 (0) |
5 (16.1) 3 (27.3) |
25 (80.6) 8 (72.7) |
0.75 |
DBP genotype not assigned for one case (Hae III genotype hh, Sty I genotype Ss)
Vitamin D status does not vary with DBP variant in Gujarati Asians
We have previously shown that vitamin D deficiency associates with susceptibility to TB in Gujarati Asians [15]. Since then, others have reported that serum 25(OH)D concentrations vary with Gc genotype, being lowest in carriers of the Gc-2 allele [8, 9]. We were interested to determine whether serum 25(OH)D concentrations varied according to Gc genotype in Gujarati Asians. Since serum samples were not collected prospectively for this purpose, we conducted a retrospective search for results of routinely performed assays of serum 25(OH)D concentration for all Gujarati Asian participants. Results were available for 84/123 cases and 55/140 controls. Vitamin D deficiency (serum 25(OH)D < 20 nmol/l) was more common among TB cases vs. controls (affecting 60/84 vs. 31/55) but this difference did not attain statistical significance (P=0.07). No significant differences in median serum 25(OH)D concentration or prevalence of vitamin D deficiency were observed between individuals with different genotype (median serum 25(OH)D, 17.0 vs. 12.0 vs. 10.0 nmol/l for Gc1/1, Gc1/2 and Gc2/2 genotypes respectively, P=0.28; prevalence of vitamin D deficiency, 35/58 vs. 43/63 vs. 13/18 deficient for Gc1/1, Gc1/2 and Gc2/2 genotypes respectively, P=0.53).
The association between Gc genotype and susceptibility to TB in Gujarati Asians varies according to vitamin D status
We next investigated whether association between Gc genotype and susceptibility to TB in Gujarati Asians varied by vitamin D status by performing separate contingency analyses for vitamin D deficient participants and non-deficient participants (Table 6). Among vitamin D deficient Gujarati Asians we found that Gc genotype was strongly associated with susceptibility to TB, with genotype 2/2 present in 13/60 vitamin D deficient cases vs. 0/31 vitamin D deficient controls (P=0.01). In contrast, no association between Gc genotype and susceptibility to TB was seen among Gujarati Asians with serum 25(OH)D ≥ 20 nmol/l (P=0.36). Statistical analysis to determine whether Gc genotype and vitamin D status interact to influence susceptibility to TB was indeterminate, as the zero value for vitamin D deficient controls with Gc2/2 genotype in the contingency table (Table 6) resulted in an undefined odds ratio.
Table 6.
Frequency of DBP genotypes, Gujarati TB cases vs. controls, stratified by vitamin D status
| Serum 25(OH)D | DBP genotype, n (%) | P | |||
|---|---|---|---|---|---|
| Gc2/2 | Gc2/1 | Gc1/1 | |||
| <20 nmol/l | Cases (n=60) Controls (n=31) |
13 (21.7) 0 (0) |
26 (43.3) 17 (54.8) |
21 (35.0) 14 (45.2) |
0.01 |
| ≥20 nmol/l | Cases (n=24) Controls (n=24) |
3 (12.5) 2 (8.3) |
12 (50) 8 (33.3) |
9 (37.5) 14 (58.3) |
0.36 |
Carriage of the Gc2 allele associates with increased IFN-γ release from PPD-stimulated whole blood of Gujarati Asian TB contacts
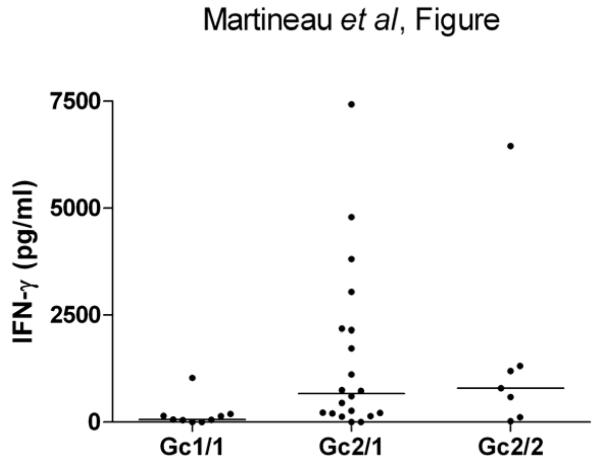
Finally we investigated whether IFN-γ responses to mycobacterial antigens varied according to Gc genotype in a group of 36 Gujarati Asian TB contacts whose characteristics are presented in Table 7. No significant differences in sex ratio, BCG status, tuberculin skin test reactivity, site of exposure or vitamin D status were observed between participants of different Gc genotype, although a statistically significant difference in mean age was observed between participants of different genotype (55.3 yr vs. 41.3 yr vs. 34.9 yr for genotypes Gc2/2, Gc2/1 and Gc1/1 respectively, P = 0.03). Median PPD-stimulated IFN-γ concentration was higher among TB contacts of Gc2/2 and Gc2/1 genotype vs. those of Gc1/1 genotype (790.8 pg/ml vs. 663.5 pg/ml vs. 60.5 pg/ml respectively, P=0.02; Figure); no difference in ESAT-6 or CFP-10-stimulated IFN-γ concentration was observed between TB contacts of different genotype (Table 7).
Table 7.
Characteristics of functional study participants by Gc genotype
| Variable | Gc2/2 (n=7) | Gc2/1 (n=20) | Gc1/1 (n=9) | P |
|---|---|---|---|---|
| Mean age, years (s.d.) | 55.3 (7.8) | 41.3 (15.0) | 34.9 (16.7) | 0.03 |
| Male sex, no. (%) | 3 (42.9) | 11 (55.0) | 3 (33.3) | 0.54 |
| BCG scar present, no. (%) | 6 (85.7) | 15 (75.0) | 8 (88.9) | 0.63 |
| UK born, no. (%) | 3 (42.9) | 6 (30.0) | 3 (33.3) | 0.35 |
| Tuberculin skin test reactive, proportion (%)* |
0/5 (0) | 5/13 (38.5) | 0/5 (0) | 0.06 |
| Household exposure, no. (%) | 4 (57.1) | 17 (85.0) | 6 (66.6) | 0.27 |
| 25(OH)D < 20 nmol/L, no. (%) | 3 (42.9) | 12 (60.0) | 5 (55.6) | 0.73 |
| Median serum 25(OH)D, nmol/l (IQ range) |
25.0 (13.2 to 43.0) |
18.4 (9.5 to 27.9) |
17.5 (7.1 to 37.0) |
0.63 |
| Median PPD-stimulated IFN-γ, pg/ml (IQ range) |
790.8 (118.6 to 1311.0) |
663.5 (208.3 to 2162.0) |
60.5 (22.7 to 170.4) |
0.02 |
| Median ESAT-6-stimulated IFN-γ, pg/ml (IQ range) |
16.6 (0.0 to 324.9) |
53.2 (20.7 to 233.7) |
52.9 (0.0 to 154.3) |
0.60 |
| Median CFP-10-stimulated IFN- γ, pg/ml (IQ range) |
0.0 (0.0 to 23.6) | 53.1 (0.0 to 263.1) |
0.0 (0.0 to 62.8) |
0.29 |
Reactive tuberculin status defined as Heaf grade 3 or 4, or Heaf grade 2 in the absence of a BCG scar; negative tuberculin status defined as Heaf grade 0 or 1, or Heaf grade 2 in the presence of a BCG scar. Tuberculin skin test results were available for 5/7 Gc2/2, 13/20 Gc2/1 and 5/9 Gc1/1 participants.
Figure.
PPD-stimulated Interferon-γ release in functional study participants by Gc genotype
Discussion
We have demonstrated an association between the Gc genotype of vitamin D binding protein and susceptibility to TB in Gujarati Asians living in London. Stratification of this analysis by vitamin D status revealed that this association was restricted to participants with profound vitamin D deficiency.
No association between carriage of the Gc2 allele and susceptibility to TB was observed among populations studied in Rio and Cape Town. Gc2 allele frequency in these populations was lower than in Gujarati Asians, and absolute numbers of Gc2 carriers were therefore small (Table 4); our study may have been underpowered to detect an association in these populations. An alternative explanation for the lack of association seen in Rio and Cape Town is that populations in Rio (latitude 22°S) and Cape Town (latitude 33°S) are likely to have significantly more exposure to UVB than those living in London (latitude 51°N). Profound vitamin D deficiency is therefore likely to be much less common in these settings. If the association between Gc2 allele and susceptibility to TB is restricted to individuals with profound vitamin D deficiency, as we postulate, then low prevalence of vitamin D deficiency in Rio and Cape Town may explain non-replication of the association in these settings. This explanation may also account for lack of association between Gc genotype and susceptibility to TB in other populations previously studied in Kuwait[16] and India [17].
Individuals carrying the Gc2 allele have previously been reported to have lower circulating concentrations of both DBP and 25(OH)D [8, 9, 18], phenomena that have been attributed to the fact that the gene product of Gc2 allele is metabolized faster than that of Gc1 alleles [19]. Vitamin D deficient carriers of the Gc2 allele may therefore have particularly low circulating concentrations of 25(OH)D-DBP complex. Receptor-mediated endocytosis of 25(OH)D-DBP complex has previously been shown to be essential for induction of vitamin D-mediated biological activity in both renal and mammary cells [20, 21]. If 25(OH)D-DBP complex is similarly required for initiation of vitamin D-inducible antimycobacterial responses then reduced circulating concentrations of this complex in vitamin D deficient carriers of the Gc2 allele could explain the association that we report. Further studies are required to establish whether circulating concentrations of 25(OH)D-DBP complex vary according to Gc allele, and to investigate whether receptor-mediated endocytosis of 25(OH)D-DBP complex is essential for induction of vitamin D-inducible antimycobacterial responses.
Our finding of striking ethnic variation in Gc allele frequency is in keeping with published literature reporting that populations with deeply pigmented skin have higher frequencies of the 1F allele [22]. Reports that cutaneous penetration of UVB is decreased in individuals with pigmented skin [23] and that the Gc1 variants of DBP have greater affinity for 25-hydroxyvitamin D than the Gc2 variant [24] raise the possibility that Gc1 variants may carry a survival advantage in persons with pigmented skin due to their superior delivery of 25(OH)D to the target tissues. If this is the case, then the observation of increasing frequency of the Gc2 allele in Caucasian populations implies that possession of this allele confers a survival benefit in conditions where solar radiation is limited. Consistent with this hypothesis, possession of the Gc2 allele has been associated with decreased risk of several other pathologies including breast cancer [9], chronic obstructive pulmonary disease [25] and fractures [26]. We also observed that alleles of the loci at codons 416 and 420 were in linkage disequilibrium in Gujaratis, whites and ethnically admixed populations, but not in Xhosa or black participants. This phenomenon has been demonstrated for other coding single nucleotide polymorphisms in multiethnic populations, and may be attributed to a ‘bottleneck’ experienced by Asian and European populations migrating from Africa 800 to 1,600 generations ago [27].
Our functional study revealed that TB contacts carrying the Gc2 allele had significantly higher PPD-stimulated IFN-γ responses than Gc1 homozygotes, who demonstrated low or undetectable IFN-γ responses to PPD; all TB contacts had low or undetectable IFN-γ responses to RD-1-encoded antigens. ESAT-6 and CFP-10 are secreted early in infection, and reversion of IFN-γ responses to these antigens with persistence of tuberculin reactivity has been previously reported in longitudinal studies of TB contacts, and may represent latent TB infection [28]. Our data may therefore indicate that Gc1 homozygotes are relatively resistant to acquisition of latent TB infection, and that Gc2 allele carriers are more susceptible.
Our study has some limitations. Gujarati Asian ethnicity was self-assigned, and although the community is genetically homogenous [15] the possibility of ethnic admixture cannot be excluded. Serum 25(OH)D concentrations were only available for 68% of Gujarati cases vs. 39% of Gujarati controls. This difference presumably arose because clinicians had a lower threshold for testing vitamin D status in TB cases than in TB contacts. Since the Gc2/2 genotype was less common among controls than cases, the absolute number of Gc2/2 controls with known vitamin D status was small (n=2). However, the proportion of participants for whom 25(OH)D was available did not differ by Gc genotype, and the results of this analysis cannot therefore be attributed to selection bias. Larger studies with prospective evaluation of circulating concentrations of 25(OH)D should be performed in populations at risk of vitamin D deficiency to determine whether profound vitamin D deficiency and Gc2 genotype interact to increase susceptibility to TB.
Acknowledgements
This work was supported by the Scadding-Morriston Davies Joint Fellowship in Respiratory Medicine awarded to ARM, a BEIT Fellowship awarded to STA, the Wellcome Trust (Refs. 066321 and 072070) and FIOCRUZ/Brazilian Ministry of Health. The funding bodies played no part in the design or conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. ARM had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
We thank Ms. Dina Shah at the Department of Infection and Tropical Medicine, Northwick Park Hospital, for her assistance in locating patient records for this study; Dr Sandra Rainbow and colleagues at the Department of Clinical Biochemistry, Northwick Park Hospital, for performing 25(OH)D assays; and Professor Robert Walton and Dr Barbara Boucher of Barts and The London Medical School for helpful discussions and review of the manuscript.
References
- 1.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37(1):113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 2.Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, Packe GE, Davidson RN, Eldridge SM, Maunsell ZJ, Rainbow SJ, Berry JL, Griffiths CJ. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176(2):208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 3.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 4.Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, Davidson RN, Sorensen OE, Kampmann B, Griffiths CJ, Wilkinson RJ. IFN-{gamma}- and TNF-Independent Vitamin D-Inducible Human Suppression of Mycobacteria: The Role of Cathelicidin LL-37. J Immunol. 2007;178(11):7190–7198. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 5.Coussens A, Timms PM, Boucher BJ, Venton TR, Ashcroft AT, Skolimowska KH, Newton SM, Wilkinson KA, Davidson RN, Griffiths CJ, Wilkinson RJ, Martineau AR. 1alpha,25-dihydroxyvitamin D inhibits matrix metalloproteinases induced by Mycobacterium tuberculosis infection. Immunology. 2008 doi: 10.1111/j.1365-2567.2008.03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleve H, Constans J. The mutants of the vitamin-D-binding protein: more than 120 variants of the GC/DBP system. Vox Sang. 1988;54(4):215–225. doi: 10.1111/j.1423-0410.1988.tb03908.x. [DOI] [PubMed] [Google Scholar]
- 7.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP) Hum Genet. 1993;92(2):183–188. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- 8.Lauridsen AL, Vestergaard P, Hermann AP, Brot C, Heickendorff L, Mosekilde L, Nexo E. Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif Tissue Int. 2005;77(1):15–22. doi: 10.1007/s00223-004-0227-5. [DOI] [PubMed] [Google Scholar]
- 9.Abbas S, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, Flesch-Janys D, Chang-Claude J. The Gc2 allele of the vitamin D binding protein is associated with a decreased postmenopausal breast cancer risk, independent of the vitamin d status. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1339–1343. doi: 10.1158/1055-9965.EPI-08-0162. [DOI] [PubMed] [Google Scholar]
- 10.WHO . Provisional guidelines for the diagnosis and classification of the EPI target diseases for primary health care, surveillance and special studies. WHO; Geneva: 1983. [Google Scholar]
- 11.Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, Packe GE, Davidson RN, Griffiths CJ, Wilkinson RJ. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117(7):1988–1994. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Compston JE. Vitamin D deficiency: time for action. Evidence supports routine supplementation for elderly people and others at risk. Bmj. 1998;317(7171):1466–1467. doi: 10.1136/bmj.317.7171.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scholvinck E, Wilkinson KA, Whelan AO, Martineau AR, Levin M, Wilkinson RJ. Gamma interferon-based immunodiagnosis of tuberculosis: comparison between whole-blood and immunospot methods. J Clin Microbiol. 2004;42(2):829–831. doi: 10.1128/JCM.42.2.829-831.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewontin RC. The interaction of selection and linkage. I. General considerations: Heterotic models. Genetics. 1964;49:49–67. doi: 10.1093/genetics/49.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355(9204):618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 16.Bahr GM, Eales LJ, Nye KE, Majeed HA, Yousof AM, Behbehani K, Rook GA. An association between Gc (vitamin D-binding protein) alleles and susceptibility to rheumatic fever. Immunology. 1989;67(1):126–128. [PMC free article] [PubMed] [Google Scholar]
- 17.Papiha SS, Agarwal SS, White I. Association between phosphoglucomutase (PGM1) and group-specific component (Gc) subtypes and tuberculosis. J Med Genet. 1983;20(3):220–222. doi: 10.1136/jmg.20.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauridsen AL, Vestergaard P, Nexo E. Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clin Chem. 2001;47(4):753–756. [PubMed] [Google Scholar]
- 19.Kawakami M, Blum CB, Ramakrishnan R, Dell RB, Goodman DS. Turnover of the plasma binding protein for vitamin D and its metabolites in normal human subjects. J Clin Endocrinol Metab. 1981;53(6):1110–1116. doi: 10.1210/jcem-53-6-1110. [DOI] [PubMed] [Google Scholar]
- 20.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96(4):507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 21.Rowling MJ, Kemmis CM, Taffany DA, Welsh J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J Nutr. 2006;136(11):2754–2759. doi: 10.1093/jn/136.11.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamboh MI, Ferrell RE. Ethnic variation in vitamin D-binding protein (GC): a review of isoelectric focusing studies in human populations. Hum Genet. 1986;72(4):281–293. doi: 10.1007/BF00290950. [DOI] [PubMed] [Google Scholar]
- 23.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 24.Constans J, Viau M, Bouissou C. Affinity differences for the 25-OH-D3 associated with the genetic heterogeneity of the vitamin D-binding protein. FEBS Lett. 1980;111(1):107–111. doi: 10.1016/0014-5793(80)80772-1. [DOI] [PubMed] [Google Scholar]
- 25.Horne SL, Cockcroft DW, Dosman JA. Possible protective effect against chronic obstructive airways disease by the GC2 allele. Hum Hered. 1990;40(3):173–176. doi: 10.1159/000153926. [DOI] [PubMed] [Google Scholar]
- 26.Lauridsen AL, Vestergaard P, Hermann AP, Moller HJ, Mosekilde L, Nexo E. Female premenopausal fracture risk is associated with gc phenotype. J Bone Miner Res. 2004;19(6):875–881. doi: 10.1359/JBMR.040133. [DOI] [PubMed] [Google Scholar]
- 27.Reich DE, Cargill M, Bolk S, Ireland J, Sabeti PC, Richter DJ, Lavery T, Kouyoumjian R, Farhadian SF, Ward R, Lander ES. Linkage disequilibrium in the human genome. Nature. 2001;411(6834):199–204. doi: 10.1038/35075590. [DOI] [PubMed] [Google Scholar]
- 28.Hill PC, Brookes RH, Fox A, Jackson-Sillah D, Jeffries DJ, Lugos MD, Donkor SA, Adetifa IM, de Jong BC, Aiken AM, Adegbola RA, McAdam KP. Longitudinal assessment of an ELISPOT test for Mycobacterium tuberculosis infection. PLoS Med. 2007;4(6):e192. doi: 10.1371/journal.pmed.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]