Abstract
A method is presented for protein secondary structure prediction based on a neural network. A training phase was used to teach the network to recognize the relation between secondary structure and amino acid sequences on a sample set of 48 proteins of known structure. On a separate test set of 14 proteins of known structure, the method achieved a maximum overall predictive accuracy of 63% for three states: helix, sheet, and coil. A numerical measure of helix and sheet tendency for each residue was obtained from the calculations. When predictions were filtered to include only the strongest 31% of predictions, the predictive accuracy rose to 79%.
Full text
PDF
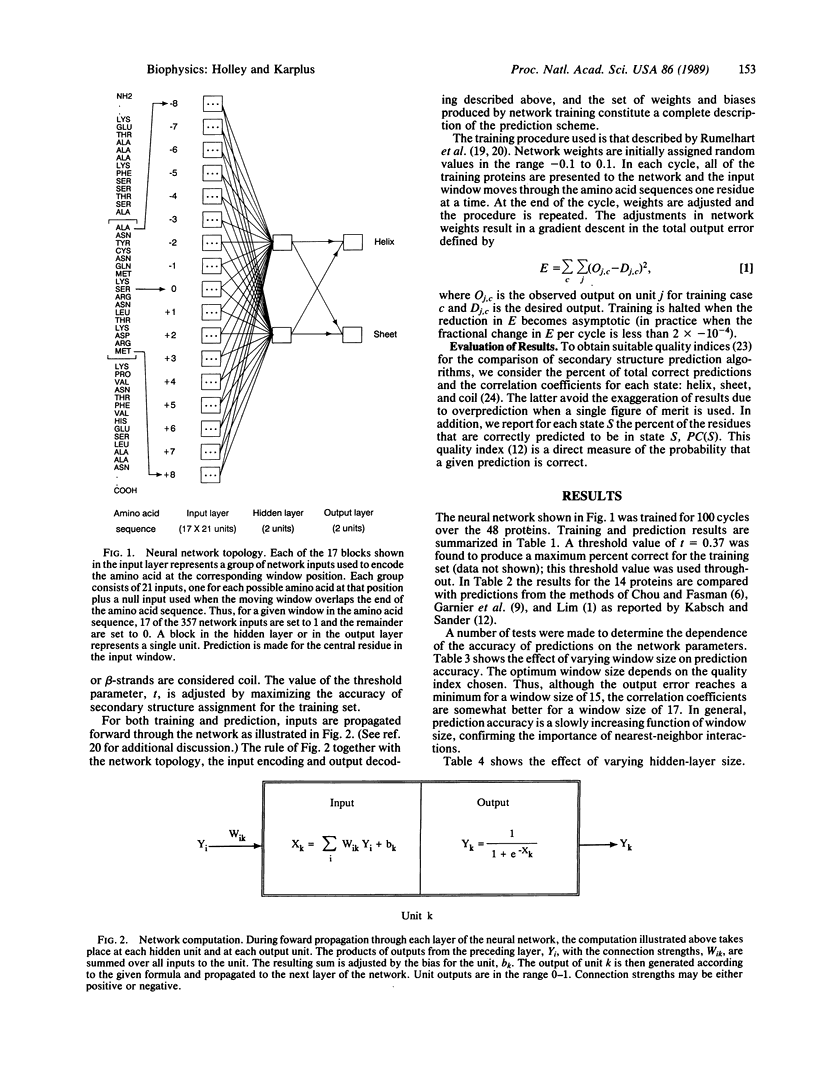
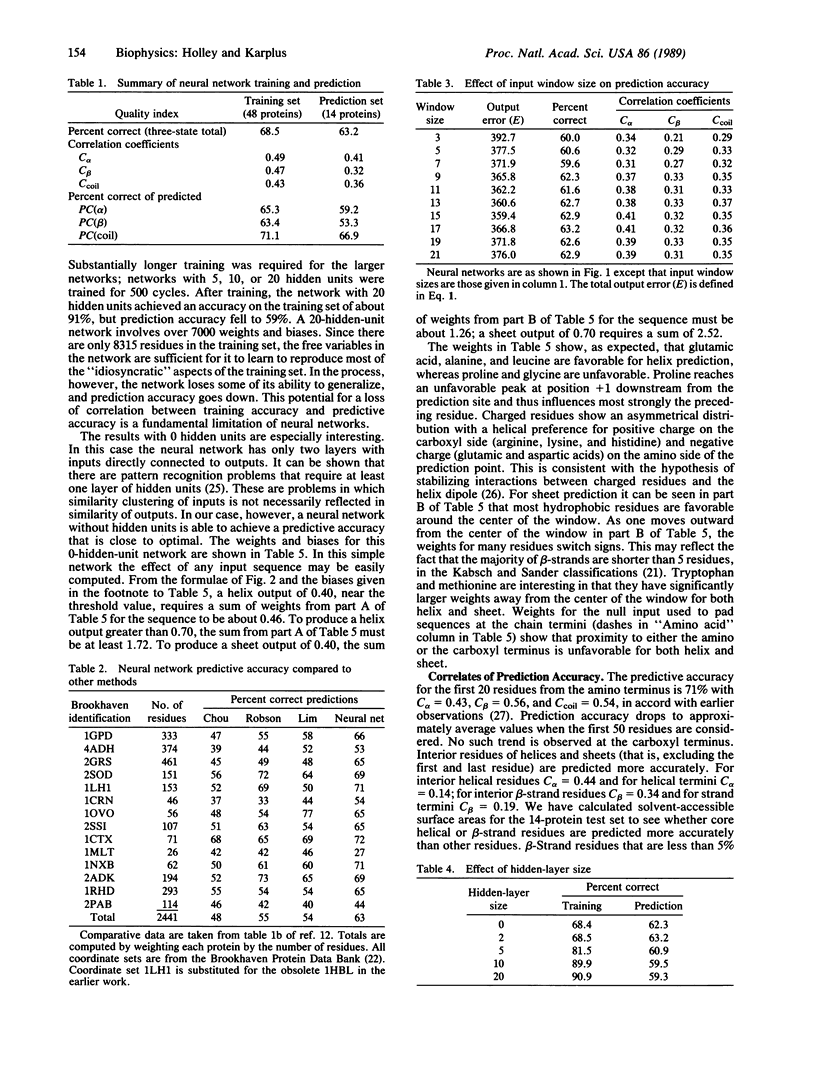
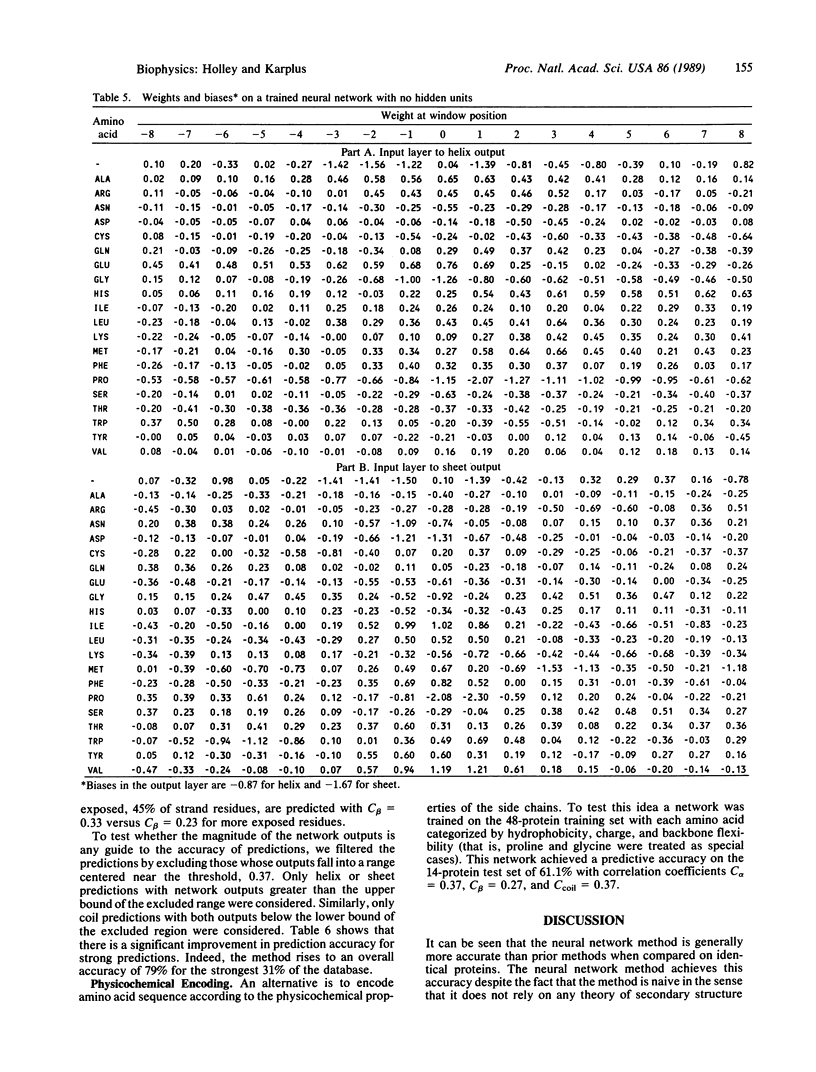

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Schwarz J., Schwarz J. An assessment of protein secondary structure prediction methods based on amino acid sequence. Biochim Biophys Acta. 1976 Aug 9;439(2):261–273. doi: 10.1016/0005-2795(76)90062-3. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Brain S. D., Tippins J. R., Morris H. R., MacIntyre I., Williams T. J. Potent vasodilator activity of calcitonin gene-related peptide in human skin. J Invest Dermatol. 1986 Oct;87(4):533–536. doi: 10.1111/1523-1747.ep12455620. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Scheraga H. A. Assessment of some problems associated with prediction of the three-dimensional structure of a protein from its amino-acid sequence. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1221–1225. doi: 10.1073/pnas.72.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Abarbanel R. M., Kuntz I. D., Fletterick R. J. Turn prediction in proteins using a pattern-matching approach. Biochemistry. 1986 Jan 14;25(1):266–275. doi: 10.1021/bi00349a037. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Niermann T., Kirschner K. Prediction of secondary structure by evolutionary comparison: application to the alpha subunit of tryptophan synthase. Proteins. 1987;2(2):118–129. doi: 10.1002/prot.340020206. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gibrat J. F., Garnier J., Robson B. Further developments of protein secondary structure prediction using information theory. New parameters and consideration of residue pairs. J Mol Biol. 1987 Dec 5;198(3):425–443. doi: 10.1016/0022-2836(87)90292-0. [DOI] [PubMed] [Google Scholar]
- Hopfield J. J. Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J. J., Tank D. W. Computing with neural circuits: a model. Science. 1986 Aug 8;233(4764):625–633. doi: 10.1126/science.3755256. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. How good are predictions of protein secondary structure? FEBS Lett. 1983 May 8;155(2):179–182. doi: 10.1016/0014-5793(82)80597-8. [DOI] [PubMed] [Google Scholar]
- Levin J. M., Robson B., Garnier J. An algorithm for secondary structure determination in proteins based on sequence similarity. FEBS Lett. 1986 Sep 15;205(2):303–308. doi: 10.1016/0014-5793(86)80917-6. [DOI] [PubMed] [Google Scholar]
- Lim V. I. Algorithms for prediction of alpha-helical and beta-structural regions in globular proteins. J Mol Biol. 1974 Oct 5;88(4):873–894. doi: 10.1016/0022-2836(74)90405-7. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Comparison of the predicted and observed secondary structure of T4 phage lysozyme. Biochim Biophys Acta. 1975 Oct 20;405(2):442–451. doi: 10.1016/0005-2795(75)90109-9. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R., Scheraga H. A. Improvements in the prediction of protein backbone topography by reduction of statistical errors. Biochemistry. 1979 Feb 20;18(4):697–704. doi: 10.1021/bi00571a023. [DOI] [PubMed] [Google Scholar]
- Nagano K. Triplet information in helix prediction applied to the analysis of super-secondary structures. J Mol Biol. 1977 Jan 15;109(2):251–274. doi: 10.1016/s0022-2836(77)80033-8. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Ooi T. Amino acid sequence homology applied to the prediction of protein secondary structures, and joint prediction with existing methods. Biochim Biophys Acta. 1986 May 12;871(1):45–54. doi: 10.1016/0167-4838(86)90131-7. [DOI] [PubMed] [Google Scholar]
- Qian N., Sejnowski T. J. Predicting the secondary structure of globular proteins using neural network models. J Mol Biol. 1988 Aug 20;202(4):865–884. doi: 10.1016/0022-2836(88)90564-5. [DOI] [PubMed] [Google Scholar]
- Schulz G. E. A critical evaluation of methods for prediction of protein secondary structures. Annu Rev Biophys Biophys Chem. 1988;17:1–21. doi: 10.1146/annurev.bb.17.060188.000245. [DOI] [PubMed] [Google Scholar]
- Shoemaker K. R., Kim P. S., York E. J., Stewart J. M., Baldwin R. L. Tests of the helix dipole model for stabilization of alpha-helices. Nature. 1987 Apr 9;326(6113):563–567. doi: 10.1038/326563a0. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An attempt to evaluate the influence of neighboring amino acids (n-1) and (n+1) on the backbone conformation of amino acid (n) in proteins. Use in predicting the three-dimensional structure of the polypeptide backbone of other proteins. J Mol Biol. 1973 Mar 25;75(1):13–31. doi: 10.1016/0022-2836(73)90526-3. [DOI] [PubMed] [Google Scholar]
- Zvelebil M. J., Barton G. J., Taylor W. R., Sternberg M. J. Prediction of protein secondary structure and active sites using the alignment of homologous sequences. J Mol Biol. 1987 Jun 20;195(4):957–961. doi: 10.1016/0022-2836(87)90501-8. [DOI] [PubMed] [Google Scholar]


