Abstract
Estrogen is central to many physiological processes throughout the human body. We have previously shown that the G protein-coupled receptor GPR30/GPER, in addition to classical nuclear estrogen receptors (ERα/β), activates cellular signaling pathways in response to estrogen. In order to distinguish between the actions of classical estrogen receptors and GPR30, we have previously characterized a selective agonist of GPR30, G-1 (1). To complement the pharmacological properties of G-1, we sought to identify an antagonist of GPR30 that displays similar selectivity against the classical estrogen receptors. Here we describe the identification and characterization of a G-1 analog, G15 (2) that binds to GPR30 with high affinity and acts as an antagonist of estrogen signaling through GPR30. In vivo administration of G15 reveals that GPR30 contributes to both uterine and neurological responses initiated by estrogen. The identification of this antagonist will accelerate the evaluation of the roles of GPR30 in human physiology.
Introduction
Estrogens play an important role in many areas of human physiology (including reproduction and the immune, vascular and nervous systems) as well as disease states such as cancer, depression and reproductive disorders1,2. Estrogen has long been known to act through soluble nuclear receptors that function as ligand-activated transcription factors. However, in addition to gene regulation, estrogen also mediates rapid signaling events, more commonly associated with growth factor and G protein-coupled receptors3. Recent studies reveal that GPR30 (International Union of Basic and Clinical Pharmacology designation: GPER), an intracellular transmembrane G protein-coupled estrogen receptor, mediates numerous aspects of cellular signaling ranging from calcium mobilization to EGFR transactivation to gene regulation4. The classical nuclear estrogen receptors (ERα/β) appear to overlap with GPR30 not only in many of their cellular and physiological responses4 but also in their ligand specificity5, making pharmacologic resolution of individual receptor functions challenging. For example, 17β-estradiol (3), 4-hydroxytamoxifen (4) and ICI182,780 (5) each bind to GPR30 in addition to classical estrogen receptors, though with different outcomes with respect to agonism and antagonism6-8. Whereas 17β-estradiol, 4-hydroxytamoxifen and ICI182,780 all activate GPR30, 17β-estradiol is an ERα agonist, 4-hydroxytamoxifen is a selective estrogen receptor modulator (SERM) and ICI182,780 is a pure ERα antagonist9. Interestingly, until recently, GPR30-specific ligands were unknown.
In 2006, we described a highly selective GPR30 agonist named G-1 that shows no detectable activity towards the classical estrogen receptors10. This compound activates multiple cellular signaling pathways via GPR30 and has been used to examine the cellular and physiological actions of GPR30. Cellular effects include activation of calcium mobilization in cancer cells10, LHRH neurons11 and hypothalamic neurons12, spinal neuron depolarization13, protein kinase Cε activation14 and phosphatidyl inositol-3-kinase (PI3K) activation10, gene expression15,16, proliferation15,17, oocyte meitotic arrest18 and primordial follicle formation19. G-1 has also been used to probe the role of GPR30 in vivo with reported effects including estrogen-induced thymic atrophy20, experimental autoimmune encephalomyelitis21 and vascular regulation22. In each of these animal models, the G-1-mediated effects were absent in GPR30 knockout mice, establishing the selectivity of this compound for GPR30. Thus, the availability of a selective GPR30 agonist has, in a very brief time, greatly advanced our understanding of the biological functions of GPR30.
Unfortunately, to date, antagonists of GPR30 have not been identified. To better understand the actions of GPR30, we identified a selective GPR30 antagonist using a combination of virtual and biomolecular screening. The compound is related in structure to the agonist G-1 and binds to GPR30 but not ERα or ERβ. Cellular assays demonstrate that this antagonist prevents both estrogen- and G-1-mediated mobilization of intracellular calcium in ER-negative breast cancer cells. Furthermore, estrogen-mediated GPR30-dependent PI3K activation is blocked, whereas no effect on either ERα- or ERβ-mediated PI3K activation in response to estrogen is observed. In vivo studies utilizing both the agonist and antagonist reveal that GPR30 contributes to estrogen-mediated proliferation of the uterine epithelium and plays an important role in the anti-depressive effects of estrogen. The introduction of this first GPR30-selective antagonist should provide additional avenues for characterizing the physiological functions of GPR30.
RESULTS
Virtual & biomolecular screening and chemical synthesis
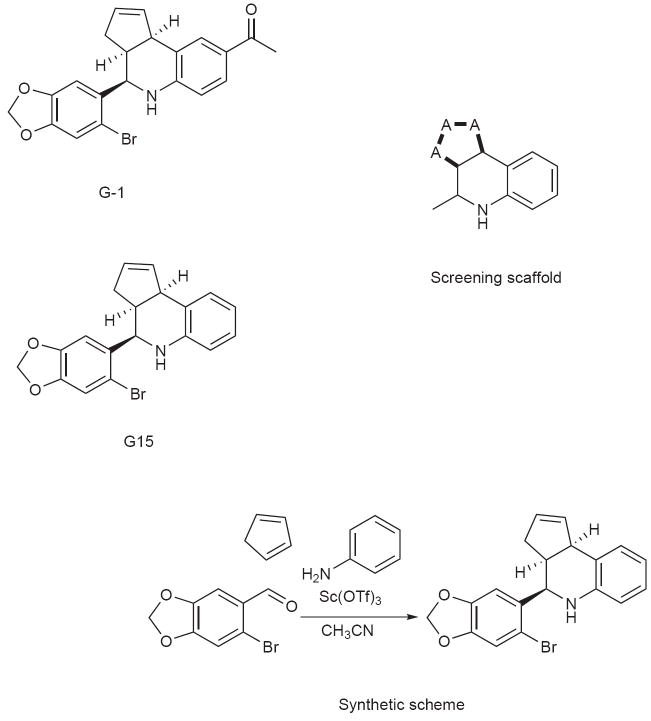
We recently employed a combination of virtual and biomolecular screening to identify the first GPR30-specific ligand, a substituted dihydroquinoline, named G-110 (Fig. 1a). To identify potentially novel GPR30-specific ligands, we again employed virtual screening to identify G-1-like structures of interest from the NIH Molecular Libraries Small Molecule Repository (MLSMR). We performed a SMARTS substructure search (Daylight Theory Manual, Daylight Chemical Informations Systems Inc., http://www.daylight.com/dayhtml/doc/theory/theory.smarts.html) of the MLSMR (consisting of 144,457 molecules at the time of the search, March 2007) for compounds containing the core scaffold of G-1 (Fig. 1b) using a custom JAVA program built using the OpenEye OEJava toolkit (OEChem - Java Theory Manual, OpenEye Scientific Software Inc., http://www.eyesopen.com/docs/html/javaprog/). The search identified 64 molecules, of which 57 were obtained from the MLSMR.
Figure 1.
Structure of G-1 (a), the substructure utilized for virtual screening of the MLSMR (where “A” means any atom (except hydrogen) and thick black lines mean any type of bond) (b) and G15 (c).
To accomplish the primary biomolecular screen for GPR30 antagonism, we utilized calcium mobilization in GPR30-expressing SKBr3 cells and tested for the ability of the compound to block cellular activation by estrogen. Primary screening of the 57 G-scaffold containing compounds from the MLSMR yielded 8 that showed some inhibition of estrogen-mediated calcium mobilization in cells expressing GPR30. Of particular interest was one compound (designated G15) that closely resembled G-1 but lacked the ethanone moiety of the molecule (Fig. 1c). Based on the structural overlap of G-1 with estrogen and in particular the similar, though not identical spacing of oxygen atoms at the extremes of the molecules, we speculated that the ketone functionality of G-1 might play an important role as a hydrogen bond acceptor by inducing conformational changes that activate GPR30. For this reason, in parallel to the virtual screening efforts, G15 was also chemically synthesized as a possible antagonist candidate.
The tetrahydro-3H-cyclopenta[c]quinoline scaffold of G-1 is synthetically accessible via the versatile three-component Povarov cyclization. We evaluated a series of reaction conditions employing protic and Lewis acid catalysts to optimize reaction rate, yield, and diastereoselectivity for the construction of G-1-like derivatives. Our optimized one-step procedure employing the catalyst Sc(OTf)3 in acetonitrile23 resulted in rapid reaction times, high product yield and enhanced selectivity favoring the syn diastereomeric products. The synthesis of G15 from aniline, 6-bromopiperonal and cyclopentadiene is illustrated in Fig. 1d. Precipitation from dichloromethane/methanol gave analytically pure G15 in yields exceeding 85% as a racemic mixture of syn diastereomers. The syn diastereomer is distinguished by the 1H-NMR coupling pattern of H-4 (4.65 ppm) with a coupling constant of 3.25 Hz that is characteristic for the syn orientation of the cyclopentene ring and phenyl group. The product was fully characterized by NMR spectroscopy, and HPLC-MS with positive ion detection (showing the correct molecular ion (MH+)) as well as UV detection (PDA/λmax= 294 nm), yielding a single peak.
G15 inhibits cellular signaling through GPR30
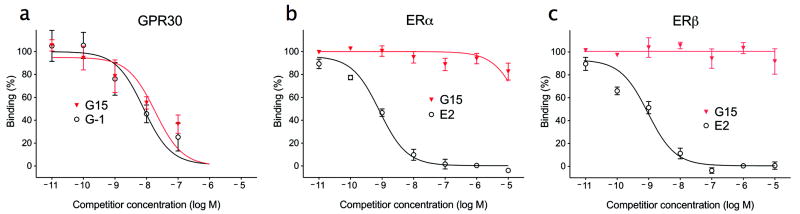
Chemically synthesized G15 was subjected to multiple cellular and physiological assays in order to characterize its biological effects. Competitive binding assays using endogenous GPR30 and a novel iodinated GPR30-selective G-1 analog (manuscript in preparation), demonstrated that G15 binds to GPR30 with an affinity of approximately 20 nM (Fig. 2a). This compares to an affinity for G-1, utilizing the same assay, of approximately 7 nM, similar to our previously reported affinity of G-1 for recombinant GPR30 of 11 nM 10 and reported affinities for 17β-estradiol between 3-6 nM 6,7. Thus removal of the ethanone moiety resulted in a decrease in relative binding affinity of approximately 3 fold. Additional competitive binding studies to assess interactions with ERα and ERβ revealed that similar to G-1, G15 displays little binding to ERα or ERβ at concentrations up to 10 μM, where estrogen competes with a Ki of approximately 0.3-0.5 nM (Figs. 2b and c). These results reveal that G15, like G-1, displays high affinity for GPR30 with minimal binding to ERα and ERβ (Ki > 10 μM).
Figure 2.
Ligand binding properties of G15. Ligand binding affinities of 17β-estradiol, G-1 and G15 for GPR30, ERα and ERβ. For GPR30 (a), Hec50 cells, which endogenously express GPR30 but neither ERα nor ERβ, were incubated with trace quantities of an iodinated G-1 derivative and the indicated concentration of either G-1 (μ) or G15 (τ) as competitor. For ERα and ERβ, COS7 cells were transfected with either ERα-GFP (b) or ERβ-GFP (c). For the latter, competitive ligand binding assays were performed using 10 nM E2-Alexa633 and the indicated concentration of either 17β-estradiol (μ) or G15 (τ). Data indicate the mean ± s.e.m. of at least three separate experiments.
Evaluation of the functional capabilities of G15 with respect to the rapid mobilization of intracellular calcium demonstrated that G15 alone was incapable of inducing a response in SKBr3 breast cancer cells, which are ERα and ERβ negative but express GPR30, whereas stimulation by either estrogen or G-1 induced a response (Fig. 3a). In contrast, stimulation of the cells with G-1 or estrogen subsequent to G15 exposure substantially reduced the response to G-1 or estrogen (Fig. 3a). There was however no inhibition of the calcium response mediated by ATP through endogenous purinergic receptors, indicating the antagonistic effect is specific to GPR30. Inhibition of G-1-mediated calcium mobilization in SKBr3 cells by G15 was dose-dependent, yielding an IC50 of approximately 185 nM (Fig. 3b), whereas inhibition of E2-mediated calcium mobilization yielded a similar IC50 of approximately 190 nM (Fig. 3c).
Figure 3.
G15 antagonism of intracellular calcium mobilization by GPR30. (a) The effect of G15 on the subsequent mobilization of calcium by G1, E2 or ATP was evaluated using indo1-AM-loaded SKBr3 cells. G15 (1 μM, red line) or vehicle (ethanol, black line) was added at 20 sec. (first arrow). G-1 (200 nM), 17β-estradiol (E2, 100 nM) or ATP (1μM, a purinergic receptor control) was added at 80 sec. (second arrow). (b) Dose response profile of G-1-stimulated SKBr3 cells to increasing concentrations of G15. (c) Dose response profile of 17β-estradiol-stimulated SKBr3 cells to increasing concentrations of G15. In panels b and c, G-1 and 17β-estradiol were used at 100 nM and 30 nM, respectively, concentrations that yield approximately the half-maximal calcium response for each ligand (approximately 25% that of the full ATP response). Data in panel a are representative of at least three independent experiments. Data in panels b and c represent the mean ± s.e.m. from at least three separate experiments.
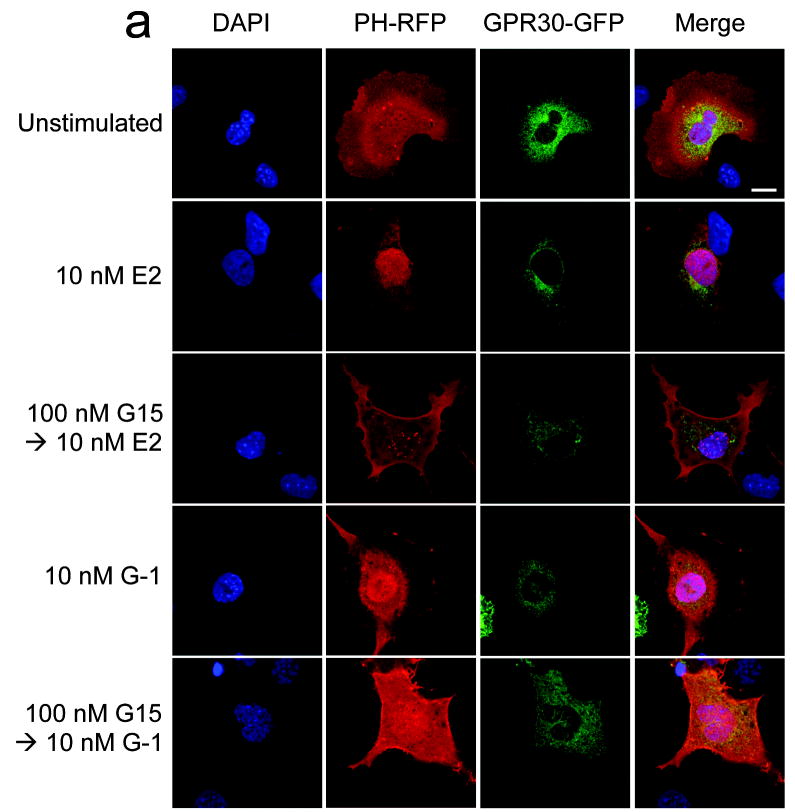
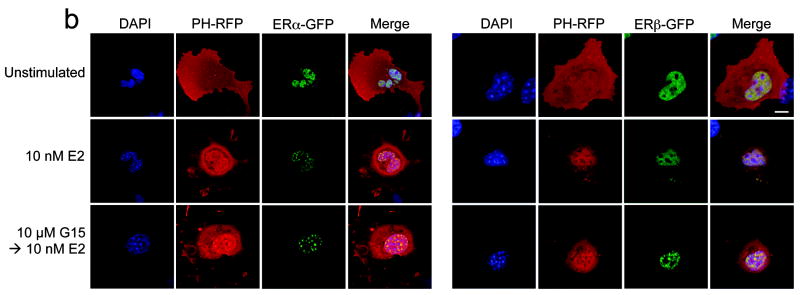
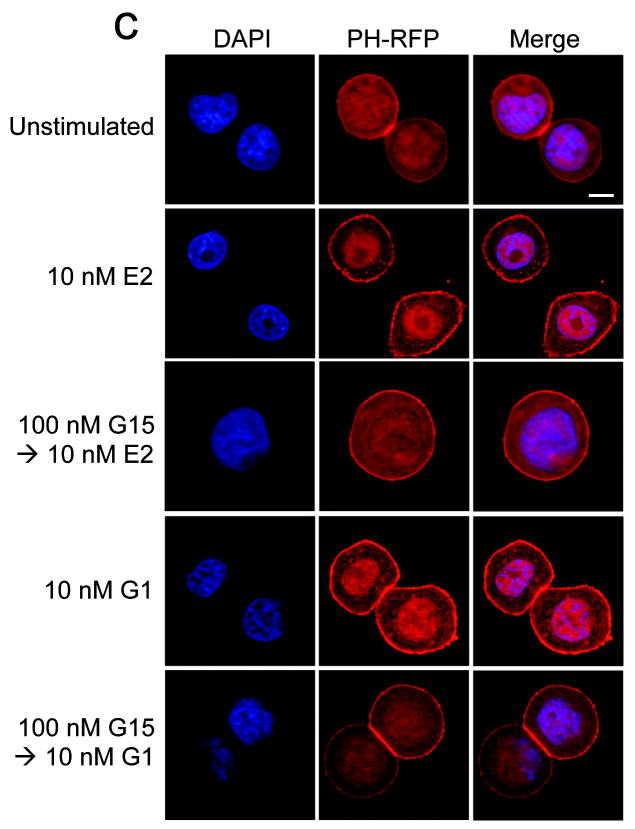
In addition to intracellular calcium mobilization, we have demonstrated that estrogen stimulation of ERα, ERβ or GPR30 results in the nuclear accumulation of PIP3 as a result of PI3K activation6, revealed by the translocation of an Akt PH domain-fluorescent protein fusion protein reporter24. To determine whether G15 similarly inhibits GPR30-mediated PI3K activation, we examined the activation of PI3K in receptor-transfected COS7 cells, where estrogen stimulates the nuclear accumulation of PIP3 through all three receptors and G-1 selectively activates GPR30 but not ERα or ERβ. Not only was G15 capable of inhibiting the G-1-mediated activation of PI3K in GPR30-transfected cells, it also effectively blocked the estrogen-mediated response in GPR30-transfected cells (Fig. 4a) but had no effect on the estrogen-mediated response in ERα or ERβ-transfected cells, even at concentrations 100-fold greater than that required to inhibit GPR30 (Fig. 4b). To determine whether G15 inhibits PI3K activation in cells endogenously expressing GPR30, we examined PI3K activation in SKBr3 breast cancer cells. As in GPR30-transfected cells, G15 was able to inhibit both estrogen and G-1 stimulation of PI3K (Fig. 4c). In total, these results demonstrate that G15 can selectively inhibit GPR30.
Figure 4.
G15 antagonism of PI3K activation by GPR30. The activity of G15 was evaluated using COS7 cells transfected with Akt-PH-mRFP1 and either GPR30-GFP (a), ERα-GFP or ERβ-GFP (b) or SKBr3 cells transfected with Akt-PH-mRFP1 (c). 17β-estradiol, G-1 and G15 were used at the indicated concentrations. The white bar in upper panel of (a-c) denotes 10 μm for all images. Data are representative of three independent experiments.
G15 inhibits GPR30-mediated function in vivo
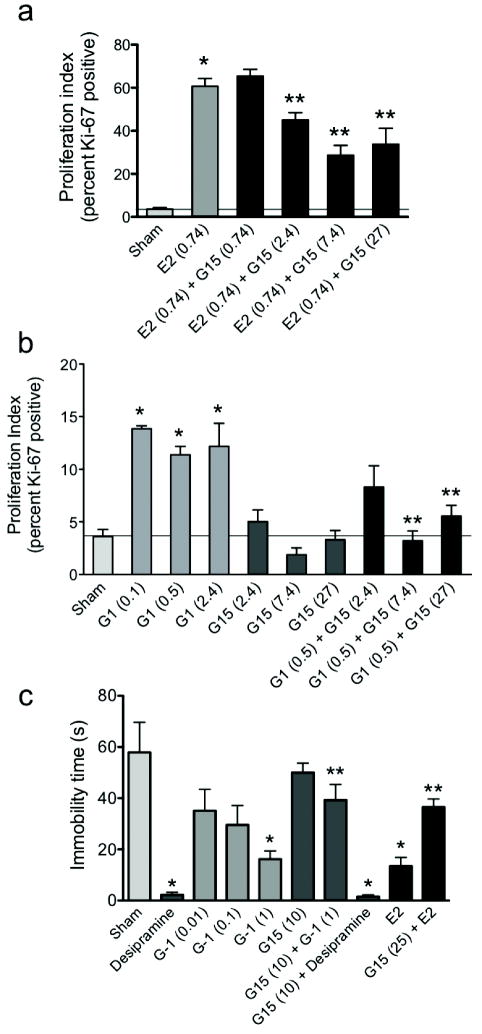
One goal of developing GPR30-specific agonists and antagonists is the elucidation of the roles of GPR30 in normal and disease physiology. One of the best characterized assays for estrogenic activity is the uterine response in the mouse, where uterine water content (i.e. imbibition) and epithelial cell proliferation are highly responsive to estrogen treatment, particularly following ovariectomy25. In the ovariectomized mouse model, a single injection of estrogen (E2) led to a 17-fold increase in the proliferative index of uterine epithelia relative to control, as measured by immunodetection of Ki-67 protein (Fig. 5a). Here we show that the GPR30 agonist G-1 also increases proliferation, by 3-4 fold over sham (Fig. 5b), and that there is little difference in proliferation rates across a 25-fold dose range, suggesting a maximal response was achieved. In contrast, treatment with the GPR30 antagonist G15 alone did not alter proliferation relative to sham injections (Fig. 5b). When mice were treated with G15 plus E2 (Fig. 5a), proliferation was reduced by approximately 50% in a dose-dependent manner, being maximal at a 10-fold molar excess of G15. G15 treatment also blocked G-1-induced proliferation in a dose-dependent manner (Fig. 5b), being maximal at a 15-fold molar excess of G15. These results suggest that GPR30 contributes to a specific estrogenic response, proliferation. Neither G-1 nor G15 had any effect on uterine wet weight or imbibition, evaluated by measuring uterine weight and by microscopic evaluation of histologic sections (not shown). In conclusion, GPR30 appears to contribute to the proliferative response in the uterus, while another response, imbibition, appears to be solely mediated by ERα26.
Figure 5.
Effects of G15 on physiological responses mediated by GPR30. Epithelial uterine cell proliferation was assessed in the presence of E2 or E2 + G15 (a) or in the presence of G-1, G15, or G-1 + G15 (b) in ovariectomized female C57Bl6 mice. Compounds (amount in parentheses indicates nmol/mouse). Proliferation of uterine epithelium was quantitated by immunofluorescence using anti-Ki-67 antibody. (c) Immobility in adult male ICR mice was assessed as an indicator of depression. Mice were suspended from the tip of the tail and the total amount of time each animal remained immobile during a 6-min period was recorded. Compounds (amount in parentheses indicates nmol/mouse; desipramine and soluble E2 were used at 10mg/kg and 5 mg/kg respectively) were administered intraperitoneally. Each group consisted of 10-12 animals. For all panels, results are expressed as mean ± s.e.m., and statistical significance (P<0.05) was assessed by student’s t test: *, significantly different than sham; **, significantly different than E2 or G-1, respectively.
Clinical observations suggest that vulnerability to depression in the female population is associated with hormonal fluctuations, in which estrogens may play an important role. For example, chronic treatment of women with E2 or conjugated equine estrogens attenuated depressive symptoms during peri-menopausal and postpartum periods27,28. Several animal models have been developed to evaluate putative anti-depressants29,30 and have demonstrated the anti-depressive effects of estrogenic compounds31. Among these, the tail suspension test32,33 is a convenient model in which many antidepressants reduce the duration of immobility, suggesting this parameter is an index of antidepressant activity34. Since GPR30 expression has been demonstrated in the male (as well as female) brain12, male mice were used to evaluate the potential neurological effects of G-1 and G15, given that behavioral and neurochemical depression studies are carried out almost exclusively in male mice34. The antidepressant action of G-1 was compared to that of E2 and the tricyclic antidepressant desipramine, which markedly reduced immobility time, when compared to control vehicle-injected animals (Fig. 5c). G-1 dose-dependently decreased immobility time, whereas pretreatment of the mice with G15, which alone had no significant effect on immobility time, significantly attenuated the effects of both G-1 and E2. Pretreatment of the mice with G15 did not influence the immobility time of subsequent desipramine treatment. Together, these results suggest a neurological role for GPR30 in the regulation of depression.
DISCUSSION
In this paper, we described the synthesis and characterization of the first GPR30 antagonist, G15. Binding studies demonstrated that G15 exhibited only moderately reduced binding to GPR30 (about 3-fold) compared to G-1, and yet no significant binding to either ERα or ERβ at concentrations as high as 1-10 μM. Functional assays revealed that G15 blocked both estrogen- and G-1-mediated mobilization of intracellular calcium in ER-negative SKBr3 breast cancer cells. In addition, GPR30-dependent PI3K activation by either estrogen or G-1 was blocked by prior incubation with G15. However, G15 was unable to prevent estrogen-mediated PI3K activation through either ERα or ERβ. In vivo studies demonstrated that G15 completely blocked uterine epithelial cell proliferation mediated by GPR30 in response to G-1 but only partially inhibited the estrogen-mediated response (presumably occurring through activation of all estrogen receptors). Finally, we established that the anti-depressive effects of estrogen appear to be mediated through GPR30, in that G-1 recapitulated the effects of estrogen and that G15 inhibited the anti-depressive effects of both G-1 and estrogen.
The selectivity of G15 towards GPR30 in cellular assays is consistent with the selectivity of G-1 for GPR30 in cells expressing both GPR30 and classical estrogen receptors as well as the stimulatory effects of estrogen in cells expressing only GPR30. Given the similarity in structure between G15 and G-1, with the difference being the lack of an ethanone moiety in G15, we suggest that G-1 activates GPR30 in a similar manner to the way in which estrogen activates classical estrogen receptors and presumably GPR30. Crystal structures of estrogen-bound ERα reveal extensive hydrogen bonding networks between the hydroxyl groups of the estrogen and receptor hydrogen bond donors and acceptors35. Assuming that G-1 activates GPR30 through similar networks of hydrogen bonds at the distal ends of the molecule, removal of one hydrogen bond acceptor could allow binding to occur without the agonist-induced receptor conformational changes required for activation.
Although estrogen mediates effects on most physiological systems, including the nervous, immune and vascular systems, its most appreciated role is in reproduction. In the uterus, a variety of cellular and molecular responses, including imbibition, proliferation, and induction of gene expression, are mediated by estrogen. Whereas ERα plays a major role in these responses26, ERβ appears to play no role in imbitition, although it plays a role in the suppression of uterine epithelial proliferation36. Here, we have demonstrated that the GPR30-specific antagonist G15 is capable of partially inhibiting estrogen-dependent uterine epithelial proliferation, but not imbibition (wet weight increase), suggesting that GPR30, in addition to ERα, plays a role in promoting uterine epithelial proliferation. Our results are in contrast to a recent paper that reported no effect of G-1 on proliferation in the uterus37; however in that report, G-1 effects on uterine epithelial proliferation were not quantitated. It is possible that a 3-fold difference in proliferation was not detected by visual inspection alone, particularly when compared to the massive response to estrogen. In contrast to the restricted role of GPR30 in uterine responses to estrogen, GPR30 appears to contribute in a significant way to the anti-depressive effects of estrogen with G-1 fully recapitulating the estrogen-mediated effects, and G15 equally inhibiting both estrogen- and G-1-mediated responses.
Although estrogen mediates the full range of uterine responses, including proliferation, imbibition, immune responses and gene expression, other estrogenic compounds have been observed to regulate these responses differentially. For example, DES (6) is weaker than estrogen in inducing uterine eosinophilia, imbibition and proliferation, equal to estrogen in mediating epithelial hypertrophy and stronger than estrogen in inducing the reduction of epithelial cell height and myometrial cell hypertrophy38. In addition, genistein (7) has only a limited ability to induce proliferation whereas estrogen-regulated genes are fully induced39. Finally, the ERα-selective compound PPT (8) is less effective in stimulating imbibition and the expression of complement component 3 and glucose-6-phosphate dehydrogenase but is as effective as estrogen in regulating lactoferrin, androgen receptor, and progesterone receptor expression40. Since genistein has been shown to bind and activate GPR3041, it is unclear whether the varying effects of genistein and other compounds on distinct aspects of uterine physiology are due to either differential activation of classical estrogen receptor(s) or complex combinatorial effects on multiple estrogen receptors, including GPR30.
In conclusion, we report the identification and preliminary characterization of the first selective GPR30 antagonist. The discovery of this high affinity GPR30-selective antagonist that does not bind significantly to classical nuclear estrogen receptors has yielded novel insights into the physiological roles of GPR30 in the reproductive and nervous systems. Future studies utilizing GPR30-selective agonists and antagonists will further define the role of GPR30 in vivo and open the door to the generation of diagnostics and therapeutics directed at individual estrogen receptors.
METHODS
Chemical synthesis and characterization of G15
The compound G15 (4-(6-Bromo-benzo[1,3]dioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline) was synthesized using an optimized one-step procedure similar to Kobayashi et. al.23 A catalytic amount of Sc(OTf)3 (0.049 g, 0.1mmol, 10 mol%) in anhydrous acetonitrile (1 mL) was added to a mixture of 6-bromopiperanal (0.229 g, 1.00 mmol), aniline (0.093 g, 1.0 mmol) and freshly distilled cyclopentadiene (0.33 g, 5.0 mmol) in acetonitrile (3 mL). The reaction was stirred at ambient temperature (~23 °C) for 3 h with monitoring of product formation by thin layer chromatography using 80% hexane/ethyl acetate eluent (Rf 0.6). The volatiles were removed in vacuo. The residue was dissolved in methylene chloride (10 mL) and then precipitated by drop-wise addition of methanol (5 mL), and the product was isolated by filtration and washed with additional methanol to give the product as a colorless solid (321mg, 87%): mp 178-180° C.
Spectroscopic characterization of G15 yielded the following: 1H NMR (300 MHz, DMSO-d6): δ 7.23 (s, 1H), 7.13 (s, 1H), 6.97 (d, J = 7.9 Hz, 1H), 6.87 (ddd, J = 7.3, 7.3, 1.3 Hz, 1H), 6.68 (dd, J = 7.9, 1.3 Hz, 1H), 6.60 (ddd, J = 7.3, 7.3, 1.3 Hz, 1H), 6.1 (d, J = 0.9 Hz, 1H), 6.06 (d, J = 0.9 Hz, 1H), 5.88-5.82 (m, 1H), 5.60-5.53 (m, 2H), 4.65 (d, J = 3.2 Hz, 1H), 3.98 (d, J = 9.8 Hz, 1H), 3.07-2.95 (m, 1H), 2.48-2.38 (m, 1H), 1.69-1.59 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 147.5, 147.2, 145.3, 134.6, 134.0, 130.3, 129.0, 126.3, 126.2, 119.4, 116.1, 113.0, 112.9, 108.1, 101.7, 56.7, 46.1, 42.2, 31.4. FT-IR (KBr, cm-1): 3338(w), 2887(w), 1605(w), 1587(s), 1473(s). HRMS (m/z): calcd. C19H16BrNO2 requires MH+, 370.0443, found MH+, 370.0435. Anal (C19H15BrNO2) C, H, N.
G15 was analyzed by standard LC-MS methods with electrospray positive ion detection. The LC-MS chromatogram showed the correct molecular (MH+) ion as well as a single peak with UV-Vis λmax 294 nm. Only one diastereomer was obtained; examination of the 1H-NMR data shows H-4 (4.65 ppm) with a coupling constant of 3.2 Hz, indicating a cis orientation of the cyclopentene and phenyl group, in agreement with the all-cis stereochemistry of similar reaction products of cyclopentadiene established previously. Thus G15 is a racemic but diastereomerically pure compound.
Ligand binding assays
Binding assays for ERα and ERβ were performed as previously described6. Briefly, COS7 cells were transiently transfected with either ERα-GFP or ERβ-GFP). Following serum starvation for 24 h, cells (~5×104) were incubated with G-15 for 20 min. in a final volume of 10 μL prior to addition of 10 μL 20 nM E2-Alexa633 in saponin-based permeabilization buffer. Following 10 min at RT, cells were washed once with 200 μL PBS/2%BSA, resuspended in 20 μL and 2 μL samples were analyzed on a DAKO Cyan flow cytometers using HyperCyt™as described42. For GPR30 binding, a radioiodinated derivative of G-1, 1-{2-[4-(6-Bromo-benzo[1,3]dioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethyl}-3-(3-iodo-phenyl)-urea, was used (see Supplementary Information, manuscript in preparation). Briefly, Hec50 cells were cultured in phenol-red free DMEM/F-12 containing 10% charcoal-stripped FBS, plated in 24-well tissue culture plates and grown to 80% confluence. Wells were rinsed with PBS and cells were incubated with competitor (G-1 or G15) for 30 minutes prior to addition of approximately 0.5-1 μCi of radioligand. The 125I radiolabeled ligand was prepared from the corresponding tributylstannane using Iodo-gen beads (Pierce) following the manufacturer’s recommended protocol. Complete details of the synthesis and radiolabeling will be described elsewhere (manuscript in preparation). Wells were incubated at 37°C for 1 hour, rinsed with PBS and radioactivity collected by ethanol extraction and counted in a Wallac Wizard 1480 gamma counter (Perkin Elmer, Gaithersburg, MD).
Intracellular calcium mobilization
SKBr3 cells (1 × 107) were incubated in HBSS containing 3 μM indo1-AM (Invitrogen) and 0.05% pluronic acid for 1 h at RT. Cells were then washed twice with HBSS, incubated at RT for 20 min, washed again with HBSS, resuspended in HBSS at a density of 108 cells/mL and kept on ice until assay, performed at a density of 2 × 106 cells/mL. Ca++ mobilization was determined ratiometrically using λex 340 nm and λem 400/490 nm at 37°C in a spectrofluorometer (QM-2000-2, Photon Technology International) equipped with a magnetic stirrer. The relative 490nm/400nm ratio was plotted as a function of time.
PI3K activation
The PIP3 binding domain of Akt fused to mRFP1 (PH-mRFP1) was used to localize cellular PIP3. COS7 cells (cotransfected with GPR30-GFP or ERα/β-GFP and PH-mRFP1) or SKBr3 (transfected with PH-mRFP1) cells were plated on coverslips and serum starved for 24 h followed by stimulation with ligands as indicated. The cells were fixed with 2% PFA in PBS, washed, mounted in Vectashield and analyzed by confocal microscopy using a Zeiss LSM510 confocal fluorescent microscope.
Mouse uterine estrogenicity assay
C57Bl6 female mice (Harlan) were ovariectomized at 10 weeks of age. E2, G-1, and G15 were dissolved in absolute ethanol at 1 mg/mL (E2 and G-1 were diluted to 10 μg/mL in ethanol, G15 was diluted to 50 μg/mL in ethanol). For treatment with all three compounds, 10 μL was added to 90 μL aqueous vehicle (0.9% NaCl with 0.1% albumin and 0.1% Tween-20). Ethanol alone (10 μL) was added to 90 μL aqueous vehicle as control (sham). At 12 days post-ovariectomy, mice were injected subcutaneously at 5:00 pm with 100 μL consisting of 1) sham; 2) 200 ng E2 (0.74 nmol); 3) 40, 200, or 1000 ng G-1 (0.1, 0.5, or 2.4 nmol, respectively); 4) 272, 900, 2725, or 10000 ng G15 (2.4, 7.4, or 27 nmol, respectively) or 5) G15 combined with E2 or G-1 (at the same concentrations as used individually: G-1 was used at 200 ng (0.5 nmol) in all G-1 + G15 combination experiments). The doses of G15 were chosen to represent an approximately 1:1, 1:3.3, 1:10, and 1:35-fold molar excess relative to E2. Eighteen hr after injection, mice were sacrificed and uteri were dissected, fixed in 4% paraformaldehyde, and embedded in paraffin. Five-micron sections were placed on slides, and proliferation in uterine epithelia was quantitated by immunofluorescence using anti-Ki-67 antibody (LabVision) followed by goat anti-mouse IgG conjugated to Alexa488 (Invitrogen). Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI). At least 4 animals per treatment were analyzed, and the Ki-67 immunodetection was repeated three times per mouse.
Mouse Depression assay
Adult male ICR mice, weighing 20-25 g, were used. Animals were maintained at room temperature, with free access to tap water and standard diet, under a 10:14 light/dark cycle (lights on 8:00h) and were housed 5/cage. Mice were acclimated to the laboratory for at least one hour before testing and used only once. All experiments were conducted during the light phase, between 8:30 and 14:30 h. Procedures used in this study were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. The procedure was similar to that described by Steru et al. 33. Mice were isolated and suspended 35 cm above the floor by an adhesive tape placed 1 cm from the tip of the tail. The mouse was 15 cm away from the nearest object. The total amount of time each animal remained immobile (mice were considered immobile only when they hung passively and completely motionless) during a 6-min period was recorded (in seconds) as immobility time. Each animal received two successive injections (0.1 mL/mouse) in order to eliminate any possible bias comparing single-compound treatments to dual-compound treatments. G1 and G15 were first dissolved in DMSO and diluted with saline; the final concentration in DMSO was 1 mM. Desipramine and E2 (cyclodextrin-encapsulated, 4-5.5% E2) were dissolved in saline solution and DMSO was added to a final concentration of 1 mM. An appropriate vehicle-treated group (saline with 1 mM DMSO) was included as a control (sham). All solutions were freshly prepared before each experimental series. Independent groups of mice (n=12-16) were treated with two consecutive intraperitoneal injections as follows: vehicle solution + vehicle solution (sham group); vehicle + G-1 (indicated amount in nmol); vehicle + desipramine (10mg/kg); G15 (10nmol/mouse) + desipramine (10mg/kg); G15 (10nmol/mouse) + G-1 (1nmol/mouse); vehicle + G15 (10nmol/mouse); vehicle + soluble E2 (5 mg/kg); G15 (25nmol/mouse) + soluble E2 (5 mg/kg). The second compound was injected 15 min (7 min for E2) after the first injection and the tail suspension test performed 30 min after the second injection.
Supplementary Material
Acknowledgments
This work was supported by NIH grants CA118743 and CA127731, and grants from the Oxnard and Stranahan Foundations to ERP, by the New Mexico Molecular Libraries Screening Center (NIH MH074425) to LAS, the New Mexico Tobacco Settlement fund to TIO, the New Mexico Cowboys for Cancer Research to JBA and NIH grants R37 NS18710 to NJD and HL90804 to EB. Flow cytometry data and confocal images in this study were generated in the Flow Cytometry and Fluorescence Microscopy Facilities, which received support from the University of New Mexico Health Sciences Center and the University of New Mexico Cancer Center as detailed: http://hsc.unm.edu/crtc/microscopy/Facility.html. In vivo data were generated with the support by the UNM Cancer Center Animal Models & Imaging Core.
References
- 1.Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–376. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- 2.Lange CA, Gioeli D, Hammes SR, Marker PC. Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Annu Rev Physiol. 2007;69:171–199. doi: 10.1146/annurev.physiol.69.031905.160319. [DOI] [PubMed] [Google Scholar]
- 3.Fu XD, Simoncini T. Extra-nuclear signaling of estrogen receptors. IUBMB Life. 2008;60:502–510. doi: 10.1002/iub.80. [DOI] [PubMed] [Google Scholar]
- 4.Prossnitz ER, et al. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 5.Prossnitz ER, Sklar LA, Oprea TI, Arterburn JB. GPR30: a novel therapeutic target in estrogen-related disease. Trends Pharmacol Sci. 2008;29:116–123. doi: 10.1016/j.tips.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 7.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G-protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 8.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 9.Ariazi EA, Ariazi JL, Cordera F, Jordan VC. Estrogen receptors as therapeutic targets in breast cancer. Curr Top Med Chem. 2006;6:181–202. [PubMed] [Google Scholar]
- 10.Bologa CG, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 11.Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G-Protein Coupled Receptor 30 (GPR30) in Rapid Action of Estrogen in Primate LHRH Neurons. Mol Endocrinol. 2009 doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brailoiu E, et al. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 13.Dun SL, et al. Expression of estrogen receptor GPR30 in the rat spinal cord and in autonomic and sensory ganglia. J Neurosci Res. 2009 doi: 10.1002/jnr.21980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn J, et al. GPR30 estrogen receptor agonists induce mechanical hyperalgesia in the rat. Eur J Neurosci. 2008;27:1700–1709. doi: 10.1111/j.1460-9568.2008.06131.x. [DOI] [PubMed] [Google Scholar]
- 15.Albanito L, et al. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–1866. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- 16.Prakash Pandey D, et al. Estrogenic GPR30 signalling induces proliferation and migration of breast cancer cells through CTGF. EMBO J. 2009 doi: 10.1038/emboj.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teng J, Wang ZY, Prossnitz ER, Bjorling DE. The G protein-coupled receptor GPR30 inhibits human urothelial cell proliferation. Endocrinology. 2008;149:4024–4034. doi: 10.1210/en.2007-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang Y, Dong J, Thomas P. Estrogen signaling characteristics of Atlantic croaker G protein-coupled receptor 30 (GPR30) and evidence it is involved in maintenance of oocyte meiotic arrest. Endocrinology. 2008;149:3410–3426. doi: 10.1210/en.2007-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Prossnitz ER, Roy SK. G protein-coupled receptor 30 expression is required for estrogen stimulation of primordial follicle formation in the hamster ovary. Endocrinology. 2008;149:4452–4461. doi: 10.1210/en.2008-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, et al. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 2008;22:636–648. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C, et al. Membrane estrogen receptor regulates experimental autoimmune encephalomyelitis through up-regulation of programmed death 1. J Immunol. 2009;182:3294–3303. doi: 10.4049/jimmunol.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas E, et al. Regulatory Role of G Protein-Coupled Estrogen Receptor for Vascular Function and Obesity. Circ Res. 2009 doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi S, Ishitani H, Nagayama S. Lanthanide triflate catalyzed imino Diels-Alder reactions; convenient synthesis of pyridine and quinoline derivatives. Synthesis. 1995:1195–1202. [Google Scholar]
- 24.Balla T, Varnai P. Visualizing cellular phosphoinositide pools with GFP-fused protein-modules. Sci STKE. 2002;2002:PL3. doi: 10.1126/stke.2002.125.pl3. [DOI] [PubMed] [Google Scholar]
- 25.Owens JW, Ashby J. Critical review and evaluation of the uterotrophic bioassay for the identification of possible estrogen agonists and antagonists: in support of the validation of the OECD uterotrophic protocols for the laboratory rodent. Organisation for Economic Co-operation and Development. Crit Rev Toxicol. 2002;32:445–520. doi: 10.1080/20024091064291. [DOI] [PubMed] [Google Scholar]
- 26.Hewitt SC, Harrell JC, Korach KS. Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol. 2005;67:285–308. doi: 10.1146/annurev.physiol.67.040403.115914. [DOI] [PubMed] [Google Scholar]
- 27.Epperson CN, Wisner KL, Yamamoto B. Gonadal steroids in the treatment of mood disorders. Psychosom Med. 1999;61:676–697. doi: 10.1097/00006842-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Genazzani AR, Spinetti A, Gallo R, Bernardi F. Menopause and the central nervous system: intervention options. Maturitas. 1999;31:103–110. doi: 10.1016/s0378-5122(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 29.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 30.Willner P. Animal models of depression: an overview. Pharmacol Ther. 1990;45:425–455. doi: 10.1016/0163-7258(90)90076-e. [DOI] [PubMed] [Google Scholar]
- 31.Estrada-Camarena E, Fernandez-Guasti A, Lopez-Rubalcava C. Antidepressant-like effect of different estrogenic compounds in the forced swimming test. Neuropsychopharmacology. 2003;28:830–838. doi: 10.1038/sj.npp.1300097. [DOI] [PubMed] [Google Scholar]
- 32.Steru L, et al. The automated Tail Suspension Test: a computerized device which differentiates psychotropic drugs. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11:659–671. doi: 10.1016/0278-5846(87)90002-9. [DOI] [PubMed] [Google Scholar]
- 33.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 34.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Tanenbaum DM, Wang Y, Williams SP, Sigler PB. Crystallographic comparison of the estrogen and progesterone receptor’s ligand binding domains. Proc Natl Acad Sci U S A. 1998;95:5998–6003. doi: 10.1073/pnas.95.11.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wada-Hiraike O, et al. Role of estrogen receptor beta in uterine stroma and epithelium: Insights from estrogen receptor beta-/- mice. Proc Natl Acad Sci U S A. 2006;103:18350–18355. doi: 10.1073/pnas.0608861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otto C, et al. GPR30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008 doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 38.Grunert G, Porcia M, Tchernitchin AN. Differential potency of oestradiol-17 beta and diethylstilboestrol on separate groups of responses in the rat uterus. J Endocrinol. 1986;110:103–114. doi: 10.1677/joe.0.1100103. [DOI] [PubMed] [Google Scholar]
- 39.Diel P, et al. The differential ability of the phytoestrogen genistein and of estradiol to induce uterine weight and proliferation in the rat is associated with a substance specific modulation of uterine gene expression. Mol Cell Endocrinol. 2004;221:21–32. doi: 10.1016/j.mce.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Frasor J, et al. Response-specific and ligand dose-dependent modulation of estrogen receptor (ER) alpha activity by ERbeta in the uterus. Endocrinology. 2003;144:3159–3166. doi: 10.1210/en.2002-0143. [DOI] [PubMed] [Google Scholar]
- 41.Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Ramirez S, Aiken CT, Andrzejewski B, Sklar LA, Edwards BS. High-throughput flow cytometry: validation in microvolume bioassays. Cytometry A. 2003;53:55–65. doi: 10.1002/cyto.a.10035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.