Abstract
The rat dorsolateral striatum (DLS) has been implicated in habit formation. Previous studies in our laboratory found that as animals acquired a motor habit or remained goal-directed, tested by reward devaluation, the vast majority of DLS neurons decreased firing rates during the same responses over training days. However, mixed results have been reported in the literature regarding whether DLS neurons exhibit cue-reactivity. In the present study, we reanalyzed a sample of DLS head movement neurons in a task in which habitual behavior was acquired (dataset of Tang et al, 2007) and found that somatic sensorimotor as well as nonsomatomotor neurons of the DLS exhibited no cue-evoked firing. A second sample of DLS neurons related to licking in a task in which goal-directed behavior occurred (dataset of Tang et al, 2009) was also reanalyzed for cue-evoked correlates. Although behavior was cue-guided, lick neurons did not exhibit cue-evoked firing. Given the complete absence of cue-related firing during habitual or goal-directed behavior, adaptations in DLS firing patterns may be regulated by movement-related learning rather than nonsomatosensory cues, consistent with convergent S1 and M1 afferents to the region. Striatal cue reactivity in the rat is likely mediated within the dorsomedial and ventromedial striatum, in line with associative and limbic afferents to these regions, respectively.
Keywords: habit, striatum, putamen, stimulus, response, accumbens, conditioned stimulus
Introduction
Habitual behaviors are the motor expression of learned stimulus-response associations. When behavior is habitual, it becomes resistant to manipulations of the consequences of actions, such as devaluation of the reward [17]. Given their resistance to reward devaluation, habits may be an important factor in addiction or other psychological disorders.
The rat dorsolateral striatum (DLS) has been implicated in habit formation [50]. The DLS contains a somatic sensorimotor map, with neurons related to head and neck, forelimb, hindlimb and vibrissae dorsally, and, neurons related to tongue more ventrally [7], [12]. These neurons exhibit robust, unconditioned increases in firing rate during somatosensory stimulation or movement of the related body part, reflective of their convergent S1 and M1 afferents [22], [27], [38].
Mixed results have been reported regarding whether rat DLS neurons change firing rates in response to learned nonsomatosensory cues. One recent report described phasic activations of DLS neurons in response to conditioned cues [44] while another found a “paucity” of cue-related activity [4]. However, in both investigations, no identification was made of any neuron's unconditioned relation to sensory stimulation or movement of specific body parts, limiting interpretations of cue-evoked firing. A previous investigation in our laboratory suggested an absence of cue-evoked activity in rat DLS neurons [8]. This study recorded DLS forelimb neurons across days of training in a skilled forelimb lever press task under the control of an auditory discriminative stimulus. These neurons were characterized by an initial robust forelimb-movement firing correlate that declined over training days. However, neurons did not exhibit phasic activity in response to the discriminative stimulus that set the occasion for the forelimb movement.
The present experiment reexamined two DLS neuron populations for possible cue-related activity. Prior reports of these neurons examined movement-related firing across days of training [45] [46]. In the head movement paradigm, animals were trained on a fixed ratio (FR) 3 vertical head movement task. Possible cue-evoked activity was analyzed in the present study by comparing firing at the completion of i) the third vertical movement, which was signaled by an audible cue simultaneous with water delivery, with ii) the first two vertical head movements which were unsignaled. In the lick movement paradigm, possible cue-evoked activity was analyzed by comparing firing before and after an audible cue signaled water delivery, noncontingent to the animals behavior.
Methods
Subjects and surgery
Male Long-Evans rats (n=37, 300–330 g; Charles River, Wilmington, MA, USA) were prepared for chronic single-unit recording of DLS neurons exhibiting unconditioned correlations specifically with vertical head movement (n=26) or licking (n=11). Details of these samples of rats, electrophysiological procedures, behavioral paradigm, videotape analysis, and histological analysis have been described previously [c.f. 45 and 46]. Protocols were performed in compliance with the Guide for the Care and Use of Laboratory Animals (NIH, Publications 865–23) and were approved by the Institutional Animal Care and Use Committee, Rutgers University.
Head Movement chamber
In a clear Plexiglas chamber (length 35 cm × width 17 cm × height 40 cm), a water trough was attached to the front wall, centered 8.5 cm from each sidewall, raised 1.5 cm from the floor, and extended 6.5 cm from the front wall into the chamber. A Plexiglas insertion, with left and right side walls 4 cm high and angled at 45 degrees, was placed along the long axis of the chamber to keep the rat's body centered, with head forward facing the water trough and camera (see below) during the experiment. A solenoid valve was used to deliver single drops of water (5 μL) to the water trough. In order to avoid recording electrical artifacts from solenoid discharge, the solenoid and its power supply were physically remote from the animal, 60 cm away, shielded by two walls of a heavy metal rack, with the solenoid encased inside a grounded metal box. To ensure that the animal could nevertheless hear each water delivery, a speaker was placed 40 cm overhead, on a perforated portion of the chamber's ceiling. Each computer pulse (50 msec) delivered to the solenoid valve simultaneously activated a tone (50 msec duration, 1000 Hz, 60 dB, <1.0 msec rise time to full volume) via the speaker, henceforth referred to as the ‘cue’. No evidence of any solenoid artifact was detected in detailed examinations of single unit recordings, waveforms or peri-event time histograms (PETH).
Two light-emitting diodes (LEDs) were mounted 9 mm apart vertically on the headstage of the harness. A camera (Computar series 3500, 60 Hz sampling rate) was placed 20 cm in front of the chamber to monitor the LEDs, signaling the 2-dimensional position of the head every 16.7 msec throughout the experiment (spatial resolution 1 mm). On the vertical scale, a value of 0 corresponded to the floor of the chamber. A value of 90–95 mm for the upper LED corresponded to a level position of the head while the animal was standing on all four limbs. Light signals from the LEDs were fed into a tracker box and, along with neural signals, into a microcomputer where signals were sampled, digitized and stored for offline analysis using Datawave Acquisition software (Datawave Technologies, Longmont, CO).
Head movement task
Each training session (2 h per session, one session per day for 14 consecutive days) began with the removal of a false Plexiglas floor, exposing the water trough to the rat. A criterion (operant) vertical head movement was defined as: (i) an upward head movement; (ii) at least 41 mm long; (iii) with the upper LED starting <94 mm and ending >135 mm on the vertical scale; and (iv) the time from the head's (LED's) crossing 94 mm to crossing 135 mm was < 1 s. For each criterion movement, detection was operationally defined as the time when the upper LED crossed 135 mm, and was time-stamped (16.7 msec resolution). Prior to sessions 2 and 13, animals were presented with their daily allotment of water (∼13 mL) for devaluation testing. Following consumption, animals were immediately placed into the head movement task under normal experimental circumstances.
Lick chamber
A water spout was positioned outside the front wall of a transparent, Plexiglas recording chamber (23.5 cm ×17.4 cm × 43 cm). Silicon tubing was used to connect a container filled with tap water, and a solenoid to a stainless steel drinking spout located 2 mm from the chamber. There was a small hole in the Plexiglas (7 mm diameter) to allow the rat's tongue access to water drops. A photo lick sensor, consisting of fiber optic below and above the spout, was used to register individual licks. The presence or absence (during a lick) of the light beam was recorded and time stamped every 16.6 msec (Datawave Technologies, Longmont, CO), in synchrony with the time stamping of neural waveforms. Water delivery was controlled by a TTL pulse from the computer that opened the solenoid for 35 msec, delivering one drop of approximately 5 μl through the spout (one water-delivery). An audible tone (3 KHz, 60 dB, <1.0 msec rise time to full volume) was sounded through a speaker mounted 40 cm above the chamber, for the duration that the solenoid was open to make the activation of the solenoid audible to the animal. Water delivery, licks and neural firing were processed and stored by a microcomputer.
Lick task
Rats were trained in a licking task for 14 consecutive sessions (2 hours per session, one session per day). Each rat was placed in the chamber and a recording harness was attached to the microwire array. Following the preliminary exam (below), experimentation began. The experiment consisted of six water-on periods (15 min) alternating with six water-off periods (5 min). During water on periods, water deliveries were pseudorandomly presented to the rat ranging from 6 to 12 sec, mean = 9 sec. Water was not delivered during water off periods. The solenoid click/audible tone compound cue was presented simultaneously with water delivery. During the two-hour session, the rat received approximately 3 ml water. After each session, the rat was given another 10 ml water to reach the daily amount of water (13 ml) needed to maintain stable weight. Prior to sessions 2 and 13, a subset of animals were presented with their daily allotment of water (∼13 mL) for devaluation testing (n=4). Two additional animals not implanted with electrodes were also devalued prior to sessions 2 and 13. Following consumption, animals were immediately placed into the lick task under normal experimental circumstances.
Determination of neuron types
Prior to the experiments, a complete sensorimotor examination [7], [12], [13], was conducted on every microwire that exhibited neural activity (signal-to-noise ratio > 3 : 1) to determine whether firing was phasically related to sensorimotor activity of any body part. Four types of neurons were recorded: (i) upward head movement neurons, i.e. DLS neurons that exhibited increased activity during upward movement; (ii) downward head movement neurons, i.e. DLS neurons that exhibited increased activity during downward movement; (iii) lick neurons that increased activity during licking determined by manually delivered water drops on the chamber floor; and (iv) nonsomatomotor neurons, i.e. DLS neurons that exhibited spontaneous activity yet did not respond to active movement, cutaneous probing, or passive manipulation of any body part [11]. The prior reports detail additional criteria [45] [46].
Analysis of cue-evoked activity
Head movement task
The present experiment reexamined the DLS neuron sample [45] for possible cue-evoked firing across training days. In this paradigm, animals were trained on a FR3 vertical head movement task. The first two criterion head movements were unsignaled while the third criterion movement was reinforced via delivery of a drop of water to the trough via the solenoid, signaled simultaneously by presentation of the conditioned stimulus cue. In order to test for cue learning, the latency to emit a criterion head movement was analyzed over days of FR3 performance. In order to test for the utilization of a counting strategy during FR3 performance, we analyzed a subset of criterion head movements (mean was approximately 20% of all criterion movements during asymptotic performance) after which animals briefly held the head in an elevated, stable position (height) after the criterion was detected (although this was not required of the animal). Each such occurrence of stable head position following a criterion movement was sorted into one cell of a 3×4 spreadsheet consisting of three levels of height (LED values 126-135, 130-143, 135-151) and four levels of duration (0-50 msec, 50-100 msec, 100-150 msec, > 150 msec) over the last four training sessions in which asymptotic behavior occurred [45]. Each cell tallied the number of occurrences in which the head was held after detection, just below, surrounding, or above detection (135) for different durations, respectively. One spreadsheet was tabulated for each of the three movements of the FR3 schedule for each session analyzed. If animals utilized a counting strategy, the height, duration, or their interaction should differ on the third movement relative to the first and second.
Both outcome variables (latency and number of head positions) were analyzed as a function of a set of categorical fixed effect independent variables (e.g., day, movement number, height, interactions) using a mixed model ANOVA using SAS PROC GLIMMIX (SAS Institute Inc., 2005). Outcome variables were highly skewed and therefore theorized to be gamma distributed rather than normally distributed. Thus, a gamma distribution with a log link was specified for the outcome variable in the mixed ANOVAs. Outcome variables were collected on multiple occasions from each subject, and thus, subject was specified as a random effects variable. The solution for the latency mixed ANOVA model was estimated using maximum pseudo-likelihood marginal expansion while the head position mixed ANOVA model was estimated using maximum likelihood with adaptive quadrature. Because the data were not normally distributed, standard errors were computed using the first order residual empirical (sandwich) estimator for the latency analysis and design-adjusted MBN empirical estimator for the head position analysis. All other default settings in PROC GLIMMIX were maintained. Pairwise comparisons (Scheffe adjusted) were computed for significant interactions.
A PETH was constructed around the detection of all criterion movements that occurred third in the FR3 sequence. Detection of this criterion movement was the node of the PETH, which also corresponded to the onsets of the cue and water delivery. Separate PETHs were constructed using detection of the first and second criterion movements as nodes. Each PETH typically contained a thousand or more daily criterion head movements. Using the PETHs, analyses of cue-evoked activity were limited to the 150 msec following detection, in order to exclude any firing related to potential cue-induced head movements, which can occur at ≥150 msec latency in response to auditory cues [25]. Cue-evoked firing of striatal medium spiny neurons has been demonstrated to occur within 150 msec of cue onset [14], [25], [49]. Only data from days in which rats performed on an FR3 schedule of reinforcement were analyzed.
Two ratios calculated the standardized change in firing rates using the PETHs. The “movement ratio” consisted of a ‘B/A+B’ formula in which ‘A’ was defined as the average firing rate during the 150 msec following detection of the first unsignaled criterion head movement and ‘B’ was defined as the average firing rate during the 150 msec following detection of the second unsignaled criterion head movement. Because DLS neuron firing related to sensory or motor activity exhibits substantial trial-to-trial variability [41], the movement ratio represented any spontaneous differences in firing rate in the absence of the conditioned stimulus cue. The “cue ratio” consisted of a ‘B/A+B’ formula in which ‘A’ was defined as the average firing rate during the 150 msec following detection of the second unsignaled successful criterion head movement and ‘B’ was defined as the average firing rate during the 150 msec following detection of the third (signaled) criterion head movement, containing the conditioned stimulus cue onset at time zero. The relative homogeneity of criterion head movements provided behavioral equivalence [40] between signaled versus unsignaled criterion head movements, enabling us to isolate cue-evoked firing. Note that cue-evoked firing was not assessed in this particular analysis via any comparisons of firing before versus after cue onset because of the ongoing head movement, with which these neurons are correlated.
Three two-level hierarchical linear models (HLM; Level 1: Equation 1, Level 2: Equation 2-5) [42], one for each type of DLS neuron (upward, downward, or nonsomatomotor), compared the regression growth lines of each individual neuron across the movement and cue ratios over training sessions. Equation 1 describes the linear growth model that was fitted for each individual neuron by regressing the standardized change in firing rate of each neuron on all session values separately for both the movement and cue ratios. In Equation 1, the session variable was group mean centered [42] by subtracting the mean of the session values (session mean = 7.5) from the individual session values (1 ≤ t ≤ 14) in order to reduce the nonessential multicollinearity in the interaction term for ratio and session. The level 1 model of the HLM was:
| (1) |
where StdChgFRtji is the B/(A+B) standardized change of firing rate of the ith neuron for the jth dummy coded, within-subjects ratio variable across all t sessions, π0i is the centered intercept for the ith neuron, π1i and π2i are the slopes of the regression of StdChgFRtji on ratio and session, respectively, π3i is the parameter associated with the interaction of ratio and session, and etji is the error term for the ith neuron.
The level 2 model of the HLM was:
| (2) |
| (3) |
| (4) |
| (5) |
where π0i through π3i are parameters from Equation 1, β00 through β30 were the estimated average values of π0i through π3i, respectively, and r0i through r3i were the estimated variances of π0i through π3i, respectively. Since the session variable was centered, β00 is the estimated grand mean for the 0 dummy coded ratio group, which in this case was movement ratio. Because of the 0-1 dummy coding of the ratio variable, π1i is the estimated average difference in standardized change in firing across all neurons between the movement and cue ratios. π2i is the estimated average level of standardized change in firing across all neurons at the centering value of 7.5. π3i is the term associated with the interaction of ratio and session.
Two final tests of cue-evoked firing compared the firing rates of neurons from matched sets of stable head positions following detection of a criterion head movement. The first analysis compared the firing rates of DLS neurons during stable head positions following a criterion movement according to i) three levels of height (LED values 126-135, 130-143, 135-151), ii four levels of duration (0-50 msec, 50-100 msec, 100-150 msec, > 150 msec), and iii) the three movements of the FR3 schedule for neurons recorded during the last four sessions of training. For 0-50, 50-100, 100-150, and >150 msec duration movements, firing rates were analyzed during 50, 100, 150, and 200 msec following detection, respectively. Firing rates were analyzed with a mixed ANOVA (SAS PROC GLIMMIX (SAS Institute Inc., 2005)). A gamma distribution with a log link was specified for firing rate and neuron was specified as a random effects variable. The solution was estimated using maximum likelihood with adaptive quadrature. The standard errors were computed using the design-adjusted MBN empirical estimator. All other default settings in PROC GLIMMIX were maintained
The second analysis sorted firing rates of each occurrence of stable head position following a criterion movement into one cell of a 3×4 spreadsheet consisting of three levels of height (LED values 126-135, 130-143, 135-151) and four levels of duration (0-50 msec, 50-100 msec, 100-150 msec, > 150 msec),for neurons recorded during the last four sessions of training. For each neuron, one spreadsheet was generated for each movement of the FR3 schedule for each session analyzed. Each cell of the 3×4 table contained the average firing rate of all occurrences of stable head positions exhibiting that specific height and duration. Firing rates were analyzed per duration of stable head position as specified in the above first analysis. Cells with < 5 occurrences were not included in the tables. Subsequently, two position ratio tables were created, one including and the other not including the cue. Cells of the position ratio table not including the cue were calculated by a ‘B/A+B’ ratio from average firing rates of the 3×4 table of movements one (‘A’) and two (‘B’). Cells of the position ratio table including the cue were calculated by a ‘B/A+B’ ratio from average firing rates of the 3×4 table of movements two (‘A’) and three (‘B’). That is, the firing rates of neurons during specific heights and durations of stable head positions (i.e., each cell) were calculated separately, creating two tables of ‘B/A+B’ values. Finally, all cells of the position ratio table including the cue and all cells of the position ratio table not including the cue were individually averaged over the last four days of training. This created one average position ratio without the cue and one average position ratio with the cue per neuron that was compared with paired t-tests for each neuron type.
Lick task
For every neuron, a PETH was constructed around the cued water delivery. Any cue node in which a lick occurred was removed from analysis; thus all cues that were analyzed occurred while the tongue was proximal to the fiberoptic detector. Nonetheless, PETHs typically contained the maximum 600 trials per day. Each neuron's daily average firing rates were collected over the 100 msec before and after cue presentation. A 100 msec firing window was utilized due to observed lick reaction times in response to the auditory cue at this latency but never preceding 100 msec. A B/(A+B) ratio calculated the standardized change in firing rate following the cue compared with the pre-cue period for the valued and devalued groups. ‘A’ was defined as the average firing rate over the 100 msec prior to cue presentation and ‘B’ was defined as the average firing rate during the 100 msec following cue presentation. A mixed model ANOVA using SAS PROC GLIMMIX (SAS Institute Inc., 2005) compared standardized changes of lick neurons in response to the cue in valued and devalued groups across sessions. A gamma distribution with a log link was specified for the outcome variable in the mixed ANOVA. Since the outcome variable was collected on multiple occasions from each neuron, neuron was specified as a random effects variable. The solution for the mixed ANOVA model was estimated using maximum pseudo-likelihood marginal expansion and standard errors were computed using the first order residual empirical estimator. All other default settings in PROC GLIMMIX were maintained. Post-hoc simple effects were computed for any overall significant interactions.
Results
Head movement task
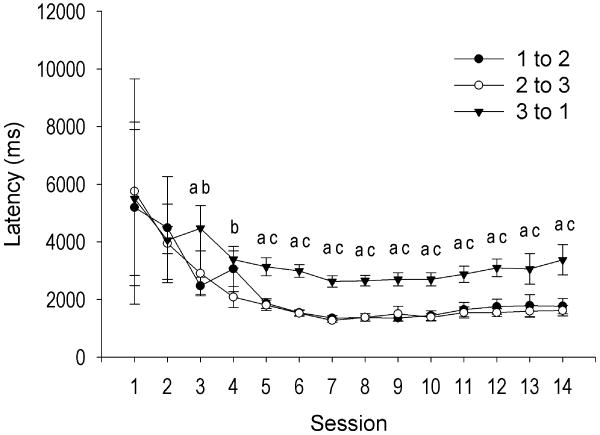
Acquisition of the task, improved movement efficiency, and demonstration of habit formation are described in detail in a previous report [45]. Briefly, animals rapidly acquired FR3 criterion head-movements within three sessions. While the conditioned stimulus did not precede the operant response, the conditioned cue likely acquired value as training proceeded. Excluding satiety sessions for devalued animals, the latency to emit criterion head movements was measured between the first and second, second and third, and third and first criterion head movements, where the cue occurred. The mixed ANOVA yielded significant main effects for movement number (F(2, 752) = 40.06, p < .0001) and day (F(23, 752) = 11.37, p < .0001), but the latency to emit a criterion head movement depended on the previous criterion head movement as well as training day, interaction of movement number × day, F(25, 752) = 39.46, p < .0001. Pairwise comparisons revealed no significant difference on days 1 and 2 but sparse significance on days 3 and 4 (Figure 1). However, emerging on day 5 and stable throughout the remainder of training, the latency to emit a criterion head movement was significantly longer following the third criterion movement than after the first (all |t| > 5.11, p < .0002) or second (all |t| > 4.18, p < .0002) criterion movements. (Figure 1). Between days 5 and 14, animals averaged 1588.83 ± 188.59 msec between the first and second, 1516.85 ± 157.74 msec between the second and third, and 2921.40 ± 300.85 msec between the third and next (first) criterion head movement. There was no difference between latencies of criterion movements one and two from days 5 through 14 (all |t| < 1.41, p > .05) (Figure 1). Given the increased latency to emit a criterion head movement following cue presentation (third movement), but not after the first or second criterion movements that were not followed by the cue, animals likely learned that the cue predicted reward delivery.
Figure 1.
Average (+SEM) latency to emit a criterion head movement during FR3 performance over fourteen training days. Movement three but not movement one or two was followed by the cue. Prior to day 5, weak and sparse differences were observed between latencies to emit a criterion head movement. On day 5, animals exhibited an increased latency to emit a criterion head movement following the cue (3 to 1) compared with the first (1 to 2) or second (2 to 3) criterion head movements while latencies between the first and second (1 to 2 vs. 2 to 3) movements did not differ. a = latency of movements 1 to 2 and 3 to 1 significantly different at p < .0002, b = latency of movements 2 to 3 and 3 to 1 significantly different at p < .05, c = latency of movements 2 to 3 and 3 to 1 significantly different at p < .0002.
Videotape analysis revealed that rats did not move toward the magazine after each head movement. Instead, rats typically made several criterion and noncriterion head movements until the cue was presented at the detection of the third criterion head movement, at which time rats emitted an oblique head movement toward the trough and consumed the reward. Nevertheless, in order to determine if animals utilized a counting strategy that did not involve cue processing, the number of stable head positions following individual criterion head movements was measured in terms of height and duration over the last four days of training. That is, after approximately 20% of criterion movements, animals tended to hold the head briefly in an elevated, stable position following criterion detection. While the mixed ANOVA yielded a significant main effect of duration, F(3, 2248) = 33.12, p < .0001, a significant duration × height interaction was revealed, R(6, 2248) = 3.57, p < .0016. Pairwise comparisons revealed that animals emitted significantly more 0-50 msec movements than 50-100 msec or 100-150 msec movements across every level of height (all t > 6.44, p < .0001). All other pairwise comparisons of duration within each height level did not significantly differ (all t < 2.76, p > .05). There was no other significant main effect (height, session, movement) or interaction (duration × movement, movement × height, duration × session, movement × session, height × session, duration × movement × height, duration × movement × session, movement × height × session, duration × movement × height × session), all F < 0.19, p > .05. These data demonstrate that animals did not emit different numbers of stable head position durations or heights following the third criterion movement compared with following the first or second criterion head movements.
Of rats that were satiated early and late in training (n=6), criterion head movements were reduced less during the late devaluation test compared with the early devaluation test (Wilcoxon signed-ranks test, p < .05), indicating animals formed a habit [see 45].
Of 68 single neurons recorded over sessions, 43 neurons (63%) exhibited neural activity recorded throughout all 14 training sessions. The mean duration of stable recording was 12.4 ± 0.3 sessions. Since head-movement neurons are directionally sensitive [7], we separately examined upward-associated head movement neurons (up neurons) and downward-associated head movement neurons (down neurons) across days. In addition, nonsomatomotor DLS neurons were examined for possible cue-induced firing.
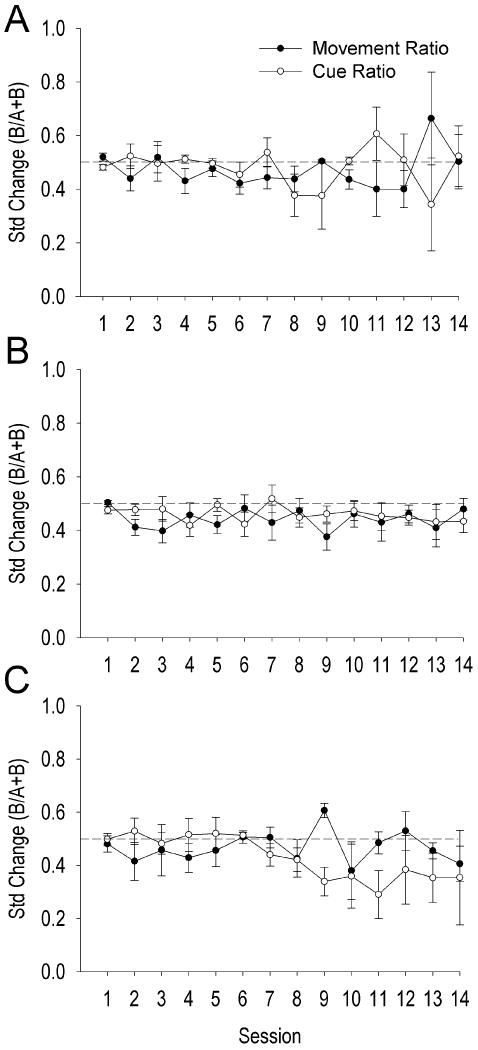
Movement- and cue-ratios of nonsomatomotor neurons (n=14, 21.86% of DLS neurons) did not significantly change over training days, t(13) = 0.185, p > .05 (Table 1). There was no significant effect of firing ratio (t(13) = 1.104, p > .05) or session × firing ratio interaction (t(34) = -0.261, p > .05). Thus, nonsomatomotor neurons of the DLS did not fire differentially to the presence or absence of the conditioned stimulus cue (Figure 2A).
Table 1. Estimated fixed and random effects (Equations 2-5) of ratio and session on standardized changes of nonsensorimotor DLS neuron firing rates according to the hierarchical linear model.
Nonsomatomotor DLS neurons exhibited no significant differences in movement- versus cue- ratio, change over sessions, or interaction of firing ratio with training sessions.
| Fixed Effects | |||
|---|---|---|---|
| Parameter | Parameter Estimate with SEM | |t|a | |
| Grand Mean of Movement Ratio | β 00 | 0.459 ± 0.021 | 21.633*** |
| Ratio | β 10 | 0.027 ± 0.024 | 1.104 |
| Session | β 20 | 0.001 ± 0.005 | 0.185 |
| Ratio × Session | β 30 | -0.002 ± 0.006 | 0.261 |
| Random Effects | |||
| Parameter | Variance Estimate | χ2 b | |
| Grand Mean of Movement Ratio (β 00) | r0i | 0.002 | 17.862 |
| Ratio Slope (β 10) | r1i | 0.000 | 10.273 |
| Session Slope (β 20) | r2i | 0.000 | 5.057 |
| Ratio × Session Slope (β 30) | r3i | 0.000 | 10.508 |
Note: The chi-square statistics reported are based on 13 of 14 neuronal units that had sufficient data for computation. All reported values in the table have been rounded.
Approximate df = 13.
df = 13.
P < 0.001;
P < 0.01;
P < 0.05.
Figure 2.
Average (+SEM) standardized change of nonsomatomotor (A), upward-associated (B), and downward-associated (C) DLS neurons for movement and cue ratios across training days. Dotted line at 0.5 indicates no change in firing rate.
Movement- and cue-ratios of up neurons (n = 35, 54.69% of DLS neurons) did not significantly change over training days, t(34) = 0.018, p > .05 (Table 2). There was no significant effect of firing ratio (t(34) = 0.385, p > .05) or session × firing ratio interaction (t(34) = -0.712, p > .05). Thus, up neurons did not fire differentially to the presence or absence of the conditioned stimulus cue (Figure 2B).
Table 2. Estimated fixed and random effects (Equations 2-5) of ratio and session on standardized changes of upward DLS neuron firing rates according to the hierarchical linear model.
Upward DLS neurons exhibited no significant differences in movement-versus cue- ratio, change over sessions, or interaction of firing ratio with training sessions.
| Fixed Effects | |||
|---|---|---|---|
| Parameter | Estimate | |t| | |
| Grand Mean of Movement Ratio | β 00 | 0.442 ± 0.020 | 21.715*** |
| Ratio | β 10 | -0.010 ± 0.027 | 0.378 |
| Session | β 20 | 0.000 ± 0.003 | 0.018 |
| Ratio × Session | β 30 | -0.003 ± 0.005 | 0.712 |
| Random Effects | |||
| Parameter | Variance Estimate | χ2 b | |
| Grand Mean of Movement Ratio (β 00) | r0i | 0.010 | 125.529*** |
| Ratio Slope (β 10) | r1i | 0.016 | 134.124*** |
| Session Slope (β 20) | r2i | 0.000 | 58.689** |
| Ratio × Session Slope (β 30) | r3i | 0.000 | 78.247*** |
Note: The chi-square statistics reported are based on 32 of 39 neuronal units that had sufficient data for computation. All reported values in the table have been rounded.
Approximate df = 34.
df = 31.
P < 0.001;
P < 0.01;
P < 0.05.
Movement- and cue-ratios of down neurons (n = 15, 23.44% of DLS neurons) did not significantly change over training days, t(14) = 0.292, p > .05 (Table 3). There was no significant effect of firing ratio (t(14) = -0.865, p > .05) or session × firing ratio interaction (t(14) = 1.982, p > .05). Thus, down neurons did not fire differentially to the presence or absence of the conditioned stimulus cue (Figure 2C).
Table 3. Estimated fixed and random effects (Equations 2-5) of ratio and session on standardized changes of downward DLS neuron firing rates according to the hierarchical linear model.
Downward DLS neurons exhibited no significant differences in movement- versus cue- ratio, change over sessions, or interaction of firing ratio with training sessions.
| Fixed Effects | |||
|---|---|---|---|
| Parameter | Estimate | |t| | |
| Grand Mean of Movement Ratio | β 00 | 0.467 ± 0.024 | 19.654*** |
| Ratio | β 10 | -0.030 ± 0.034 | 0.865 |
| Session | β 20 | 0.001 ± 0.005 | 0.292 |
| Ratio × Session | β 30 | -0.018 ± 0.009 | 1.982 |
| Random Effects | |||
| Parameter | Variance Estimate | χ2 b | |
| Grand Mean of Movement Ratio (β 00) | r0i | 0.005 | 42.927*** |
| Ratio Slope (β 10) | r1i | 0.009 | 42.188*** |
| Session Slope (β 20) | r2i | 0.000 | 30.978** |
| Ratio × Session Slope (β 30) | r3i | 0.001 | 46.420*** |
Note: The chi-square statistics reported are based on 13 of 15 neuronal units that had sufficient data for computation. All reported values in the table have been rounded.
Approximate df = 13.
df = 12.
P < 0.001;
P < 0.01;
P < 0.05.
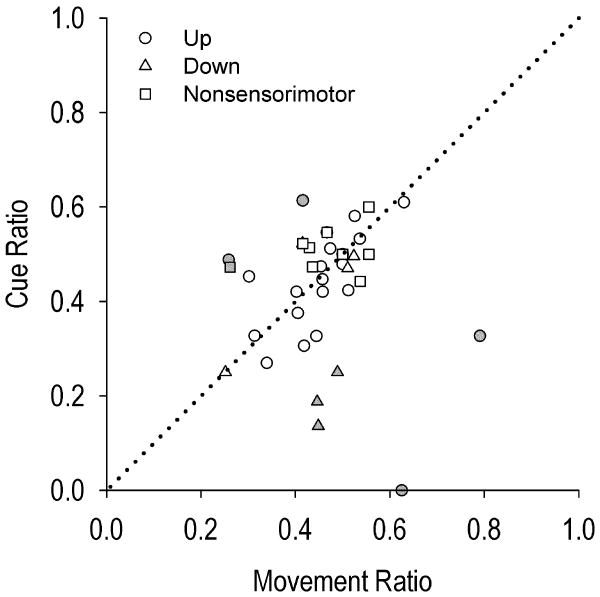
Given that devaluation by satiety decreased responding early but not late in training [45], we examined if cue-evoked firing was expressed during habitual behavior late in training. A three (up, down, nonsomatomotor neuron) × two (cue-ratio, movement-ratio) mixed ANOVA analyzing the average firing rates over the last four days of training yielded no significant difference in firing between the presence versus the absence of the cue for all three types of recorded neurons, evidenced by the non-significant interaction effect of neuron type × firing ratio (F(2, 37) = 1.671, p > .05) and non-significant main effect of firing ratio (F(2, 37) = 1.202, p > .05). Further, no significant differences were observed between DLS neuron types, F(2, 37) = 2.388, p > .05. Figure 3 displays the average cue ratio by the average movement ratio during the last four days of training for each neuron that was recorded at least once during these late training days. Plotted points for the vast majority of neurons (n=32; 80% of all neurons) lie near the line of no difference.
Figure 3.
Scatterplot of movement ratio (x-axis) by cue ratio (y-axis), averaged over late (last four) days of training. Dotted diagonal line represents no difference between the two ratios, i.e., no cue-evoked change in firing. See Figure 4 for gray shaded neurons.
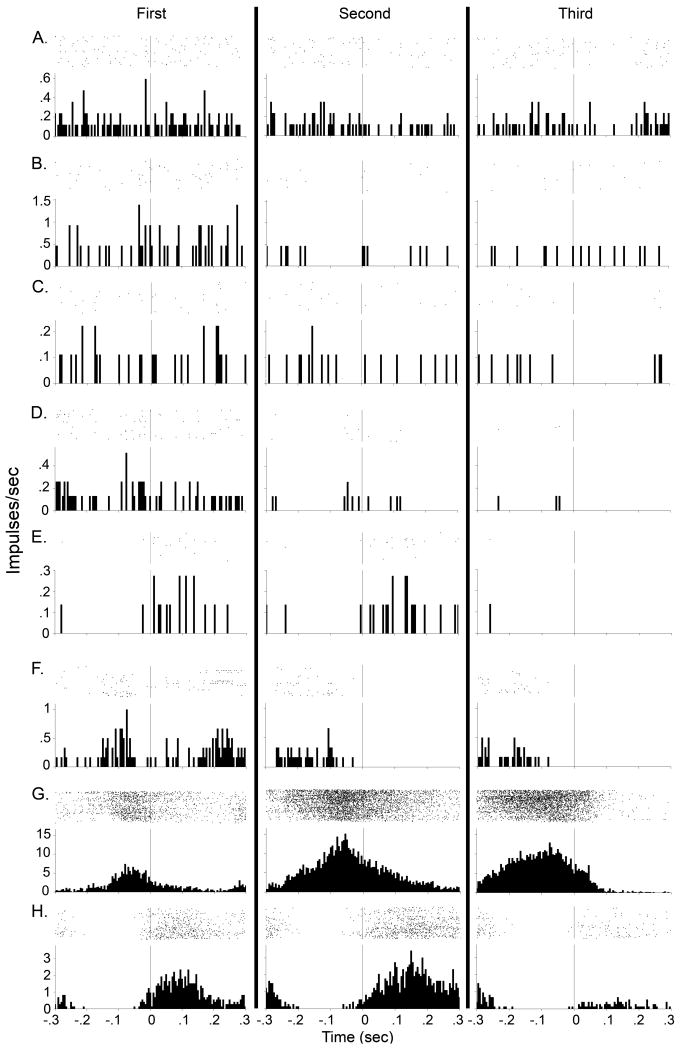
The remaining 8 neurons showed relatively greater deviation in firing from the line of no difference (shaded in gray in Figure 3B). For each of these neurons, a representative day over the last four days' training underwent more rigorous examination (Figure 4). Analyses of firing rates around cue onset provided little evidence that firing was cue-evoked. Most of these neurons exhibited spurious changes in firing rate during the 150 msec following each of the three criterion head movements, with or without the cue (Figure 4A-E). Despite thousands of criterion head movements, these neurons exhibited very few spikes during the first post-criterion head movement. Further, the same or fewer spikes were recorded following the second and third post-criterion head movements. Other neurons exhibited firing characteristics likely related to individual movements or movement sequences [1] (Figure 4F-H). One up neuron exhibited spurious firing rates during the 150 msec following the first criterion movement but not on the second or third criterion movements (Figure 4F). One down neuron's firing across the three movements was attributed to differences in firing rate prior to onset of downward movement rather than the presence of the cue (Figure 4G). A different down neuron exhibited decreased firing rates following the third criterion movement/cue onset relative to the first two unsignaled criterion head movements. (Figure 4H). This might indicate suppression of motor-correlated firing by the presence of the cue. However, that direction of firing was inconsistent with primarily (80%) increases in firing rate of medium spiny neurons in response to cues in the medial striatum [25], [49]. In general, firing during cue presentation was negligible in comparison to the clear auditory-evoked firing of neurons demonstrated in other striatal subregions [1], [23], [25], [28], [31], [49].
Figure 4.
A representative raster and PETH during one of the last four days of training is presented for each outlier neuron shaded gray in Figure 3 (each row). Each row of the rasters represents one criterion head movement. Each dot of the rasters represents one action potential. Each PETH represents the average firing rate of the DLS neuron over all criterion movements (first, second or third movements of the FR3 schedule) in that session. Vertical line at time zero in raster and PETH represents detection of criterion head movement. Column 1 represents firing rates during the first unsignaled criterion head movement. Column 2 represents firing rates during the second unsignaled criterion head movement. Column 3 represents firing rates during the third (signaled) criterion head movement, with the conditioned stimulus cue presented at time zero. Columns are separated by solid black lines. PETH bin size is 5 ms. For each neuron, first, second, and third column contains the same number of trials in each PETH, which were 1683 (A), 433 (B), 1791 (C), 1547 (D), 1206 (E), 1459 (F), 1459 (G), and 1299 (H). Nonsomatomotor neuron is A; upward-associated neurons are B, D, F G; downward-associated neurons are C, E, H.
The final analyses examined firing during the subsets of matched stable head positions following each criterion head movement according to three levels of height and four levels of duration, collapsing the last four training sessions. The mixed ANOVAs for up, down, and nonsomatomotor DLS neurons did not yield any significant main effects (duration, movement, height) or interactions (all F < 0.14, p > .05). Furthermore the average position ratio with the cue did not differ from the average position ratio without the cue for any cell type (all |t| < 1.11, p > .05). Thus, matched sets in which the head was in the same, stable position (matching heights and durations) exhibited firing rates that did not differ whether the cue was presented or not.
Lick task
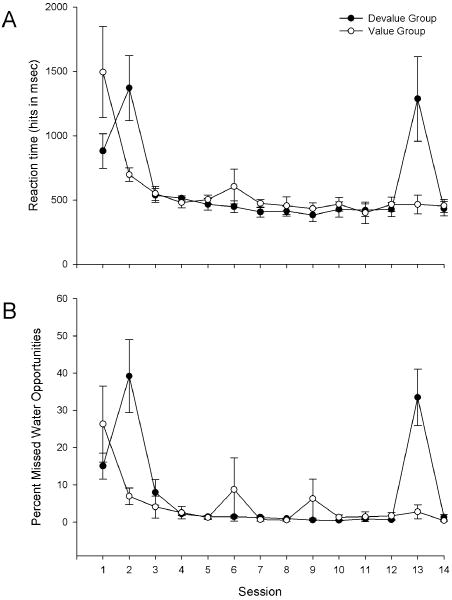
Acquisition of behavior and tests of habit formation are described in detail in a previous report [46]. Briefly, animals rapidly acquired the lick task, engaging in greater than 10,000 licks on the first day of training. Furthermore, both groups decreased average lick reaction time (hits only) in response to the auditory cue to roughly 450 msec (main effect of session, F(11,136) = 64.29, p < 0.0001, no main effect of group), demonstrating cue learning. However, animals differed in their reaction time depending on devaluation (session × group interaction F(11, 136) = 24.68, p < 0.0001) (Figure 5A). Devaluation on days 2 (F(1,136) = 15.27, p < 0.0001) and 13 (F(1,136) = 10.84, p < 0.01) nearly tripled reaction time and no significant difference existed between groups on any other days (p > .05). Furthermore, while both groups decreased the percent of missed water opportunities to roughly 1% over sessions (main effect of session F(11,136) = 33.04, p < 0.0001, no main effect of group), devalued animals exhibited significantly more misses than valued rats (session × group interaction F(11, 136) = 6.52, p < 0.0001) on devalued days 2 (F(1,136) = 9.60, p < 0.01) and 13 (F(1,136) = 9.82, p < 0.01) but not on any other days (p > 0.05) (Figure 5B). The increased misses by the devalued group likely contributed to the significantly reduced number of licks compared to the nondevalued group both early and late in training, indicating animals did not form a habit [46].
Figure 5.
Average (+SEM) reaction time of hits (A) following cue presentation and percent of nonresponded cue-presentations (B). Devaluation increased reaction time as well as number of nonresponded (misses) cue-presentations.
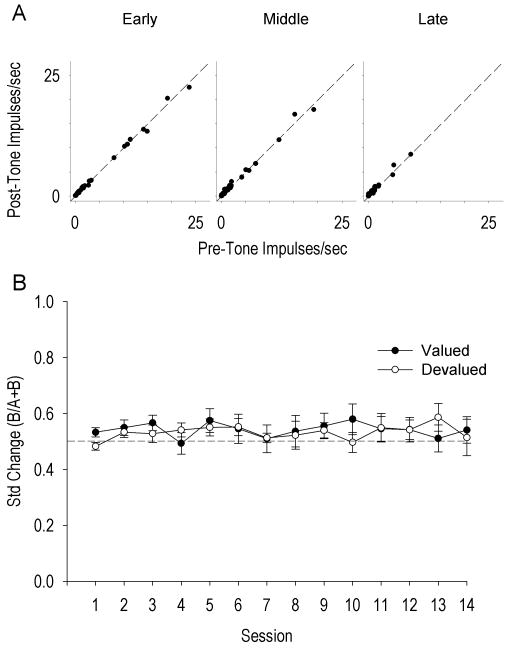
Of 29 neurons, 19 (66%) exhibited neural activity recorded throughout all 14 training sessions. The mean stable recording for all neurons was 12.2 ± 0.5 sessions. An initial analysis of potential cue-related firing examined pre-cue versus post-cue firing over three levels of training (early - average of days 1, 3, 4, and 5; middle - average of days 6, 7, 8, and 9; late - average of days 10, 11, 13, and 14). The repeated measures ANOVA yielded a significant main effect of training, F(2,132) = 4.44, p < .05, but no main effect of cue, F(1,132) = 0.32, p > .05, or cue × training interaction, F(2,132) = 0.09, p > .05, indicating that firing in general decreased over training but showed no change in response to the cue (Figure 6A). This analysis did not include devaluation sessions because all neurons were pooled together. However, given the decreased responding of the devalued group during the cue-guided lick task, cue-evoked firing may exist between devalued and valued groups during devaluation sessions. A mixed ANOVA analyzing the standardized changes in firing rate in response to the auditory cue revealed a significant main effect of session, F(13, 295) = 3.45, p < 0.0001, no overall group effect F(1, 295) = 1.56 p > 0.05, and a significant session × group interaction, F(13, 295) = 2.14, p < 0.05. Post-hoc simple comparisons revealed a significant difference between groups on day 1 of training F(1, 295) = 5.16, p < 0.05, but not on any subsequent training day, including devaluation days (p > 0.05) (Figure 6B). Thus, despite the difference in responding between groups on devaluation days during cue-guided behavior, cue-evoked firing was absent. Furthermore, cue-evoked firing did not emerge over training sessions (Figure 6B).
Figure 6.
A. Scatterplot of averaged pre-tone (x-axis) by post-tone (y-axis) firing rates averaged over early (days 1, 3, 4, and 5), middle (days 6, 7, 8, and 9), and late (days 10, 11, 12, 14) training days for all lick neurons. Dotted black lines represent no change in firing between pre and post tone firing rates, not a regression line. B. Average (+SEM) standardized change of DLS lick neurons for devalued and valued groups across training days. Dotted line at 0.5 indicates no change in firing rate between pre-tone and post-tone conditions.
Discussion
The DLS has been linked with learning habitual movements [45], [46], [50]. However, mixed results have been reported regarding whether the DLS is responsive to environmental stimuli [4], [8], [33], [44], which has implications for habitual or goal-directed behavior. In the head movement task, behavior was initially goal-directed but after extended training became habitual. Although head movement responses were self-initiated rather than cue-initiated, animals learned that the cue signaled reward delivery as indicated by the emergence over training days of an increased latency to emit a criterion head movement following cue presentation compared with nonpresentation. Furthermore, following criterion head movement detection, the duration and height of head positions did not differ between the three movements. This result indicates that animals did not utilize a counting strategy, in which the height and duration of the head would have been decreased on the third head movement relative to the first and second head movements. In spite of cue learning, DLS neurons did not change firing rates in response to the cue. Nevertheless, because the head movement was not cue-initiated, we analyzed a recent report of lick-related DLS neurons during cue guided licking [46].
In spite of cue-learning, as demonstrated by decreased reaction time to lick in response to the auditory cue, cue-reactivity was also absent in DLS lick neurons. This result suggests that the lack of cue-reactivity in DLS head movement and nonsomatomotor neurons was not due to a lack of cue-initiated movements. Indeed, a recent investigation reported a “paucity” of DLS neurons exhibiting cue-reactivity during win-stay maze performance [4]. The present results extend the results of Berke and colleagues [4] in that DLS neurons did not exhibit cue reactivity during goal-directed or habitual behavior.
We have previously demonstrated that discriminative stimuli do not change firing rates of rat DLS forelimb-associated neurons [8]. Thus, the lack of cue-evoked firing was not due to the difference of conditioned stimulus or discriminative stimulus cue, or of the body part with which firing was correlated. The present results are consistent with the previously reported properties of type IIb neurons of the primate putamen [33]. These neurons exhibit sensorimotor correlates, but not cue correlates, and represent roughly two-thirds of neurons the primate putamen [33]. The present study corroborates the findings of this report regarding type IIb neurons (e.g., neurons related to head or tongue movement) and extends them to nonsomatomotor neurons of the DLS. Similar to somatic sensorimotor DLS neurons, i) nonsomatomotor neurons exhibited decreased firing during upward head movements over training sessions (Tang et al, 2007), and ii) no cue correlates, nor did any develop as animals formed a habit. The nonsomatomotor neurons of the DLS reported herein cannot be classified as type I neurons [33], because firing rates of nonsomatomotor neurons were low. Nonsomatomotor neurons cannot be classified as type IIa neurons either because they did not exhibit pre-movement correlates [33]. It is possible that nonsomatomotor neurons encode other sensory modalities or somatomotor correlates not revealed by the head-movement task or our somatomotor examination. Further investigations of DLS are needed to determine the precise role of “nonsomatomotor” neurons.
It is not surprising that DLS neurons did not display cue correlates because auditory or limbic projections largely avoid the rat DLS [29], [47]. Instead, DLS integrates SI, SII, MI, and sensorimotor thalamic projections [5], [6], [17], [20], [21], [22]. Due to these afferents, DLS exhibits somatic sensorimotor responsiveness prior to any task learning [7] [8] [15] [16] [33-36] [45] [46]. However, with extended training on tasks that induce behaviors that are habitual or remain goal-directed, DLS firing during the same movements predominantly declines [45] [46]. Thus, if cue-related firing were to emerge over training days, it might be inappropriately interpreted by downstream pallidal and nigral regions either as somatosensation/movement or a change in task conditions [see 46].
We and others have found cue correlates more medially in the dorsal striatum [14], [23], [25], [28], [31], [49]. This structure can be delineated from the DLS by calbindin staining [6] and due to its receiving projections from the medial agranular cortex and posterior parietal cortex [10], [11], [17] [43], it has been suggested to be the “associative striatum” as opposed to the lateral “sensorimotor striatum” [4]. Interestingly, unconditional auditory sensory responses have been reported in the dorsocentral striatum [14] suggesting that this region of the striatum is not simply a transition zone between medial and lateral compartments of the dorsal striatum.
The present results differ from a previous report by Takahashi and colleagues [44]. In that report, experimenters trained rats to make two new odor associations (CS+ with sucrose and CS- with quinine) and reversed these associations daily for several months. DLS neurons were recorded and reported to exhibit slowly developing cue-evoked activity over a single session. Since the Takahashi report [44] did not test for sensorimotor correlates in their recorded DLS sample it is possible that “cue-evoked” firing was due to somatosensory, motor, or sequence correlates of those neurons. For instance, many neurons of the DLS are sensitive to vibrissal, facial somatosensation, oral, and/or facial musculature [7], [12] and thus could be affected by active sniffing--a suggestion validated by diverse sniffing behaviors rodents exhibit during differential behavioral tasks as well as in response to olfactory cues [30], [51].
The present results may differ from cue-reactivity in the primate putamen. For example, primate type I tonically active neurons are responsive to cues [2]. Further, type IIa neurons of the primate putamen have been observed to exhibit robust cue-evoked firing in response to a solenoid click at 50-65 msec latency [34], as similarly used in the present experiments. It is possible that the rat exhibits cue-reactivity slightly more medially in striatum than the DLS. For instance, the solenoid-reactive putamen cells recorded by Kimura [42] tended to occur in the ventromedial and dorsolateral parts of the putamen where movement-related neurons were rare and microstimulation elicited few behavior correlates.
It is possible that DLS movement-related firing is modulated by cues. In the primate, a subpopulation of putamen neurons exhibits differential firing to similar movements when cue guided, self-initiated or memory-guided [35]. In the rat, DLS neurons were reported to exhibit enhanced firing during reward-port head removal when preceded by a cue [32]. However, the possibility existed in that investigation that the head removal movement was different (slant, direction, velocity, etc) between cued and uncued conditions. Any differences in movement would alter firing in at least head movement DLS neurons, but the somatomotor properties of recorded neurons were not reported in that study. Demonstration that the exact same movement differed between cued and uncued conditions might suggest an influence of the hypothesized “limbic/cognitive/motor interface” from the cue-reactive medial shell [25] to the somatic sensorimotor DLS [26].
Given the absence of cue responsiveness in the most lateral portions of rat striatum, cue processing may occur primarily medially, both dorsally and ventrally [23], [25], [31] and gain access to motor response circuitry via laterally spiraling mesencephalic and thalamocortical connections [26]. Intermediate among these subregions is the nucleus accumbens core, which while lacking unconditioned motor correlates, develops correlations with movements that lead to rewards, and has stronger relations with these learned movements than the medial accumbens shell [9], [24]. Learned motor correlates of core neurons, as well as dorsomedial striatal neurons [24], [31] exhibit both increases as well as decreases in firing rates correlated with reward-related movements. In contrast, most laterally in striatum, unconditioned (i.e., unlearned) motor correlates are always reflected as increases in firing rate [7], [12], [15], [16], [33], [36], [48]. Furthermore, the vast majority of DLS neurons decline firing rates during the same movements across training days [45] [46] whereas both cue [25] and motor [25] firing correlates have been observed in accumbal neurons in spite of one month not performing the task. Thus, medial-lateral differences in the role of striatal subregions in cue and motor learning and performance may exist in addition to their differences in encoding of unconditioned movements. Further research is needed to explore the possibility of whether cue reactivity of neurons in the medial striatum, learned motor correlates of the ventromedial striatum (core), and unconditioned motor correlates of the lateral striatum, concomitantly influence downstream premotor neurons before and after overtraining.
Acknowledgments
This study was supported by the National Institute on Drug Abuse Grants DA 06886, DA 004551, DA 0026252. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank David J. Barker, Linda King, Patrick Grace, Kevin Coffey, Harry Ting, Jaya Sandoghar for technical assistance.
Abbreviations
- DLS
dorsolateral striatum
- M1
motor cortex
- S1
somatosensory cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aldridge JW, Berridge KC. Coding of serial order by neostriatal neurons: a “natural action” approach to movement sequence. J Neurosci. 1998;18(7):2777–87. doi: 10.1523/JNEUROSCI.18-07-02777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate's striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci. 1994;14(6):3969–84. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199(1):43–52. doi: 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Berke JD, Breck JT, Eichenbaum H. Striatal versus hippocampal representations during win-stay maze performance. J Neurophysiol. 2009;101:1575–1587. doi: 10.1152/jn.91106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown LL, Sharp FR. Metabolic mapping of rat striatum: somatotopic organization of sensorimotor activity. Brain Research. 1995;686(2):207–222. doi: 10.1016/0006-8993(95)00457-2. [DOI] [PubMed] [Google Scholar]
- 6.Brown LL, Smith DM, Goldbloom LM. Organizing principles of cortical integration in the rat neostriatum: corticostriate map of the body surface is an ordered lattice of curved laminae and radial points. J Comp Neurol. 1998;392(4):468–488. [PubMed] [Google Scholar]
- 7.Carelli RM, West MO. Representation of the body by single neurons in the dorsolateral striatum of the awake, unrestrained rat. J Comp Neurol. 1991;309(2):231–249. doi: 10.1002/cne.903090205. [DOI] [PubMed] [Google Scholar]
- 8.Carelli RM, Wolske M, West MO. Loss of lever press-related firing of rat striatal forelimb neurons after repeated sessions in a lever pressing task. J Neurosci. 1997;17(5):1804–1814. doi: 10.1523/JNEUROSCI.17-05-01804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JY, Sawyer SF, Lee RS, Woodward DJ. Electrophysiological and pharmacological evidence for the role of the nucleus accumbens in cocaine self-administration in freely moving rats. J Neurosci. 1994;14(3 Pt 1):1224–44. doi: 10.1523/JNEUROSCI.14-03-01224.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheatwood JL, Corwin JV, Reep RL. Overlap and interdigitation of cortical and thalamic afferents to dorsocentral striatum in the rat. Brain Res. 2005;1036:90–100. doi: 10.1016/j.brainres.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 11.Cheatwood JL, Reep RL, Corwin JV. The associative striatum: cortical and thalamic projections to the dorsocentral striatum in rats. Brain Res. 2003;968:1–14. doi: 10.1016/s0006-8993(02)04212-9. [DOI] [PubMed] [Google Scholar]
- 12.Cho J, West MO. Distributions of single neurons related to body parts in the lateral striatum of the rat. Brain Res. 1997;756(1-2):241–246. doi: 10.1016/s0006-8993(97)00143-1. [DOI] [PubMed] [Google Scholar]
- 13.Cho J, Duke D, Manzino L, Sonsalla PK, West MO. Dopamine depletion causes fragmented clustering of neurons in the sensorimotor striatum: evidence of lasting reorganization of corticostriatal input. J Comp Neurol. 2002;452(1):24–37. doi: 10.1002/cne.10349. [DOI] [PubMed] [Google Scholar]
- 14.Cromwell HC, Klein A, Mears RP. Single unit and population responses during inhibitory gating of striatal activity in freely moving rats. Neuroscience. 2007;146(1):69–85. doi: 10.1016/j.neuroscience.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crutcher MD, DeLong MR. Single cell studies of the primate putamen. I. Functional organization. Exp Brain Res. 1984a;53(2):233–43. doi: 10.1007/BF00238153. [DOI] [PubMed] [Google Scholar]
- 16.Crutcher MD, DeLong MR. Single cell studies of the primate putamen. II. Relations to direction of movement and pattern of muscular activity. Exp Brain Res. 1984b;53(2):244–58. doi: 10.1007/BF00238154. [DOI] [PubMed] [Google Scholar]
- 17.Deniau JM, Menetrey A, Charpier S. The lamellar organization of the rat substantia nigra pars reticulata: segregated patterns of striatal afferents and relationship to the topography of corticostriatal projections. Neuroscience. 1996;73(3):761–781. doi: 10.1016/0306-4522(96)00088-7. [DOI] [PubMed] [Google Scholar]
- 18.Dickinson A. Actions and habits: the development of behavioural autonomy. Phil Trans R Soc Lond B. 1985;308:67–78. [Google Scholar]
- 19.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flaherty AW, Graybiel AM. Corticostriatal transformations in the primate somatosensory system. Projections from physiologically mapped body-part representations. J Neurophysiol. 1991;66(4):1249–63. doi: 10.1152/jn.1991.66.4.1249. [DOI] [PubMed] [Google Scholar]
- 21.Flaherty AW, Graybiel AM. Input-output organization of the sensorimotor striatum in the squirrel monkey. J Neurosci. 1994;14(2):599–610. doi: 10.1523/JNEUROSCI.14-02-00599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flaherty AW, Graybiel AM. Two input systems for body representations in the primate striatal matrix: experimental evidence in the squirrel monkey. J Neurosci. 1993;13(3):1120–37. doi: 10.1523/JNEUROSCI.13-03-01120.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardiner TW, Kitai ST. Single-unit activity in the globus pallidus and neostriatum of the rat during performance of a trained head movement. Experimental Brain Research. 1992;88(3):517–530. doi: 10.1007/BF00228181. [DOI] [PubMed] [Google Scholar]
- 24.Ghitza UE, Fabbricatore AT, Prokopenko VF, West MO. Differences between accumbens core and shell neurons exhibiting phasic firing patterns related to drug-seeking behavior during a discriminative-stimulus task. J Neurophysiol. 2004;92(3):1608–14. doi: 10.1152/jn.00268.2004. [DOI] [PubMed] [Google Scholar]
- 25.Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci. 2003;23(19):7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20(6):2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffer ZS, Alloway KD. Organization of corticostriatal projections from the vibrissal representations in the primary motor and somatosensory cortical areas of rodents. J Comp Neurol. 2001;439(1):87–103. doi: 10.1002/cne.1337. [DOI] [PubMed] [Google Scholar]
- 28.Homayoun H, Moghaddam B. Differential representation of Pavlovian-instrumental transfer by prefrontal cortex subregions and striatum. Eur J Neurosci. 2009;29:1461–1476. doi: 10.1111/j.1460-9568.2009.06679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelley AE, Domesick VB, Nauta WJ. The amygdalostriatal projection in the rat--an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7(3):615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- 30.Kepecs A, Uchida N, Mainen ZF. Rapid and precise control of sniffing during olfactory discrimination in rats. J Neurophysiol. 2007;98:205–213. doi: 10.1152/jn.00071.2007. [DOI] [PubMed] [Google Scholar]
- 31.Kimchi EY, Laubach M. Dynamic encoding of action selection by the medial striatum. J Neurosci. 2009;29(10):3148–3159. doi: 10.1523/JNEUROSCI.5206-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimchi EY, Torregrossa MM, Taylor JR, Laubach M. Neuronal correlates of instrumental learning in the dorsal striatum. J Neurophysiol. 2009;102:475–489. doi: 10.1152/jn.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura M. Behaviorally contingent property of movement-related activity of the primate putamen. J Neurophysiol. 1990;63(6):1277–1296. doi: 10.1152/jn.1990.63.6.1277. [DOI] [PubMed] [Google Scholar]
- 34.Kimura M. Behavioral modulation of sensory responses of primate putamen neurons. Brain Res. 1992;578(1-2):204–214. doi: 10.1016/0006-8993(92)90249-9. [DOI] [PubMed] [Google Scholar]
- 35.Kimura M, Aosaki T, Hu Y, Ishida A, Watanabe K. Activity of primate putamen neurons is selective to the mode of voluntary movement: visually guided, self-initiated or memory-guided. Exp Brain Res. 1992;89:473–477. doi: 10.1007/BF00229870. [DOI] [PubMed] [Google Scholar]
- 36.Liles SL. Activity of neurons in putamen during active and passive movements of wrist. J Neurophysiol. 1985;53(1):217–36. doi: 10.1152/jn.1985.53.1.217. [DOI] [PubMed] [Google Scholar]
- 37.Marsden CD. The mysterious motor function of the basal ganglia: the Robert Wartenberg Lecture. Neurology. 1982;32(5):514–39. doi: 10.1212/wnl.32.5.514. [DOI] [PubMed] [Google Scholar]
- 38.McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29(3):503–37. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 39.Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, Knowlton BJ. The neural correlates of motor skill automaticity. J Neurosci. 2005;25(22):5356–64. doi: 10.1523/JNEUROSCI.3880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porrino LJ. Functional consequences of acute cocaine treatment depend on route of administration. Psychopharmacol (Berl) 1993;112:343–51. doi: 10.1007/BF02244931. [DOI] [PubMed] [Google Scholar]
- 41.Prokopenko VF, Pawlak AP, West MO. Fluctuations in somatosensory responsiveness and baseline firing rates of neurons in the lateral striatum of freely moving rats: effects of intranigral apomorphine. Neuroscience. 2004;125(4):1077–82. doi: 10.1016/j.neuroscience.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 42.Raudenbush SW, Bryk AS, Cheong YF, Congdon R. HLM: Hierarchical linear and nonlinear modeling (Version 6) [Computer software] Chicago, IL: Scientific Software International; 2008. [Google Scholar]
- 43.Reep RL, Cheatwood JL, Corwin JV. The associative striatum: organization of cortical projections to the dorsocentral striatum in rats. J Comp Neurol. 2003;467:271–292. doi: 10.1002/cne.10868. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi Y, Roesch MR, Stalnaker TA, Schoenbaum G. Cocaine exposure shifts the balance of associative encoding from ventral to dorsal striatum. Front Integr Neurosci. 2007:1–11. doi: 10.3389/neuro.07/011.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang C, Pawlak AP, Prokopenko V, West MO. Changes in activity of the striatum during formation of a motor habit. Eur J Neurosci. 2007;25(4):1212–1227. doi: 10.1111/j.1460-9568.2007.05353.x. [DOI] [PubMed] [Google Scholar]
- 46.Tang CC, Root DH, Duke DC, Zhu Y, Teixeria K, Ma S, Barker DJ, West MO. Decreased firing of striatal neurons related to licking during acquisition and overtraining of a licking task. J Neurosci. 2009;29(44):13952–13961. doi: 10.1523/JNEUROSCI.2824-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 48.West MO. Anesthetics eliminate somatosensory-evoked discharges of neurons in the somatotopically organized sensorimotor striatum of the rat. J Neurosci. 1998;18(21):9055–68. doi: 10.1523/JNEUROSCI.18-21-09055.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.West MO, Michael AJ, Knowles SE, Chapin JK, Woodward DJ. Striatal unit activity and the linkage between sensory and motor events. In: Schneider JS, Lidsky TI, editors. Basal Ganglia and Behavior: Sensory Aspects of Motor Functioning. Toronto: Hans Huber, Inc.; 1987. pp. 27–36. [Google Scholar]
- 50.Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19(1):181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- 51.Youngentob SL, Mozell MM, Sheehe PR, Hornung DE. A quantitative analysis of sniffing strategies in rats performing odor detection tasks. Physiology & Behavior. 1987;41(1):59–69. doi: 10.1016/0031-9384(87)90131-4. [DOI] [PubMed] [Google Scholar]