Abstract
Rationale
Cardioprotective signaling mediates anti-apoptotic actions through multiple mechanisms including maintenance of mitochondrial integrity. Pim-1 kinase is an essential downstream effector of AKT-mediated cardioprotection but the mechanistic basis for maintenance of mitochondrial integrity by Pim-1 remains unexplored. This study details anti-apoptotic actions responsible for enhanced cell survival in cardiomyocytes with elevated Pim-1 activity.
Objective
The purpose of this study is to demonstrate that the cardioprotective kinase Pim-1 acts to inhibit cell death by preserving mitochondrial integrity in cardiomyocytes.
Methods and Results
A combination of biochemical, molecular, and microscopic analyses demonstrate beneficial effects of Pim-1 upon mitochondrial integrity. Pim-1 protein level increases in the mitochondrial fraction with a corresponding decrease in the cytosolic fraction of myocardial lysates from hearts subjected to 30 minutes of ischemia followed by 30 minutes of reperfusion. Cardiac-specific overexpression of Pim-1 results in higher levels of anti-apoptotic Bcl-XL and Bcl-2 compared to samples from normal hearts. In response to oxidative stress challenge Pim-1 preserves the inner mitochondrial membrane potential (ΔΨm). Ultrastructure of the mitochondria is maintained by Pim-1 activity, which prevents swelling induced by calcium overload. Finally, mitochondria isolated from hearts created with cardiac-specific overexpression of Pim-1 show inhibition of cytochrome c release triggered by a truncated form of pro-apoptotic Bid.
Conclusion
Cardioprotective action of Pim-1 kinase includes preservation of mitochondrial integrity during cardiomyopathic challenge conditions, thereby raising the potential for Pim-1 kinase activation as a therapeutic interventional approach to inhibit cell death by antagonizing pro-apoptotic Bcl-2 family members that regulate the intrinsic apoptotic pathway.
Keywords: Pim-1, mitochondria, cardiomyocyte, apoptosis
Introduction
Cardiovascular disease (CVD) is the leading cause of death among men and women and affects approximately 33% of the US population.1 A direct correlation between the decline in heart function and loss of cardiomyocytes via apoptosis involving the mitochondria occurs in cardiomyopathy, myocardial ischemia reperfusion (I/R), and congestive heart failure.2-9 Specifically, myocardial I/R injury generates calcium overload and oxidative stress, which initiate the intrinsic apoptotic pathway through activation of the mitochondrial permeability transition pore (mPTP). The ensuing chain of events result in dramatic changes to mitochondrial morphology associated with uncoupling of the electron transport chain (ETC), depolarization of the inner membrane, matrix swelling and unfolding of the cristae, and ultimately outer membrane rupture with release of pro-apoptotic cytochrome c.10-15 Release of cytochrome c into the cytosol consequently activates apoptotic protease-activating factor (Apaf-1) that mediates caspase cascade programmed cell death.16 Thus, preservation of mitochondrial integrity is essential in designing molecular strategies to enhance cardiomyocyte cell survival by blunting injury attributed to cardiomyopathic insult.
Cardioprotection mediated by survival kinase signal transduction acts through multiple mechanisms including preservation of mitochondrial integrity.17 Numerous studies have documented anti-apoptotic actions of the serine / threonine kinase AKT which acts in part through protecting mitochondrial structure and function.17 However, the cardioprotective action of AKT signaling depends, at least in part, upon downstream induction of Pim-1 kinase.18 Pim-1 overexpression in cardiomyocytes results in enhanced cell survival, while loss of Pim-1 results in increased apoptotic cell death.18-20 Pim-1 antagonizes mitochondrial outer membrane permeabilization (MOMP) associated with release of several pro-apoptotic factors, including cytochrome c. Pim-1 suppresses MOMP by impacting upon Bcl-2 family members through a combination of inhibiting pro-apoptotic proteins as well as activating anti-apoptotic proteins. Specifically, cardiomyocytes over expressing Pim-1 exhibit increased levels of anti-apoptotic members Bcl-2 and Bcl-XL in conjunction with elevated phosphorylation and inactivation of pro-apoptotic Bad.18 These data suggest that cardioprotective effects of Pim-1 are linked to mitochondrial preservation, but fail to directly assess the impact of Pim-1 activity upon mitochondrial structure and function. Therefore, the role of Pim-1 in protection of mitochondrial integrity in cardiomyocytes was examined by multiple approaches. Taken together, our findings indicate that Pim-1 translocates to the mitochondria in response to I/R injury, enhances mitochondrial resistance to inner membrane depolarization, attenuates mitochondrial swelling, and inhibits cytochrome c release. These findings have important implications for AKT-mediated cardioprotective signaling as well as molecular therapeutic interventional strategies to blunt cell death via preservation of mitochondrial integrity.
Materials and Methods
Expanded Methods are available in the Online Data Supplement at http://circres.ahajournals.org.
Generation of Transgenic Animals
Transgenic mice were generated as previously described.21 Transgenics generated in the FVB/NJ strain (non-transgenic, NTG) express wild-type 34kDa human Pim-1 transgene (Pim WT) fused downstream of enhanced green fluorescent protein (eGFP). Similarly, the K79M kinase dead mutant (Pim DN) was fused downstream of eGFP. Transgene expression is regulated by the alpha Myosin Heavy Chain (α-MHC) promoter (a kind gift from Dr. Jeffrey Robbins, Cincinnati Children's Hospital Medical Center) for cardiomyocyte specificity. All experimental procedures were approved by San Diego State University and the Institutional Animal Care and Use Committee.
Ex Vivo Ischemia Reperfusion (I/R) Treatment
Ex vivo I/R treatment of mouse hearts was performed as previously described 22 with additional details provided in the supplement.
Mitochondrial Isolation
NTG, Pim WT, and Pim DN mice were anesthetized with 120 mg/kg ketamine and 5 mg/kg xylazine followed by excision of the heart. Isolated hearts were washed in sterile PBS (Mg and Ca2+ free), minced in 2 ml homogenization buffer (250 mmol/L sucrose, 10 mmol/L MOPS-pH 7.4, 1 mmol/L EGTA, 2mmol/L MgCl2, 0.1% BSA-fatty acid free) and briefly homogenized using a glass-teflon dounce. The homogenized mixture was centrifuged at 600 × g for 5 minutes and supernatant was decanted and centrifuged at 3000 × g for 10 minutes. The mitochondrial pellet was resuspended in 100 μl homogenization buffer. All procedures were performed on ice. Total mitochondria protein was quantified using a Bradford assay.
Neonatal Rat Cardiomyocyte (NRCM)s Culture/Infections
Isolation of NRCMs were performed as previously described 23-25 with details provided in the supplement.
Mitochondrial Swelling Assay
Sixty μg of isolated mouse heart mitochondria from NTG, Pim WT, and Pim DN (n = at least 5) in swelling buffer (250 mmol/L sucrose, 10 mmol/L MOPS, 5 μmol/L EGTA, 2 mmol/L MgCl2, 5 mmol/L KH2PO4, 5 mmol/L pyruvate, 5 mmol/L malate) were incubated with 150 μm calcium chloride (CaCl2) or 1 μmol/L cyclosporin A (CsA) in a final volume of 200 μl in a 96 well plate for 20 minutes. Absorbance was read every 30 seconds at 520 nm.
Transmission Electron Microscopy (TEM)
The protocol was adapted from a previous fixative protocol for isolated mitochondria 26 with additional details in the supplement.
Cytochrome c Assay
Isolated mouse heart mitochondria from NTG, Pim WT, and Pim DN (60 μg, (n = 5) in swelling buffer were incubated with 50 μl activated truncated Bid (tBid) protein at a final volume of 200 μl on a 96-well plate. The plate was immediately spun down at 3000 × g for 20 min to separate treated mitochondria mixture into supernatant and mitochondria pellet fractions. Sample buffer (1mol/L Tris-Hcl pH 6.8, 50% glycerol, 10% SDS, 0.288 mol/L β-mercaptoethanol, 1% bromophenol blue) was added to the fractions and the samples were boiled for 10 minutes to denature.
Western Blot Analysis
Immunoblotting was performed as described previously 27 with additional details in the supplement.
Statistical Analysis
Statistical analysis was performed using student's T test and ANOVA (one-way and two-way) for comparison where indicated. Komogorov-Smirnov (KS) test was performed to compare the distribution of mitochondrial diameter size obtained from TEM, reported as the maximum difference between the cumulative distribution (D). P values of < 0.05 were considered statistically significant.
Results
Pim-1 translocates to mitochondria in response to I/R injury
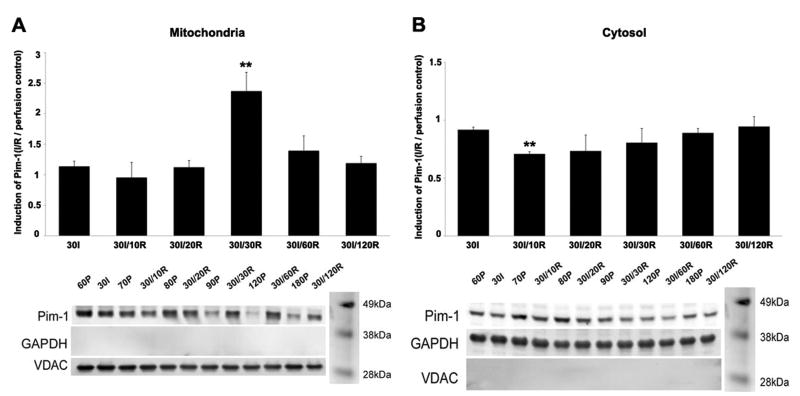
Endogenous Pim-1 (44 kDa) level in mitochondrial and cytosolic fractions of NTG heart lysates was assessed under conditions of Ischemia (I) or I/R with Perfusion (P) samples served as the control (Figure 1). The mitochondrial fraction exhibited increased Pim-1 levels starting at 30I/20R and up to 30I/120R time point compared to 30I. A significant 2.3-fold increase of Pim-1 expression was observed at 30I/30R (Figure 1A). A corresponding decrease of Pim-1 levels was also observed in the cytosolic fraction from 30I/10R up to 30I/120R compared to 30I. Specifically at 30I/10R in the cytosol, Pim-1 levels significantly decreased by 25% (Figure 1B).
Figure 1. Induction of Pim-1 in response to ex vivo I/R injury.
Mitochondrial (A) and cytosolic (B) fractions from NTG whole hearts challenged by ischemia (I), ischemia/reperfusion (I/R), or perfusion (P) as control probed for Pim-1 levels by immunoblot analyses. Corresponding quantitative graphs represent Pim-1 induction (fold change) determined by comparing Pim-1 levels (normalized to loading control, mitochondrial fraction = VDAC and cytosolic fraction = GAPDH) to corresponding perfusion control. Results represented as mean ± SEM, N = 3. Student's T test: **P<0.02 for 30I vs. 30I/30R in the mitochondrial fraction and 30I vs. 30I/10R in the cytosolic fraction.
Pim-1 enhances expression of anti-apoptotic Bcl-XL and Bcl-2
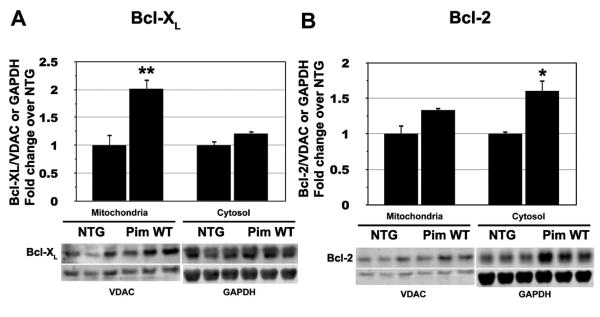
BCL-2 family members Bcl-XL and Bcl-2 were assayed for protein expression level in mitochondrial and cytosolic fractions of lysates prepared from either NTG or Pim WT hearts (Figure 2). Bcl-XL show a significant 2-fold increase in the mitochondrial fraction prepared from Pim WT compared to NTG samples, although levels of Bcl-XL were comparable in the cytosol of both NTG and Pim WT samples (Figure 2A). In comparison, Bcl-2 levels show a significant 2-fold increase in the cytosolic fraction of Pim WT relative to NTG samples, while Bcl-2 levels remain similar in the mitochondria (Figure 2B). In order to address the adverse effects of Pim-DN, mitochondrial and cytosolic fractionations were compared between NTG and Pim-DN for Bcl-2 and Bcl-XL expression. As shown in supplemental figure I, Bcl-2 levels show a significant 0.5-fold decrease in the cytosolic fraction of Pim DN relative to NTG samples. In addition, Bcl-XL and Bcl-2 show a 1.4-fold increase in both mitochondrial and cytosolic fraction of Pim WT compared to NTG samples in response to I/R injury (Supplemental Figure II). To further investigate the mechanistic role of the BCL-2 family members in mediating the protective effects of Pim-1 in cardiomyocytes, we used small interfering RNA (siRNA) to knock down the expression of Bcl-XL, Bcl-2 or both proteins. Knockdown of Bcl-XL and Bcl-2 led to a significant increase of apoptotic cells in GFP overexpressing cells after initiation of apoptosis with 0.5 mmole/L staurosporine. Interestingly, Pim-1 overexpressing cardiomyocytes were still protected from apoptosis after knockdown of Bcl-2, Bcl-XL or both proteins (Supplemental Figure III). Collectively these findings indicate that Pim-1 elevates expression of anti-apoptotic BCL-2 family members in both mitochondrial and cytoplasmic cellular compartments, although a significant inhibition of Bcl-2 family members is still possible without diminution of the anti-apoptotic action of Pim-1.
Figure 2. Anti-apoptotic Bcl-2 family members are upregulated in Pim WT hearts.
Bcl-XL (A) and Bcl-2 (B) from mitochondrial and cytosolic fractions of NTG mouse hearts probed by immunoblot analyses to determine protein expression levels. Quantitative graphs represent relative Bcl-XL and Bcl-2 levels normalized to VDAC (mitochondrial fraction) or GAPDH (cytosolic fraction). Results are represented as mean ± SEM, n = at least 3 hearts. Student's T test: **P<0.02 for NTG vs. Pim WT Bcl-XL levels in the mitochondria fraction. *P<0.05 for NTG vs. Pim WT Bcl-2 levels in the cytosolic fraction.
Mitochondria show inhibition of inner membrane depolarization by Pim-1
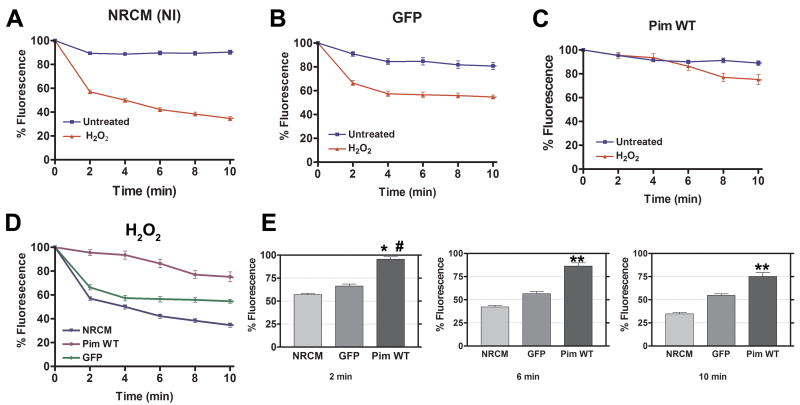
Non-infected cardiomyocytes (NRCM-NI) and cardiomyocytes infected with adenovirus expressing either GFP or GFP-tagged wild-type Pim-1 (Pim WT) were labeled with TMRE and challenged by oxidative stress (100 μmol/L H2O2) to assess preservation of mitochondrial inner membrane potential (ΔΨm). Maintenance of ΔΨm in cardiomyocytes from Pim WT, GFP, and the NRCM-NI groups under normal conditions is evident by TMRE fluorescence intensity above 80% throughout the 10-minute time course (NRCM-NI = 90.3%, GFP = 80.8%, Pim WT = 89.0%; Figure 3A-C). NRCM and GFP groups challenged with H2O2 show dramatic decreases in fluorescence within 2 minutes leading to significantly lower fluorescence intensities at all time points examined indicative of mitochondrial inner membrane depolarization (Figure 3A-B,D-E). Strikingly, TMRE fluorescence intensity in Pim WT exposed to H2O2 is also maintained above 80% for up to 8 minutes, after which 75.2% of fluorescence intensity remained at 10 minutes of exposure (Figure 3C). A control experiment using NRCM treated with CCCP, an uncoupler (100 nmol/L), evokes a response similar to H2O2-induced oxidative stress (65.4% TMRE florescence intensity within 4 min), confirming the decrease of TMRE fluorescence can be attributed to mitochondrial inner membrane depolarization (Supplemental Figure IV).
Figure 3. Mitochondrial inner membrane depolarization in cardiomyocytes induced by H2O2 is delayed by Pim-1 expression.
TMRE fluorescence of NRCMs under control conditions (untreated) versus oxidative stress challenge (H2O2; 100 μmol/L). NRCM (NI) = non-infected NRCM (A), GFP = NRCM infected with GFP adenovirus (B), Pim WT = NRCM infected with Pim-1 adenovirus (C), and combined plot comparing results of A, B, and C is shown in D. Quantitative graphs comparing H2O2 treated NRCM, GFP, and Pim WT at 2, 6, and 10 minutes (E). Results are represented as mean ± SEM. N = at least 3 independent experiments. One-way ANOVA: *P< 0.001 for NRCM (NI) vs. Pim WT at 2 minutes. #P<0.001 for Pim WT vs. GFP at 2 minutes. **P<0.001 for NRCM (NI) vs. GFP vs. Pim WT at 6 and 10 minutes.
Mitochondrial swelling is attenuated by Pim-1
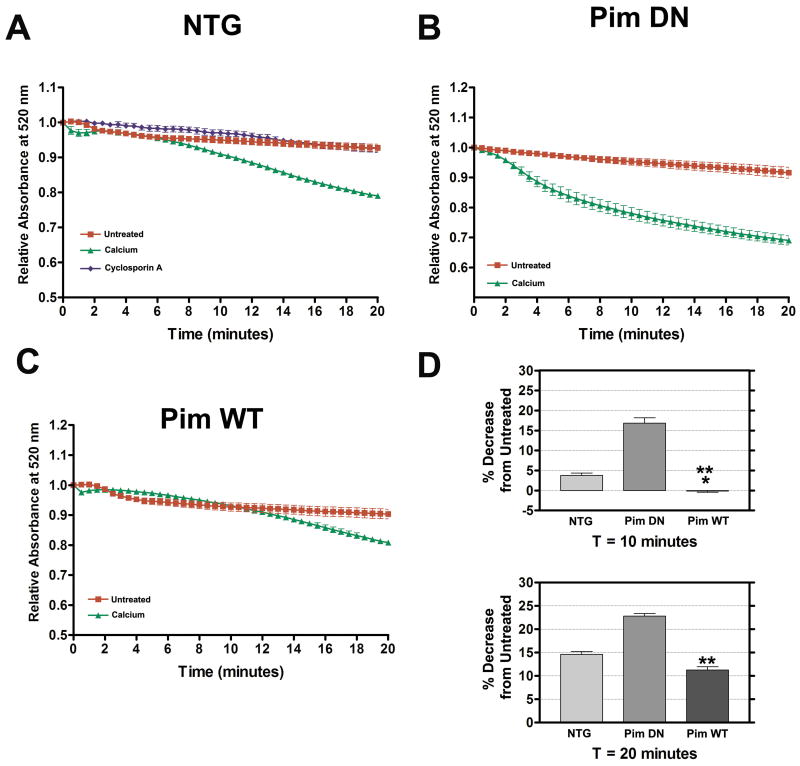
Mitochondria were isolated from Pim WT, Pim DN, and NTG hearts, challenged with calcium overload stimulus (150 μmol/L CaCl2) and the rate of mitochondrial swelling determined by light scattering. Samples of untreated Pim WT, Pim DN, and NTG mitochondria show stable absorbance at 520 nm throughout the 20-minute time course (Figure 4A-C). However, Pim DN and NTG mitochondria challenged with calcium overload show decreased absorbance at 8 minutes and 2 minutes respectively indicative of swelling (Figure 4A-B). At 8 minutes, calcium treated Pim DN mitochondria showed an accelerated rate of swelling as seen by the dramatic decrease in absorbance at 14.3% compared to untreated Pim DN mitochondria, while NTG had a 1.5% decrease compared to untreated NTG mitochondria. In contrast, Pim WT mitochondria maintain relatively stable absorbance indicative of resistance to calcium-induced swelling reading for up to 12 minutes after which absorbance starts to decrease (Figure 4C). The greatest difference in swelling is observed between the calcium treated isolated mitochondrial samples (NTG, Pim WT, and Pim DN) at 14 minutes. Calcium treated Pim WT mitochondria exhibited a 4.1% decrease of absorbance compared to untreated Pim WT mitochondria, whereas calcium treated NTG mitochondria had an 8.7% decrease compared to untreated NTG mitochondria and calcium treated Pim DN mitochondria with 19.9% compared to untreated Pim DN mitochondria. By the end of the 20-minute time course the Pim WT mitochondria exposed to calcium exhibit only an 11.3% decrease of absorbance relative to untreated Pim WT mitochondria (Figure 4D), whereas calcium treated NTG mitochondria had a 14.7% decrease compared to untreated NTG mitochondria, while calcium treated Pim DN mitochondria had the highest rate of calcium-induced mitochondrial swelling with a 22.1% decrease (Figure 4D). As a control, NTG mitochondria treated with the mPTP inhibitor CsA (1 μmol/L) prevented mitochondrial swelling and maintained a stable absorbance reading (Figure 4A).
Figure 4. Pim-1 overexpression attenuates calcium-induced mitochondrial swelling.
Rate of mitochondrial swelling of untreated, calcium treated (150 μmol/L CaCl2), and CsA treated (1 μmol/L CsA) isolated NTG mitochondria (A). Rate of mitochondrial swelling of untreated and calcium treated mitochondria from Pim DN (B) or Pim WT (C). Quantitative graphs comparing calcium treated NTG, Pim DN, and Pim WT's percentage decrease of 520 nm absorbance reading at 10 minutes and 20 minutes (D). Results represented as mean ± SEM. N = at least 5. One-way ANOVA: *P<0.01 for NTG vs. Pim WT at 10 minutes. **P<0.001 for NTG vs. Pim DN and Pim DN vs. Pim WT at 10 minutes; NTG vs. Pim DN v. Pim WT at 20 minutes.
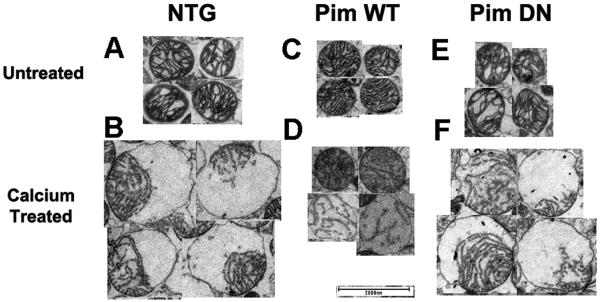
Mitochondrial ultrastructure was directly visualized to confirm the effects of calcium-induced swelling in the NTG, Pim WT, and Pim DN samples. Untreated mitochondrial samples isolated from Pim WT, Pim DN, or NTG hearts show intact cristae uniformly distributed across the organelle (Figure 5A,C,E). However, calcium challenged NTG and Pim DN mitochondria show evidence of matrix swelling represented by unfolded cristae localized at one pole of the organelle (Figure 5 B,F). Interestingly, examples of distressed mitochondria could be observed in Pim DN preparations that include disfigured mitochondria in untreated samples as well as massive swelling with inner membrane ruptured through the outer membrane in while calcium treated Pim DN mitochondria (Supplemental Figure V). In comparison, protective effects were evident in samples from Pim WT mitochondria that resist calcium-induced matrix swelling and contain either intact or only partially folded cristae following challenge (Figure 5D).
Figure 5. Representative electron micrographs of mitochondria under normal or calcium overload conditions.
Untreated mitochondrial preparations from NTG (A), Pim WT (C), or Pim DN (E) compared to calcium challenged NTG (B), Pim WT (D), or Pim DN (F). Magnification at 2700X, scale bar set at 2000 nm.
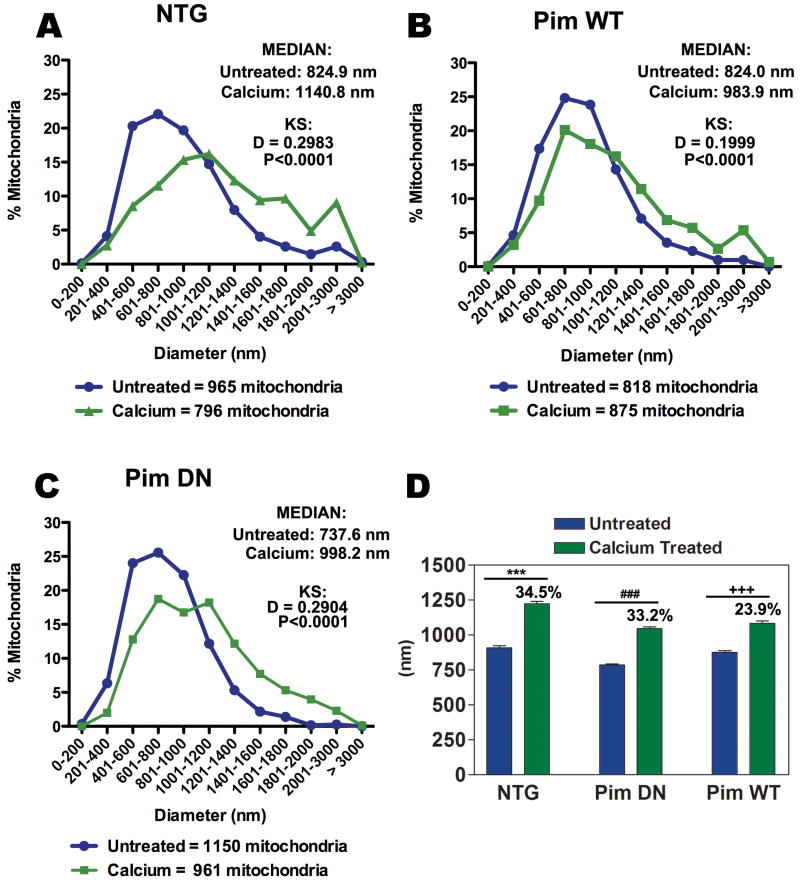
Morphometric determinations were assessed by measuring the diameter (nm) of each mitochondria from TEM micrographs and comparing the maximum shift of diameter distribution (D) between untreated and calcium treated samples using KS test (Figure 6). Mitochondria from untreated NTG samples possess a median diameter of 825.0 nm, but exposure to calcium resulted in a 34.5% increase in diameter (D = 0.2983) indicative of matrix swelling (Figure 6 A, D). The median diameter of Pim DN mitochondria is 737.6 nm with a 33.2% increase in diameter size (D = 0.2904) (Figure 6 C,D). While Pim WT mitochondria normally show a median diameter of 824.0 nm comparable to NTG samples, the calcium treated Pim WT mitochondria show inhibition of swelling relative to NTG samples with a 23.9% average increase in diameter (D = 0.1999) (Figure 6 B and D). Although the diameter size between calcium treated Pim WT and calcium treated Pim DN appear to be relatively similar (calcium treated Pim WT = 1083.99 ± 17.29, calcium treated Pim DN = 1045.38 ± 13.86 nm), untreated Pim DN have a significantly smaller initial size in diameter compared to untreated Pim WT (untreated Pim WT = 875.27 ± 12.60, untreated Pim DN = 785.10 ± 9.05) (Fig. 6d). This resulted in calcium treated Pim DN with a larger percentage increase of 33.2% in diameter size compared to Pim WT with 23.9% (Fig. 6d), consistent with our data that showed calcium treated Pim DN with a significantly greater rate of swelling compared to calcium treated NTG and calcium treated PIM WT (Fig. 4d).
Figure 6. Morphometric comparison of mitochondrial diameter resulting from exposure to calcium overload.
Quantitative graphs displaying the percentage of total untreated and calcium treated mitochondria with corresponding diameter in nm (A-C). The median of each sample is listed in the upper right corner of each graph with reported diameter shift (D) and significance (P) value using KS test for NTG (A), Pim WT (B), and Pim DN (C) mitochondrial preparations from 2 separate hearts. Average diameter length represented as mean ± SEM with corresponding percentage increase when treated with calcium (D). One-way ANOVA: ***P<0.001 for untreated NTG vs. calcium treated NTG, ###P<0.001 for untreated Pim DN vs. calcium treated Pim DN, +++P<0.001 for untreated Pim WT vs. calcium treated Pim WT.
Cytochrome c release from mitochondria is prevented by Pim-1
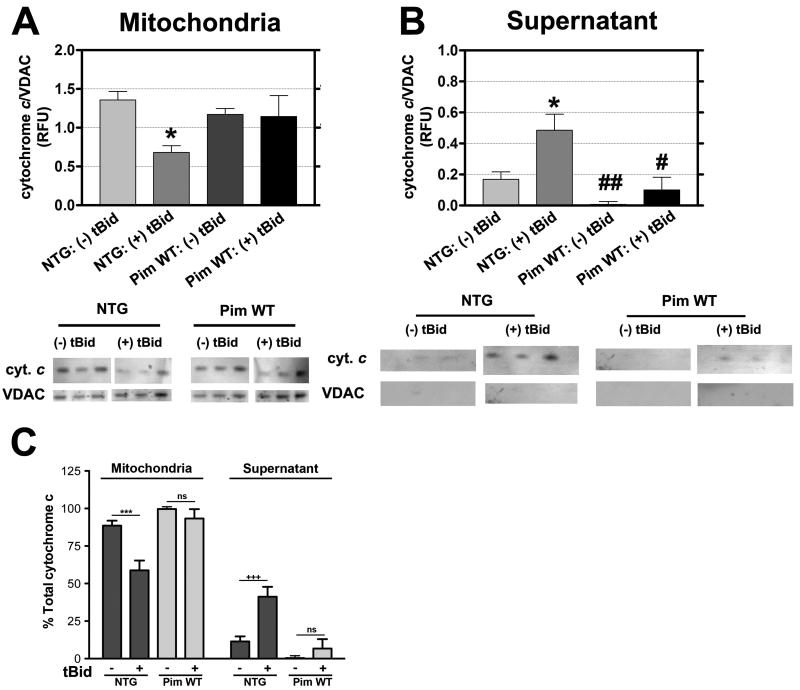
Mitochondrial preparations from NTG or Pim WT hearts were challenged with tBid to induce cytochrome c release from mitoplasm into the cytosolic fraction indicating loss of membrane integrity and proapoptotic signaling (Figure 7). Mitochondrial preparations from Pim WT possess 99.4% of the total cytochrome c and show modest release after tBid challenge (91.9% versus 8.1% in the mitochondrial pellet versus supernatant, respectively). In comparison, while untreated NTG mitochondria retain 89.0% of total cytochrome c in the assay, the tBid challenge prompts release into the supernatant and decreases the percentage retained in the mitochondrial pellet to 58.3% with a corresponding increase to 41.6% in the supernatant fraction (Figure 7C). Collectively, these results demonstrate a higher degree of resistance to tBid induced release in mitochondria isolated from Pim WT hearts.
Figure 7. Pim-1 overexpression decreases cytochrome c release from mitochondria.
Preparations of NTG versus Pim WT under normal conditions (- tBid) or challenged with tBid (+ tBid) and subsequently separated into mitochondrial pellet (A) or supernatant (B) fractions for immunoblot analysis to quantitate cytochrome c levels. Corresponding quantitative graph is normalized to VDAC as a loading control. Results are represented as mean ± SEM. N = 5 hearts. Two-way ANOVA: *P<0.05 for untreated NTG vs. tBid treated NTG in mitochondrial and supernatant fraction, #P<0.01 for tBid treated NTG vs. tBid treated Pim WT in supernatant fraction, ##P<0.001 for tBid treated NTG vs. untreated Pim WT in supernatant fraction. (C) Corresponding percentage of total cytochrome c in mitochondrial and supernatant fractions of untreated and tBid treated isolated NTG and Pim WT mitochondria. Two-way ANOVA: ***P<0.001 for untreated NTG vs. tBid treated NTG in mitochondrial fraction, +++P<0.001 for untreated NTG vs. tBid treated in supernatant fraction, ns = not significant for untreated Pim WT vs. tBid treated Pim WT in mitochondrial and supernatant fraction.
Discussion
Mitochondria are key regulators in the intrinsic apoptotic pathway and recent studies also view mitochondria as a target for cardioprotection.2-9 In response to a variety of stress signals to the heart, mitochondria undergo dramatic changes in morphology that ultimately result in the release of several pro-apoptotic factors including cytochrome c to trigger activation of caspase cascade programmed cell death.9 Dynamic regulation of this intrinsic apoptotic pathway depends upon Bcl-2 family proteins.28 When activated, pro-apoptotic members Bax and Bak translocate from the cytosol to the outer mitochondrial membrane in order to mediate cytochrome c release from the mitochondria through MOMP.9 Bad and Bid are pro-apoptotic BH3-only proteins that also localize to the outer mitochondrial membrane upon activation, assisting MOMP activation by antagonizing anti-apoptotic effects of Bcl-2 and Bcl-XL.9, 29 Bcl-2 and Bcl-XL sequester pro-apoptotic tBid and Bad at the mitochondria, resulting in the prevention of Bax and Bak translocation and activation.30 Bak can also be directly sequestered by Bcl-XL at the mitochondria.31
Pim-1 is part of a family of survival kinases that function downstream of JAK/STAT and AKT signaling.17-18, 32 Pim-1 enhances cell survival by regulating members of the Bcl-2 family 33,34, targets pro-apoptotic Bcl-2 family members, and is an upstream regulator of Bcl-2 and Bcl-XL expression.35 Bcl-XL and Bcl-2 expression levels elevated in mitochondrial versus cytosolic fractions (respectively) of Pim WT hearts as shown in Figure 2 are consistent with initial findings using cultured cardiomyocytes with elevated Pim-1 activity.18 Although Pim-1's protection of the mitochondria is mediated by the upregulation of BCL-2 family proteins that are primarily located in the cytosol, an upregulation of Bcl-XL was observed in the mitochondrial fraction (Figure 2a). It has been previously reported that the 33kD version of Pim-1 upregulated Bcl-2 levels while the 44 kD version was shown to antagonize the pro-apoptotic effects in cells overexpressing Bax.36 The additional presence of Pim-1 expression in the mitochondria could therefore supplement the inhibition of the apoptotic intrinsic pathway by targeting Bcl-XL in the mitochondria and further preventing Bax/Bak activation of MOMP. Previous studies have also reported that Bcl-2 can directly interact with Bax in the cytosol and prevent Bax translocation to the mitochondria.37 The upregulation of cytosolic Bcl-2 observed in Pim WT hearts (Figure 2b, Supplemental Figure II) could play a role of sequestering Bax in response to stress and I/R injury. Pim-1 cardioprotection also may involve inhibition of pro-apoptotic Bad via increased phosphorylation18 as previously reported to occur at both residues Ser112 34 and Ser136. 35 Phosphorylation at the regulatory site Ser112 prevents Bad from binding and inhibiting Bcl-XL and Bcl-2 34,35, whereas phosphorylation at Ser136 signals Bad to be sequestered by 14-3-3 and prevent activation of apoptosis. 38,39 Since Pim-1 expression inhibits tBid induced cytochrome c release (Figure 7), blunting of MOMP activation is a plausible mechanism for the observed preservation of mitochondrial integrity by Pim-1 through combined inhibition of pro-apoptotic molecules and enhancing expression levels of anti-apoptotic Bcl-2 family members.
Pim-1 mediates an anti-apoptotic effect through Bcl-2 family members including increasing expression as well as phosphorylation of pro-apoptotic proteins, but knockdown experiments of Bcl-2 and Bcl-XL suggest that Pim-1 may work through both Bcl-2 dependent and independent mechanisms that could not be deciphered in our experiments. It is possible that Bcl-2 and Bcl-XL can still provide a substantial protective effect if levels are not completely eliminated by siRNA treatment in our studies. Pim-1 phosphorylation of Bad in combination with residual Bcl-2 and Bcl-XL in our system could supply enough protective effect to inhibit staurosporine-mediated apoptosis. Alternatively, Bcl-2 independent mechanisms may also contribute to Pim-1 mediated protection, such as phosphorylation of mitochondrial hexokinase II.40 Alternate mechanisms of protection and cell integrity mediated by Pim-1 are a subject of ongoing studies.
In addition to MOMP, activation of mPTP during calcium overload-dependent necrotic cell death can also triggers release of cytochrome c from mitochondria. 41,42 I/R injury generates an increase in cytosolic calcium and oxidative stress in cardiomyocytes that subsequently triggers opening of the mPTP pore, inner membrane depolarization and mitochondrial swelling, and eventual rupture of the outer membrane and release of cytochrome c. 10-15,41 Pim-1 may play a direct role in preventing calcium overload during reperfusion by decreasing cell calcium loading. Overexpression of Pim-1 enhances calcium handling and reuptake in part by increased sarco/endoplasmic reticulum Ca2+ ATP-ase 2a (SERCA2a) and sodium/calcium exchanger (NCX) expression.18 While it is uncertain whether Pim-1 interferes with ROS formation, previous studies have shown that the presence of oxidative stress induced Pim-1 protein and mRNA expression43 as well as increased phosphorylated Pim-1 protein levels.44 In addition, recent findings have shown Pim-1's protective effects from oxidative stress-induced apoptosis by inhibiting apoptosis signaling kinase1 (ASK1) and the caspase-3 cascade.45
Although mPTP activation and MOMP independently regulate the intrinsic apoptotic pathway, recent studies suggest a connection between the two during key events of inner membrane depolarization and mitochondrial swelling. Bcl-XL and Bcl-2 not only promote cell survival by binding and inhibiting pro-apoptotic Bcl-2 family members 31,46, but also prevent cytochrome c release by regulating the inner mitochondrial membrane potential.47 Indeed, Pim-1 overexpression in cardiomyocytes protects ΔΨm in the face of oxidative stress challenge (Figure 3). Thus, Pim-1's enhancement of Bcl-XL and Bcl-2 expression levels may also participate in prevention of inner membrane depolarization in both MOMP and mPTP activation.
Preservation of morphology and size in Pim WT isolated mitochondria (Figures 4-6) reinforces the postulate that overexpression of Pim-1 protects mitochondrial structural integrity. Recent studies show that Bax also plays a role in regulating calcium concentrations at the endoplasmic reticulum and sarcoplasmic reticulum (ER/SR). Consequently, activation of Bax results in overabundance of calcium taken up by mitochondria and ultimately triggering activation of mPTP.36 Bcl-XL expression raised by Pim-1 also functions to inhibit Bax's generation of calcium overload, thereby suppressing mitochondrial swelling and disruption of the outer mitochondrial membrane.
Loss of Pim-1 activity has also been found to produce deleterious consequences in the context of cardiac-specific Pim DN expression in transgenic mice.48 The participation of mitochondria in the cardiomyopathic phenotype of Pim DN hearts is supported by observations in this report of increased rate of calcium induced swelling and disruption of structural integrity in mitochondria when Pim DN was present (Figures 4,5 and Supplemental Figure V). Future studies may uncover additional links between the loss of inducible cardioprotection in Pim-1 knockout mice 18 and the destabilization of mitochondrial integrity in this report.
Another subject to be explored is Pim-1's relation to mitochondrial fusion and fission events. Recent studies point to regulation of mitochondrial morphology as a mechanism to mediate apoptosis where fission can occur during apoptosis resulting in formation of “small and round mitochondrial fragments”.49 Pro-apoptotic Bcl-2 family members are implicated in this process, as Bax colocalizes and activates dynamin-related protein-1 (DRP-1), a fission protein located at scission sites at the foci of the outer mitochondrial membrane. 9,49 Our findings provide initial insights regarding Pim-1's inhibition of Bax activity by enhancing Bcl-XL expression levels and preventing tBid induced cytochrome c release (Figures 1 and 7), and ongoing studies are needed to correlate Pim-1's cardioprotective role with mitochondrial fusion and fission.
Pim-1 offers many intriguing cardioprotective actions that may prove useful as a therapeutic agent for myocardial repair and protection from cardiac failure. For example, overexpression of Pim-1 blunts infarction injury in the myocardium, while inactivation of Pim-1 increases infarction injury and fibrosis. 17,18,21 In addition, hypertrophic remodeling is inhibited by Pim-1 leading to improved hemodynamic function 17,21 that may be due in part to enhanced calcium dynamics and cardiac contractility through increased expression of SERCA2a.18,21 We now add preservation of mitochondrial structure and function to the mechanistic basis for Pim-1 mediated cardioprotection that promotes cardiomyocytes survival by inhibition of MOMP and mPTP activation. By targeting the mitochondria, Pim-1 can serve as a therapeutic intervention in the treatment of cardiomyopathy damage by blunting cell death through the intrinsic apoptotic pathway.
Novelty and Significance
What is Known?
Pim-1 is a cardioprotective kinase that inhibits cell death and cardiomyocyte hypertrophy induced by pathologic injury without apparent maladaptive side effects.
Myocardial regeneration is enhanced using cardiac stem cells genetically engineered to overexpress Pim-1.
Protection of mitochondrial integrity is critical for cellular function and survival.
What New Information does this article contribute?
Pim-1 kinase preserves mitochondrial integrity, thereby enhancing cellular survival.
Multiple types of pathologic challenge including oxidative stress, calcium overload, and pro-apoptotic cascades are similarly inhibited by Pim-1 activity.
The protective effects of Pim-1 kinase are mediated by both mitochondrial-dependent and mitochondrial-independent mechanisms.
Identification of the Pim-1 cardioprotective kinase challenges us to examine long-standing observations regarding the anti-apoptotic effects of the Akt signaling cascade. Now that we appreciate the role of Pim-1 for enhancing survival downstream of Akt, understanding the mechanism of Pim-1-mediated cellular protection in the cardiac context is critical to assess therapeutic utility and potential clinical relevance to treat heart disease. In this report we demonstrate that Pim-1 activity enhances resistance to pathologic insults that compromise mitochondrial integrity, such as calcium overload, oxidative stress, and pro-apoptotic signaling. Enhanced mitochondrial integrity was observed in both intact cardiomyocytes and purified mitochondrial preparations. Furthermore, loss of Pim-1 activity correlated with enhanced susceptibility to pathologic challenge. The protective effect of Pim-1 appears mediated through a combination of mitochondrial dependent and independent actions. Based upon these findings, preservation of mitochondrial integrity is an important mechanism for the cardioprotective actions of Pim-1. This salutary influence provides a basis for using Pim-1 as a molecular interventional strategy to protect myocardial structure and function and may explain enhanced regenerative and reparative capacity of hearts and stem cells engineered to express Pim-1 kinase.
Supplementary Material
Acknowledgments
The authors wish to thank all members of the Sussman laboratory for their helpful discussions and technical support.
Sources of Funding: N.A.G. is supported by the Rees-Stealy Foundation and the American Heart Association Predoctoral Training Grant. M.A.S. is supported by National Institutes of Health grants 5R01HL067245, 1R01HL091102, 1P01HL085577, 1R37HL091102 and 1P01AG023071.
Abbreviations
- ΔΨm
mitochondrial inner membrane potential
- a-MHC
alpha myosin heavy chain
- ANP
atrial natriuretic peptide
- AP
alkaline phosphatase
- Apaf-1
apoptotic protease-activating factor
- ASK1
apoptosis signaling kinase1
- CCCP
carbonyl cyanide 3-chlorophenylhydrazone
- CsA
cyclosporin A
- CVD
cardiovascular disease
- CY5
cyanine5
- eGFP
enhanced green fluorescent protein
- ER/SR
endoplasmic reticulum/sarcoplasmic reticulum
- ETC
electron transport chain
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GFP
green fluorescent protein
- HRP
horseradish peroxidase
- I/R
ischemia/reperfusion
- KHB
Krebs-Henseleit buffer
- KS
Komogorov-Smirnov test
- MOMP
mitochondrial outer membrane permeabilization
- mPTP
mitochondrial permeability transition pore
- NCX
sodium/calcium exchanger
- NRCM
neonatal rat cardiomyocyte
- NRCM-NI
non-infected neonatal rat cardiomyocyte
- NTG
non-transgenic, wildtype
- Pim DN
expressing K79M dominant negative mutant of Pim-1
- Pim WT
expressing 34kDa Pim-1 transgene
- ROS
reactive oxygen species
- SERCA2a
sarco/endoplasmic reticulum Ca+2 ATP-ase 2a
- siRNA
small interfering RNA
- tBid
activated truncated Bid
- TEM
transmission electron microscopy
- TMRE
tetramethylrhodamine ethyl ester
- VDAC
voltage activated anion channel
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, Mc Dermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics—2007 update: a Statistics Subcommitte. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, Dal Bello B, Semigran MJ, Bielsa-Masdeu A, Dec GW, Israels S, Ballester M, Virmani R, Saxena S, Kharbanda S. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci USA. 1999;96:8144–8149. doi: 10.1073/pnas.96.14.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steenbergen C, Afshari CA, Petranka JG, Collins J, Martin K, Bennett L, Haugen A, Bushel P, Murphy E. Alterations in apoptotic signaling in human idiopathic cardiomyopathic hearts in failure. Am J Physiol Heart Circ Physiol. 2003;284:H268–H276. doi: 10.1152/ajpheart.00707.2002. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, Gambert SR, Cigola E, Anversa P. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol. 1996;28:2005–2016. doi: 10.1006/jmcc.1996.0193. [DOI] [PubMed] [Google Scholar]
- 6.Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation. 1997;95:320–323. doi: 10.1161/01.cir.95.2.320. [DOI] [PubMed] [Google Scholar]
- 7.Guerra S, Leri A, Wang X, Finato N, Di Loreto C, Beltrami CA, Kajstura J, Anversa P. Myocyte death in the failing human heart is gender dependent. Circ Res. 1999;85:856–866. doi: 10.1161/01.res.85.9.856. [DOI] [PubMed] [Google Scholar]
- 8.Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 9.Gustafsson AB, Gottlieb RA. BCL-2 family members and apoptosis, taken to heart. AM J Physiol Cell Physiol. 2007;292:C45–51. doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- 10.Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta. 1998;1366:79–94. doi: 10.1016/j.bbabio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Halestrap AP, Kerr PM, Javadov S, Woodfield KY. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta. 1998;1366:79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 12.Jennings RB, Reimer KA. The cell biology of acute myocardial ischemia. Annu Rev Med 42. 1991:225–246. doi: 10.1146/annurev.me.42.020191.001301. [DOI] [PubMed] [Google Scholar]
- 13.Appleyard RF, Cohn LH. Myocardial stunning and reperfusion injury in cardiac surgery. J Card Surg. 1993;8:316–324. doi: 10.1111/j.1540-8191.1993.tb01332.x. [DOI] [PubMed] [Google Scholar]
- 14.Suleiman MS, Halestrap AP, Griffiths EJ. Mitochondria: a target for myocardial protection. Pharmacol Ther. 2001;89:29–46. doi: 10.1016/s0163-7258(00)00102-9. [DOI] [PubMed] [Google Scholar]
- 15.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 16.Zou H, Li Y, Liu X, Wang X. An APAF1-cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto S, Rubio M, Sussman MA. Nuclear and mitochondrial signalling Akts in cardiomyocytes. Cardiovasc Res. 2009;82:272–285. doi: 10.1093/cvr/cvp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, Esposito G, Delucchi F, Arcarese M, Alvarez R, Siddiqi S, Emmanuel GN, Wu W, Fischer K, Martindale JJ, Glembotski CC, Leri A, Kajstura J, Magnuson N, Berns A, Beretta RM, Houser SR, Schaefer EM, Anversa P, Sussman MA. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 19.Bachmann M, Moroy T. The serine/threonine kinase PIM-1. Int J Biochem Cell Biol. 2005;37:726–730. doi: 10.1016/j.biocel.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Bhattacharya N, Weaver M, Petersen K, Meyer M, Gapter L, Magnuson NS. Pim-1: a serine/threonine kinase with a role in cell survival, proliferation, differentiation, and tumorigenesis. J Vet Sci. 2001;2:167–179. [PubMed] [Google Scholar]
- 21.Muraski JA, Fischer KM, Wu W, Cottage CT, Quijada P, Mason M, Din S, Gude N, Alvarez R, Jr, Rota M, Kajstura J, Wang Z, Schaefer E, Chen X, MacDonnel S, Magnuson N, Houser SR, Anversa P, Sussman MA. Pim-1 kinase antagonizes aspects of myocardial hypertrophy and compensation to pathological pressure overload. Proc Natl Acad Sci USA. 2008;105(37):13889–94. doi: 10.1073/pnas.0709135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison LE, Whittaker RJ, Klepper RE, Wawrousek EF, Glembotski CC. Roles for αB-crystallin and HSPB2 in protecting the myocardium from ischemia-reperfusion-induced damage in a KO mouse model. Am J Physiol Heart Circ Physiol. 2004;286:H847–855. doi: 10.1152/ajpheart.00715.2003. [DOI] [PubMed] [Google Scholar]
- 23.Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, Nurzynska D, Kajstura J, Leri A, Anversa P, Sussman MA. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res. 2004;94:884–891. doi: 10.1161/01.RES.0000124394.01180.BE. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharya N, Wang Z, Davitt C, McKenzie IF, Xing PX, Magnuson NS. Pim-1 associates with protein complexes necessary for mitosis. Chromosoma. 2002;111:80–95. doi: 10.1007/s00412-002-0192-6. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs MD, Black J, Futer O, Swenson L, Hare B, Fleming M, Saxena K. Pim-1 Ligand-bound Structures Reveal the Mechanism of Serine/Threonine Kinase Inhibition by LY294002. J Biol Chem. 2005;280:13728–13734. doi: 10.1074/jbc.M413155200. [DOI] [PubMed] [Google Scholar]
- 26.von Ahsen O, Renken C, Perkins G, Kluck RM, Bossy-Wetzel E, Newmeyer DD. Preservation of mitochondrial structure and function after Bid- or Bax-mediated cytochrome c release. J Cell Biol. 2000;150:1027–36. doi: 10.1083/jcb.150.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato T, Muraski J, Chen Y, Tsujita Y, Wall J, Glembotski CC, Schaefer E, Beckerle M, Sussman MA. Atrial natriuretic peptide promotes cardiomyocyte survival by cGMP- dependent nuclear accumulation of zyxin and Akt. J Clin Invest. 2005;115:2716–2730. doi: 10.1172/JCI24280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green DR, Kroemer G. Pharmacological manipulation of cell death: clinical applications in sight? J Clin Investig. 2005;115:2610–2617. doi: 10.1172/JCI26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13:1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- 30.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-XL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 31.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammerman PS, Fox CJ, Birnbaum MJ, Thompson CB. Pim and Akt oncogenes are independent regulators of hematopoietic cell growth and survival. Blood. 2005;105:4477–4483. doi: 10.1182/blood-2004-09-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aho TL, Sandholm J, Peltola KJ, Mankonen HP, Lilly M, Koskinen PJ. Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett. 2004;571:43–49. doi: 10.1016/j.febslet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 34.Macdonald A, Campbell DG, Toth R, McLauchlan H, Hastie CJ, Arthur JS. Pim kinases phosphorylate multiple sites on Bad and promote 14-3-3 binding and dissociation from Bcl-XL. BMC Cell Biol. 2006;7:1. doi: 10.1186/1471-2121-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. 14-3-3 Proteins and Survival Kinases Cooperate to Inactivate BAD by BH3 Domain Phosphorylation. Mol Cell. 2000;6:44–51. [PubMed] [Google Scholar]
- 36.Lilly M, Sandholm J, Cooper JJ, Kosikinen PJ, Kraft A. The Pim-1 serine kinase prolongs survival and inhibits apoptosis-related mitochondrial dysfunction in part through a bcl-2 dependent pathway. Oncogene. 1999;18:4022–4031. doi: 10.1038/sj.onc.1202741. [DOI] [PubMed] [Google Scholar]
- 37.Murphy KM, Ranganathan V, Farnsworth ML, Kavallaris M, Lock RB. Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Diff. 2000;7:102–111. doi: 10.1038/sj.cdd.4400597. [DOI] [PubMed] [Google Scholar]
- 38.Chiang CW, Kanies C, Kim KW, Fang WB, Parkhurst C, Xie M, Henry T, Yang E. Protein Phosphatase 2A Dephosphorylation of Phosphoserine 112 Plays the Gatekeeper Role for BAD-Mediated Apoptosis. Mol Cell Biol. 2003;23:6350–6362. doi: 10.1128/MCB.23.18.6350-6362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci USA. 1999;92:7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyamoto S, Rubio M, Sussman MA. Nuclear and mitochondrial signalling Akts in cardiomyocytes. Cardiovasc Res. 2009;82:272–285. doi: 10.1093/cvr/cvp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–44. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–62. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 43.Katakami N, Kaneto H, Hao H, Umayahara Y, Fujitani Y, Sakamoto K, Gorogawa S, Yasuda T, Kawamori D, Kajimoto Y, Matsuhisa M, Yutani C, Hori M, Yamasaki Y. Role of Pim-1 in smooth muscle cell proliferation. J Biol Chem. 2004;279:54742–54749. doi: 10.1074/jbc.M409140200. [DOI] [PubMed] [Google Scholar]
- 44.Wan J, Winn LM. In utero exposure to benzene increases embryonic c-Myb and Pim-1 protein levels in CD-1 mice. Toxicol Appl Pharmacol. 2008;228:326–333. doi: 10.1016/j.taap.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Gu JJ, Wang Z, Reeves R, Magnuson NS. PIM1 phosphorylates and negatively regulates ASK1-mediated apoptosis. Oncogene. 2009;28:4261–4271. doi: 10.1038/onc.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 47.Nutt LK, Pataer A, Pahler J, Fang B, Roth J, McConkey DJ, Swisher SG. Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. J Biol Chem. 2002;277:9219–9225. doi: 10.1074/jbc.M106817200. [DOI] [PubMed] [Google Scholar]
- 48.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 49.Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.