Abstract
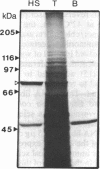
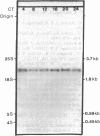
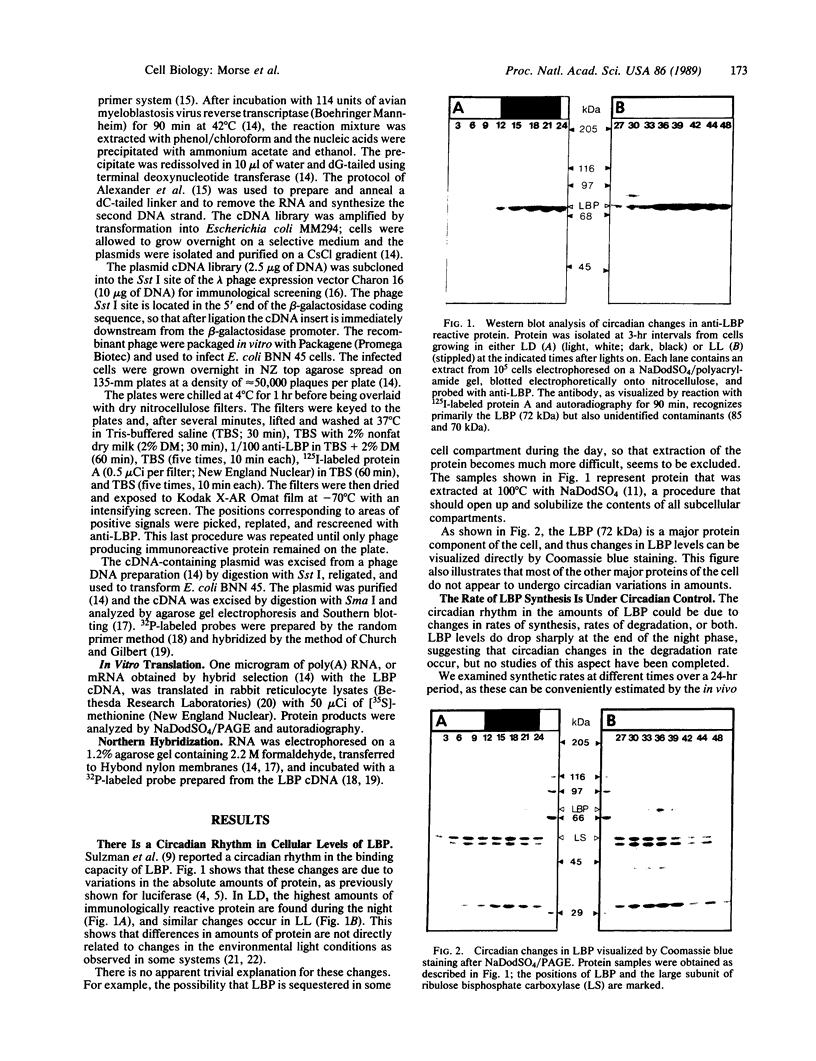
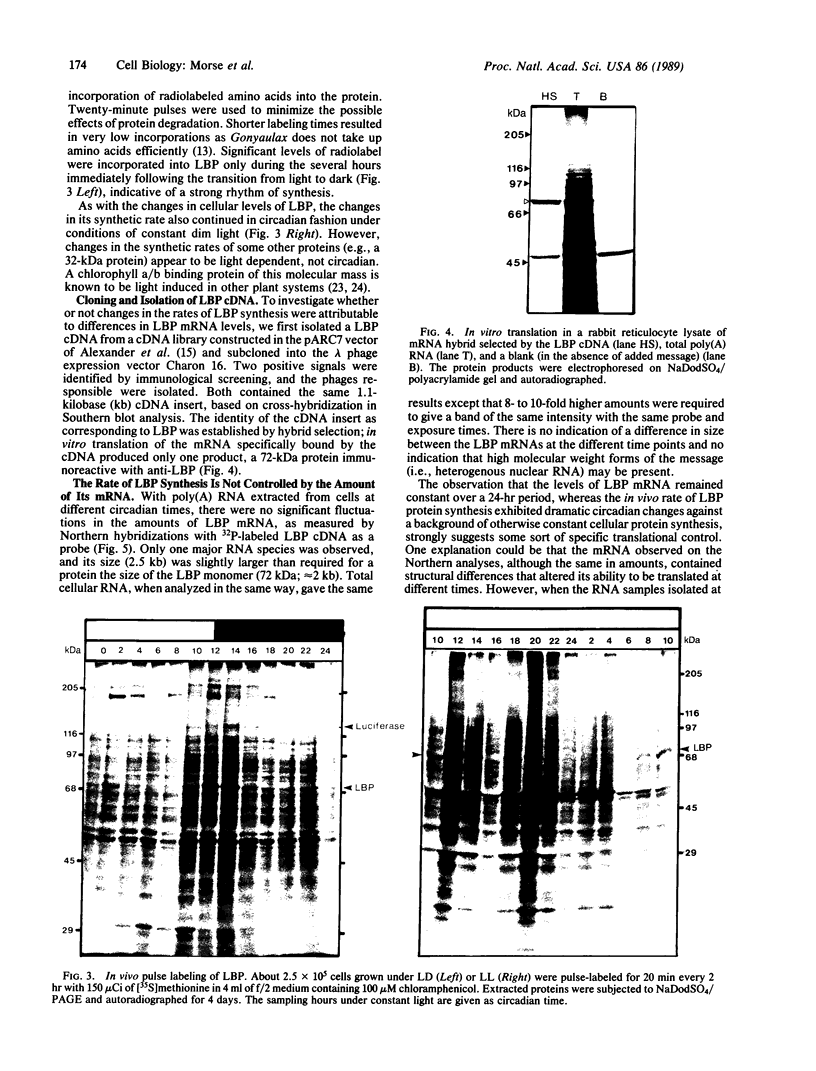
A 10-fold circadian variation in the amount of luciferin binding protein (LBP) in the marine dinoflagellate Gonyaulax polyedra is reported. This protein binds and stabilizes luciferin, the bioluminescence substrate. In early night phase, when bioluminescence is increasing and LBP levels are rising in the cell, pulse labeling experiments show that LBP is being rapidly synthesized in vivo. At other times, the rate of LBP synthesis is at least 50 times lower, while the rate of synthesis of most other proteins remains the same. The LBP mRNA levels, as determined by in vitro translations and by RNA (Northern) hybridizations, do not vary over the daily cycle, indicating that circadian control of bioluminescence in this species is mediated by translation.
Full text
PDF
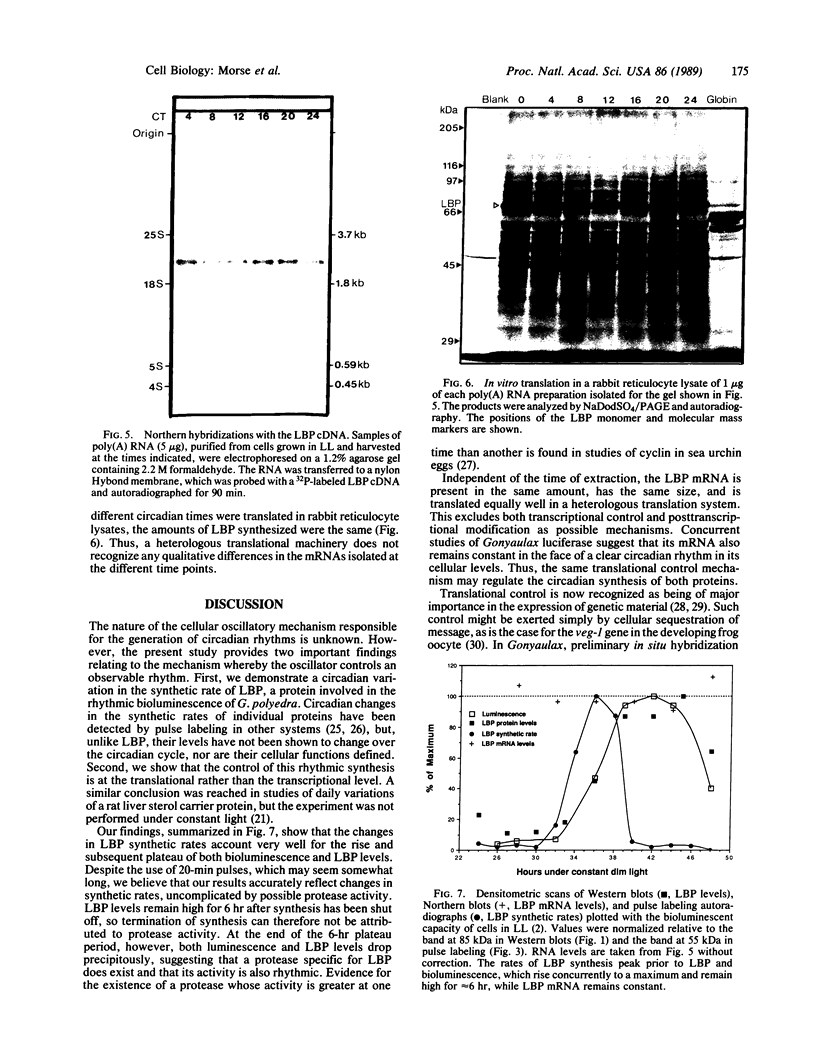

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. C., McKnight T. D., Williams B. G. A simplified and efficient vector-primer cDNA cloning system. Gene. 1984 Nov;31(1-3):79–89. doi: 10.1016/0378-1119(84)90197-5. [DOI] [PubMed] [Google Scholar]
- Bode V. C., Desa R., Hastings J. W. Daily Rhythm of Luciferin Activity in Gonyaulax polyedra. Science. 1963 Sep 6;141(3584):913–915. doi: 10.1126/science.141.3584.913. [DOI] [PubMed] [Google Scholar]
- Brown C. R. Avian sociobiology: population ecology of the cooperatively breeding acorn woodpecker. Science. 1987 Dec 11;238(4833):1590–1591. doi: 10.1126/science.238.4833.1590. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap J. C., Hastings J. W. The biological clock in Gonyaulax controls luciferase activity by regulating turnover. J Biol Chem. 1981 Oct 25;256(20):10509–10518. [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feldman J. F. Lengthening the period of a biological clock in Euglena by cycloheximide, an inhibitor of protein synthesis. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1080–1087. doi: 10.1073/pnas.57.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel M., Hastings J. W. A substrate-binding protein in the Gonyaulax bioluminescence reaction. Arch Biochem Biophys. 1971 Jan;142(1):310–321. doi: 10.1016/0003-9861(71)90289-x. [DOI] [PubMed] [Google Scholar]
- Hartwig R., Schweiger M., Schweiger R., Schweiger H. G. Identification of a high molecular weight polypeptide that may be part of the circadian clockwork in Acetabularia. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6899–6902. doi: 10.1073/pnas.82.20.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma K., Hastings J. W. Cell cycle synchronization of Gonyaulax polyedra by filtration: quantized generation times. J Biol Rhythms. 1988 Spring;3(1):49–58. doi: 10.1177/074873048800300104. [DOI] [PubMed] [Google Scholar]
- Johnson C. H., Inoué S., Flint A., Hastings J. W. Compartmentalization of algal bioluminescence: autofluorescence of bioluminescent particles in the dinoflagellate Gonyaulax as studied with image-intensified video microscopy and flow cytometry. J Cell Biol. 1985 May;100(5):1435–1446. doi: 10.1083/jcb.100.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. H., Roeber J. F., Hastings J. W. Circadian changes in enzyme concentration account for rhythm of enzyme activity in gonyaulax. Science. 1984 Mar 30;223(4643):1428–1430. doi: 10.1126/science.223.4643.1428. [DOI] [PubMed] [Google Scholar]
- KARAKASHIAN M. W., HASTINGS J. W. THE EFFECTS OF INHIBITORS OF MACROMOLECULAR BIOSYNTHESIS UPON THE PERSISTENT RHYTHM OF LUMINESCENCE IN GONYAULAX. J Gen Physiol. 1963 Sep;47:1–12. doi: 10.1085/jgp.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakashian M. W., Schweiger H. G. Evidence for a cycloheximide-sensitive component in the biological clock of Acetabularia. Exp Cell Res. 1976 Mar 15;98(2):303–312. doi: 10.1016/0014-4827(76)90442-0. [DOI] [PubMed] [Google Scholar]
- Kirk M. M., Kirk D. L. Translational regulation of protein synthesis, in response to light, at a critical stage of Volvox development. Cell. 1985 Jun;41(2):419–428. doi: 10.1016/s0092-8674(85)80015-5. [DOI] [PubMed] [Google Scholar]
- Klein R. R., Mullet J. E. Regulation of chloroplast-encoded chlorophyll-binding protein translation during higher plant chloroplast biogenesis. J Biol Chem. 1986 Aug 25;261(24):11138–11145. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire G., Gold L., Yarus M. Autogenous translational repression of bacteriophage T4 gene 32 expression in vitro. J Mol Biol. 1978 Nov 25;126(1):73–90. doi: 10.1016/0022-2836(78)90280-2. [DOI] [PubMed] [Google Scholar]
- McGarry T. J., Lindquist S. The preferential translation of Drosophila hsp70 mRNA requires sequences in the untranslated leader. Cell. 1985 Oct;42(3):903–911. doi: 10.1016/0092-8674(85)90286-7. [DOI] [PubMed] [Google Scholar]
- McGuire D. M., Olson C. D., Towle H. C., Dempsey M. E. Translational control of the circadian rhythm of liver sterol carrier protein. J Biol Chem. 1984 May 10;259(9):5368–5371. [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Ochoa S. Regulation of protein synthesis initiation in eucaryotes. Arch Biochem Biophys. 1983 Jun;223(2):325–349. doi: 10.1016/0003-9861(83)90598-2. [DOI] [PubMed] [Google Scholar]
- Olesiak W., Ungar A., Johnson C. H., Hastings J. W. Are protein synthesis inhibition and phase shifting of the circadian clock in Gonyaulax correlated? J Biol Rhythms. 1987 Summer;2(2):121–138. doi: 10.1177/074873048700200204. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Poutre C. G., Fox T. D. PET111, a Saccharomyces cerevisiae nuclear gene required for translation of the mitochondrial mRNA encoding cytochrome c oxidase subunit II. Genetics. 1987 Apr;115(4):637–647. doi: 10.1093/genetics/115.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman B. S., Strumwasser F. Phase shifting the circadian rhythm of neuronal activity in the isolated Aplysia eye with puromycin and cycloheximide. Electrophysiological and biochemical studies. J Gen Physiol. 1976 Oct;68(4):359–384. doi: 10.1085/jgp.68.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell D. J., Alexander D. C., Williams B. G., Etzler M. E. cDNA cloning and in vitro synthesis of the Dolichos biflorus seed lectin. Eur J Biochem. 1987 Sep 1;167(2):227–231. doi: 10.1111/j.1432-1033.1987.tb13327.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks D. L., Melton D. A. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell. 1987 Dec 4;51(5):861–867. doi: 10.1016/0092-8674(87)90109-7. [DOI] [PubMed] [Google Scholar]