Abstract
Brucellosis is a widespread zoonotic disease that is also a potential agent of bioterrorism. Current serological assays to diagnose human brucellosis in clinical settings are based on detection of agglutinating anti-LPS antibodies. To better understand the universe of antibody responses that develop after B. melitensis infection, a protein microarray was fabricated containing 1,406 predicted B. melitensis proteins. The array was probed with sera from experimentally infected goats and naturally infected humans from an endemic region in Peru. The assay identified 18 antigens differentially recognized by infected and non-infected goats, and 13 serodiagnostic antigens that differentiate human patients proven to have acute brucellosis from syndromically similar patients. There were 31 cross-reactive antigens in healthy goats and 20 cross-reactive antigens in healthy humans. Only two of the serodiagnostic antigens and eight of the cross-reactive antigens overlap between humans and goats. Based on these results, a nitrocellulose line blot containing the human serodiagnostic antigens was fabricated and applied in a simple assay that validated the accuracy of the protein microarray results in the diagnosis of humans. These data demonstrate that an experimentally infected natural reservoir host produces a fundamentally different immune response than a naturally infected accidental human host.
Author Summary
Brucellosis is a bacterial disease transmitted from infected animals to humans. This disease often presents as a prolonged but non-specific illness primarily characterized as fever without specific organ localization. Because infections can result after ingestion (typically from unpasteurized animal milk or milk products from goats, cattle or sheep) or inhalation (important because of bioterrorism potential) of small numbers of organisms, the bacteria that cause brucellosis are potential biological warfare agents. Here, a protein microarray containing 1406 Brucella melitensis proteins was used to study the antibody response of experimentally infected goats and naturally infected humans in B. melitensis infection. Goats recognized 18 proteins and humans recognized 13 proteins as serodiagnostic antigens; antibody detection of only two of these antigens was shared by goats and humans, suggesting either fundamentally different immune responses or different responses in relation to mode or setting of infection. The human serodiagnostic antigens were evaluated in a simple nitrocellulose line blot assay, which validated the protein microarray results. The approach described here will lead to the development of new diagnostics for brucellosis and other infectious diseases, and aid in understanding the human and animal host immune response to pathogenic organisms.
Introduction
Brucellosis is a zoonotic infectious disease endemic in regions around the world where agricultural, animal husbandry and vaccination practices have not controlled infection among livestock reservoirs [1]–[3]. The reservoirs of Brucella melitensis, the most virulent species affecting humans, include goats and sheep [4], especially in Peru and the Middle East [3]. Identification of goat, sheep and other animal sources of infection have long used agglutination tests, although newer tests are being developed and applied in the veterinary setting [5]–[7]. Commonly used screening tests do not necessarily differentiate between vaccination and infection in goats ([8]; summarized in [6]). By themselves, the Rose Bengal and other agglutination tests cannot be used exclusively to diagnose human brucellosis because while sensitive and specific for first episodes of brucellosis, these tests can be problematic in differentiating acute, chronic and relapsing forms of brucellosis in humans living in endemic regions [9]–[12], and typically require titration and differentiation of IgM from IgG antibodies either in solid phase formats or by use of the mercaptoethanol test [1], [3], [13]–[16].
The current knowledge of protein antigens recognized by humans and reservoir animals is limited to a relatively small number of immunogenic Brucella abortus proteins recognized by cattle, mice and sheep and limited studies on human and goat recognition of Brucella melitensis antigens [9]–[11], [17]–[33]. No individual antigen has proven to be of sufficient diagnostic utility to replace the LPS-based tests. Indeed, antibodies to smooth LPS have been observed to arise sooner in the course of brucellosis compared to known antigens or groups of uncharacterized cytoplasmic protein antigens [15], [34]–[43], especially if treatment is initiated early after clinical presentation [43]. We tested the hypothesis that the immune response to B. melitensis infection of natural reservoir host (goat) and accidental host (humans) is similar despite potentially different routes of infection. For this we constructed a protein microarray consisting of 1406 B. melitensis proteins and probed with a collection of sera from naturally infected and control human sera from Lima Peru, and goats experimentally infected with virulent B. melitensis 16M.
Materials and Methods
Ethics statement
Human sera were obtained from patients enrolled in a prospective clinical study of brucellosis in Lima, Peru. The human subjects part of the study was approved by the Humans Research Protections Committee of the University of California San Diego, the Comite de Ética of Universidad Peruana Cayetano Heredia, Lima, Peru and the Comite de Ética of Asociación Benéfica PRISMA, Lima, Peru, all of whom have maintained federal wide assurances with the United States Department of Health and Human Services. All patients provided written informed consent prior to enrollment in the study, and signed consent forms have been stored in locked files in study offices at UPCH and AB PRISMA, Lima, Peru.
Goat sera were obtained from previously stored samples from experimentally infected goats under Institutional Animal Care and Use protocols approved by Texas A&M University, College Station, Texas, USA. Animals were housed in an outdoor, restricted access, large-animal isolation facility operated under guidelines approved by the United States Department of Agriculture/Animal and Plant Health Inspection Service (USDA/APHIS). At the termination of the experiments, adult animals were euthanized by captive bolt. All animals were disposed of by University approved protocols.
Gene amplification and cloning
Genes were amplified and cloned using high-throughput PCR and recombination method as described previously [44]. ORFs from Brucella melitensis 16M genomic DNA were identified using GenBank NC_003317 and NC_003318, amplified using gene specific primers containing 33bp nucleotide extension complementary to ends of linearized pXT7 vector. Homologous recombination takes place between the PCR product and pXT7 vector in competent DH5a cells. The recombinant plasmids were isolated from this culture using QIAprep 96 Turbo kit (Qiagen). Around one quarter of the cloned genes were sequenced and verified that the correct sequence was inserted. The resulting fusion proteins also harbor a hemagglutinin epitope at 3′ end and polyhistidine at the 5′ end.
Microarray printing and staining
Plasmids were expressed at 30°C in 5 hour- in vitro transcription/translation E. coli system (RTS 100 kits from Roche), according to the manufacturer's instructions. For microarrays, 15 µl of reaction was mixed with 5 µl 0.2% Tween 20 to give a final concentration of 0.05% Tween 20, and 15-µl volumes were transferred to 384-well plates and printed onto nitrocellulose coated glass FAST slides (Whatman) using Omni Grid 100 microarray printer (Genomic Solutions). Protein expression and printing was monitored by immunoprobing with anti-polyhistidine (clone His-1, Sigma) and anti-hemagglutinin (clone 3F10, Roche). For all array staining, sera samples were diluted to 1∶200 in Protein Array Blocking Buffer (Schleicher & Schuell). Slides were first blocked for 30 min in protein array-blocking buffer before incubation with primary antibody at 4°C overnight with agitation. The slides are then washed extensively and incubated in biotin-conjugated secondary antibody (Jackson Immuno Research) diluted 1/200 in blocking buffer. After washing, bound antibodies are detected by incubation with streptavidin-conjugated SureLight® P-3 (Columbia Biosciences). The slides are washed and air dried after brief centrifugation. Slides were scanned and analyzed using a Perkin Elmer ScanArray Express HT microarray scanner. Intensities are quantified using QuantArray software. All signal intensities are corrected for spot-specific background.
Brucella melitensis serum samples
Human sera tested in this study were obtained from the following patient groups: patients confirmed (by positive blood culture) to have acute brucellosis in Lima, Peru; from culture-negative, Rose Bengal-positive patients presenting with brucellosis-compatible syndromes; Rose Bengal-negative patients referred by their physicians for possible brucellosis; and ambulatory, apparently healthy control patients from the north Lima neighborhood of Puente Piedra where brucellosis is known to occur with risk factors similar to those in the rest of Lima. No patients in this study were known to be directly exposed to goats; risk factors for all were reported to be ingestion of unpasteurized goat's milk products, the typical risk factor in Lima for acquisition of brucellosis. All patients included in this study had their first known episode of brucellosis, with clinical presentation within 1–3 weeks of onset of symptoms. The patient samples were as follows: 42 serum samples from B. melitenis culture-positive patients all of whom were positive by the Rose Bengal screening test and had tube agglutination tests > = 1/160; and 18 samples from culture negative, Rose Bengal serology-positive patients. These latter 18 samples were from culture negative individuals diagnosed with brucellosis and treated according to standard antibiotic therapy within 2 days of serum sampling. Additional control patient samples included 13 sera from Rose Bengal-negative patients, 44 samples from ambulatory healthy controls from north Lima where brucellosis occasionally affects patients, and sera from humans in the U.S. where brucellosis is not found.
Goat sera tested in this study were positive (B. melitensis 16M-infected) and negative (uninfected) controls from a previously conducted vaccine safety study [23] in which pregnant, card-test negative angora goats were inoculated with B. melitensis. Goats were experimentally infected with 1×107 CFU of Brucella melitensis strain 16M by bilateral conjunctival instillation at 110 days' gestation, and sera were collected 8 weeks after infection. As an additional negative control, 15 serum samples from a specific pathogen-free goat flock were obtained (Capralogics, Inc, Hardwick, MA).
Immunostrip printing and probing
Thirteen plasmids of interest were expressed in five hour in vitro transcription-translation reactions (RTS 100 E. coli HY Kit from Roche) according to the manufacturer's instructions. VIG was obtained from ADi as a gift, and the concentration of VIG was diluted to 0.05 mg/ml. Proteins were printed on Optitran BA-S 85 0.45 µm Nitrocellulose membrane (Whatman) using BioJet dispenser (BioDot) at 1 µl/cm, and cut into 3 mm strips. Individual strips were then blocked in 10% non fat dry milk dissolved in 10 mM Tris (pH 8.0) and 150 mM NaCl containing 0.05% (v/v) Tween 20 buffer for 30 minutes. Prior to immunostrip probing, forty two culture positive and forty four Peruvian naive sera were diluted to 1/250 in 10% non fat dry milk solution containing 20% E. coli lysate (McLab) and incubated for 30 minutes with constant mixing at room temperature. Each strip was then incubated with pretreated sera overnight at 4°C with gentle mixing. Strips were then washed five times in Tris buffer containing 0.05% (v/v) tween 20, and then incubated for 1 hour at room temperature in alkaline phosphatase conjugated donkey anti-human immunoglobulin (anti-IgG, Fcγ fragment-specific, Jackson ImmunoResearch), diluted to 1/5000 in tris buffer containing 0.05% (v/v) tween 20. Strips were then washed extensively and reactive bands were visualized by incubating with 1-step Nitro-Blue Tetrazolium Chloride/5-Bromo-4-Chloro-3′-Indolyphosphate p-Toluidine Salt (NBT/BCIP) developing buffer (Thermo Fisher Scientific) for 1 minute at room temperature. Strips were scanned with Hewlett-Packard scanner, and were quantified using Image J software.
Data analysis
All analysis was performed using the R statistical environment (http://www.r-project.org). It has been noted in the literature that data derived from microarray platforms is heteroskedatic [45]–[48]. This mean-variance dependence has been observed in the arrays presented in this manuscript [49], [50]. In order to stabilize the variance, the vsn method [51] implemented as part of the Bioconductor suite (www.bioconductor.org) is applied to the quantified array intensities. In addition to removing heteroskedacity, this procedure corrects for non-specific noise effects by finding maximum likelihood shifting and scaling parameters for each array such that control probe variance is minimized. This calibration has been shown to be effective on a number of platforms [52]–[54]. Normalized data is retransformed with the ‘sinh’ function to allow visualization and discussion at an approximate raw scale.
Diagnostic biomarkers between groups were determined using a Bayes regularized t-test adapted from Cyber-T for protein arrays [47], [48], which has been shown to be more effective than other differential expression techniques [55]. To account for multiple testing conditions, the Benjamini and Hochberg (BH) method was used to control the false discovery rate [56]. Multiplex classifiers were constructed using linear and non-linear Support Vector Machines (SVMs) using the “e1071” R package. SVM is a supervised learning method that has been successfully applied to microarray data characterized by small samples sizes and a large number of attributes [50], [57]. The SVM approach, as any other supervised classification approach, uses a training dataset to build a classification model and a testing set to validate the model. To generate unbiased training and testing sets, leave one out cross-validation (LOOCV) was used. With this methodology, each data point is tested with a classifier trained using all of the remaining data points. Plots of receiver operating characteristic (ROC) curves were made with the ‘ROCR” R package.
Results
Gene amplification, cloning and protein expression
A set of 1406 ORFs from Brucella melitensis 16M was selected for this study. We picked 1009 antigens based on their Psort information and B cell epitope prediction score, and 397 ORFs were randomly selected. The ORFs were amplified from Brucella melitensis 16M (Bm) genomic DNA and cloned using the high throughput recombination method previously described [44]. About one-fourth of the cloned genes were sequenced and >99% of sequenced clones had the correct sequence in frame with correct orientation. Bm ORFs cloned in pXT7 vector were expressed under T7 promoter in the E. coli in vitro transcription/translation system, and printed in duplicates on microarrays as described in Methods and 97.4% of the proteins were positive for the His tag ( Fig. 1a ), and 95.4% were positive for HA tags.
Figure 1. Construction of a B. melitensis Protein Microarray.
Arrays were printed containing 1406 B. melitensis proteins, positive and negative control spots. Proteins were printed in duplicates. Each array contains positive control spots printed from 6 serial dilutions of human and mouse IgG, 6 serial dilutions of EBNA1 protein, and “No DNA” negative control spots. (A) The array was probed with anti-His antibody as described in Materials and Methods, to confirm the expression and printing of over 95% proteins. (B) Comparison of arrays probed with Peruvian naïve serum and Culture positive serum. The arrays were read in a laser confocal scanner, analyzed, and the data normalized as described in Materials and Methods. The signal intensity of each antigen is represented by rainbow palette of blue, green, red and white by increasing signal intensity.
Immune screening with goat serum samples
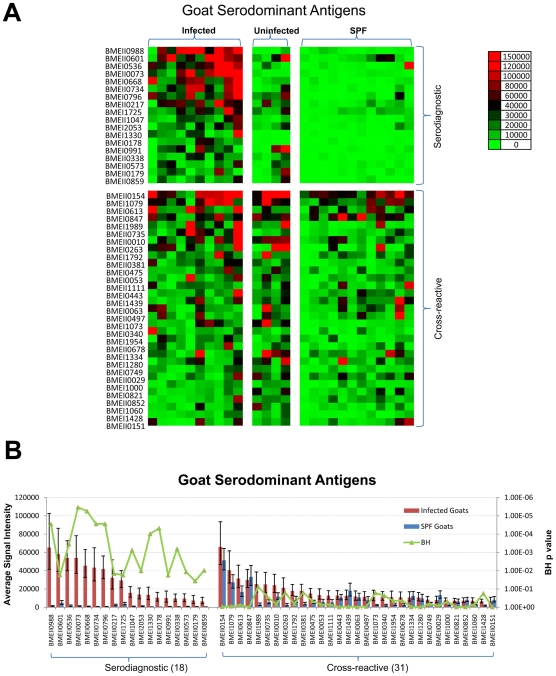
Bm protein arrays were probed with sera from experimentally infected goats, naïve goats from the same pasture, and specific pathogen free (SPF) goats from a different location. Reactivity of sera from the individual goats is shown as a heat map with samples grouped according to their description ( Fig. 2a ). Data were analyzed using methods described elsewhere [58]. Serodominant antigens are defined as antigens with mean signal intensity greater than the mean plus two standard deviations above the negative controls. Serodiagnostic antigens are significantly differentially reactive serodominant antigens with adjusted Cyber-T p-values between infected and SPF goats <0.05. All of the sera, whether from infected, uninfected or naïve goats, reacted similarly to the cross-reactive antigens (p-value >0.05). A set of 49 antigens were identified to be serodominant among 1406 antigens tested. Of these, 18 antigens were serodiagnostic, and reacted differentially between infected goats and SPF goats (p-value <0.05). The remaining 31 serodominant antigens reacted similarly among all goats ( Fig. 2a, 2b ).
Figure 2. Probing a collection of B. melitensis infected, uninfected, and SPF control goat sera and discovery of goat serodiagnostic antigens.
Arrays containing 1406 B. melitensis proteins were probed with goat sera organized into 3 groups as described in the text. (A). Heatmap showing normalized intensity with red strongest, bright green weakest and black in between. The antigens are in rows and are grouped to serodiagnostic and cross-reactive antigens. The goat samples are in columns and sorted left to right by increasing average intensity to serodiagnostic antigens. (B) The mean sera reactivity of the 1406 antigens was compared between the Infected and SPF Naive groups. Antigens with Benjamini Hochberg corrected p-value less than 0.05 are organized to the left and cross-reactive antigens to the right. The 18 most reactive serodiagnostic and 31 of the most reactive cross-reactive antigens are shown.
Human antigenic profile
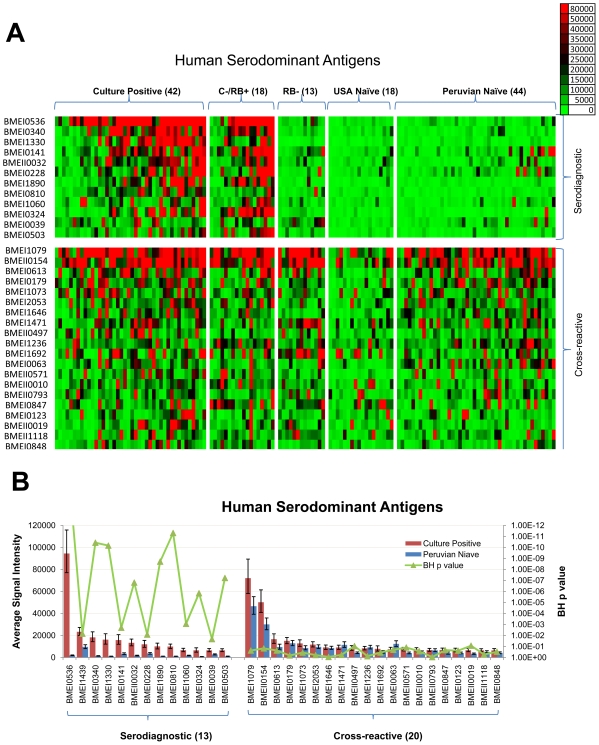
Bm protein arrays were also probed with sera from acute brucellosis patients in Lima, Peru obtained within 1–3 weeks of the onset of symptoms. All patients in this study, as is true of virtually all patients from Lima [3], [59]–[62], were infected with B.. melitensis biovar 1. Sera from Bm culture-positive humans ( Fig. 1b ) showed pronounced reactivity against several antigens compared to unexposed individuals. A set of 33 antigens was identified to be serodominant among 1406 antigens tested ( Fig. 3a, 3b ). Of these, 13 antigens were serodiagnostic, and reacted differentially between naïve and culture positive patients from Peru (p-value<.05). The same antigens also reacted robustly with individuals diagnosed Rose Bengal positive but negative by blood culture for B. melitensis. For some of these subjects, treatment with antibiotics may have resulted in a negative blood culture for B. melitensis. The elevated antibody response from a few individuals in the Peruvian naïve group might be indicative of past exposure to similar proteins in environmental bacteria, or to a past subclinical Brucella infection. We also identified 20 cross-reactive antigens that reacted similarly among all human samples, whether from naïve individuals or individuals diagnosed to be infected and use of these antigens in serodiagnostic tests can therefore be selectively avoided.
Figure 3. Probing a collection of B. melitensis human sera and discovery of human serodiagnostic antigens.
Arrays were probed with human sera organized into 5 groups: Culture Positive, Culture Negative/Rose Bengal Positive, Rose Bengal Negative, USA Naïve, and Peruvian Naïve, as described in the text. (A). Heatmap showing normalized intensity with red strongest, bright green weakest and black in between. The antigens are in rows and are grouped to serodiagnostic and cross-reactive antigens. The human samples are in columns and sorted left to right by increasing average intensity to serodiagnostic antigens. (B) The mean sera reactivity of the 1406 antigens was compared between the Culture Positive and Peruvian Naive groups. Antigens with Benjamini Hochberg corrected p-value less than 0.05 are organized to the left and cross-reactive antigens to the right. The 13 most reactive serodiagnostic and 31 of the most reactive cross-reactive antigens are shown. C−/RB+, Culture Positive and Rose Bengal negative; RB−, Rose Bengal negative. Numbers in () are case numbers from each group.
Identification of serodiagnostic antigens
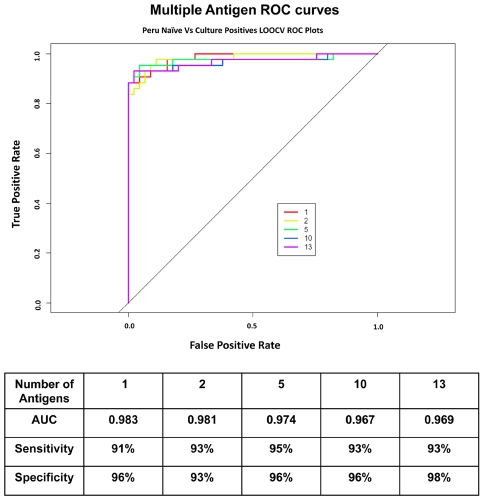
To establish a collection of antigens able to accurately distinguish brucellosis cases from controls, leave one out cross-validation (LOOCV) receiver operating characteristic (ROC) curves were generated for individual serodiagnostic antigens to assess the ability to separate the control and disease cases ( Fig. 4 ). The serodiagnostic antigens were ordered by decreasing single antigen area under the curve (AUC). The top ten ORFs all have an AUC greater than 0.734 (Table 1), with BP26 (BMEI0536; AUC 0.983; Benjamini and Hochberg adjusted Cyber-T p-value<10e-16) giving the best single antigen discrimination with sensitivity and specificity 91% and 96% ( Fig. 4 ), respectively. We used kernel methods and support vector machines [47], [63] to build linear and nonlinear classifiers. As input to the classifier, we used the highest-ranking 1, 2, 5, 10, 13 ORFs on the basis of single antigen AUC. The results show that increasing the antigen number from 2 to 5 produced an improvement in sensitivity and specificity ( Fig. 4 ). This classifier yielded a high sensitivity and specificity rate of 95% and 96%, respectively.
Figure 4. Multiple Antigen LOOCV ROC curves.
The LOOCV ROC graphs show classifiers with increasing number of human serodiagnostic antigens. Overall, the sensitivity and specificity for array test is over 95%.
Table 1. Common and specific Brucella melitensis antigens for humans and goats.
| B. melitensis 16M | B. abortus 2308 | B. suis 1330 | AUC | Product Name | Reference for other Brucella species | |
| Serodiagnostic for both Humans and Goats | BMEI0536 | BAB1_1494 | BR1475 | 0.983 | 26 kDa periplasmic immunogenic protein bp26 | {Cloeckaert, 1996; Connolly, 2006; Lindler, 1996; Yang, 2005} |
| BMEI1330 | BAB1_0635 | BR0611 | 0.870 | Protease Do | {Roop, 1994} | |
| Serodiagnostic for Humans only | BMEI1439 | BAB1_0522 | BR0497 | 0.715 | Chromosome Segregation | |
| Protein SMC | ||||||
| BMEI0340 | BAB1_1707 | BR1695 | 0.889 | Omp16 lipoprotein | {Tibor, 1994} | |
| BMEI0141 | BAB1_1922 | BR1922 | 0.734 | 2-oxoglutarate dehydrogenase, E2 component, dihydrolipoamide succinyltransferase | ||
| BMEII0032 | BAB2_0061 | BRA0062 | 0.849 | VirB8 | ||
| BMEI0228 | BAB1_1830 | BR1822 | 0.719 | Hypothetical protein | ||
| BMEI1890 | BAB1_0051 | BR0054 | 0.866 | Transporter | ||
| BMEI0810 | BAB1_1199 | BR1177 | 0.918 | COG1434 Uncharacterized conserved protein | ||
| BMEI1060 | BAB1_0927 | BR0909 | 0.763 | DsbA Protein-disulfide isomerase | ||
| BMEI0324 | BAB1_1726 | BR1714 | 0.822 | COG1360 Flagellar Motor Protein | ||
| BMEI0039 | BAB1_2033 | BR2032 | 0.700 | Acetyl CoA carboxylase, carboxyltransferase, alpha subunit | ||
| BMEI0503 | BAB1_1528 | BR1510 | 0.865 | COG1607 Acyl-CoA Hydrolase | ||
| Serodiagnostic for Goats only | BMEII0988 | BAB2_0943 | BRA0260 | 0.942 | Copper-containing nitrite reductase NirK | |
| BMEII0601 | BAB2_0558 | BRA0682 | 0.917 | ABC amino acid transporter, periplasmic binding protein | ||
| BMEII0073 | BAB2_0019 | BRA0020 | 1.000 | Hypothetical protein | ||
| BMEI0668 | BAB2_0441 | BRA0797 | 1.000 | Calcium binding protein Asp24 | {Lin, 1995} | |
| BMEII0734 | BAB2_0699 | BRA0538 | 0.983 | Oligopeptide binding protein precursor | ||
| BMEI0796 | BAB1_0294 | BR0263 | 0.950 | TRAP transporter solute receptor Bcsp31 | {Mayfield, 1988} | |
| BMEII0217 | BAB2_1043 | BRA1084 | 0.908 | ABC dipeptide transport protein, periplasmic component | ||
| BMEI1725 | BAB1_0226 | BR0225 | 0.967 | COG1732 glycine betaine-binding protein | ||
| BMEII1047 | BAB2_0190 | BRA0196 | 0.967 | 10kDa chaperonin groES | {Connolly, 2006} | |
| BMEI2053 | BAB1_2075 | BR2074 | 0.892 | Calcium binding protein | ||
| BMEI0178 | BAB1_1885 | BR1885 | 0.967 | Hypothetical protein | ||
| BMEI0991 | BAB1_1009 | BR0990 | 0.842 | Rare Lipoprotein A | ||
| BMEII0338 | BAB2_0275 | BRA0960 | 0.958 | ABC transporter periplasmic | ||
| BP, lipoprotein | ||||||
| BMEII0573 | BAB2_0527 | BRA0712 | 0.917 | Transcriptional regulator, RpiR family | ||
| BMEII0179 | BAB2_1078 | BRA1120 | 0.858 | Zn binding protein | ||
| BMEII0859 | BAB2_0812 | BRA0409 | 0.883 | ABC dipeptide transport system, periplasmic component |
Validation of serodiagnostic accuracy with immunostrips
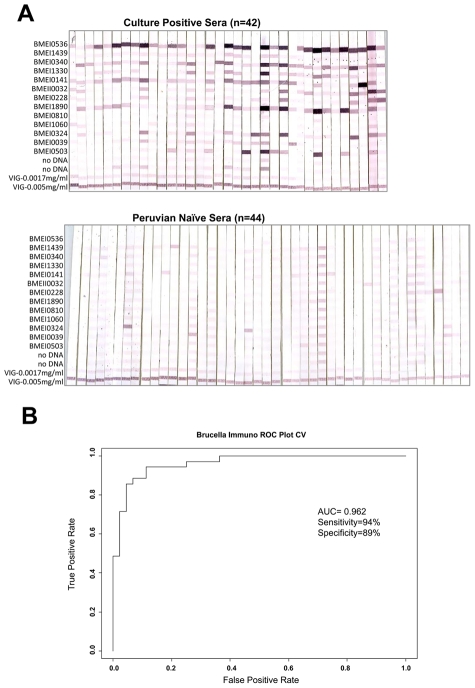
To test the feasibility of using the serodiagnostic antigens in an alternative analytical assay, thirteen serodiagnostic proteins were printed onto Nitrocellulose membranes using a BioDot jet dispenser. The paper was then cut into 3 mm strips ( Fig. 5a ). The individual strips were probed with 42 different culture positive sera and 44 Peruvian naive sera. Brucellosis patients reacted strongly against the serodiagnostic antigens with variable signal intensity among the patients. Naïve samples showed much lower reactivity against these serodiagnostic antigens. To assess the ability of antigens to separate disease and healthy controls, the LOOCV ROC curve was also generated ( Fig. 5b ). The ROC curve shows that this probing test yielded a high AUC of 0.962 with sensitivity rate of 94% and specificity rate of 89%. Thus, thirteen differentially reactive serodiagnostic antigens identified by microarray analysis in immunostrip format validated the list of serodiagnostic antigens to correctly classify B. melitensis positive sera.
Figure 5. Immunostrips probing.
(a)Thirteen serodiagnostic antigens were printed onto nitrocellulose paper in adjacent stripes using a BioDot jet dispenser as described in Materials and Methods. Strips were probed with Culture Positive or Peruvian naive sera diluted 1/200 followed by alkaline phosphatase conjugated secondary antibody and enzyme substrate. Weak reactivity in the naïve healthy controls can be distinguished from the strong reactivity in infected group. (b). The LOOCV ROC curve was generated and sensitivity and specificity of immunostrips probing test is 94% and 89%, respectively.
The sensitivity and specificity of the top 5 serodiagnostic antigens discovered using the protein microarrays had sensitivity and specificity of 95% and 96%, better than that of the 13 antigens on the immunostrips (94% and 89%).
Comparing antigenic proteins among humans and goats
Both humans and animals can be infected by Bm. In the present study, goats were infected by B. melitensis strain 16M which would be expected to be virtually identical to the strains infected by patients in Lima given the limited diversity of the strains found there [3], [59]–[62]. To better understand the differences in the immune response to Bm infection between humans and goats, we compared serodominant antigens for both humans and goats. In the current study, two antigens are found to be serodiagnostic for both humans and goats ( Fig. 6 , Table 1 ). The top antigen on the list, BMEI0536 (Bp26 protein) is a 26KD periplasmic immunogenic protein which was simultaneously identified by three nonrelated research groups as an immunodominant antigen in infected cattle, sheep, goats, and humans [17], [19], [27], [35]. Use of an indirect ELISA to detect antibodies in brucellosis patients (n = 20) and uninfected controls (n = 35) yielded a sensitivity of 0.9 and specificity of 0.91 (not shown). Another serodiagnostic protein for both humans and goats was Protease DO, also designated as HtrA [31]. Use of an indirect ELISA to BMEI1330 yielded a sensitivity of 0.84 and a specificity of 0.99. Thus, the ELISA data were consistent with values determined using immunostrips and with the proteome array data. There are 11 antigens exclusively useful for human brucellosis diagnosis and 16 antigens exclusively for goats. Most of these are membrane proteins, lipoproteins, transporter proteins, proteins with signal peptide and proteins related to pathogenicity. We also identified 8 common cross-reactive antigens for both humans and goats, and 12 exclusively for humans and 23 for goats ( Fig. 6 , Table 2 ).
Figure 6. Serodiagnostic and cross-reactive antigens for humans and goats.
Table 2. Cross-reactive Brucella melitensis antigens for humans and goats.
| B. melitensis | Product Name | |
| Cross- reactive for both Humans and Goats | BMEI1079 | Lipoprotein NlpD |
| BMEII0154 | Flagellar motor protein | |
| BMEI0613 | Protease Do | |
| BMEI1073 | Glucose-inhibited division protein A | |
| BMEII0497 | Enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase | |
| BMEI0063 | Hypothetical membrane spanning protein | |
| BMEII0010 | Hypothetical membrane associated protein | |
| BMEI0847 | Protein-export membrane protein | |
| Cross- reactive for Humans only | BMEI0179 | Hypothetical protein transporter |
| BMEI2053 | ||
| BMEI1646 | Acriflavin resistance protein E | |
| BMEI1471 | 4-Amino-4-deoxychorismate lyase | |
| BMEI1236 | Hypothetical exported proline-rich protein | |
| BMEI1692 | Flagellar protein FlgJ | |
| BMEII0571 | Acetolactate synthase IolD | |
| BMEII0793 | Multidrug resistance efflux pump | |
| BMEI0123 | Peptidyl-prolyl cis-trans isomerase | |
| BMEII0019 | Stomatin like protein | |
| BMEII1118 | Multidrug resistance protein A | |
| BMEI0848 | Probable carnitine operon oxidoreductase CaiA | |
| Cross-reactive for Goats only | BMEI1989 | Phosphate-binding periplasmic protein periplasmic oligopeptide-binding protein precursor |
| BMEII0735 | ||
| BMEI0263 | Leu-, Ile-, Val-, Thr-, and Ala-binding protein precursor | |
| BMEI1792 | Hypothetical protein | |
| BMEII0381 | Acriflavin resistance protein E | |
| BMEI0475 | Cytochrome C1 | |
| BMEI0053 | Cation-transporting ATPase PacS | |
| BMEII1111 | Hypothetical protein | |
| BMEI0443 | Hypothetical protein | |
| BMEI1439 | Chromosome segregation protein SMC2 | |
| BMEI0340 | Peptidoglycan-associated lipoprotein Omp16 | |
| BMEI1954 | ABC transporter substrate binding protein | |
| BMEII0678 | Lipoyltransferase | |
| BMEI1334 | Cytochrome C-type biogenesis protein CycH | |
| BMEI1280 | Hypothetical cytosolic protein | |
| BMEI0749 | DNA-directed RNA polymerase beta subunit | |
| BMEII0029 | Type IV secretion apparatus protein VirB5 | |
| BMEI1000 | Hypothetical protein | |
| BMEI0821 | Hypothetical protein | |
| BMEII0852 | Succinoglycan biosynthesis transport protein | |
| BMEI1060 | DsbA Protein-disulfide isomerase | |
| BMEI1428 | Ribonuclease III | |
| BMEII0151 | Flagellar Mring protein FliF |
Discussion
Here we report a large scale analysis showing that the humoral immune responses against B. melitensis protein antigens differ between humans naturally infected by consuming Brucella melitensis-contaminated, unpasteurized goat's milk products, and goats experimentally infected with B. melitensis by conjunctival instillation. These observations show that a natural reservoir host and the accidental human host have fundamentally different immune responses against this zoonotic pathogen. These data have implications for the practical development of diagnostics and reflect basic differences in host pathogen interactions and disease pathogenesis.
In addition, we demonstrate that a systematic, genome-wide analysis proved to identify protein antigens recognized by humans and animals not previously identified using Western blot or genomic library immunoscreening. Further, by virtue of being found to react with antibodies, the protein array technology is able to provide strong evidence of the comprehensive set of proteins expressed in vivo within a mammalian host by B. melitensis. As with our published experience with viral, bacterial and protozoal genomes expressed using protein microarray technology [44], [49], [50], [58], [64]–[66], conformation-dependent epitopes seem not to present problems with data interpretation or comprehensiveness of antigen discovery. This is likely because the polyclonal antibody response to protein antigens after infection detects both linear and 3-dimensional epitopes. The B. melitensis proteins placed onto the array, while expressed heterologously in a bacterial system, likely reflect a mix of conformationally correct as well as misfolded epitopes both of which are capable of binding specific antibodies.
Serological diagnosis of both human and animal brucellosis can suffer from the inability to distinguish new from previous infection (in the case of humans [1]) and differentiation of vaccination from new infection (in the case of animals [8]). In the absence of known exposure history in endemic regions, there is the possibility of mistaken diagnosis and overtreatment [10]. Current assays are based primarily on identification of antibodies to LPS in patient serum. Since Brucella LPS is cross-reactive with several other species, including E. coli O157:H7, Yersinia enterocolitica O9, and Francisella tularensis (although the clinical presentations of infectious caused by these agents are quite different), identification of diagnostic protein antigens may facilitate the development of more specific serodiagnostic assays [21], [67], [68]. The top 5 serodiagnostic antigens discovered using the protein microarrays had sensitivity and specificity of 95% and 96%, better than that of the 13 antigens on the immunostrips (94% and 89%), which in turn was roughly comparable to that of smooth LPS-based tests used in the Rose Bengal, lateral flow, and ELISA formats. In the present study however, the sensitivity of the serodiagnostic protein antigens could not be compared to that of the Rose Bengal test because we did not confirm any brucellosis cases among Rose Bengal negative patients by culture.
One interesting finding of this study was the difference in background reactivity to B. melitensis proteins in uninfected individuals from endemic vs. non-endemic areas. In Peru, control subjects tended to have higher background reactivity to Brucella antigens, compared to US control subjects (Fig. 3a). Consideration of these differences would be important for the development of diagnostic assays intended for use in both endemic and non-endemic regions of the world. The degree of variability between subjects differs depending on the infection and the results for Brucella reported here are similar to those that we obtained from patients with melioidosis [64], [65] and Lyme disease.
Our results with the B. melitensis proteome array represent the first large-scale analysis of B. melitensis proteins that are immunogenic in the context of naturally acquired human infections. In the case of the present study, the identified risk factor for human infection was ingestion of B. melitensis-contaminated, unpasteurized goat's milk products. In other epidemiological contexts, B. melitensis can be contracted by direct exposure to infected animals, not only goats, but also sheep and cattle [1], [3]. Further, we compared the set of proteins identified using human patient sera with the set that was immunogenic in the animal reservoir for zoonotic disease, the goat. Two proteins, BMEI0536 (Bp26) and BMEI1330 (HtrA/DegP), were immunogenic in the context of both infections. These results are in good agreement with previous reports on these antigens from other Brucella spp. Roop et al. [31] showed that HtrA was recognized by serum from goats, cattle and mice experimentally infected with B. abortus and by serum of dogs infected with B. canis. HtrA/DegP is a periplasmic serine protease that contributes to survival following stresses including oxidative damage. Bp26 has been proposed as a diagnostic antigen for detection of B. melitensis infection in sheep and B. abortus in cattle [17], [19]. The Omp16 lipoprotein (BMEI0340), originally identified as an immunogenic protein of B. abortus [32], was recognized by patient sera, but was found to be reactive in both infected and uninfected goats. Our results differ from those of Letesson et al., who reported no reactivity of uninfected goats to Omp16 [25]. This difference may reflect exposure of goats used in our study to other pathogens or to environmental bacteria expressing a cross-reactive antigen. The identification of known immunogenic Brucella proteins on the proteome array provided confirmation that our approach could identify both known and novel immunogenic proteins.
In addition to these well-characterized antigens, our study identified several novel serodiagnostic antigens specific for human B. melitensis patients (Table 1). These included BMEI1439 (SMC), an ATPase shown in other bacterial species to be involved in condensation and segregation of replicating chromosomes [29]. Further, the bacterial cell envelope proteins VirB8 (BMEII0032), DsbA (BMEI1060), and an uncharacterized transporter (BMEI1890), were immunogenic in patients. Three metabolic enzymes, acetyl coA carboxylase, (BMEI0503), Acetyl CoA carboxylase (BMEI0039 and 2-oxoglutarate dehydrogenase (BMEI0141) also represent novel serodiagnostic antigens for human brucellosis. The finding that these proteins are immunogenic suggests that they are expressed during B. melitensis infection of humans.
A group of 16 antigens was found to be serodiagnostic for goats, but not humans (Table 1). These included 7 predicted transporters, as well as a zinc-binding protein and two binding proteins for calcium: Asp24 (BMEI0668) [26], and a second, uncharacterized calcium-binding protein (BMEI2053). The chaperonin GroES, shown to be seroreactive in a patient suffering from B. suis infection [20], was serodiagnostic in goats, as well as Bscp31 (BMEI0796), a well-known seroreactive protein [28].
The differences in the patterns of goat and human seroreactivity to B. melitensis proteins could be explained in several different ways. First, the group of seroreactive proteins in goats and humans gives some insight into the metabolic pathways expressed during infection in these hosts. The large number of immunogenic proteins with predicted function in nutrient uptake suggests that B. melitensis utilizes peptides and amino acids for growth during infection. Three serodiagnostic B. melitensis proteins (BMEI0340, BMEI0141 and BMEI0178), have been found to be upregulated during intracellular infection by B. suis or B. abortus [18], [24], suggesting that they may play a role in adaptation to intracellular life in the host. Second, the immunogenetics of immune responses to B. melitensis infection may be related to species differences between humans and goats but further comment on this possibility is limited by the lack of available data. Third, while humans in this study were thought to have been infected by ingestion of infected goat's milk products containing a small inoculum, the goats were infected with a high dose (107 CFU) of B. melitensis strain 16M via the conjunctiva. Both dose and route of inoculum may have contributed to the differential antigen recognition between goats and humans, and, in fact, among humans as well. Finally, while it seems unlikely that the different patterns of immune response variation between goats and humans were due to protein expression differences by different strains of B. melitensis, this possibility must be considered as well.
The results presented here represent an analysis of 1406 proteins of 3198 predicted proteins in the B. melitensis 16M genome. Of these 1406 proteins, we only observed less than two fold enrichment of serodiagnostic antigens in the 1009 selected versus randomly selected antigens (not shown). Completion of the proteome array is currently underway, which will allow a more complete genome-level analysis of all immunogenic B. melitensis proteins. The subset of diagnostic antigens identified here provided an initial estimated accuracy rate of 95% for diagnosis of human cases and it is likely that this set of antigens will form the basis of a new and accurate serodiagnostic assay for human brucellosis. The clinical and veterinary utility of the protein antigens discovered in this study for diagnosis of acute and chronic brucellosis awaits validation in prospective studies in endemic regions.
Acknowledgments
We thank Kalina Campos for outstanding microbiological work, and thank Paula Maguina of the University of California San Diego who made essential research contributions in terms of ethics management and international coordination as well as logistical support for this project.
Footnotes
PLF is an inventor on patent applications pertaining to this work and owns stock in a company (Anitigen Discovery Inc.) that has licensed the technology. XL is an inventor on patent applications pertaining to this work and is employed at a company (Anitigen Discovery Inc.) that has licensed the technology. WJWM is employed at company (Anitigen Discovery Inc.) that has licensed technology pertaining to this work.
This work is supported by US National Institutes of Health grants U01AI078213, U54AI065359, and U01AI075420, and SBIR grant 1R43AI06816601A1. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005;352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 2.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 3.Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 4.Young EJ. An overview of human brucellosis. Clin Infect Dis. 1995;21:283–289; quiz 290. doi: 10.1093/clinids/21.2.283. [DOI] [PubMed] [Google Scholar]
- 5.World Organisation for Animal Health. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; 2009. [Google Scholar]
- 6.Ramirez-Pfeiffer C, Diaz-Aparicio E, Gomez-Flores R, Rodriguez-Padilla C, Morales-Loredo A, et al. Use of the Brucella melitensis native hapten to diagnose brucellosis in goats by a rapid, simple, and specific fluorescence polarization assay. Clin Vaccine Immunol. 2008;15:911–915. doi: 10.1128/CVI.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez-Pfeiffer C, Diaz-Aparicio E, Rodriguez-Padilla C, Morales-Loredo A, Alvarez-Ojeda G, et al. Improved performance of Brucella melitensis native hapten over Brucella abortus OPS tracer on goat antibody detection by the fluorescence polarization assay. Vet Immunol Immunopathol. 2008;123:223–229. doi: 10.1016/j.vetimm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Aparicio E, Marin C, Alonso-Urmeneta B, Aragon V, Perez-Ortiz S, et al. Evaluation of serological tests for diagnosis of Brucella melitensis infection of goats. J Clin Microbiol. 1994;32:1159–1165. doi: 10.1128/jcm.32.5.1159-1165.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan WJ, MacKinnon DJ, Lawson JR, Cullen GA. The rose bengal plate agglutination test in the diagnosis of brucellosis. Vet Rec. 1969;85:636–641. doi: 10.1136/vr.85.23.636. [DOI] [PubMed] [Google Scholar]
- 10.Jennings GJ, Hajjeh RA, Girgis FY, Fadeel MA, Maksoud MA, et al. Brucellosis as a cause of acute febrile illness in Egypt. Trans R Soc Trop Med Hyg. 2007;101:707–713. doi: 10.1016/j.trstmh.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 11.Al Dahouk S, Tomaso H, Nockler K, Neubauer H, Frangoulidis D. Laboratory-based diagnosis of brucellosis–a review of the literature. Part II: serological tests for brucellosis. Clin Lab. 2003;49:577–589. [PubMed] [Google Scholar]
- 12.Gomez MC, Nieto JA, Rosa C, Geijo P, Escribano MA, et al. Evaluation of seven tests for diagnosis of human brucellosis in an area where the disease is endemic. Clin Vaccine Immunol. 2008;15:1031–1033. doi: 10.1128/CVI.00424-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orduna A, Almaraz A, Prado A, Gutierrez MP, Garcia-Pascual A, et al. Evaluation of an immunocapture-agglutination test (Brucellacapt) for serodiagnosis of human brucellosis. J Clin Microbiol. 2000;38:4000–4005. doi: 10.1128/jcm.38.11.4000-4005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casao MA, Navarro E, Solera J. Evaluation of Brucellacapt for the diagnosis of human brucellosis. J Infect. 2004;49:102–108. doi: 10.1016/j.jinf.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Mantecon MA, Gutierrez P, del Pilar Zarzosa M, Duenas AI, Solera J, et al. Utility of an immunocapture-agglutination test and an enzyme-linked immunosorbent assay test against cytosolic proteins from Brucella melitensis B115 in the diagnosis and follow-up of human acute brucellosis. Diagn Microbiol Infect Dis. 2006;55:27–35. doi: 10.1016/j.diagmicrobio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Bosilkovski M, Katerina S, Zaklina S, Ivan V. The role of Brucellacapt test for follow-up patients with brucellosis. Comp Immunol Microbiol Infect Dis. 2009 doi: 10.1016/j.cimid.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Rossetti OL, Arese AI, Boschiroli ML, Cravero SL. Cloning of Brucella abortus gene and characterization of expressed 26-kilodalton periplasmic protein: potential use for diagnosis. J Clin Microbiol. 1996;34:165–169. doi: 10.1128/jcm.34.1.165-169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al Dahouk S, Jubier-Maurin V, Scholz HC, Tomaso H, Karges W, et al. Quantitative analysis of the intramacrophagic Brucella suis proteome reveals metabolic adaptation to late stage of cellular infection. Proteomics. 2008;8:3862–3870. doi: 10.1002/pmic.200800026. [DOI] [PubMed] [Google Scholar]
- 19.Cloeckaert A, Debbarh HS, Vizcaino N, Saman E, Dubray G, et al. Cloning, nucleotide sequence, and expression of the Brucella melitensis bp26 gene coding for a protein immunogenic in infected sheep. FEMS Microbiol Lett. 1996;140:139–144. doi: 10.1016/0378-1097(96)00169-3. [DOI] [PubMed] [Google Scholar]
- 20.Connolly JP, Comerci D, Alefantis TG, Walz A, Quan M, et al. Proteomic analysis of Brucella abortus cell envelope and identification of immunogenic candidate proteins for vaccine development. Proteomics. 2006;6:3767–3780. doi: 10.1002/pmic.200500730. [DOI] [PubMed] [Google Scholar]
- 21.Corbel MJ, Stuart FA, Brewer RA. Observations on serological cross-reactions between smooth Brucella species and organisms of other genera. Dev Biol Stand. 1984;56:341–348. [PubMed] [Google Scholar]
- 22.Gor D, Mayfield JE. Cloning and nucleotide sequence of the Brucella abortus groE operon. Biochim Biophys Acta. 1992;1130:120–122. doi: 10.1016/0167-4781(92)90476-g. [DOI] [PubMed] [Google Scholar]
- 23.Kahl-McDonagh MM, Elzer PH, Hagius SD, Walker JV, Perry QL, et al. Evaluation of novel Brucella melitensis unmarked deletion mutants for safety and efficacy in the goat model of brucellosis. Vaccine. 2006;24:5169–5177. doi: 10.1016/j.vaccine.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Lamontagne J, Forest A, Marazzo E, Denis F, Butler H, et al. Intracellular adaptation of Brucella abortus. J Proteome Res. 2009;8:1594–1609. doi: 10.1021/pr800978p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Letesson JJ, Tibor A, van Eynde G, Wansard V, Weynants V, et al. Humoral immune responses of Brucella-infected cattle, sheep, and goats to eight purified recombinant Brucella proteins in an indirect enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 1997;4:556–564. doi: 10.1128/cdli.4.5.556-564.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin J, Ficht TA. Protein synthesis in Brucella abortus induced during macrophage infection. Infect Immun. 1995;63:1409–1414. doi: 10.1128/iai.63.4.1409-1414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindler LE, Hadfield TL, Tall BD, Snellings NJ, Rubin FA, et al. Cloning of a Brucella melitensis group 3 antigen gene encoding Omp28, a protein recognized by the humoral immune response during human brucellosis. Infect Immun. 1996;64:2490–2499. doi: 10.1128/iai.64.7.2490-2499.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayfield JE, Bricker BJ, Godfrey H, Crosby RM, Knight DJ, et al. The cloning, expression, and nucleotide sequence of a gene coding for an immunogenic Brucella abortus protein. Gene. 1988;63:1–9. doi: 10.1016/0378-1119(88)90540-9. [DOI] [PubMed] [Google Scholar]
- 29.Pogliano K, Pogliano J, Becker E. Chromosome segregation in Eubacteria. Curr Opin Microbiol. 2003;6:586–593. doi: 10.1016/j.mib.2003.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rolan HG, den Hartigh AB, Kahl-McDonagh M, Ficht T, Adams LG, et al. VirB12 is a serological marker of Brucella infection in experimental and natural hosts. Clin Vaccine Immunol. 2008;15:208–214. doi: 10.1128/CVI.00374-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roop RM, 2nd, Fletcher TW, Sriranganathan NM, Boyle SM, Schurig GG. Identification of an immunoreactive Brucella abortus HtrA stress response protein homolog. Infect Immun. 1994;62:1000–1007. doi: 10.1128/iai.62.3.1000-1007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tibor A, Weynants V, Denoel P, Lichtfouse B, De Bolle X, et al. Molecular cloning, nucleotide sequence, and occurrence of a 16.5-kilodalton outer membrane protein of Brucella abortus with similarity to pal lipoproteins. Infect Immun. 1994;62:3633–3639. doi: 10.1128/iai.62.9.3633-3639.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X, Hudson M, Walters N, Bargatze RF, Pascual DW. Selection of protective epitopes for Brucella melitensis by DNA vaccination. Infect Immun. 2005;73:7297–7303. doi: 10.1128/IAI.73.11.7297-7303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baldi PC, Miguel SE, Fossati CA, Wallach JC. Serological follow-up of human brucellosis by measuring IgG antibodies to lipopolysaccharide and cytoplasmic proteins of Brucella species. Clin Infect Dis. 1996;22:446–455. doi: 10.1093/clinids/22.3.446. [DOI] [PubMed] [Google Scholar]
- 35.Salih-Alj Debbarh H, Cloeckaert A, Bezard G, Dubray G, Zygmunt MS. Enzyme-linked immunosorbent assay with partially purified cytosoluble 28-kilodalton protein for serological differentiation between Brucella melitensis-infected and B. melitensis Rev.1-vaccinated sheep. Clin Diagn Lab Immunol. 1996;3:305–308. doi: 10.1128/cdli.3.3.305-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassataro J, Delpino MV, Velikovsky CA, Bruno L, Fossati CA, et al. Diagnostic usefulness of antibodies against ribosome recycling factor from Brucella melitensis in human or canine brucellosis. Clin Diagn Lab Immunol. 2002;9:366–369. doi: 10.1128/CDLI.9.2.366-369.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassataro J, Pasquevich K, Bruno L, Wallach JC, Fossati CA, et al. Antibody reactivity to Omp31 from Brucella melitensis in human and animal infections by smooth and rough Brucellae. Clin Diagn Lab Immunol. 2004;11:111–114. doi: 10.1128/CDLI.11.1.111-114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Contreras-Rodriguez A, Seleem MN, Schurig GG, Sriranganathan N, Boyle SM, et al. Cloning, expression and characterization of immunogenic aminopeptidase N from Brucella melitensis. FEMS Immunol Med Microbiol. 2006;48:252–256. doi: 10.1111/j.1574-695X.2006.00145.x. [DOI] [PubMed] [Google Scholar]
- 39.Delpino MV, Cassataro J, Fossati CA, Baldi PC. Antibodies to the CP24 protein of Brucella melitensis lack diagnostic usefulness in ovine brucellosis. Vet Microbiol. 2003;93:101–107. doi: 10.1016/s0378-1135(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 40.Estein SM, Baldi PC, Bowden RA. Comparison of serological tests based on outer membrane or internal antigens for detecting antibodies to Brucella ovis in infected flocks. J Vet Diagn Invest. 2002;14:407–411. doi: 10.1177/104063870201400508. [DOI] [PubMed] [Google Scholar]
- 41.Goldbaum FA, Rubbi CP, Wallach JC, Miguel SE, Baldi PC, et al. Differentiation between active and inactive human brucellosis by measuring antiprotein humoral immune responses. J Clin Microbiol. 1992;30:604–607. doi: 10.1128/jcm.30.3.604-607.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verstreate DR, Creasy MT, Caveney NT, Baldwin CL, Blab MW, et al. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982;35:979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallach JC, Miguel SE, Baldi PC, Guarnera E, Goldbaum FA, et al. Urban outbreak of a Brucella melitensis infection in an Argentine family: clinical and diagnostic aspects. FEMS Immunol Med Microbiol. 1994;8:49–56. doi: 10.1111/j.1574-695X.1994.tb00424.x. [DOI] [PubMed] [Google Scholar]
- 44.Davies DH, Liang X, Hernandez JE, Randall A, Hirst S, et al. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc Natl Acad Sci U S A. 2005;102:547–552. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durbin BP, Hardin JS, Hawkins DM, Rocke DM. A variance-stabilizing transformation for gene-expression microarray data. Bioinformatics. 2002;18(Suppl 1):S105–110. doi: 10.1093/bioinformatics/18.suppl_1.s105. [DOI] [PubMed] [Google Scholar]
- 46.Ideker T, Thorsson V, Siegel AF, Hood LE. Testing for differentially-expressed genes by maximum-likelihood analysis of microarray data. J Comput Biol. 2000;7:805–817. doi: 10.1089/10665270050514945. [DOI] [PubMed] [Google Scholar]
- 47.Baldi P, Brunak SR. Bioinformatics: the machine learning approach. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- 48.Baldi P, Long AD. A Bayesian framework for the analysis of microarray expression data: regularized t -test and statistical inferences of gene changes. Bioinformatics. 2001;17:509–519. doi: 10.1093/bioinformatics/17.6.509. [DOI] [PubMed] [Google Scholar]
- 49.Sundaresh S, Doolan DL, Hirst S, Mu Y, Unal B, et al. Identification of humoral immune responses in protein microarrays using DNA microarray data analysis techniques. Bioinformatics. 2006;22:1760–1766. doi: 10.1093/bioinformatics/btl162. [DOI] [PubMed] [Google Scholar]
- 50.Sundaresh S, Randall A, Unal B, Petersen JM, Belisle JT, et al. From protein microarrays to diagnostic antigen discovery: a study of the pathogen Francisella tularensis. Bioinformatics. 2007;23:i508–518. doi: 10.1093/bioinformatics/btm207. [DOI] [PubMed] [Google Scholar]
- 51.Huber W, von Heydebreck A, Sultmann H, Poustka A, Vingron M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics. 2002;18(Suppl 1):S96–104. doi: 10.1093/bioinformatics/18.suppl_1.s96. [DOI] [PubMed] [Google Scholar]
- 52.Kreil DP, Karp NA, Lilley KS. DNA microarray normalization methods can remove bias from differential protein expression analysis of 2D difference gel electrophoresis results. Bioinformatics. 2004;20:2026–2034. doi: 10.1093/bioinformatics/bth193. [DOI] [PubMed] [Google Scholar]
- 53.Barbacioru CC, Wang Y, Canales RD, Sun YA, Keys DN, et al. Effect of various normalization methods on Applied Biosystems expression array system data. BMC Bioinformatics. 2006;7:533. doi: 10.1186/1471-2105-7-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarkar D, Parkin R, Wyman S, Bendoraite A, Sather C, et al. Quality assessment and data analysis for microRNA expression arrays. Nucleic Acids Res. 2009;37:e17. doi: 10.1093/nar/gkn932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choe SE, Boutros M, Michelson AM, Church GM, Halfon MS. Preferred analysis methods for Affymetrix GeneChips revealed by a wholly defined control dataset. Genome Biol. 2005;6:R16. doi: 10.1186/gb-2005-6-2-r16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 57.Doran M, Raicu DS, Furst JD, Settimi R, Schipma M, et al. Oligonucleotide microarray identification of Bacillus anthracis strains using support vector machines. Bioinformatics. 2007;23:487–492. doi: 10.1093/bioinformatics/btl626. [DOI] [PubMed] [Google Scholar]
- 58.Doolan DL, Mu Y, Unal B, Sundaresh S, Hirst S, et al. Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics. 2008;8:4680–4694. doi: 10.1002/pmic.200800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nockler K, Maves R, Cepeda D, Draeger A, Mayer-Scholl A, et al. Molecular epidemiology of Brucella genotypes in patients at a major hospital in central Peru. J Clin Microbiol. 2009;47:3147–3155. doi: 10.1128/JCM.00900-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Espinosa BJ, Chacaltana J, Mulder M, Franco MP, Blazes DL, et al. Comparison of culture techniques at different stages of brucellosis. Am J Trop Med Hyg. 2009;80:625–627. [PubMed] [Google Scholar]
- 61.Smits HL, Espinosa B, Castillo R, Hall E, Guillen A, et al. MLVA genotyping of human Brucella isolates from Peru. Trans R Soc Trop Med Hyg. 2009;103:399–402. doi: 10.1016/j.trstmh.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Mendoza-Nunez M, Mulder M, Franco MP, Maas KS, Castaneda ML, et al. Brucellosis in household members of Brucella patients residing in a large urban setting in Peru. Am J Trop Med Hyg. 2008;78:595–598. [PubMed] [Google Scholar]
- 63.Girosi F. An Equivalence Between Sparse Approximation and Support Vector Machines. Neural Comput. 1998;10:1455–1480. doi: 10.1162/089976698300017269. [DOI] [PubMed] [Google Scholar]
- 64.Barbour AG, Jasinskas A, Kayala MA, Davies DH, Steere AC, et al. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect Immun. 2008;76:3374–3389. doi: 10.1128/IAI.00048-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Felgner PL, Kayala MA, Vigil A, Burk C, Nakajima-Sasaki R, et al. A Burkholderia pseudomallei protein microarray reveals serodiagnostic and cross-reactive antigens. Proc Natl Acad Sci U S A. 2009;106:13499–13504. doi: 10.1073/pnas.0812080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davies DH, Molina DM, Wrammert J, Miller J, Hirst S, et al. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics. 2007;7:1678–1686. doi: 10.1002/pmic.200600926. [DOI] [PubMed] [Google Scholar]
- 67.Behan KA, Klein GC. Reduction of Brucella species and Francisella tularensis cross-reacting agglutinins by dithiothreitol. J Clin Microbiol. 1982;16:756–757. doi: 10.1128/jcm.16.4.756-757.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chart H, Okubadejo OA, Rowe B. The serological relationship between Escherichia coli O157 and Yersinia enterocolitica O9 using sera from patients with brucellosis. Epidemiol Infect. 1992;108:77–85. doi: 10.1017/s0950268800049529. [DOI] [PMC free article] [PubMed] [Google Scholar]