Abstract
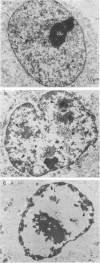
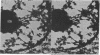
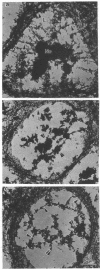
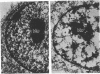
The maintenance of normal chromatin morphology requires ongoing RNA synthesis. We have examined the role of RNA in chromatin organization, using selective detergent extraction of cells, RNA synthesis inhibitors, and enzymatic digestion of nuclear RNA. Comparison of extracted and unextracted cells showed that the important features of chromatin architecture were largely unchanged by the extraction procedure. Normally, chromatin was distributed in small heterochromatic regions and dispersed euchromatic strands. Ribonucleoprotein granules were dispersed throughout the euchromatic regions. Exposure to actinomycin led to the redistribution of chromatin into large clumps, leaving large empty spaces and a dense clustering of the remaining ribonucleoprotein granules. When the nuclei of extracted cells were digested with RNase A, there was a rearrangement of chromatin similar to but more pronounced than that seen in cells exposed to actinomycin. The inhibitor 5,6-dichloro-1-beta-D-ribofuranosylbenzimidizole also inhibits RNA synthesis but by a different mechanism that leaves no nascent RNA chains. The drug had little effect on chromatin after brief exposure but resembled actinomycin in its effect at longer times. We also examined the structure of the nuclear matrix to which most heteronuclear RNA remains associated. Pretreatment of cells with actinomycin or digestion of the nuclear matrix with RNase A caused the matrix fibers to collapse and aggregate. The experiments show a parallel decay of chromatin and of nuclear matrix organization with the depletion of nuclear RNA and suggest that RNA is a structural component of the nuclear matrix, which in turn may organize the higher order structure of chromatin.
Full text
PDF
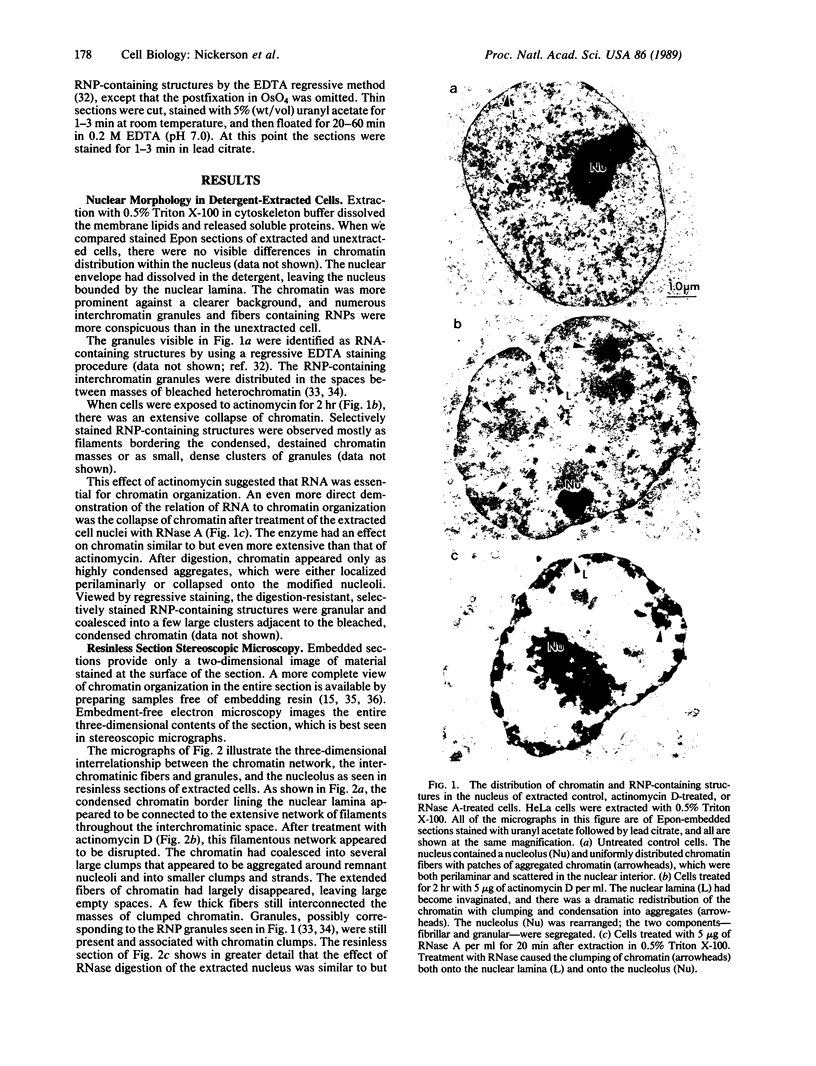

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
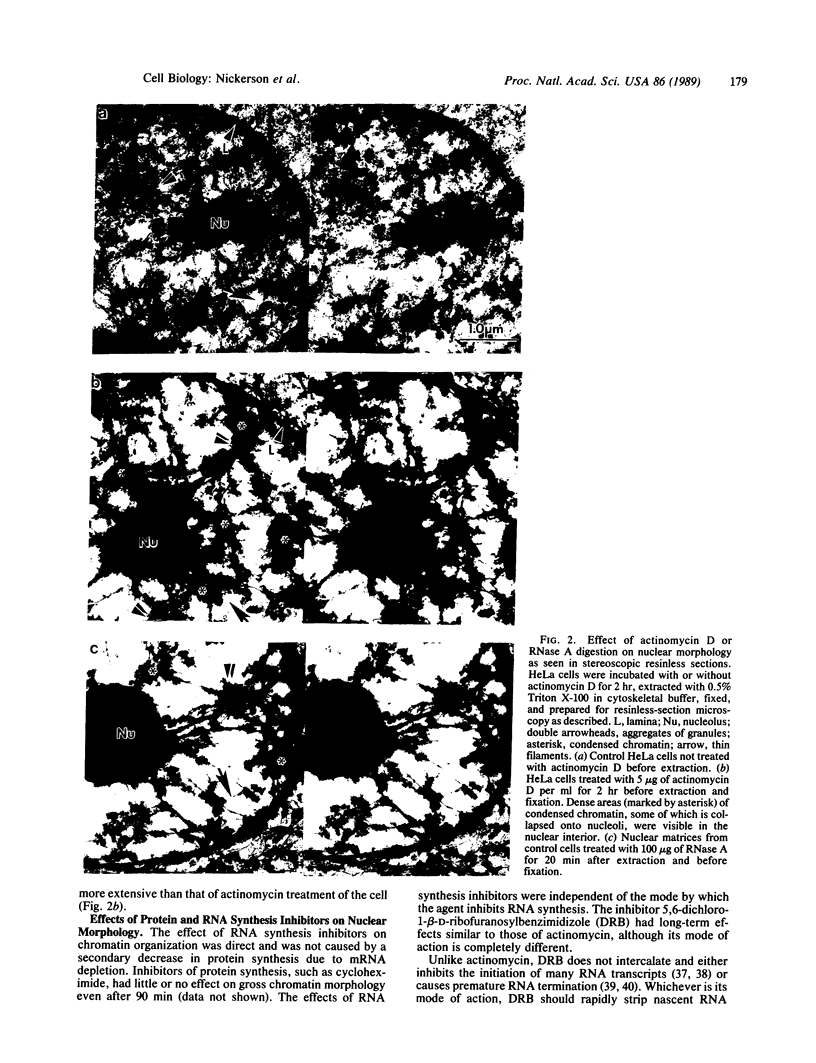
- Ashburner M. Ecdysone induction of puffing in polytene chromosomes of Drosophila melanogaster. Effects of inhibitors of RNA synthesis. Exp Cell Res. 1972;71(2):433–440. doi: 10.1016/0014-4827(72)90313-8. [DOI] [PubMed] [Google Scholar]
- Barrack E. R. The nuclear matrix of the prostate contains acceptor sites for androgen receptors. Endocrinology. 1983 Jul;113(1):430–432. doi: 10.1210/endo-113-1-430. [DOI] [PubMed] [Google Scholar]
- Berezney R., Buchholtz L. A. Dynamic association of replicating DNA fragments with the nuclear matrix of regenerating liver. Exp Cell Res. 1981 Mar;132(1):1–13. doi: 10.1016/0014-4827(81)90076-8. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Identification of a nuclear protein matrix. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1410–1417. doi: 10.1016/0006-291x(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear protein matrix: association with newly synthesized DNA. Science. 1975 Jul 25;189(4199):291–293. doi: 10.1126/science.1145202. [DOI] [PubMed] [Google Scholar]
- Berezney R. Fractionation of the nuclear matrix. I. Partial separation into matrix protein fibrils and a residual ribonucleoprotein fraction. J Cell Biol. 1980 Jun;85(3):641–650. doi: 10.1083/jcb.85.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
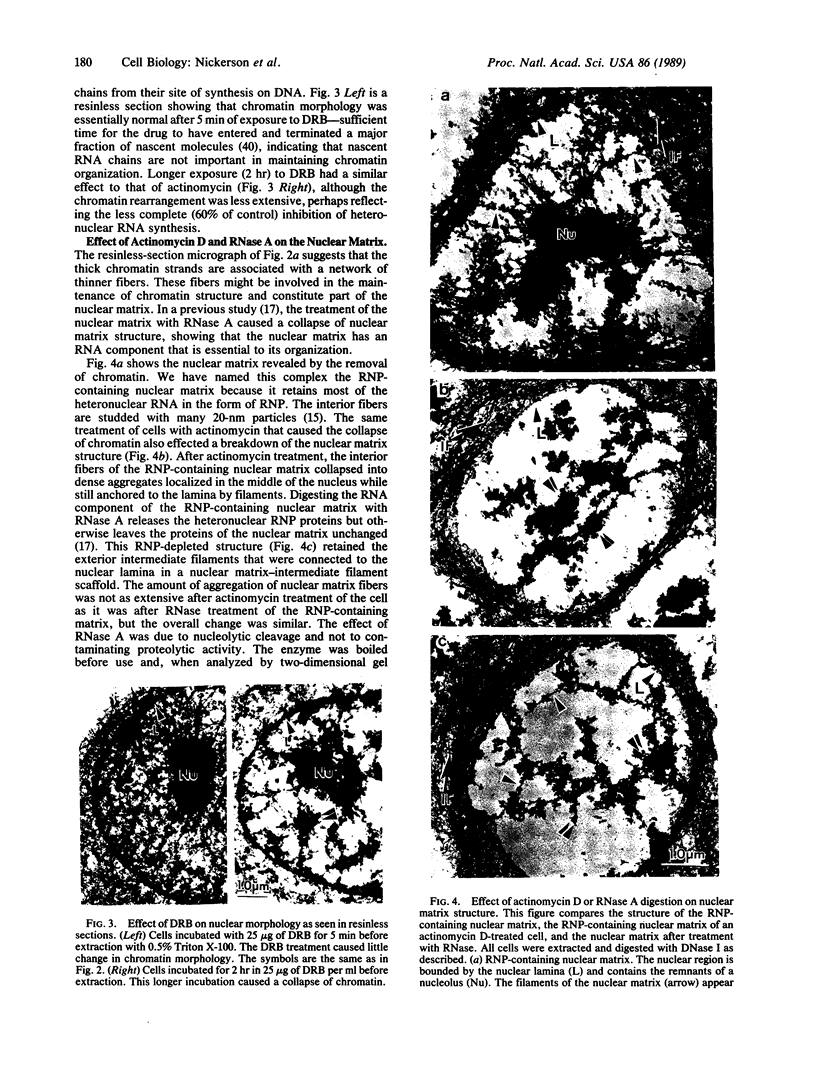
- Bernhard W. A new staining procedure for electron microscopical cytology. J Ultrastruct Res. 1969 May;27(3):250–265. doi: 10.1016/s0022-5320(69)80016-x. [DOI] [PubMed] [Google Scholar]
- Bourgeois C. A., Laquerriere F., Hemon D., Hubert J., Bouteille M. New data on the in-situ position of the inactive X chromosome in the interphase nucleus of human fibroblasts. Hum Genet. 1985;69(2):122–129. doi: 10.1007/BF00293281. [DOI] [PubMed] [Google Scholar]
- Capco D. G., Krochmalnic G., Penman S. A new method of preparing embeddment-free sections for transmission electron microscopy: applications to the cytoskeletal framework and other three-dimensional networks. J Cell Biol. 1984 May;98(5):1878–1885. doi: 10.1083/jcb.98.5.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capco D. G., Wan K. M., Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982 Jul;29(3):847–858. doi: 10.1016/0092-8674(82)90446-9. [DOI] [PubMed] [Google Scholar]
- Cremer T., Cremer C., Baumann H., Luedtke E. K., Sperling K., Teuber V., Zorn C. Rabl's model of the interphase chromosome arrangement tested in Chinese hamster cells by premature chromosome condensation and laser-UV-microbeam experiments. Hum Genet. 1982;60(1):46–56. doi: 10.1007/BF00281263. [DOI] [PubMed] [Google Scholar]
- Cremer T., Cremer C., Schneider T., Baumann H., Hens L., Kirsch-Volders M. Analysis of chromosome positions in the interphase nucleus of Chinese hamster cells by laser-UV-microirradiation experiments. Hum Genet. 1982;62(3):201–209. doi: 10.1007/BF00333519. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G., McGhee J. D. Structure of the 30 nm chromatin fiber. Cell. 1986 Feb 14;44(3):375–377. doi: 10.1016/0092-8674(86)90456-3. [DOI] [PubMed] [Google Scholar]
- Fey E. G., Krochmalnic G., Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol. 1986 May;102(5):1654–1665. doi: 10.1083/jcb.102.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E. G., Wan K. M., Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J Cell Biol. 1984 Jun;98(6):1973–1984. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser N. W., Sehgal P. B., Darnell J. E. DRB-induced premature termination of late adenovirus transcription. Nature. 1978 Apr 13;272(5654):590–593. doi: 10.1038/272590a0. [DOI] [PubMed] [Google Scholar]
- Hens L., Baumann H., Cremer T., Sutter A., Cornelis J. J., Cremer C. Immunocytochemical localization of chromatin regions UV-microirradiated in S phase or anaphase. Evidence for a territorial organization of chromosomes during cell cycle of cultured Chinese hamster cells. Exp Cell Res. 1983 Nov;149(1):257–269. doi: 10.1016/0014-4827(83)90397-x. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., McCready S. J., Cook P. R. RNA is synthesized at the nuclear cage. Nature. 1981 Aug 6;292(5823):552–555. doi: 10.1038/292552a0. [DOI] [PubMed] [Google Scholar]
- Kumara-Siri M. H., Shapiro L. E., Surks M. I. Association of the 3,5,3'-triiodo-L-thyronine nuclear receptor with the nuclear matrix of cultured growth hormone-producing rat pituitary tumor cells (GC cells). J Biol Chem. 1986 Feb 25;261(6):2844–2852. [PubMed] [Google Scholar]
- Lewis M., Helmsing P. J., Ashburner M. Parallel changes in puffing activity and patterns of protein synthesis in salivary glands of Drosophila. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3604–3608. doi: 10.1073/pnas.72.9.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long B. H., Huang C. Y., Pogo A. O. Isolation and characterization of the nuclear matrix in Friend erythroleukemia cells: chromatin and hnRNA interactions with the nuclear matrix. Cell. 1979 Dec;18(4):1079–1090. doi: 10.1016/0092-8674(79)90221-6. [DOI] [PubMed] [Google Scholar]
- Manuelidis L. Individual interphase chromosome domains revealed by in situ hybridization. Hum Genet. 1985;71(4):288–293. doi: 10.1007/BF00388453. [DOI] [PubMed] [Google Scholar]
- Miller T. E., Huang C. Y., Pogo A. O. Rat liver nuclear skeleton and ribonucleoprotein complexes containing HnRNA. J Cell Biol. 1978 Mar;76(3):675–691. doi: 10.1083/jcb.76.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. M., Vogelstein B., Coffey D. S. A fixed site of DNA replication in eucaryotic cells. Cell. 1980 Feb;19(2):527–536. doi: 10.1016/0092-8674(80)90527-9. [DOI] [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Puvion E. Relationship between chromatin and perichromatin granules in cadmium-treated isolated hepatocytes. J Ultrastruct Res. 1981 Mar;74(3):341–350. doi: 10.1016/s0022-5320(81)80125-6. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold G. A., Cremer T., Hager H. D., Davies K. E., Müller C. R., Yang T. Sex chromosome positions in human interphase nuclei as studied by in situ hybridization with chromosome specific DNA probes. Hum Genet. 1984;67(3):317–325. doi: 10.1007/BF00291361. [DOI] [PubMed] [Google Scholar]
- Robinson S. I., Nelkin B. D., Vogelstein B. The ovalbumin gene is associated with the nuclear matrix of chicken oviduct cells. Cell. 1982 Jan;28(1):99–106. doi: 10.1016/0092-8674(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Ross D. A., Yen R. W., Chae C. B. Association of globin ribonucleic acid and its precursors with the chicken erythroblast nuclear matrix. Biochemistry. 1982 Feb 16;21(4):764–771. doi: 10.1021/bi00533a029. [DOI] [PubMed] [Google Scholar]
- Simmen R. C., Means A. R., Clark J. H. Estrogen modulation of nuclear matrix-associated steroid hormone binding. Endocrinology. 1984 Sep;115(3):1197–1202. doi: 10.1210/endo-115-3-1197. [DOI] [PubMed] [Google Scholar]
- Small D., Nelkin B., Vogelstein B. The association of transcribed genes with the nuclear matrix of Drosophila cells during heat shock. Nucleic Acids Res. 1985 Apr 11;13(7):2413–2431. doi: 10.1093/nar/13.7.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I., Hand R., Caliguiri L. A. Action of dichlorobenzimidazole riboside on RNA synthesis in L-929 and HeLa cells. J Cell Biol. 1976 May;69(2):229–240. doi: 10.1083/jcb.69.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef M. A cytochemical study of interchromatin granules. J Ultrastruct Res. 1979 Oct;69(1):121–133. doi: 10.1016/s0022-5320(79)80047-7. [DOI] [PubMed] [Google Scholar]
- Winicov I., Button J. D. Dichlorobenzimidazole-riboside inhibition of nuclear RNA accumulation initiated with gamma-thio analogues of ATP and GTP. Eur J Biochem. 1982 May 17;124(2):239–244. doi: 10.1111/j.1432-1033.1982.tb06583.x. [DOI] [PubMed] [Google Scholar]
- Wolosewick J. J. The application of polyethylene glycol (PEG) to electron microscopy. J Cell Biol. 1980 Aug;86(2):675–661. doi: 10.1083/jcb.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandomeni R., Bunick D., Ackerman S., Mittleman B., Weinmann R. Mechanism of action of DRB. III. Effect on specific in vitro initiation of transcription. J Mol Biol. 1983 Jul 5;167(3):561–574. doi: 10.1016/s0022-2836(83)80098-9. [DOI] [PubMed] [Google Scholar]
- Zeitlin S., Parent A., Silverstein S., Efstratiadis A. Pre-mRNA splicing and the nuclear matrix. Mol Cell Biol. 1987 Jan;7(1):111–120. doi: 10.1128/mcb.7.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen C. A., van Venrooij W. J. hnRNA and its attachment to a nuclear protein matrix. J Cell Biol. 1981 Mar;88(3):554–563. doi: 10.1083/jcb.88.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]