Abstract
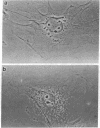
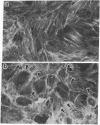
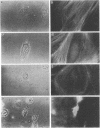
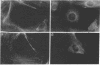
Tumor necrosis factor (TNF) is cytotoxic to certain transformed cells, whereas normal cells are resistant to its effects. The resistance of normal cells can often be overcome by treatment with inhibitors of transcription or translation such as actinomycin D or cycloheximide (CHI), suggesting that normal cells produce a protein(s) that protects them from TNF-induced cytolysis. In this report, we examine the mechanism of cytolysis in a 3T3-like mouse cell line, C3HA, which was sensitized to TNF by treatment with CHI. We found that an early change in TNF/CHI-treated cells was a significant loss of stress fibers in perinuclear areas of the cytoplasm. The disruption of microfilaments, which was observed within 15 min of treatment, was not seen in untreated cells or in cells treated with either TNF or CHI alone. The dissolution of microfilaments spread peripherally over time and preceded other TNF/CHI-induced effects such as cytoplasmic "boiling," decrease in cell volume, and lysis of the plasma membrane. The breakdown of stress fibers occurred without a change in microtubules or intermediate filaments. Cytochalasin E, which disrupts microfilaments, induced cytolysis of TNF-treated cells even in the absence of CHI; however, demecolcine, which depolymerizes microtubules, did not sensitize cells to TNF. We propose that the TNF-induced cytolysis of certain cell types is preceded by a selective disruption of the microfilament lattice.
Full text
PDF


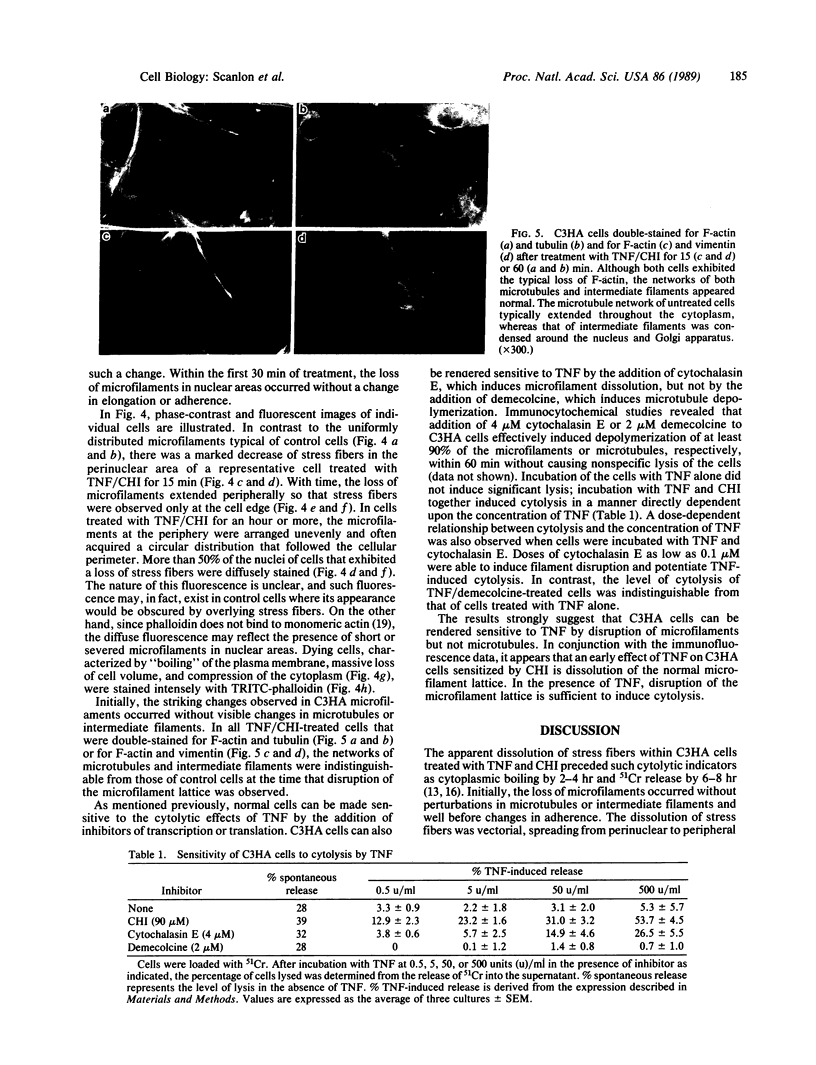

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anghileri L. J., Robert J. Effects of tumor necrosis factor on tumor cell plasma membrane permeability. Tumori. 1987 Jun 30;73(3):269–271. doi: 10.1177/030089168707300310. [DOI] [PubMed] [Google Scholar]
- Beresini M. H., Lempert M. J., Epstein L. B. Overlapping polypeptide induction in human fibroblasts in response to treatment with interferon-alpha, interferon-gamma, interleukin 1 alpha, interleukin 1 beta, and tumor necrosis factor. J Immunol. 1988 Jan 15;140(2):485–493. [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapes S. K., Gooding L. R. Evidence for the involvement of cytolytic macrophages in rejection of SV40-induced tumors. J Immunol. 1985 Sep;135(3):2192–2198. [PubMed] [Google Scholar]
- Chen M. J., Holskin B., Strickler J., Gorniak J., Clark M. A., Johnson P. J., Mitcho M., Shalloway D. Induction by E1A oncogene expression of cellular susceptibility to lysis by TNF. Nature. 1987 Dec 10;330(6148):581–583. doi: 10.1038/330581a0. [DOI] [PubMed] [Google Scholar]
- Dealtry G. B., Naylor M. S., Fiers W., Balkwill F. R. DNA fragmentation and cytotoxicity caused by tumor necrosis factor is enhanced by interferon-gamma. Eur J Immunol. 1987 May;17(5):689–693. doi: 10.1002/eji.1830170517. [DOI] [PubMed] [Google Scholar]
- Fletcher W. H., Shiu W. W., Ishida T. A., Haviland D. L., Ware C. F. Resistance to the cytolytic action of lymphotoxin and tumor necrosis factor coincides with the presence of gap junctions uniting target cells. J Immunol. 1987 Aug 1;139(3):956–962. [PubMed] [Google Scholar]
- Gooding L. R., Elmore L. W., Tollefson A. E., Brady H. A., Wold W. S. A 14,700 MW protein from the E3 region of adenovirus inhibits cytolysis by tumor necrosis factor. Cell. 1988 May 6;53(3):341–346. doi: 10.1016/0092-8674(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Gooding L. R. Specificities of killing by T lymphocytes generated against syngeneic SV40 transformants: studies employing recombinants within the H-2 complex. J Immunol. 1979 Mar;122(3):1002–1008. [PubMed] [Google Scholar]
- Hahn T., Toker L., Budilovsky S., Aderka D., Eshhar Z., Wallach D. Use of monoclonal antibodies to a human cytotoxin for its isolation and for examining the self-induction of resistance to this protein. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3814–3818. doi: 10.1073/pnas.82.11.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey P. A., Chaponnier C., Lind S. E., Zaner K. S., Stossel T. P., Yin H. L. Interactions of gelsolin and gelsolin-actin complexes with actin. Effects of calcium on actin nucleation, filament severing, and end blocking. Biochemistry. 1985 Jul 2;24(14):3714–3723. doi: 10.1021/bi00335a046. [DOI] [PubMed] [Google Scholar]
- Kirstein M., Baglioni C. Tumor necrosis factor induces synthesis of two proteins in human fibroblasts. J Biol Chem. 1986 Jul 25;261(21):9565–9567. [PubMed] [Google Scholar]
- Kobayashi Y., Utsunomiya N., Nakanishi M., Osawa T. Early transmembrane events in tumor necrosis factor and lymphotoxin-induced cytotoxicity. Immunol Lett. 1987 May;15(1):53–57. doi: 10.1016/0165-2478(87)90076-9. [DOI] [PubMed] [Google Scholar]
- Kull F. C., Jr, Cuatrecasas P. Possible requirement of internalization in the mechanism of in vitro cytotoxicity in tumor necrosis serum. Cancer Res. 1981 Dec;41(12 Pt 1):4885–4890. [PubMed] [Google Scholar]
- Laster S. M., Wood J. G., Gooding L. R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988 Oct 15;141(8):2629–2634. [PubMed] [Google Scholar]
- Leopardi E., Friend D. S., Rosenau W. Target cell lysis: ultrastructural and cytoskeletal alterations. J Immunol. 1984 Dec;133(6):3429–3436. [PubMed] [Google Scholar]
- Ostrove J. M., Gifford G. E. Stimulation of RNA synthesis in L-929 cells by rabbit tumor necrosis factor. Proc Soc Exp Biol Med. 1979 Mar;160(3):354–358. doi: 10.3181/00379727-160-40449. [DOI] [PubMed] [Google Scholar]
- Schmid D. S., Hornung R., McGrath K. M., Paul N., Ruddle N. H. Target cell DNA fragmentation is mediated by lymphotoxin and tumor necrosis factor. Lymphokine Res. 1987 Summer;6(3):195–202. [PubMed] [Google Scholar]
- Tsujimoto M., Yip Y. K., Vilcek J. Tumor necrosis factor: specific binding and internalization in sensitive and resistant cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7626–7630. doi: 10.1073/pnas.82.22.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderame M., Alcorta D., Egnor M., Smith K., Pollack R. Cytoskeletal F-actin patterns quantitated with fluorescein isothiocyanate-phalloidin in normal and transformed cells. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6624–6628. doi: 10.1073/pnas.77.11.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Holtmann H., Engelmann H., Nophar Y. Sensitization and desensitization to lethal effects of tumor necrosis factor and IL-1. J Immunol. 1988 May 1;140(9):2994–2999. [PubMed] [Google Scholar]
- Wieland T., Faulstich H. Amatoxins, phallotoxins, phallolysin, and antamanide: the biologically active components of poisonous Amanita mushrooms. CRC Crit Rev Biochem. 1978 Dec;5(3):185–260. doi: 10.3109/10409237809149870. [DOI] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Zaner K. S., Stossel T. P. Ca2+ control of actin gelation. Interaction of gelsolin with actin filaments and regulation of actin gelation. J Biol Chem. 1980 Oct 10;255(19):9494–9500. [PubMed] [Google Scholar]