Abstract
Hypertension is a mechanism-based toxic effect of drugs that inhibit the vascular endothelial growth factor signaling pathway (VSP). Substantial evidence exists for managing hypertension as a chronic condition, but there are few prospectively collected data on managing acute hypertension caused by VSP inhibitors. The Investigational Drug Steering Committee of the National Cancer Institute convened an interdisciplinary cardiovascular toxicities expert panel to evaluate this problem, to make recommendations to the Cancer Therapy Evaluation Program on further study, and to structure an approach for safe management by treating physicians. The panel reviewed: the published literature on blood pressure (BP), hypertension, and specific VSP inhibitors; abstracts from major meetings; shared experience with the development of VSP inhibitors; and established principles of hypertension care. The panel generated a consensus report including the recommendations on clinical concerns summarized here. To support the greatest possible number of patients to receive VSP inhibitors safely and effectively, the panel had four recommendations: 1) conduct and document a formal risk assessment for potential cardiovascular complications, 2) recognize that preexisting hypertension will be common in cancer patients and should be identified and addressed before initiation of VSP inhibitor therapy, 3) actively monitor BP throughout treatment with more frequent assessments during the first cycle of treatment, and 4) manage BP with a goal of less than 140/90 mmHg for most patients (and to lower, prespecified goals in patients with specific preexisting cardiovascular risk factors). Proper agent selection, dosing, and scheduling of follow-up should enable maintaining VSP inhibition while avoiding the complications associated with excessive or prolonged elevation in BP.
Box 1. Summary recommendations
Conduct and document a formal risk assessment for potential cardiovascular complications before vascular endothelial growth factor signaling pathway (VSP) inhibitor treatment. The assessment should include standardized blood pressure measurements (two separate sessions are suggested) and thorough history and examination to assess specific cardiovascular risk factors, and directed laboratory studies as indicated. (Table 2 summarizes the risk factors.) The purpose of this evaluation is to guide the physician and patient in determining the appropriate intensity of monitoring and control of blood pressure elevations. This provides an important opportunity to address comorbidities that through more attentive management could help prolong the patient's life and support more aggressive anticancer therapy.
Recognize that preexisting hypertension will be common in cancer patients and should be identified and addressed before initiation of VSP inhibitor therapy. Given the suspected importance of pretreatment intervention in the management of VSP inhibitor–induced blood pressure elevations, properly collected, objective, office measurements or more thorough evaluations for isolated office hypertension (also known as “white coat hypertension”) should guide the risk assessment rather than patient and/or physician speculation and dismissal.
Actively monitor blood pressure throughout treatment with more frequent assessments during the first cycle of treatment. The first cycle is typically when the bulk of the blood pressure elevation is expected to occur and when most patients unexpectedly present with elevations warranting treatment even in the absence of preexisting cardiovascular risk factors.
The goal for hypertension control in patients receiving VSP inhibitor therapy is a maximum blood pressure of 140/90 mmHg, and efforts to reach this goal should begin before initiation of VSP inhibitor therapy. The recommendation for a goal of maintaining blood pressure less than 140/90 mmHg is based on prudence and consistency with general guidelines. As per the risk stratification considerations, targets should be adjusted lower for patients with multiple preexisting risk factors for adverse consequences of high blood pressure. For example, for patients with diabetes and/or chronic kidney disease, a goal blood pressure of less than 130/80 mmHg is the current public health recommendation.
Manage blood pressure elevations aggressively to avoid the development of complications associated with excessive/prolonged elevations. Management requires attention to proper agent selection, dosing, and scheduling of follow-up to ensure efficacy and to control adverse effects of the antihypertensive agent. The panel suggests that at any time, if the oncologist or responsible medical team member has any difficulty in helping the patient progress to the goal blood pressure of 140/90 mmHg, consultation with the local hypertension specialist (cardiologist, nephrologist, endocrinologist, or certified hypertension specialist) should be obtained promptly.
Inhibiting angiogenesis is an effective approach to cancer therapy, but it has been associated with cardiovascular toxic effects. At times, adverse events such as hypertension and heart failure have led to treatment cessation and even life-threatening consequences (1–7). Cancer patients have often been excluded from studies of cardiovascular disease, and patients with clinically significant cardiovascular disease have been excluded from studies of new cancer therapies. Consequently, the capacity for determining the incidence or prevalence of cardiovascular toxic effects of anticancer agents and to determine their optimal management has been limited. Oncologists and cardiovascular medicine specialists have increasingly recognized that the prevention and management of these toxic effects is important for these potentially life-sustaining anticancer agents to benefit the greatest possible number of patients (8–14).
The Investigational Drug Steering Committee of the National Cancer Institute (NCI) formed a Cardiovascular Toxicities Panel joining members of its Angiogenesis Task Force with experts in the management of hypertension and cardiovascular toxic effects in cancer patients to generate consensus recommendations to optimize risk assessment, monitoring, and safe administration of new agents, despite the currently limited data specific to this medical problem. This Commentary has been written for oncologists and cancer researchers and also for a general medical audience, including primary care physicians and cardiovascular medicine specialists. Given the limited data specific to management of cardiovascular toxic effects of vascular endothelial growth factor (VEGF) signaling pathway (VSP) inhibitors, this is not a guidelines document but rather a collection of principles to guide safer more expansive use of these drugs and recommendations for the individual treating physician (see Box 1 for a summary of the panel's recommendations).
Hypertension: A Toxic Effect of VEGF Signaling Pathway Inhibitors
Angiogenesis (the generation of new branches of blood vessels from preexisting vessels) is a complex process of numerous molecules and cells within tissues. Drugs can inhibit angiogenesis by many mechanisms. The term VSP inhibitor includes agents that within their typical therapeutic dose range block downstream signaling of the soluble ligand, VEGF, and its primary cognate receptor on endothelial cells, VEGF receptor-2 (VEGFR2). VSP inhibitors constitute a subclass of angiogenesis inhibitors with four agents approved for marketing by multiple regulatory bodies worldwide: bevacizumab (Avastin), sorafenib (Nexavar), sunitinib (Sutent), and pazopanib (Votrient).
VEGF binding VEGFR2 activates the receptor's kinase function, triggering multiple downstream signaling cascades. These cascades are associated with different VEGF effects including increased capillary permeability, production of nitric oxide (leading to vascular smooth muscle relaxation), endothelial cell proliferation, migration, and survival under stress. Bevacizumab is a monoclonal antibody that binds VEGF. It is currently approved in combination with different chemotherapeutic regimens for the treatment of advanced breast, colorectal, and lung cancer, in combination with interferon alpha for kidney cancer, and for glioblastoma as a single agent. Sorafenib has been approved as a single agent in the treatment of hepatocellular and renal cancer, whereas sunitinib has been approved as single agent therapy for the treatment of renal and gastrointestinal stromal tumors. Pazopanib was more recently approved as another acceptable agent for treatment of renal cell carcinoma. In addition to blocking the kinase activity of VEGFR2, these small molecules also block kinases in tumor cells, cardiomyocytes, and other cells too. Several additional VSP inhibitors are in the later stages of clinical development, including aflibercept (VEGF Trap), axitinib (AG-013736), cediranib (AZD2171, Recentin), motesanib (AMG 706), and vandetanib (ZD6474, Zactima).
Blood pressure (BP) elevation is an effect common to all VSP inhibitors, with hypertension reported as an adverse event in every trial of these drugs (Table 1). BP regulation entails complex physiology, and the detailed mechanisms by which VSP inhibitors elevate BP in humans remain undetermined. Some evidence suggests that two effects of VSP inhibition on the systemic vasculature contribute to BP elevation: 1) increased vascular tone because of decreased nitric oxide production and 2) increased peripheral resistance because of endothelial cell damage and dysfunction (24–27). Although there are limited data on which directly to base recommendations for effective pretreatment evaluation, on-treatment surveillance, and management of hypertension throughout the VSP inhibitor treatment course, it is important to address this increasingly common clinical problem. Hemorrhage, thrombosis, nephrotoxicity, and cardiac toxic effects are also increasingly recognized adverse events of VSP inhibitors, but because BP elevations are more common and easier to address, this Commentary focuses on BP management.
Table 1.
Incidence of any and clinically significant hypertension in clinical trials of vascular endothelial growth factor signaling pathway (VSP) inhibitors*
| Agent | First author, year (reference) | Total No. of patients treated | Total incidence, % | Incidence of grade ≥ 3, % | Incidence of any grade hypertension in the comparator group, % |
| Aflibercept | Tew, 2007 (15) | 162 | 46 | 18 | NA |
| Axitinib† | Rugo, 2007 (16) | 167 | 30 | 5 | 5 |
| Bevacizumab‡ | Hurwitz, 2004 (17) | 790 | 22 | 11 | 8 |
| Cediranib† | Hirte, 2008 (18) | 49 | 72 | 33 | NA |
| Motesanib | Sherman, 2008 (19) | 93 | 56 | 25 | NA |
| Pazopanib‡ | Hutson, 2007 (20) | 161 | 37 | 8 | NA |
| Sorafenib† | Escudier, 2007 (21) | 902 | 17 | 4 | 2 |
| Sunitinib† | Motzer, 2007 (22) | 735 | 24 | 8 | 1 |
| Vandetanib | Arnold, 2007 (23) | 106 | 21 | 2 | 9 |
For each agent, one of the larger studies with that drug and the reported incidence of hypertension by the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) is listed. For some agents, data from a randomized placebo-controlled trial are not yet available. Note the differences in reported rates, and grades cannot be accurately compared across trials because of differing clinical setting and the CTCAE versions used, but in every trial with a comparator arm, the incidence of hypertension is higher with the addition of the VSP inhibitor. NA = not applicable (no untreated comparator group).
Based on CTCAE version 3.0 (grade 3 hypertension is defined as “requiring more than one drug or more intensive therapy than previously”).
Based on CTCAE version 2.0 (grade 3 hypertension is defined as “requiring initiation or increase medication”).
Principles for BP Management in Patients Receiving VSP Inhibitor Therapy
Shared and Divergent Goals of BP Management in Patients With Advanced Solid Tumors vs Cardiovascular Disease Prevention
Effectively lowering BP reduces morbidity and mortality from congestive heart failure, myocardial infarction, stroke, and renal insufficiency. Risk for death from these conditions is proportional to the level of BP (28). Effective control of chronic hypertension reduces the attributable 10-year mortality rate by 9% (number needed to treat is 11) (29), and so management has been typically approached as a public health problem (30–32). A public health solution requires a simple and easy clinical process. Several expert panels periodically review epidemiological and clinical trial data to produce evidence-based guidelines consistent with health-care system and regional cultural concerns.
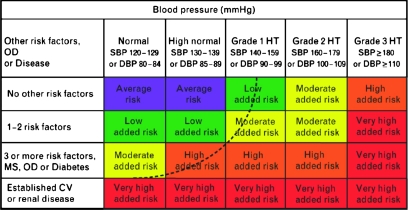
A simple algorithm that could be applied by a physician's support staff would be popular, but data support complex patient-specific evaluations and decision making. The European Societies of Cardiology and Hypertension (ESCH) (33) have described a predictive algorithm for BP control goals based on cardiovascular risk factors and adverse cardiovascular outcomes (Figure 1). In contrast to the population for which these long-term mortality risk reduction concepts have been developed, the patients for whom VSP inhibitor therapy is currently indicated have metastatic disease and limited life expectancy. Some patients might receive VSP inhibitor therapy for only a few months, and so the goals of BP management in this setting differ from those of primary prevention of long-term risk for cardiovascular disease. Nevertheless, control of BP is important for patients receiving VSP inhibitor therapy for the following five reasons:
Serious adverse events have been associated with unmanaged hypertension (1–3,5,34), and these could be prevented with control of BP before and early in the course of VSP inhibitor therapy.
These agents can cause dramatic increases in BP from pretreatment measurements (as high as 29 mmHg systolic and 27 mmHg diastolic in one prospective clinical investigation) (35) during the first week of treatment (35,36), and currently, it cannot be predicted which patients will have this magnitude of BP elevation.
Although minimalist approaches to hypertension for patients with incurable disease might be favored in certain circumstances, management of comorbidities, including hypertension, could improve overall survival. One large study (37) demonstrated that the burden of comorbidities, especially when unmanaged, affects cancer patient survival as much as stage at diagnosis.
As the indications for these agents expand to more chronic treatment courses, curative, and perhaps preventive settings, the goals of BP management then become similar to those for primary prevention of cardiovascular disease.
Active control of hypertension should allow patients to tolerate the highest effective dose of VSP inhibitor therapy and benefit from the tumor growth control for the longest period, improving quality and length of life with VSP inhibitor therapy.
Figure 1.
Stratification of cardiovascular risk and its relationship to blood pressure in adults. Reprinted with permission from Mancia et al. (33). OD = subclinical organ damage; SBP = systolic blood pressure; DBP = diastolic blood pressure, HT = hypertension; MS = metabolic syndrome; CV = cardiovascular. As per the European Societies of Cardiology and Hypertension guidelines low, moderate, high, and very high added risk refer to 10-year risk of a CV fatal or nonfatal event relative to the average population risk. These categories are associated with population and occupation-based cohorts and empiric methods of quantitative risk determination. Risk factors are boldfaced in Table 2. The dashed line indicates that the categorization of hypertension might be variable and dependent on total CV risk. For example, a patient with established CV or renal disease might have normal blood pressure limits of 129/84 mmHg, whereas someone with no other risk factors could have 150/95 mmHg as a normal blood pressure limit to achieve the same degree of cardiovascular risk reduction.
Specific Guidance for Assessment, Monitoring, and Management of Hypertension Associated With VSP Inhibitor Therapy
Rating Hypertension to Determine Intervention.
In the past, treatment-related hypertension was seldom a concern in anticancer therapeutics (38). During the development of the VSP inhibitors, hypertension surveillance, rating, and structured adverse event reporting to the NCI were through the standardized Common Terminology Criteria for Adverse Events (CTCAE) (39). This rating system categorizes “any unfavorable symptom, sign, or disease (including an abnormal laboratory finding) associated with the use of a medical treatment or procedure that may or may not be considered related to or caused by the medical treatment or procedure.” For example, “hypertension” is an adverse event term categorized under “Cardiovascular (General).” All adverse events are graded on a 5-point scale of general proportionality: 0 = no event, within normal limits; grade 1 = mild adverse event; 2 = moderate event; 3 = severe and undesirable; 4 = life threatening or disabling; and 5 = death. The document's purpose is to unify safety reporting so that reviewers can identify therapeutics, doses, or patient groups associated with unusual risk for severe adverse events. Because of its common use among clinical investigators and most practicing oncologists’ familiarity with the grading concepts, the CTCAE is often referenced in many circumstances beyond its intended use. CTCAE rating scales were not intended or validated to be instruments for grading severity of events, but most retrospective analyses of clinical trials of VSP inhibitors rely on the CTCAE grades. These grades do not reflect any typical hypertension classification system and so drawing inferences to guide management is challenging. The new CTCAE version 4 Hypertension scale has been aligned with the US National High Blood Pressure Education Program categories to improve communication among oncologists, cardiovascular medicine specialists, and primary care physicians.
For three decades, the Joint National Committee of the National Heart, Lung, and Blood Institute-sponsored National High Blood Pressure Education Program (the JNC) has reviewed data and developed recommendations for detection, evaluation, and treatment of hypertension. The current classification categorizes BP for adults aged 18 years or older as normal (<120/80 mmHg), prehypertension (120–139/80–89 mmHg) warranting intervention in patients with high risk, stage I hypertension (140–159/90–99 mmHg) warranting intervention, and stage II hypertension (≥160/100) warranting immediate attentive intervention to prevent acute symptoms. Other major organizations (32,33) have developed similar concepts, with different numerical classifications, to guide whether, when, and which intervention should begin.
The panel recognized that at this time, integrating into day-to-day clinical practice, a specific approach to BP in patients receiving VSP inhibitors presents logistical challenges. Oncologists are uniquely skilled to address numerous matters with their patients but under increasing time constraints. For the busy oncology specialist in a team environment that includes general medicine and cardiovascular disease specialists, colleagues more experienced in these matters might play a major collaborative role. For community practitioners, this process is a natural extension of good general medical care, and we highlight when risk is at a level that might warrant skills of specialists in other fields.
Pretreatment Assessment of Patient's Risk for Cardiovascular Complications.
Pretreatment risk assessment determines the likelihood that the patient will tolerate unexpected cardiovascular stress from these drugs without morbidity. It is reasonable to apply this concept of total cardiovascular risk to guide the intensity of pre-VSP inhibitor therapy evaluation and intervention, and on-treatment surveillance and intervention. Therefore, this panel recommends:
Formal evaluation and documentation of a patient's pretreatment risk for cardiovascular complications before initiation of VSP inhibitor therapy. The purpose of this assessment is not to exclude patients from potential life-extending VSP inhibitor therapy but rather to provide a systematic means by which risk for potential complications of VSP inhibitor therapy might be addressed efficiently for each patient. Most adult cancer patients are likely to have essential rather than secondary hypertension, so the identification of reversible secondary causes of hypertension is not part of this evaluation.
The pretreatment assessment should entail repeated BP measurements, a history and examination to assess specific risk factors listed in Table 2, and directed laboratory or instrument tests in Table 2 or in addition to these as clinically indicated.
Table 2.
Risk factors for adverse consequences of high blood pressure (BP)*
| Systolic BP ≥160 mmHg or diastolic BP ≥100 mmHg |
| Diabetes mellitus |
| Established CV disease including any history of: |
| Ischemic stroke, cerebral hemorrhage, or transient ischemic attack |
| Myocardial infarction, angina, coronary revascularization, or heart failure |
| Peripheral artery disease |
| Retinal hemorrhages or exudates and papilledema |
| Established or subclinical renal disease including: |
| Microalbuminuria or proteinuria (>30 mg/24 h) |
| Serum creatinine in men >1.5 mg/dL, women >1.4 mg/dL |
| Calculated or estimated glomerular filtration rate <60 mL/min/1.73 m2 |
| Subclinical organ damage previously documented by: |
| ECG or echocardiogram revealing left ventricular hypertrophy |
| Carotid ultrasound study revealing wall thickening or plaque |
| Three or more of the following CV risk factors: |
| Age (men >55 y, women >65 y) |
| Cigarette smoking |
| Dyslipidemia as measured by: |
| Total cholesterol >190 mg/dL or |
| Low-density lipoprotein cholesterol >130 mg/dL or |
| High-density lipoprotein cholesterol (men <40 mg/dL; women <46 mg/dL) or |
| Triglyceride > 150 mg/dL |
| Fasting plasma glucose >100 mg/dL |
| Family history of premature CV disease (first-degree male relative age <55 y or first-degree female relative <65 y) |
| Abdominal obesity male waist circumference >40 in; female >35 in (in persons of East Asian ancestry: male waist circumference >35 in and for women >31 in) |
Adapted, with permission, from Mancia et al. (33). CV = cardiovascular.
Considerations for Pretreatment Risk Assessment.
The initial measurement of BP is important and should be performed with an appropriately calibrated device in accord with published recommendations (40), including a correctly sized cuff and having the patient rest 5 minutes in the seated position before initial measurement (see Supplementary Appendix 1, available online). The mean of a minimum of two measurements collected at least 3 minutes apart should be used. The collection of these measurements on two separate clinic visits is typically used to diagnose hypertension in patients without cancer.
Identifying hypertension pretreatment means additional work for the patient, in terms of addressing a comorbid condition, and for the physician in assessment and management. Anecdotally, panel members noted a tendency for colleagues and patients to explain and dismiss high readings rather than address them objectively. Given the suspected importance of pretreatment intervention for VSP inhibitor–induced elevation in BP, properly collected office measurements or more thorough evaluations for isolated office hypertension/“white coat hypertension” should guide the risk assessment rather than patient or physician speculation and dismissal. Explanations for previously undiagnosed hypertension in advanced cancer patients include uncontrolled pain. Baseline measurements should be reassessed after better pain control with appropriate agents is achieved because nonsteroidal anti-inflammatory drugs can increase pressure, whereas narcotics and better pain control will lower pressure. Members of the panel noted that patients and physicians tended to take this diagnostic evaluation more seriously if, similar to preoperative clearance, the patient was referred to an existing primary care clinician or treating cardiovascular medicine specialist for the assessment.
The assessment should focus on establishing pretreatment cardiovascular risk, including identification of subclinical organ damage and concomitant clinical conditions. The assessment is simplified by the system advocated by the ESCH and summarized in Table 2. The six boldfaced items each constitute a risk factor for complications of elevated BP. We recommend stratifying patients as low risk = zero factors, high risk = one factor, and higher risk of at least two factors. Cancer patients initially evaluated for VSP inhibitor therapy could be at high risk for complications from elevated BP (Table 2), and this will only be identified by thorough clinical assessment. In patients who have not had recent routine screening for cardiovascular diseases, this evaluation will entail several tests beyond the complete blood count and metabolic profile typically reviewed for cytotoxic chemotherapy regimens. According to Table 2, for most advanced cancer patients, thorough risk assessment will include an electrocardiogram and measurements of serum cholesterol and triglycerides and of fasting plasma glucose. In many patients, echocardiogram or carotid ultrasound studies might be indicated to determine risk accurately. Registrational studies of bevacizumab, sorafenib, and sunitinib excluded patients with recent (within 6–12 months of treatment initiation) “cardiac events” or “clinically significant cardiovascular disease,” including myocardial infarction, severe or unstable angina, coronary or peripheral artery bypass grafting, symptomatic congestive heart failure, cerebrovascular accident, or transient ischemic attack. The drug labels recommend that patients should be carefully monitored for toxic effects. All of these individuals would be considered to be in the higher added risk cohort. Any patients not meeting the above criteria but classified as having higher added risk should undergo similar evaluation of the risk–benefit ratio and the most attentive risk reduction maneuvers.
The Goal BP in Patients Receiving VSP Inhibitor Therapy Is Less Than 140/90 mmHg.
The primary goal in this setting is to prevent hypertension-related toxic effects. The recommendation for maintaining BP less than 140/90 mmHg is based on prudence and consistency with JNC guidelines. For some patients in the higher added risk cohort, targets should be adjusted lower. For example, for patients with diabetes and/or chronic kidney disease, a goal BP of less than 130/80 mmHg is the current public health recommendation. For the high- and higher-risk patients, BP should be less than 140/90 mmHg before initiating VSP inhibitor therapy. If the patient reports already taking antihypertensive therapy, adherence to the regimen should be verified. If the patient is adherent and could tolerate, the dose should be titrated to achieve the 140/90 mmHg goal. If the dose is already the maximum, a second agent should be added and titrated appropriately with no less than weekly follow-up. The pace at which antihypertensive therapy is titrated is determined by the treating physician's balancing risks for delaying anticancer therapy with the threat of cardiovascular complications in the setting of incompletely controlled cardiovascular risks.
If the patient is not receiving antihypertensive therapy, begin it intending to titrate to reduce BP to goal. Often, VSP inhibitor therapy should be started shortly after the initial oncology evaluation. Shorter-acting agents with close follow-up (as frequently as every 2–4 days) might achieve the goal BP quickly, and the patient's BP could then be maintained with an equivalent dose of a longer-acting agent. If the BP goal is not achieved, it may not be necessary to delay starting VSP inhibitor therapy until antihypertensive therapy is fully titrated, as long as the BP is below the level that is likely to be associated with acute complications. Before beginning VSP inhibitor therapy, obtain objective evidence of improving BP control and plan to continue titration of the antihypertensive agent to the numerical goal as VSP inhibitor therapy begins. If there is any difficulty making progress toward achieving the goal BP of less than 140/90 mmHg, consultation with the local hypertension specialist should be obtained promptly.
In medical oncology practice, hypertension will be common, but detection depends on the thoroughness of surveillance. In 1999–2000, the most recently studied period of the National Health and Nutrition Examination Survey (NHANES), the prevalence of stage I hypertension in the entire US population was 28.7%; it was 65.4% in those aged 60 years and older. Of those found to be hypertensive, 69% were aware they had hypertension, 58.4% were receiving some medication to lower BP, and among those treated, 53% had their BP within the goal therapeutic range. NHANES is not based on cancer patients, and one might speculate that the cancer population has a lower prevalence of hypertension than the general population, but two small prospective studies of previously treated cancer patients with advanced solid tumors undergoing careful pretreatment BP assessment, mean age 56–59 years, reported rates of hypertension before VSP inhibitor therapy of 28% (35) and 29% (3), which are similar to the general population.
Monitoring BP During VSP Inhibitor Therapy.
Current NCI clinical trial protocols recommend monitoring BP weekly during the first cycle of VSP inhibitor therapy and then at least every 2–3 weeks for the duration of treatment. After the first cycle is completed and a stable BP has been achieved, depending on the level of risk for complications, the evaluation schedule might be more conveniently aligned with routine clinical evaluations or home BP monitoring. Cohort studies of sorafenib (17) and sunitinib (3) detected elevations in BP for the population during the first cycle of treatment. In more intensive studies using home BP (36) or ambulatory BP monitoring (35), initial elevations were detected during the first week of treatment. If untreated, BP may further increase to concerning levels for patients with predisposition for cardiovascular complications. Consequently, early detection and attentive management of BP elevations might prevent some of the severe complications of VSP inhibitor therapy. The easiest approaches for maintaining BP in these patients within a safe range are likely to be weekly office nursing visits or as has been implemented in many NCI-sponsored trials, home BP monitoring. Home monitoring entails a greater degree of patient education, provides the patient with an opportunity to participate actively in her or his care and therefore, for patients who can afford to purchase a certified upper arm cuff device (eg, no wrist or finger device has been certified for accuracy comparable to brachial cuff measurements), might be the preferred method. In some NCI trials, patients are provided with a BP diary, including instructions for accurate self-measurement, and numerical thresholds warranting a call to the physician's office for further instructions (see Supplementary Appendix 2, available online). For asymptomatic elevations in BP, treatment can be initiated by telephone, and evaluation for proper dosing and adverse effects of antihypertensive therapy can be scheduled after steady-state levels of the antihypertensive agent have been reached days later.
This guidance should be adjusted for younger patients who are clearly normotensive and at low baseline risk. One study of ambulatory monitoring and sorafenib (35) found the magnitude of elevation in BP in normotensive individuals to be highly variable. Some patients had no elevation in BP, and others had increases in diastolic BP greater than 20 mmHg. These changes in BP warrant management but might ordinarily go unaddressed in younger normotensive patients who are considered low risk for cardiovascular complications. In these patients, on-treatment BP measurements might be considered “normal” because they are not above 140/90 mmHg, but elevations of this magnitude within a short time frame (days to weeks) can place these patients among those at highest risk for hypertensive complications. A consensus recommendation from the panel for these patients is to focus on the magnitude of the change in BP rather than the crossing of a population-based threshold, such as 140/90 mmHg. Antihypertensive treatment should be initiated in any patient having an increase in diastolic BP of 20 mmHg and greater even if the absolute measurement is in the “normal” range. Keeping the diastolic increase within 20 mmHg of the baseline measurement should maintain a safe margin.
Finally, although bevacizumab is currently the most commonly prescribed VSP inhibitor, there are no published prospectively collected data on the time course of BP elevation. In animal models, various strategies for disrupting the VSP including VEGF-binding agents demonstrate similar effects on the normal vasculature over the same time course (41,42). Consequently, despite differences in the pharmacokinetics of bevacizumab, sorafenib, and sunitinib, in the absence of data suggesting otherwise, similar surveillance methods are appropriate.
Management of Treatment Emergent Hypertension.
Rather than recommend a specific algorithm for management of VSP inhibitor–induced hypertension, we recommend a general algorithm based on fundamental principles that experienced practitioners of BP management should find easy to implement. Patients developing stage I hypertension (≥140/90 mmHg) or increases in diastolic BP of 20 mmHg and higher from baseline should initiate antihypertensive therapy, have current therapy titrated to better control, or have another agent added. Patients frequently fast before blood collection on the day of office visits. Patients should be instructed to take their BP medication even if fasting to ensure accurate representation of the treated BP on office visits.
Panel members have routinely used many classes of agents to treat VSP inhibitor–induced hypertension, including thiazide diuretics, beta blockers, dihydropyridine and non-dihydropyridine calcium channel antagonists, angiotensin-converting enzyme inhibitors, and angiotensin receptor antagonists. Each of these classes of antihypertensive agents has successfully controlled VSP inhibitor–induced hypertension on an individual patient basis. Whether any one agent is superior to another in successful control rates remains to be determined.
Consider four matters in selecting an antihypertensive drug: 1) cancer and cancer therapeutics–specific cautions and contraindications to avoid a specific agent, 2) compelling considerations for preferring a specific agent in the general medical setting, 3) cautions and contraindications to avoid a specific agent in the general medical setting, and 4) time available to titrate to goal effect. Table 3 summarizes various classes of agents and important considerations in selection of an agent for patients in this setting (see Supplementary Appendix 3, available online, for suggestions on pairing different classes of agents). For patients who need to start VSP inhibitor therapy or return to treatment after cessation, achieving the goal BP can be more urgent than management of chronic hypertension. Oral angiotensin-converting enzyme inhibitors can relatively rapidly lower BP, whereas some dihydropyridine calcium channel antagonists, such as felodipine and amlodipine, typically take 3–5 days for BP lowering to be apparent. The treating clinician will avoid iatrogenic complications that lead to disruption of the anticancer treatment and that could harm the patient if these matters are considered before antihypertensive therapy is prescribed. It is useful to note that some members of the panel reported that VSP inhibitor–induced hypertension could typically be safely controlled with low doses of amlodipine combined with an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker.
Table 3.
Cautions, contraindications, and compelling considerations for major classes of antihypertensive drugs*
| Class of drug | Cancer-specific cautions or reasons to avoid | Basis for preferred selection | General cautions and contraindications |
| Angiotensin-converting enzyme inhibitors | Coadministration/titration with renal clearance–dependent agents (eg, cisplatin and pemetrexed); hyperkalemia | Left ventricular systolic dysfunction; diabetic nephropathy | Renovascular disease; peripheral vascular disease; renal impairment |
| Angiotensin II receptor blockers | Coadministration/titration with renal clearance–dependent agents (eg, cisplatin and pemetrexed); hyperkalemia | Intolerance of other agents, especially ACE inhibitors; left ventricular systolic dysfunction; diabetic nephropathy | Renovascular disease; peripheral vascular disease; renal impairment |
| Beta blockers | Asthenia; malaise; fatigue; QT interval prolonging drugs | Angina; history of myocardial infarction; anxiety | Bradycardia/heart block; diabetes (risk for hypoglycemia); asthma/chronic obstructive pulmonary disease (wheezing); decompensated heart failure |
| Calcium channel blockers (eg, dihydropyridines) | Lower extremity swelling | Elderly patients; isolated systolic hypertension | Preexisting edema; slow onset of action |
| Thiazide diuretics | Gout; hypercalcemia; hypokalemia; young patients (age ≤45 y); QT interval prolonging drugs | Elderly patients; isolated systolic hypertension; secondary stroke prevention; typically least expensive | Gout; documented sulfa allergy |
Adapted, with permission, from Mancia et al. (33). Diltiazem and verapamil are inhibitors of CYP3A4, an important enzyme in the metabolism of sunitinib and sorafenib. Although specific drug–drug interactions are undocumented, as general guidance, the other agent classes might be used with a greater potential safety margin.
Prescribing antihypertensive agents warrants awareness of their pharmacology so that adverse effects can be recognized and corrected and so assessment of response to therapy can be properly scheduled (a list of agents is featured in Supplementary Appendix 4, available online). The goal is to avoid complications from acute hypertension, and this goal is most likely to be achieved by attentive management at the earliest convincing sign of BP elevation above an acceptable threshold for the patient and the patient's cardiovascular risk. Excessively aggressive BP lowering or inappropriate selection of antihypertensive agents could have consequences for cancer patients that are as damaging as the cardiovascular complications of VSP inhibitors, and so physician competence and judgment are essential for successful implementation of these recommendations.
Because BP elevation is a reversible mechanism-based effect of VSP inhibition, discontinuation or where appropriate, dose reduction of the VSP inhibitor can also be used to control VSP inhibitor–induced hypertension. This approach can be more easily used with the kinase inhibitors, which are typically administered once or twice daily. Temporary cessation of kinase inhibitors can be useful when hypertension is difficult to control, when patients report symptoms of excess BP elevation (13), or when the BP elevation is one of a constellation of multiple drug-associated toxic effects. Kinase inhibitor therapy should be reinstituted at the same or lower dose once BP control and titration of antihypertensive agents is achieved to increase the likelihood for tumor control. However, if BP elevation is the only toxic effect a patient experiences, reasonable efforts (fully titrating at least two antihypertensive medications and referral to the local hypertension specialist if a BP of 140/90 mmHg still is not achieved) should be made to maintain the patient at the highest tolerable dose.
For bevacizumab and other VSP inhibitors with long half-lives, eg, aflibercept (VEGF-Trap), the drug effect is not readily withdrawn after treatment cessation and the effects of dose reduction are unknown, so stricter implementation of dose interruption and management with antihypertensives could be required. In NCI-sponsored trials, it is usually recommended that the bevacizumab dose be held for BP higher than 160 mmHg systolic or 100 mmHg diastolic or any symptomatic hypertension at the time of treatment. If BP remains uncontrolled despite active management with antihypertensives during the withholding period (reinstatement of bevacizumab treatment is delayed for >4 weeks), then discontinuation of bevacizumab should be considered. Notably, this general statement is dependent on the physician's expertise in controlling BP. Therefore, any patient who has to have a dose of bevacizumab withheld for uncontrolled hypertension despite active intervention should be referred to the local hypertension specialist to achieve the BP goal before the scheduled time for the next cycle of bevacizumab.
To achieve the BP control goals, physicians should be mindful of other agents that could magnify the BP-elevating effects of the VSP inhibitors. Excessive alcohol consumption and some agents that are commonly prescribed by oncologists also elevate BP, including nonsteroidal anti-inflammatory drugs (including cyclooxygenase 2 inhibitors), adrenal steroid hormones, erythropoietin, oral contraceptive hormones, and sympathomimetics, such as methylphenidate. If any of these agents is indicated during the course of VSP inhibitor therapy, anticipate further BP elevation and either increase the antihypertensive therapy or the frequency of BP measurements accordingly.
Finally, VSP inhibition–induced BP elevation will dissipate with discontinuation of VSP inhibition. For patients discontinuing VSP inhibitor therapy, anticipate the need to discontinue or reduce the dose of antihypertensive agents administered to control the BP elevation associated with the VSP inhibitor.
Funding
US National Cancer Institute, Investigational Drug Steering Committee initiative (cochairs: Charles Erlichman and Michael Grever) of the Clinical Trials Working Group of the National Cancer Advisory Board. The Cardiovascular Toxicities Panel was convened by the Angiogenesis Task Force (cochairs: George Wilding and Roy Herbst). M.L.M. was supported by mentored career development award 5K23CA124802 from the National Cancer Institute.
Supplementary Material
Footnotes
The sponsors had no direct role in the preparation of the manuscript.
The authors are grateful to the administrative team of Amy Gravell, Emmes Corporation, and Dr LeeAnn Jensen, Coordinating Center for Clinical Trials, Office of the Director, National Cancer Institute and the leadership of the Angiogenesis Task Force and Investigational Drug Steering Committee for supporting this effort.
G. L. Bakris receives grant and research support from the Juvenile Diabetes Research foundation, GSK, Forest Labs, and CVRx and is a consultant for GSK, Merck, Novartis, Boehringer Ingelheim, Takeda, Abbott, Walgreen’s, BMS/Sanofi, Gilead, and Forest. W. J. Elliott has received royalties from Elsevier and has served as advisor or consultant to Gilead Sciences, Nicox, Novartis Pharmaceuticals Corp, Forest Research Institute, and Sanofi-Aventis and has been a member of the speaker's bureau for Pfizer, Novartis, and Forest; none of these relationships were related to cancer therapy. J. Lindenfield is currently conducting research sponsored by Merck. S. C. Remick receives clinical research grant funding and serves on the Cardiovascular Safety Advisory Board for Oxigene, Inc. J.-B. Durand is on the speaker's bureau for Novartis. M. L. Maitland receives research funding from Bayer and Genentech to conduct studies of vascular endothelial growth factor signaling pathway inhibition on blood pressure.
References
- 1.Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354(9):980–982. doi: 10.1056/NEJMc052954. [DOI] [PubMed] [Google Scholar]
- 2.Ozcan C, Wong SJ, Hari P. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354(9):980–982. [PubMed] [Google Scholar]
- 3.Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370(9604):2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khakoo AY, Kassiotis CM, Tannir N, et al. Heart failure associated with sunitinib malate: a multitargeted receptor tyrosine kinase inhibitor. Cancer. 2008;112(11):2500–2508. doi: 10.1002/cncr.23460. [DOI] [PubMed] [Google Scholar]
- 5.Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26(32):5204–5212. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 6.Telli ML, Witteles RM, Fisher GA, Srinivas S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol. 2008;19(9):1613–1618. doi: 10.1093/annonc/mdn168. [DOI] [PubMed] [Google Scholar]
- 7.Snider KL, Maitland ML. Cardiovascular toxicities: clues to optimal administration of vascular endothelial growth factor signaling pathway inhibitors. Target Oncol. 2009;4(2):67–76. doi: 10.1007/s11523-009-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors: a review. Eur J Cancer. 2006;42(18):3127–3139. doi: 10.1016/j.ejca.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer. 2008;8(4):309–316. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 10.Jain RK, Duda DG, Willett CG, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6(6):327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Heeckeren WJ, Ortiz J, Cooney MM, Remick SC. Hypertension, proteinuria, and antagonism of vascular endothelial growth factor signaling: clinical toxicity, therapeutic target, or novel biomarker? J Clin Oncol. 2007;25(21):2993–2995. doi: 10.1200/JCO.2007.11.5113. [DOI] [PubMed] [Google Scholar]
- 12.Izzedine H, Ederhy S, Goldwasser F, et al. Management of hypertension in angiogenesis inhibitor-treated patients. Ann Oncol. 2009;20(5):807–815. doi: 10.1093/annonc/mdn713. [DOI] [PubMed] [Google Scholar]
- 13.Jain M, Townsend RR. Chemotherapy agents and hypertension: a focus on angiogenesis blockade. Curr Hypertens Rep. 2007;9(4):320–328. doi: 10.1007/s11906-007-0058-7. [DOI] [PubMed] [Google Scholar]
- 14.Lenihan DJ. Tyrosine kinase inhibitors: can promising new therapy associated with cardiac toxicity strengthen the concept of teamwork? J Clin Oncol. 2008;26(32):5154–5155. doi: 10.1200/JCO.2008.18.5439. [DOI] [PubMed] [Google Scholar]
- 15.Tew WP, Colombo N, Ray-Coquard I, et al. VEGF-Trap for patients with recurrent platinum-resistant epithelial ovarian cancer: preliminary results of a randomized, multicenter phase II study [meeting abstracts] J Clin Oncol. 2007;25(18 suppl):5508. [Google Scholar]
- 16.Rugo HS, Stopeck A, Joy AA, et al. A randomized, double-blind phase II study of the oral tyrosine kinase inhibitor axitinib (AG-013736) in combination with docetaxel compared to docetaxel plus placebo in metastatic breast cancer [meeting abstracts] J Clin Oncol. 2007;25(18 suppl):1003. [Google Scholar]
- 17.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 18.Hirte HW, Vidal L, Fleming GF, et al. A phase II study of cediranib (AZD2171) in recurrent or persistent ovarian, peritoneal or fallopian tube cancer: final results of a PMH, Chicago and California consortia trial [meeting abstracts] J Clin Oncol. 2008;26(15 suppl):5521. [Google Scholar]
- 19.Sherman SI, Wirth LJ, Droz JP, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359(1):31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 20.Hutson TE, Davis ID, Machiels JP, et al. Pazopanib (GW786034) is active in metastatic renal cell carcinoma: interim results of a phase II randomized discontinuation trial [meeting abstracts] J Clin Oncol. 2007;25(18 suppl):5031. [Google Scholar]
- 21.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 22.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 23.Arnold AM, Seymour L, Smylie M, et al. Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20. J Clin Oncol. 2007;25(27):4278–4284. doi: 10.1200/JCO.2007.12.3083. [DOI] [PubMed] [Google Scholar]
- 24.Mourad JJ, des Guetz G, Debbabi H, Levy BI. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol. 2008;19(5):927–934. doi: 10.1093/annonc/mdm550. [DOI] [PubMed] [Google Scholar]
- 25.Steeghs N, Gelderblom H, Roodt JO, et al. Hypertension and rarefaction during treatment with telatinib, a small molecule angiogenesis inhibitor. Clin Cancer Res. 2008;14(11):3470–3476. doi: 10.1158/1078-0432.CCR-07-5050. [DOI] [PubMed] [Google Scholar]
- 26.Veronese ML, Mosenkis A, Flaherty KT, et al. Mechanisms of hypertension associated with BAY 43-9006. J Clin Oncol. 2006;24(9):1363–1369. doi: 10.1200/JCO.2005.02.0503. [DOI] [PubMed] [Google Scholar]
- 27.Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension. 2009;54(3):652–658. doi: 10.1161/HYPERTENSIONAHA.109.129973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 29.Ogden LG, He J, Lydick E, Whelton PK. Long-term absolute benefit of lowering blood pressure in hypertensive patients according to the JNC VI risk stratification. Hypertension. 2000;35(2):539–543. doi: 10.1161/01.hyp.35.2.539. [DOI] [PubMed] [Google Scholar]
- 30.European Society of Hypertension-European Society of Cardiology Guidelines Committee. European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21(6):1011–1053. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 32.Williams B, Poulter NR, Brown MJ, et al. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ. 2004;328(7440):634–640. doi: 10.1136/bmj.328.7440.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28(12):1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 34.Govindarajan R, Adusumilli J, Baxter DL, El-Khoueiry A, Harik SI. Reversible posterior leukoencephalopathy syndrome induced by RAF kinase inhibitor BAY 43-9006. J Clin Oncol. 2006;24(28):e48. doi: 10.1200/JCO.2006.08.4608. [DOI] [PubMed] [Google Scholar]
- 35.Maitland ML, Kasza KE, Karrison TG, et al. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res. 2009;15(19):6250–6257. doi: 10.1158/1078-0432.CCR-09-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azizi M, Chedid A, Oudard S. Home blood-pressure monitoring in patients receiving sunitinib. N Engl J Med. 2008;358(1):95–97. doi: 10.1056/NEJMc072330. [DOI] [PubMed] [Google Scholar]
- 37.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 38.Grossman E, Messerli FH. High blood pressure. A side effect of drugs, poisons, and food. Arch Intern Med. 1995;155(5):450–460. doi: 10.1001/archinte.155.5.450. [DOI] [PubMed] [Google Scholar]
- 39.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 40.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 41.Baffert F, Le T, Sennino B, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006;290(2):H547–H559. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 42.Kamba T, Tam BY, Hashizume H, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290(2):H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.