Abstract
Background
Arsenic is a carcinogen that targets the urogenital system, including the prostate. Although the mechanisms for arsenic-induced carcinogenesis are undefined, arsenic drives overaccumulation of stem cells and cancer stem cells (CSCs) in vivo and in vitro, indicating that these cells are a key target population. Disruption of stem cell population dynamics may be critical to acquisition of cancer phenotype. We tested the hypothesis that prostate stem cells have a survival selection advantage during arsenic exposure that favors their accumulation and facilitates their malignant transformation.
Methods
Innate and acquired resistance to acute (24–72 hours of exposure) and chronic (6 weeks of exposure) arsenite-induced cytolethality and apoptosis were assessed in a human prostate stem cell line (WPE-stem) and the mature parental cell line (RWPE-1). Real-time reverse transcription–polymerase chain reaction and/or Western blot analysis was used to measure the expression of apoptosis-, stress-, and arsenic-related genes. Arsenic-, cadmium-, and N-methyl-N-nitrosourea–induced isogenic malignant transformants of RWPE-1 cells were compared for acquisition of CSC-like qualities by holoclone and sphere formation assays, growth in soft agar, and expression of CSC biomarkers. All statistical tests were two-sided.
Results
WPE-stem cells showed innate resistance to arsenic-induced cytolethality (arsenite concentration lethal to 50% of the cells [LC50] = 32.4 μM, 95% confidence interval [CI] = 31.5 to 33.3 μM) and apoptosis compared with parental RWPE-1 cells (LC50 = 10.4 μM, 95% CI = 7.4 to 13.4 μM). Compared with RWPE-1 cells, WPE-stem cells showed noticeably higher expression of antiapoptotic (ie, BCL2, MT), stress-related (ie, NFE2L2, SOD1, PRODH), and arsenic adaptation (ie, ABCC1, GSTP1) factors and noticeably lower expression of proapoptotic factors (ie, BAX, caspases 3, 7, 8, and 9). WPE-stem cells also showed hyper-adaptability to chronic arsenite exposure (5 μM, 6 weeks) compared with RWPE-1 cells (LC50 = 94.7 vs 32.1 μM, difference = 62.6 μM, 95% CI = 53.3 to 71.9 μM) at levels that in previous work induced a malignant phenotype in RWPE-1 after 30 weeks of exposure. Quantification of CSC-like cells in isogenic RWPE-1 transformants showed that marked overproduction was unique to a malignant phenotype acquired in response to arsenic exposure but not in response to cadmium or N-methyl-N-nitrosourea exposure.
Conclusions
An apparent stem cell survival advantage with regard to arsenic causes selection during malignant transformation that manifests itself as an overabundance of CSC-like cells specifically after arsenic-driven acquisition of malignant phenotype. The increased resistance to apoptosis and arsenite hyper-adaptability of WPE-stem cells suggests that arsenite transformation of RWPE-1 cells involves an increase in the number of CSC-like cells.
CONTEXT AND CAVEATS
Prior knowledge
Arsenic is a carcinogen that targets the prostate, but the precise mechanisms are not defined.
Study design
In vitro assays were used to compare a human prostate stem cell line with the mature parental nontumorigenic cell line from which it was derived with respect to their intrinsic resistance and adaptation to arsenite exposure during initial phases of arsenite-induced malignant transformation. Arsenic-, cadmium-, and N-methyl-N-nitrosourea–induced isogenic malignant transformants of the mature prostate cell line were compared for their acquisition of cancer stem cell–like qualities by holoclone and sphere formation assays, growth in soft agar, and expression of stem cell biomarkers.
Contribution
Prostate stem cells showed innate resistance to arsenic-induced cytolethality and apoptosis compared with parental cells. Compared with parental cells, stem cells showed higher expression of antiapoptotic, stress-related, and arsenic adaptation factors and lower expression of proapoptotic factors, as well as hyper-adaptability to chronic arsenite exposure. Cancer stem cell–like cells were overproduced in arsenic-induced isogenic malignant transformants of the mature prostate cell line but not in cadmium- or N-methyl-N-nitrosourea–induced transformants.
Implications
Arsenic likely targets cells that have a stem or progenitor phenotype during malignant transformation.
Limitations
Molecular markers were used to identify cancer stem cells. It is unclear if arsenic targeting of a stem cell population during carcinogenesis is generalizable to all targets of arsenic carcinogenesis. All analyses were done in cultured cell lines.
From the Editors
Inorganic arsenic is a widespread environmental contaminant and human carcinogen (1). Although its carcinogenic mechanisms remain elusive (2), arsenic targets various human tissues, including several sites in the urogenital system (1) such as the prostate (3). Chronic in vitro exposure of human cells of urogenital system origin to inorganic arsenic can induce their malignant transformation (4). In mice, in utero arsenic exposure induces oncogenesis at multiple sites within the urogenital system of the adult offspring (5). There is also evidence that arsenic has transplacental carcinogenic activity in humans (6). Fetal stem cells are thought to be key targets during transplacental chemical carcinogenesis (7).
The fact that stem cells share key properties with cancer cells fortifies the emerging concept of cancer as a stem cell–based disease (8). According to this concept, malignancies arise from a small subset of pluripotent cells that are targeted for malignant conversion, which leads to the production of cancer stem cells (CSCs) that drive the overall carcinogenic process (8). Thus, a stem cell survival selection advantage could be of critical importance to all stages of chemical carcinogenesis. Indeed, there is a strong suspicion that fetal stem cells are key targets in transplacental chemical carcinogenesis (7) because of their relative abundance and their roles in organogenesis and differentiation. In a human in vitro skin model, short-term arsenic exposure initially blocks stem cell differentiation, which may increase the pool of target stem cells available for oncogenic transformation (9). Furthermore, in utero exposure of mice to arsenite facilitated the formation of skin malignancies in adulthood that are associated with altered skin stem cell signaling and population dynamics resulting in tumors with an overabundance of skin CSCs (10). This finding implicates a targeting of stem cells in fetal skin and indicates that stem cell populations are key targets of arsenic carcinogenesis (10).
Cancer cells often are highly resistant or readily adapt to toxic insults, such as from metallochemotherapeutics, allowing them to readily avoid apoptosis. The initiation of carcinogenesis is frequently associated with the selection for cells that are resistant to apoptosis (11,12), which is a hallmark of cancer cells and a major driving force in tumor progression (13,14). Cancer cells develop multiple ways to block apoptosis, including overexpression of antiapoptotic Bcl-2 protein family members and loss of expression of proapoptotic Bax-like proteins and caspases (13–15). In addition, altered expression levels of key ATP-binding cassette (ABC) efflux transporter proteins in cancer cells are critical factors in their innate or acquired resistance to xenobiotics, including chemotherapeutic agents (16,17). Increased levels of glutathione and glutathione S-transferases (GSTs) often increase production of glutathione conjugates that are effluxed from cancer cells (16,18). Such features increase cancer cell survival and allow the expansion of malignant cell populations (13–15). For example, human cells that are chronically exposed to low levels of arsenic in vitro adapt to this metalloid by showing generalized resistance to apoptosis and adaptation to arsenic toxicity (16,18,19) as they proceed to malignant transformation (4). Although the role of stem cells in arsenic adaptation is undefined, stem cells often are resistant to chemical insult and act as a “strategic” reserve for wound repair, including repair from chemical wounding (20,21).
Most current research on resistance to toxicants or apoptosis in stem cells focuses on CSCs isolated from advanced cancers rather than those identified during the very early stage of oncogenesis and is directed at chemotherapeutic resistance (22,23). However, a limitation of such studies is the cellular heterogeneity and presumed genetic drift of advanced cancers (22). Nonetheless, factors that mediate resistance of CSCs to chemotherapeutics are similar to those that protect normal stem cells from toxic insult (22–25), which strongly suggests that CSCs are derived from normal stem cells through survival selection, although this possibility has not to our knowledge been directly tested.
We hypothesized a survival selection advantage exists for stem cells that facilitates arsenite-induced oncogenesis in key target tissues of arsenic carcinogenesis. To sequentially test this hypothesis, we compared the well-characterized prostate stem cell line, WPE-stem (26), with RWPE-1, the mature, heterogeneous nontumorigenic parental cell line from which it was derived, with respect to their intrinsic resistance and adaptation to arsenite exposure during initial phases of arsenite-induced malignant transformation. We also compared RWPE-1 cells malignantly transformed by arsenite, cadmium, or N-methyl-N-nitrosourea (MNU) to examine whether stem cell selection had occurred during transformation and, in keeping with our hypothesis, whether selection is a feature particularly pronounced with an arsenite-induced malignant phenotype.
Materials and Methods
Chemicals and Cell Culture Matrices
Sodium arsenite and p-iodonitrotetrazolium were obtained from Sigma (St Louis, MO), type IV collagen was obtained from Trevigen (Gaithersburg, MD), and fibronectin was obtained from BD Biosciences (Bedford, MD). Arsenite was dissolved in sterile distilled water to make stock solutions.
Cells and Cell Culture
All cell lines were derived and characterized by the laboratories directly involved in this study. RWPE-1 is a nontumorigenic cell line that was derived from normal human prostate epithelium (27) and has been shown to contain stem, intermediate, and differentiated cell types (26). The WPE-stem cell line was isolated from RWPE-1 cells by single-cell cloning and shows extensive characteristics of a stem cell phenotype, including overexpression of p63 and keratins 5 and 14; low expression of keratin 18, androgen receptor, and prostate-specific antigen; and a high proliferative rate and colony formation ability compared with parental RWPE-1 cells (26). WPE-stem cells also show high expression of the prostate stem cell markers ABC subfamily G member 2 (ABCG2) and the BMI1 oncoprotein compared with RWPE-1 cells, which have barely detectable levels, as well as the ability to form viable free-floating cell spheres (prostaspheres) and budding ductal-like structures from single cells in Matrigel (28), characteristics that are typical of stem cells and CSCs (29,30). Tissue culture surfaces were coated with type IV collagen and fibronectin (2.5 μg of each/cm2) unless otherwise noted. We also used three malignantly transformed cell lines (defined by their ability to form malignant tumors in mouse xenograft studies) that were derived from RWPE-1 cells in previous studies: WPE1-NB26 cells were derived after a single exposure of RWPE-1 cells to the direct-acting organic carcinogen MNU (31); CTPE cells were derived from RWPE-1 cells chronically exposed to low levels of cadmium (32); and CAsE-PE cells were derived RWPE-1 cells chronically exposed to low levels of arsenite (4). Thus, all of the cell lines used in this study share a common genetic lineage.
All cell lines were maintained in keratinocyte serum-free medium containing 50 μg/mL bovine pituitary extract, 5 ng/mL epidermal growth factor, and 1% antibiotic–antimycotic mixture (all from Gibco/Invitrogen, Rockville, MD). The culture medium was changed every 48 hours unless otherwise noted. Subconfluent cells were passaged once a week. To examine the potential for adaptation to arsenite, cells were continuously exposed for 6 weeks to 5 μM sodium arsenite, a nontoxic, environmentally relevant level of arsenite (33) that is known to produce malignant transformation of RWPE-1 cells after 30 weeks of continuous exposure (4), and were compared with untreated passage-matched control cells. Our previous work indicated that RWPE-1 cells that are exposed to this level of arsenite will start to show adaptation (ie, resistance to arsenite cytotoxicity and increased levels of ABCC1 and glutathione) to arsenic cytotoxicity between 4 and 8 weeks of continuous exposure (34) and will undergo malignant transformation after approximately 30 weeks of continuous exposure (4).
Cell Viability Assay
RWPE-1 and WPE-stem cells were exposed to various concentrations of arsenite for 72 hours. Cell viability was assessed using the trypan blue dye exclusion method (7.5 × 104 cells per well, plated in six-well plates) and by using a Cell Titer 96 AQueous One Solution Cell Proliferation Assay (MTS) kit (1.0 × 104 cells per well in 96-well plates) (Promega, Madison, WI) according to the manufacturer’s instructions. Viable cells were counted with a hemocytometer (trypan blue method) or by absorbance at 490 nm (MTS assay). The concentration that induced a 50% decrease in cell viability compared with untreated control cells (ie, the LC50) was derived from averaging multiple (n = 3) survival curves for each treatment group. Similar LC50 values were obtained with both methods (data not shown), and thus, only data from the trypan blue dye exclusion method are shown and were used to calculate LC50 values.
Apoptosis Assay
RWPE-1 and WPE-stem cells (2.0 × 106 per 100-mm dish) were plated in triplicate for each treatment and allowed to attach overnight. To obtain detectable levels of apoptosis, cells were treated with 30 μM sodium arsenite for 24 hours. Floating cells (from the supernatant) and adherent cells (collected by brief trypsin exposure) were harvested, combined, and centrifuged (300g for 5 minutes). The cell pellets were washed with phosphate-buffered saline (PBS), aliquoted (1.0 × 106 cells per tube), and centrifuged at 300g for 8 minutes (4°C). Apoptosis was detected with the use of a TACS Annexin V-FITC Apoptosis Detection Kit (Trevigen) according to the manufacturer's protocol except that we used 3 μL of Annexin V and stained for 20–30 minutes. Annexin-V–positive cells were quantified with the use of a FACSort flow cytometer (Becton-Dickinson, San Jose, CA) equipped with CellQuest software (Becton-Dickinson). The percentage of apoptotic cells in arsenite-treated samples (n = 3) compared with that in untreated control cells (n = 3) was determined using CellQuest software.
Arsenic Biokinetics
RWPE-1 and WPE-stem cells (1.0 × 106 per 100-mm dish, n = 3 dishes per treatment) were plated in complete medium, incubated overnight, and placed in fresh complete medium that lacked (control) or contained 5 μM sodium arsenite for 24 hours. The cells were washed three times with PBS, harvested by incubation in trypsin–EDTA, counted, and digested overnight in 50% perchloric acid:nitric acid (2:1 [vol/vol]) at 70°C, and the total uptake of arsenic by the cells was determined with the use of a AAnalyst 100 graphite furnace atomic absorption spectrophotometer (Perkin-Elmer, Waltham, MA). To measure arsenic efflux, the cells were incubated in medium that contained 5 μM sodium arsenite for 24 hours, washed three times with PBS, and then incubated for 24 hours in 10 mL of fresh arsenic-free medium. The cells were then washed, harvested, digested, and subjected to spectrometry as described above to determine cellular arsenic that remained. Data were normalized to cell number.
Glutathione Levels
Briefly, RWPE-1 and WPE-stem cells (2.5 × 106 per cell line) were harvested by incubation in trypsin–EDTA, counted, centrifuged (300g for 10 minutes at 4°C), washed with cold PBS, centrifuged, and resuspended in 500 μL of cold 5% metaphosphoric acid. Cells were then sonicated for 10 seconds, incubated on ice for 5 minutes, and centrifuged (12 000g for 5 minutes at 4°C). Total glutathione levels were measured with the use of a Glutathione Assay Kit (Trevigen) according to the manufacturer's protocol. Three separate flasks were used for each cell line (n = 3).
Real-Time Reverse Transcription–Polymerase Chain Reaction Analysis
Gene expression levels in each cell line (RWPE-1, WPE-stem, WPE1-NB26, CTPE, and CAsE-PE) were measured by real-time reverse transcription–polymerase chain reaction analysis. Total RNA was isolated from cell lines with the use of TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions and purified with the use of RNeasy mini kit columns (Qiagen, Valencia, CA). Purified RNA was reverse transcribed to cDNA with the use of Moloney murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA) reverse transcriptase and oligo-dT primers. Gene-specific primers (Supplementary Table 1, available online) were designed with the use of Primer Express software (Applied Biosystems). The ABsolute SYBR Green ROX Mix (ABgene, Rockford, IL) was used for amplifications. Amplification conditions were as follows: 15 minutes at 95°C, followed by 40 cycles of 95°C for 1 minute and 60°C for 1 minute. Cycle time (Ct) values for the selected genes were normalized to values for β-actin and glyceraldehyde 3-phosphate dehydrogenase in the same sample. For each cell line, samples were collected from three independent flasks (n = 3). For baseline gene expression analysis, RWPE-1 cells were used as the control for comparison with WPE-stem samples. Ct values for all controls were set at 100%.
Western Blot Analysis
Protein extracts were collected from cell lines with the use of M-PER extraction reagent (Pierce, Rockford, IL), and 25 μg of protein per lane was resolved on sodium dodecyl sulfate–polyacrylamide gels, transferred to polyvinylidene difluoride membranes, and the membranes were incubated with the following primary antibodies: anti-metallothionein (MT)-1/2 (clone E9, mouse monoclonal, 1:1000 dilution; Dako, Carpinteria, CA); anti-caspase 3 (K-19, goat polyclonal, 1:250 dilution), anti-caspase 7 (B-5, mouse monoclonal, 1:500 dilution), anti-caspase 8 (H-134, rabbit polyclonal, 1:500 dilution), and anti-caspase 9 (F-7, mouse monoclonal, 1:500 dilution; all from Santa Cruz Biotechnology, Santa Cruz, CA); anti-Bcl-2 (Bcl-2/100, mouse monoclonal, 1:1000 dilution) and anti-Bax (6A7, mouse monoclonal, 1:1000 dilution; both from BD Pharmingen, San Diego, CA); and anti-GST-π (354212, rabbit polyclonal, 1:1000 dilution; Calbiochem, San Diego, CA). The membranes were washed and incubated with horseradish peroxidase–conjugated anti-mouse, anti-rabbit, or anti-goat IgG secondary antibodies (1:1000 to 1:2000 dilution; Santa Cruz Biotechnology) as appropriate, and bound antibody was detected using Amersham ECL Western Blot Detection Reagents (GE Healthcare, Buckinghamshire, UK). Membranes were then stripped with Restore Western Blot Stripping Buffer (Pierce) and incubated with anti-β-actin antibody (JLA20, mouse monoclonal, 1:2500 dilution; Calbiochem) followed by incubation with horseradish peroxidase–conjugated anti-mouse IgG (Santa Cruz Biotechnology). Bound antibodies were quantified using ImageJ software (35). Proteins that have similar molecular weights were probed for on separate membranes. Membranes were used no more than two times before they were probed for β-actin.
Holoclone Formation
Each cell line (RWPE-1, WPE1-NB26, CTPE, and CAsE-PE) was plated (2.0 × 106 per T-75 flask) and grown until approximately 90% confluent, fed with 10 mL of medium, and incubated for 72 hours. Floating cells were recovered from the culture medium by centrifugation (600g), resuspended in complete medium, and plated in T-25 flasks for counting (7.5 × 104 cells, n = 3 flasks per cell line) in six-well plates for RNA collection (5.0 × 105 cells per well, n = 3 wells per cell line) or on chambered cover glass slides for immunocytochemistry (5.0 × 105 cells per chamber, n = 6 chambers per cell line) (see below). Plasticware and chamber slides used for these assays were not coated with type IV collagen and fibronectin. Cells were fed every 48 hours and observed for up to 10 days for the appearance of holoclones. Holoclones were counted with the use of an inverted microscope.
Immunocytochemistry
Floating cells from each cell line (RWPE-1, WPE1-NB26, CTPE, and CAsE-PE) were plated on Lab-Tek chambered cover glass chamber slides (5.0 × 105 cells per chamber; Nunc, Rochester, NY) and fed every 48 hours for 10 days. The cells were washed twice with PBS and fixed for 2 minutes in acetone:methanol (1:1 [vol/vol]). The cells were incubated for 1 hour with 1% bovine serum albumin in PBS to block nonspecific antibody binding followed by incubation for 1 hour with primary antibodies against BMI1 (ab38295, rabbit polyclonal; Abcam, Cambridge, MA), cytokeratin 5 (EP1601Y, rabbit monoclonal; Abcam), or ABCG2 (B7185, rabbit polyclonal; Sigma), each diluted 1:100 in 1% bovine serum albumin in PBS. The cells were washed three times in PBS and then incubated for 1 hour 37°C with Alexa Fluor 488 goat anti-rabbit IgG (H+L) secondary antibody (Invitrogen) diluted 1:500 in PBS containing 1% bovine serum albumin. The cells were washed three times with PBS and imaged by confocal microscopy (see below). Negative controls—cells treated similarly except that they were not exposed to primary antibody—showed no signal at the microscope settings that used to image specific fluorescence.
Confocal Microscopy
To acquire images, floating cells from each cell line (RWPE-1, WPE1-NB26, CTPE, and CAsE-PE) that were seeded in chamber slides (5.0 × 105 cells per chamber) in keratinocyte serum-free medium alone (RWPE-1, WPE1-NB26) or medium that contained 10 μM cadmium (CTPE) or 5 μM arsenite (CAsE-PE) were fixed and stained for immunocytochemistry. Chamber slides were then mounted on the stage of a Zeiss Model 510 inverted confocal laser-scanning microscope (Carl Zeiss, Inc, Thornwood, NY) and viewed through a ×40 water immersion objective (numeric aperture = 1.2), with a 488-nm laser line for excitation (Argon ion laser), a 510-nm dichroic filter, and a 500- to 550-nm band-pass emission filter. Low laser intensity was used to avoid photobleaching. Confocal images (512 × 512 × 8 bits) were acquired and electronically preserved for later use. For each sample, four to five areas of cells were randomly selected for image collection. Data reported are the results of single experiments that are representative of two to three replicate experiments.
Free-Floating Sphere Formation
RWPE-1, WPE1-NB26, CTPE, and CAsE-PE cells were plated in uncoated T-25 flasks (7.5 × 104 cells per flask) and grown for 10 days with feedings every 48 hours. To not disturb sphere formation or break up any spheres, fresh medium was gently added to each flask and no medium was aspirated. Each flask was marked with an external grid pattern comprising 16 equal portions to facilitate counting, and the total number of spheres in each flask (n = 3 flasks per cell line) was counted with the use of an inverted microscope.
Colony Formation in Soft Agar
Colonies that form when cancer cell lines are plated in agar are generally considered to be derived from CSCs (29,36). Floating RWPE-1, WPE1-NB26, CTPE, and CAsE-PE cells were separated from adherent cells, and a soft agar assay was performed using only the floating cells to examine colony formation as previously described (26). Briefly, 2 mL of 0.5% agar was allowed to harden in 35-mm dishes at room temperature under sterile conditions. A single-cell suspension containing 1.25 × 104 of the floating cells per milliliter in a mixture of 0.33% agar in complete growth medium was prepared, and 1 mL of that mixture was layered on top of the hardened agar in each dish. Three dishes for each cell line were incubated at 37°C for 21 days. Colonies were stained with p-iodonitrotetrazolium overnight at 37°C, fixed in 10% buffered formalin, and counted with the use of an inverted microscope.
Statistical Analysis
Data are presented as mean values with 95% confidence intervals (CIs). All statistical tests were two-sided, and a P value less than .05 was considered statistically significant. The Student t test was used to compare untreated passage-matched control cells with adapted cells (either RWPE-1 or WPE-stem) or to compare RWPE-1 data with WPE-stem data in certain cases. The Tukey–Kramer test after analysis of variance was used to compare CSC-like characteristics between control cells (RWPE-1) and the RWPE-1 malignant transformant cell lines (ie, WPE1-NB26, CTPE, and CAsE-PE). For LC50 determinations, treatment concentrations and response data were made linear by log10 conversion, and the 50% response (LC50) was determined by extrapolation from separate curves. The mean values were determined from these individual extrapolations. For each cell line, three independent curves were generated and used to determine mean basal LC50 values and six independent curves were generated and used to determine mean adapted LC50 values.
Results
Comparative Innate and Acquired Resistance of RWPE-1 and WPE-Stem Cells to Arsenite
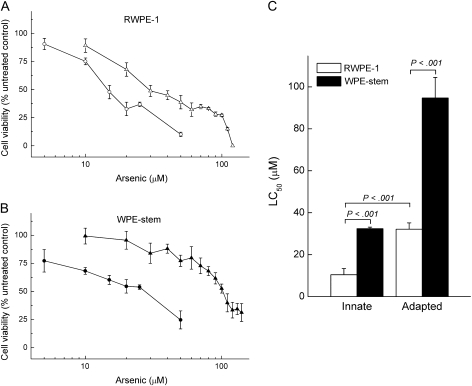
We first examined the basal cytotoxicity of arsenite in RWPE-1 and WPE-stem cells that had never been exposed to the metalloid and then the ability of cells that had been exposed to 5 μM arsenite for 6 weeks to acquire resistance (ie, adapt) to arsenite cytotoxicity. A clear difference in innate arsenite cytolethality occurred between the WPE-stem cells (LC50 = 32.4 μM, 95% CI = 31.5 to 33.3 μM) and the heterogeneous parental RWPE-1 cell line (LC50 = 10.4 μM, 95% CI = 7.4 to 13.4 μM) following short-term (ie, 72 hours) arsenite exposure (Figure 1, A and B). Furthermore, after exposure to 5 μM arsenite for 6 weeks, WPE-stem cells showed a greater ability to adapt to arsenite cytolethality than RWPE-1 cells as reflected by a higher LC50 in adapted WPE-stem cells than in adapted RWPE-1 cells (94.7 vs 32.1 μM, difference = 62.6 μM, 95% CI = 53.3 to 71.9 μM, P < .001) (Figure 1, C). Thus, it appears that the WPE-stem cells possess hyper-adaptability to arsenite compared with the parental RWPE-1 cell line.
Figure 1.
Arsenite concentration lethal to 50% of cells (LC50) values derived from in vitro assays using the trypan blue dye exclusion method. Cell viability curves for A) RWPE-1 cells and B) WPE-stem cells in response to exposure to various concentrations of arsenite for 72 hours among cells previously unexposed to arsenite (circles) and cells previously exposed to 5 μM arsenite for 6 weeks (triangles) were used to determine innate and adapted LC50 values. C) Innate and adapted LC50 values for RWPE-1 (open bars) and WPE-stem (closed bars) were derived from survival curves after log10 conversion. Data are presented as mean LC50 values (n = 3 for innate; n = 6 for adapted); error bars correspond to 95% confidence intervals. P values are two-sided (Student t test).
Resistance to Arsenite-Induced Apoptosis
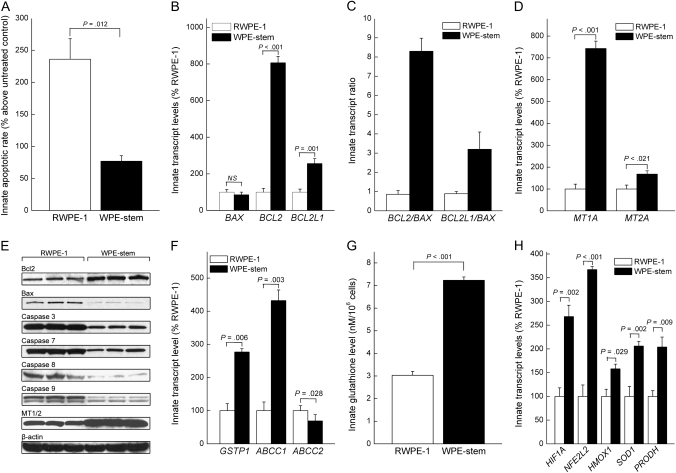
We next assessed the innate sensitivity of WPE-stem and RWPE-1 cells to arsenite-induced apoptosis (Figure 2, A). Cells were cultured for 24 hours in medium that lacked (control) or contained 30 μM sodium arsenite and then assayed for apoptosis by flow cytometry with Annexin V–FITC staining. The percentage of apoptotic cells was higher in arsenite-treated cells than in untreated cells (RWPE-1 cells: 14.5% vs 4.3%, difference = 10.2%, 95% CI = 8.2% to 12.2%, P < .001; WPE-stem cells: 6.8% vs 3.8%, difference = 3.0%, 95% CI = 1.5% to 4.6%, P = .006). The relative comparative induced level of apoptosis was much higher in arsenite-exposed RWPE-1 cells than in arsenite-exposed WPE-stem cells (increase compared with untreated control, RWPE-1 vs WPE-stem, 239% vs 78%, difference = 161%, 95% CI = 71.7% to 247.6%, P = .012) (Figure 2, A), indicating that the stem cells have an innate resistance to arsenite-induced apoptosis.
Figure 2.
Levels of arsenite-induced apoptosis and innate expression levels of apoptosis- and arsenite stress–related factors. A) Apoptotic rates in RWPE-1 and WPE-stem cells treated with 30 μM arsenite for 24 hours. Data are presented as the mean percent increase in apoptosis over the respective untreated control cells (n = 3). B) Mean transcript expression levels of various general apoptosis-related factors (n = 3). NS = not statistically significant. C) Mean BCL2/BAX and BCL2L1/BAX transcript ratios (n = 3). D) Mean MT1A and MT2A transcript levels in WPE-stem and RWPE-1 cells (n = 3). E) Western blot analyses comparing various apoptosis-related factors in RWPE-1 (left three lanes) and WPE-stem cells (right three lanes). Each lane (25 μg protein per lane) corresponds to an independently prepared protein sample (n = 3). β-Actin was used as the control for equal protein loading. F) Mean transcript expression levels of arsenic efflux–related factors in WPE-stem and RWPE-1 cells (n = 3). G) Mean innate glutathione levels in RWPE-1 and WPE-stem cells (n = 3). H) Mean transcript expression of various arsenic-specific and metabolism-related stress factors in RWPE-1 and WPE-stem cells (n = 3). For comparisons of transcript expression levels, data are presented as the percent expression vs RWPE-1 (control) cells. Error bars represent 95% confidence intervals. All P values are two-sided (Student t test).
To examine the basis for this resistance to arsenite-induced apoptosis, we analyzed the innate expression of apoptosis-related genes in untreated RWPE-1 and WPE-stem cells. The innate levels of antiapoptotic BCL2 and BCL2L1 mRNAs were 7.9-fold higher (95% CI = 7.2- to 8.6-fold higher) and 2.6-fold higher (95% CI = 2.2- to 3.0-fold higher), respectively, in the stem cells than in parental cells, whereas proapoptotic BAX mRNA levels were similar in both lines (Figure 2, B). The innate BCL2/BAX and BCL2L1/BAX mRNA ratios, which are positively associated with apoptosis resistance (37), were 8.2 (95% CI = 7.4 to 9.0) and 3.2 (95% CI = 2.3 to 4.1), respectively, in stem cells and 0.87 (95% CI = 0.67 to 1.1) and 0.89 (95% CI = 0.78 to 1.0), respectively, in parental RWPE-1 cells (Figure 2, C). MT is a negative regulator of apoptosis, the expression of which is associated with reduced arsenic toxicity (38,39). mRNAs for the predominant MT isoforms, MT1 and MT2, were overexpressed in the WPE-stem cells compared with parental RWPE-1 cells (Figure 2, D). Compared with RWPE-1 cells, WPE-stem cells showed noticeably higher expression of antiapoptotic proteins (ie, BCL2, MT1, MT2) and noticeably lower expression of proapoptotic proteins factors (ie, BAX, caspases 3, 7, 8, and 9) by western blot analysis (Figure 2, E). ABCC1 and ABCC2 are key transport proteins in arsenic efflux, which occurs via a glutathione trimer conjugate that contains GSTP1 (40,41). Compared with RWPE-1 cells, WPE-stem cells showed higher innate expression of two arsenite metabolism- and efflux-related genes, GSTP1 and ABCC1, at the transcript level (Figure 2, F) and at the protein level (data not shown). Surprisingly, ABCC2 mRNA levels were lower in the WPE-stem cells than in the parental cells. The innate glutathione concentration was 239% higher in WPE-stem cells than in RWPE-1 cells (7.2 ng/106 cells vs 3.0 nM/106 cells, difference = 4.2 ng/106 cells, 95% CI = 3.8 to 4.6 ng/106 cells, P < .001) (Figure 2, G). Innate transcript expression of several arsenic-induced stress-response genes, including NF-E2–related factor-2 (NFE2L2), superoxide dismutase-1 (SOD1), heme oxygenase-1 (HMOX1), and hypoxia-inducible factor-1α (HIF1A), and of the metabolic stress–related gene proline oxidase (PRODH) was higher in stem cells than in mature parental cells (Figure 2, H). Together, these data suggest that apoptosis-, stress-, and arsenic biokinetics–related factors contribute to the greater innate arsenic resistance of WPE-stem cells compared with the mature parental RWPE-1 cells. Arsenic uptake was similar in WPE-stem and RWPE-1 cells (not shown). However, the stem cells effluxed 1.6 times (95% CI = 1.2 to 2.0 times) as much of the arsenic that was taken in as the parental cells, possibly because they expressed higher levels of ABCC1, GSTP1, and GSH compared with the parental cells (see Figure 2).
Hyper-adaptability of Stem Cells to Arsenic
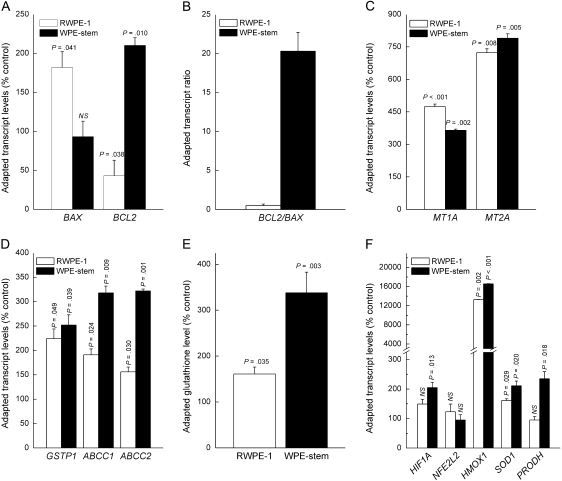
Often as a mechanism of acquired resistance to toxicity (adaptation), various genes are activated in response to continued apoptotic stimuli, metabolic stress, or specifically to arsenic exposure (16,18,19,42). After 6 weeks of exposure to 5 μM sodium arsenite, which previous work has shown results in malignant transformation of both RWPE-1 (4) and WPE-stem (28) cells, both cell lines showed changes (eg, increases in LC50 and ABCC1 and GSTP1 expression) indicative of adaptation to the toxic effects of arsenite. However, the stem cells remained more resistant to arsenite-induced apoptosis than the parental RWPE-1 cells (data not shown). Following this adaptation, only RWPE-1 cells showed increased mRNA expression of proapoptotic CASP3 compared with passage-matched untreated control cells and both cell lines showed increased mRNA expression of CASP10, although the increase was much smaller in WPE-stem cells than in parental cells (data not shown). RWPE-1 cells showed decreased BCL2 mRNA expression and increased BAX mRNA expression compared with passage-matched untreated control cells, whereas WPE-stem cells showed increased BCL2 mRNA expression and no change in BAX mRNA expression (Figure 3, A). These changes in mRNA expression increased the BCL2/BAX mRNA ratio, which is inversely related to apoptotic sensitivity (37), from an innate level of 8.2 to 19 (95% CI = 16.6 to 21.4) in the adapted stem cells, whereas the ratio remained approximately 1.0 in the adapted parental cells (Figure 3, B). Both adapted cell lines showed similar increases in GSTP1 mRNA levels compared with passage-matched untreated control cells, whereas the adapted stem cells showed a greater increase in ABCC1 mRNA (Figure 3, D) and GSH levels than did adapted parental cells (Figure 3, E). Although innate ABCC2 mRNA levels were moderately lower in the stem cells compared with the parental cells (see Figure 2, F), the adapted ABCC2 mRNA levels in the stem cells showed a much greater increase over their respective control cells (3.2-fold increase, 95% CI = 3.1- to 3.3-fold increase, P = .001) than the parental line (1.6-fold increase, 95% CI = 1.5- to 1.7-fold increase, P = .030) (Figure 3, D). In addition, both arsenite-adapted cell lines showed increased expression of HIF1A, HMOX1, SOD1, and PRODH mRNAs compared with passage-matched untreated control cells (Figure 3, F), but the arsenite-adapted stem cells often showed larger increases than the arsenite-adapted parental cells.
Figure 3.
Adapted levels of apoptosis-, arsenite efflux–, and stress-related factors in RWPE-1 and WPE-stem cells after chronic exposure to 5 μM arsenite for 6 weeks. A) Transcript levels of general apoptosis-related factors (n = 3). B) BCL2/BAX transcript ratios (n = 3). C) MT1A and MT2A transcript levels (n = 3). D) Transcript levels of key arsenic efflux–related factors (n = 3). E) Glutathione levels (n = 3). F) Transcript levels of various arsenic-specific or metabolism-related stress factors (n = 3). Note the break in the y-axis. In all cases, data are expressed as mean values with 95% confidence intervals (error bars) and, except for panel (B), represent expression in treated cells vs their respective untreated control cells. All P values are two-sided (Student t test). NS = not statistically significant.
Thus, unexposed WPE-stem cells expressed higher levels of a number of factors that would confer a greater innate resistance to arsenite compared with unexposed parental cells. Furthermore, after 6 weeks of exposure to 5 μM arsenite, the stem cells showed more marked increases in levels of most of the resistance factors that would favor survival during arsenite exposure, which implies that the stem cells are hyper-adaptable to arsenite compared with the mature parental cell line. This is not true for all carcinogens or toxicants: We found that the inorganic carcinogen, cadmium, is more toxic to WPE-stem cells than to RWPE-1 cells following subchronic (2–4 weeks) exposure to various concentrations (1–10 μM) of cadmium (E. J. Tokar, C. Kojima, Y. Sun, M. P. Waalkes, Inorganic Carcinogenesis Section, Laboratory of Comparative Carcinogenesis, National Cancer Institute and the National Institute of Environmental Health Sciences, unpublished data).
CSC-Like Production in Isogenic RWPE-1 Transformants
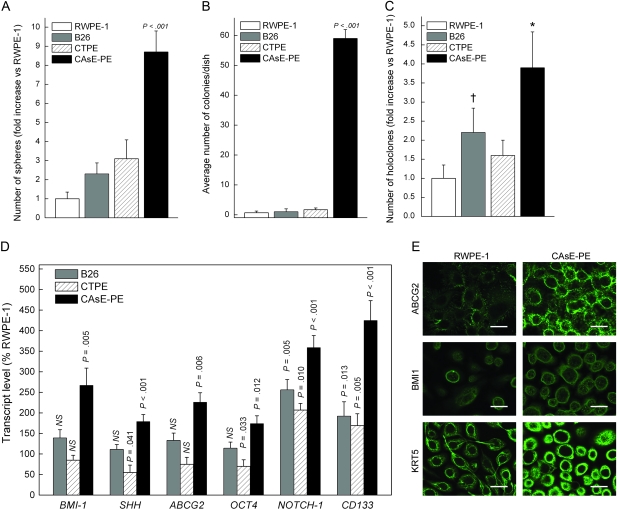
We next examined the effect of different known human carcinogens on the production of CSC-like cells during malignant transformation of the parental RWPE-1 cell line. When grown in culture, many cancer cell lines form free-floating spheres of viable cells that contain a preponderance of CSCs (30,43). Arsenite-transformed RWPE-1 cells (ie, CAsE-PE cells) formed such free-floating spheres in abundance (increase vs RWPE-1 = 8.7-fold, 95% CI = 7.6- to 9.9-fold), whereas the MNU- and cadmium-transformed cells (WPE1-NB26 and CTPE cells, respectively) formed relatively low numbers of spheres (WPE1-NB26, increase vs RWPE-1 = 2.3-fold, 95% CI = 1.7- to 2.9-fold; CTPE, increase vs RWPE-1 = 3.1-fold, 95% CI = 2.1- to 4.1-fold) (Figure 4, A). To more precisely quantify CSC-like cells produced by these cell lines, the floating spheres were dissociated into single-cell suspensions and plated in soft agar to assess their clonogenic ability (Figure 4, B). Floating CAsE-PE cells formed colonies in soft agar at a very high rate compared with floating control parental RWPE-1 cells (59 vs 0.67 colonies, difference = 58.3 colonies, 95% CI = 53.4 to 63.4 colonies, P < .001), whereas floating WPE1-NB26 (1.0 vs 0.67 colonies, difference = 0.33 colonies, 95% CI = −1.5 to 2.2 colonies, P = .643) and CTPE (1.7 vs 0.67 colonies, difference = 1.0 colonies, 95% CI = −0.31 to 2.3 colonies, P = .101) cells did not (Figure 4, B).
Figure 4.
Cancer stem cell (CSC)–like characteristics in isogenic RWPE-1 transformants. A) Free-floating sphere assay. Data are expressed as the fold increase in the number of spheres formed vs RWPE-1 cells (n = 3 flasks). CAsE-PE showed a statistically significant difference (P < .001) compared with all other cell lines. B) Quantification of colonies from soft agar assay per dish for all four cell lines (n = 3 dishes per cell line). CAsE-PE cells showed a statistically significant difference (P < .001) compared with all other cell lines. C) Formation of holoclones. Data are expressed as the fold increase in the total number of holoclones formed compared with the total number of holoclones formed by RWPE-1 cells (n = 3 flasks per cell line). Asterisk represents statistically significant difference compared with all other cell lines (P < .001). Dagger represents statistically significant difference compared with RWPE-1 cells (P = .011). D) Transcript expression of stem cell and CSC markers in holoclones. Data are expressed as a percentage of the expression in RWPE-1 cells (n = 3). NS = not statistically significant. E) Fluorescence confocal microscopic imaging of expression levels of stem cell markers in holoclones from RWPE-1 and CAsE-PE cells. Note the strong homogeneous staining in CAsE-PE images. Bars = 20 μm. All P values are two-sided, and in all cases, the Tukey–Kramer test after analysis of variance was used to compare RWPE-1 (control) with the isogenic malignant transformant cell lines. Data represent mean values and error bars correspond to 95% confidence intervals.
Holoclones—clusters of tightly packed cells that form in subconfluent cell cultures—often contain stem cells and CSCs (44,45). We next assessed the ability of floating cells from RWPE-1 (control) and the three malignant transformant cell lines to form holoclones. Floating cells from all four cell lines formed holoclones (Figure 4, C). However, CAsE-PE cells showed the greatest fold increase in total holoclones compared with RWPE-1 (3.9- vs 1-fold, difference = 2.9-fold, 95% CI = 1.7- to 4.1-fold, P < .001). Furthermore, only the CAsE-PE holoclones could be subcloned and propagated on a long-term basis (data not shown), clearly indicating that these cells possess the capacity for self-renewal, a classic hallmark of both stem cells and CSCs (8). RWPE-1 holoclones failed to continue to form after 7 weeks of culture, WPE1-NB26 holoclones after 9 weeks, and the cadmium transformant after 12 weeks (data not shown). By contrast, CAsE-PE holoclones continued to form until the study was terminated at 30 weeks (data not shown). Compared with RWPE-1 holoclones, CAsE-PE holoclones expressed higher levels of the six common stem cell and CSC marker genes examined (ie, BMI1, ABCG2, POU5F1, NOTCH1, PROM1, and SHH), whereas with the exception of NOTCH-1 and PROM1, WPE1-NB26 and CTPE cells did not (Figure 4, D). Immunocytochemical analysis of RWPE-1 and CAsE-PE holoclones revealed high and widespread expression of three common prostate stem cell markers—ABCG2, BMI1, and keratin 5—in the CAsE-PE holoclones (Figure 4, E). Thus, only arsenite-transformed CAsE-PE cells formed multiple holoclones that have the ability for indefinite passaging and an abundance of CSCs.
Discussion
We found innate resistance and hyper-adaptability to arsenite toxicity in WPE-stem cells compared with their mature, heterogeneous parental cell line, RWPE-1. This resistance and hyper-adaptability was associated with higher mRNA and protein expression of several antiapoptotic, stress-related, and arsenic adaptation factors and lower expression of proapoptotic factors. We also found that by the point of arsenite-induced malignant transformation (4), a stem cell survival selection advantage has occurred in the RWPE-1 cells that manifested itself as an arsenite-specific overabundance of CSC-like cells. These data support the hypothesis that, as part of its carcinogenic mechanism, arsenic targets stem cells for transformation, which subsequently produces cancers enriched in CSC-like cells.
For environmental chemical carcinogenesis to occur, tumor-forming cells must survive the primary exposure and any subsequent chronic exposure and retain the ability to propagate. WPE-stem cells were innately much more resistant than their mature parental RWPE-1 cells to arsenite-induced apoptosis, which is similar to the generalized apoptotic resistance of many cancer cells (13,14,46) and likely provides a survival advantage during initial chemical exposure. Furthermore, in general, the progressive acquisition of apoptotic resistance contributes to tumor development and progression (11–14). In this study, both the parental and stem cell lines showed acquired resistance (adapted) to arsenite-induced cytotoxicity following 6 weeks of exposure to an environmentally relevant level of arsenite [5 μM (32)] compared with nonadapted cells. However, the stem cells adapted to a greater extent than the parental cells, presumably because they selectively expressed many factors that contribute to apoptotic resistance, which indicates arsenite hyper-adaptability of WPE-stem cells.
The factors that contribute to apoptotic resistance are well documented (11,13,47). Caspases are proapoptotic molecules that are critical to apoptotic commitment and execution. Reduced caspase activity can perturb or even prevent apoptosis and is common in apoptotic-resistant cancers (11,13–15,47). The Bcl2 family of proteins is considered a main intracellular regulator of the apoptotic process; some Bcl2 family members inhibit apoptosis (ie, Bcl2, Bcl2l1), whereas others (eg, Bax) promote apoptosis. Higher ratios of Bcl2 or Bcl2l1 to Bax are associated with resistance to apoptosis (37), and Bcl-2 overexpression has been shown to protect stem cells against apoptosis, increase their frequency, and confer them with a competitive repopulation advantage (48). Furthermore, MT can protect cells that are exposed to metals, including arsenic, and perturb metal-induced apoptosis (16,38,49,50). Poor MT expression is associated with increased sensitivity to arsenic (39). ABC transporter proteins (ie, ABCC1, ABCC2), GSTP1, and GSH are associated with arsenic tolerance and generalized inhibition of apoptosis (16–18,51,52). In addition, ABCC1, ABCC2, GSTP1, and GSH work together to efflux arsenic out of cells (40,41), a key element in arsenic adaptation (16,18). HIF1A, HMOX1, SOD1, and NFE2L2 play important roles in innate resistance and/or adaptation to arsenic by preventing arsenic-induced oxidative damage and apoptosis (46,53–55). Increased expression of PRODH, a stress-induced enzyme that metabolizes proline and generates ATP, can maintain cellular energy levels and help cancer cells adapt to nutrient and energy limitations (42). Expression of all of these factors enhanced the intrinsic resistance of WPE-stem cells to arsenite compared with mature parental RWPE-1 cells and, taken together, would likely increase the survival selection advantage of WPE-stem cells during malignant transformation. Most cells, including RWPE-1, adapt to arsenite during periods of low-level chronic exposure (18,34,46,56,57). However, in this study, the stem cells displayed greater adaptation to arsenite than the mature parental cells.
Our data clearly show that WPE-stem cells possess an inherent capacity to survive arsenite exposure and to continue with self-renewal in the face of continuous arsenite exposure, making them ideal candidates to acquire the lesions necessary for malignant transformation, thereby becoming cells that drive tumorigenesis in arsenite-transformed RWPE-1 cells (4). Tumor-derived cancer cell lines can form free-floating spheres that contain chemoresistant and malignant CSC-like cells (30), and many cancer cell lines that are cultured for a long time form CSC-containing holoclones (45,58). The arsenite-induced malignant transformant, CAsE-PE, was the only isogenic RWPE-1 transformant that formed free-floating spheres at a higher rate than control cells and produced floating cells that formed holoclones that could be repeatedly subcloned and propagated for multiple passages, indicating that these cells possess a greater self-renewal capacity consistent with a stem cell or CSC nature (8). Moreover, we showed that CAsE-PE holoclones overexpress multiple common stem cell and CSC markers, including BMI1, SHH, ABCG2, POU5F1, NOTCH-1, and PROM1. Clonogenic assays such as colony formation in soft agar have long been used to enrich for tumor-initiating cells, and the cells that form colonies in such assays are generally considered to be CSCs (29,36). Only floating cells from CAsE-PE cells formed colonies in soft agar, whereas those from RWPE-1 cells transformed by inorganic cadmium and the direct-acting organic carcinogen MNU did not. Taken together, these data demonstrate that the selection of stem cells and the ability to greatly increase their numbers during malignant transformation is a potentially unique mechanistic feature of arsenic.
Some potential limitations of this study include the use of molecular markers to identify CSCs. Although biomarkers are widely used to identify CSCs in tumors, this practice is still somewhat controversial (59). Analysis of additional prostate CSC biomarkers (eg, CD117, Sca1) in the isogenic RWPE-1 transformants could further support the role of these cells in prostate carcinogenesis. Although in this and other (9,10) studies, arsenic appears to target a stem cell population during carcinogenesis, similar in vitro or in vivo studies are needed to determine if this phenomenon is generalizable to all targets of arsenic carcinogenesis. Finally, all analyses in this study were done in cultured cell lines. Similar studies using arsenic-induced carcinogenesis in whole animals and humans are needed to determine if these results translate to in vivo conditions.
In summary, the prostate stem cell line, WPE-stem, showed inherent resistance to apoptosis and hyper-adaptability to arsenite that was likely because of its enhanced protective molecular responses compared with the mature cell line from which it was derived. The consequence of this increased resistance to apoptosis and arsenite hyper-adaptability when arsenite transformed the heterogeneous mature RWPE-1 line was an increase in the number of CSC-like cells, suggesting that a selection for stem cells had occurred. The similarities between stem cells and cancer cells, coupled with the accumulating evidence of the key role of CSCs in carcinogenesis (8), indicate that the apoptotic resistance that characterizes most tumor cells is also an intrinsic characteristic of WPE-stem cells in response to arsenic. The innate resistance and hyper-adaptability in prostate stem cells observed in this study indicate that these cells, compared with their mature counterparts, could more readily survive arsenic exposure yet retain the critical quality of self-renewal essential to tumor formation. Emerging data indicate that normal stem cells are highly protected from the effects of toxicants and that CSCs represent a similarly small, yet highly chemoresistant, population (20,21). The characteristics of the WPE-stem cells that provide for arsenite resistance essentially mirror those in cells malignantly transformed with arsenic (4). These observations fortify the contention that arsenic likely targets cells that have a stem or progenitor phenotype during malignant transformation.
Funding
This research was supported, in part, by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research and also, in part, with federal funds from the National Cancer Institute, National Institutes of Health, under Contract NO1-CO-12400.
Supplementary Material
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. Authors declare no conflicts of interest.
E. J. Tokar and M. P. Waalkes designed research; E. J. Tokar, W. Qu, J. Liu, and W. Liu performed research; E. J. Tokar, M. M. Webber, J. M. Phang, and M. P. Waalkes analyzed data; and E. J. Tokar and M. P. Waalkes wrote the paper. The authors are solely responsible for the design of the study, the collection and analysis of data and the interpretation of the results, the preparation of the manuscript, and the decision to submit the manuscript for publication.
We thank Drs L. Keefer and C. Kojima for insightful comments; C. Bortner and M. Sifre for assistance with flow cytometry, S. Wang for assistance with confocal microscopy, and Mr M. Bell for assistance with figures.
References
- 1.International Agency for Research on Cancer. Arsenic in Drinking Water. International Agency for Research on Cancer Monographs on the Evaluation of Carcinogenic Risk to Humans. Some Drinking Water Disinfectants and Contaminants, Including Arsenic. Vol. 84. Lyon, France: IARC Press; 2004. 269–477. [PMC free article] [PubMed] [Google Scholar]
- 2.Kitchin KT. Recent advances in arsenic carcinogenesis: modes of action, animal model systems, and methylated arsenic metabolites. Toxicol Appl Pharmacol. 2001;172(3):249–261. doi: 10.1006/taap.2001.9157. [DOI] [PubMed] [Google Scholar]
- 3.Benbrahim-Tallaa L, Waalkes MP. Inorganic arsenic and human prostate cancer. Environ Health Perspect. 2008;116(2):158–164. doi: 10.1289/ehp.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achanzar WE, Brambila EM, Diwan BA, et al. Inorganic arsenite induced malignant transformation of human prostate epithelial cells. J Natl Cancer Inst. 2002;94(24):1888–1891. doi: 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- 5.Waalkes MP, Liu J, Diwan BA. Transplacental arsenic carcinogenesis in mice. Toxicol Appl Pharmacol. 2007;222(3):271–280. doi: 10.1016/j.taap.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith AH, Marshall G, Yuan Y, et al. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006;114(8):1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson LM, Diwan B, Fear N, et al. Critical windows of exposure for children's health: cancer in human epidemiological studies and neoplasms in experimental animal models. Environ Health Perspect. 2000;108(Suppl 3):573–594. doi: 10.1289/ehp.00108s3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3(12):895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 9.Patterson TJ, Rice RH. Arsenite and insulin exhibit opposing effects on epidermal growth factor receptor and keratinocyte proliferative potential. Toxicol Appl Pharmacol. 2007;221(1):119–128. doi: 10.1016/j.taap.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waalkes MP, Liu J, Germolec D, et al. Arsenic exposure in utero exacerbates skin cancer response in adulthood with contemporaneous distortion of rumor stem cell dynamics. Cancer Res. 2008;68(20):8278–8285. doi: 10.1158/0008-5472.CAN-08-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108(2):153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 12.Waalkes MP, Fox DA, States JC, et al. Metals and disorders of cell accumulation: modulation of apoptosis and cell proliferation. Toxicol Sci. 2000;56(2):255–261. doi: 10.1093/toxsci/56.2.255. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 14.McKenzie S, Kyprianou N. Apoptosis evasion: the role of survival pathways in prostate cancer progression and therapeutic resistance. J Cell Biochem. 2006;97(1):18–32. doi: 10.1002/jcb.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhivotovsky B, Orrenius S. Carcinogenesis and apoptosis: paradigms and paradoxes. Carcinogenesis. 2006;27(10):1939–1945. doi: 10.1093/carcin/bgl035. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Chen H, Miller DS, et al. Overexpression of glutathione S-transferase II and multidrug resistant transport proteins is associated with acquired arsenic tolerance to inorganic arsenic. Mol Pharmacol. 2001;60(2):302–309. doi: 10.1124/mol.60.2.302. [DOI] [PubMed] [Google Scholar]
- 17.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 18.Brambila EM, Achanzar WE, Qu W, et al. Chronic arsenic exposed human prostate epithelial cells exhibit stable arsenic tolerance: mechanistic implications of altered cellular glutathione and glutathione S-transferase. Toxicol Appl Pharmacol. 2002;183(2):99–107. [PubMed] [Google Scholar]
- 19.Zhao CQ, Young MR, Diwan BA, et al. Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc Natl Acad Sci U S A. 1997;94(20):10907–10912. doi: 10.1073/pnas.94.20.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnenberg VS, Luketich JD, Landreneau RJ, et al. Tumorigenic epithelial stem cells and their normal counterparts. Ernst Schering Found Symp Proc. 2006;5(9):245–263. doi: 10.1007/2789_2007_054. [DOI] [PubMed] [Google Scholar]
- 21.Donnenberg VS, Landreneau RJ, Donnenberg AD. Tumorigenic stem and progenitor cells: implications for the therapeutic index of anti-cancer agents. J Control Release. 2007;122(3):385–391. doi: 10.1016/j.jconrel.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 23.Shafee N, Smith CR, Wei S, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-meditated mouse mammary tumors. Cancer Res. 2008;68(9):3243–3250. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iannolo G, Conticello C, Memeo L, De Maria R. Apoptosis in normal and cancer stem cells. Crit Rev Oncol Hematol. 2008;66(1):42–51. doi: 10.1016/j.critrevonc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Matsui W, Wang Q, Barber JP, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68(1):190–197. doi: 10.1158/0008-5472.CAN-07-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tokar EJ, Ancrile BB, Cunha GR, Webber MM. Stem/progenitor and intermediate cells and the origin of human prostate cancer. Differentiation. 2005;73(9–10):463–473. doi: 10.1111/j.1432-0436.2005.00047.x. [DOI] [PubMed] [Google Scholar]
- 27.Bello D, Webber MM, Kleinman HK, et al. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis. 1997;18(6):1215–1223. doi: 10.1093/carcin/18.6.1215. [DOI] [PubMed] [Google Scholar]
- 28.Tokar EJ, Diwan BA, Waalkes MP. Arsenic transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype. Environ Health Perspect. 2010;118(1):108–115. doi: 10.1289/ehp.0901059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439(7079):993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 30.Ghods AJ, Irvin D, Liu G, et al. Spheres isolated from 9L gliosarcoma rat cell line possess chemoresistant and aggressive cancer stem-like cells. Stem Cells. 2007;25(7):1645–1653. doi: 10.1634/stemcells.2006-0624. [DOI] [PubMed] [Google Scholar]
- 31.Webber MM, Quader STA, Kleinman HK, et al. Human cell lines as an in vitro/in vivo model for prostate carcinogenesis and progression. Prostate. 2001;47(1):1–13. doi: 10.1002/pros.1041. [DOI] [PubMed] [Google Scholar]
- 32.Achanzar WE, Diwan BA, Liu J, Quader ST, Webber MM, Waalkes MP. Cadmium-induced malignant transformation of human prostate epithelial cells. Cancer Res. 2001;61(2):455–458. [PubMed] [Google Scholar]
- 33.Pi J, Yamauchi H, Kumagai Y, et al. Evidence for induction of oxidative stress caused by chronic exposure of Chinese residents to arsenic contained in drinking water. Environ Health Perspect. 2002;110(4):331–336. doi: 10.1289/ehp.02110331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coppin JF, Qu W, Waalkes MP. Interplay between cellular methyl metabolism and adaptive efflux during oncogenic transformation from chronic arsenic exposure in human cells. J Biol Chem. 2008;283(28):19342–19350. doi: 10.1074/jbc.M802942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasband WS. ImageJ. Bethesda, MD: U.S. National Institutes of Health; 1997–2005. http://rsb.info.nih.gov/ij/. Accessed January 15, 2009. [Google Scholar]
- 36.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Yu J, Park BH, et al. Role of bax in the apoptotic response to anticancer agents. Science. 2000;290(5493):989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 38.Shimoda R, Achanzar WE, Qu W, et al. Metallothionein is a potential negative regulator of apoptosis. Toxicol Sci. 2003;73(2):294–300. doi: 10.1093/toxsci/kfg095. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Cheng ML, Yang Q, et al. Blood metallothionein transcript as a biomarker for metal sensitivity: low blood metallothionein transcripts in arsenicosis patients from Guizhou, China. Environ Health Perspect. 2007;115(7):1101–1106. doi: 10.1289/ehp.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leslie EM, Haimeur A, Waalkes MP. Arsenic transport by the human multidrug resistance protein (MRP1/ABCC1): evidence that a tri-glutathione conjugate is required. J Biol Chem. 2004;279(31):32700–32708. doi: 10.1074/jbc.M404912200. [DOI] [PubMed] [Google Scholar]
- 41.Kala SV, Neely MW, Kala G, et al. The MRP2/cMOAT transporter and arsenic-glutathione complex formation are required for biliary excretion of arsenic. J Biol Chem. 2000;275(43):33404–33408. doi: 10.1074/jbc.M007030200. [DOI] [PubMed] [Google Scholar]
- 42.Pandhare J, Cooper SK, Donald SP, Phang JM. Regulation and function of proline oxidase under nutrient stress. J Cell Biochem. 2009;107(4):759–768. doi: 10.1002/jcb.22174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dubrovska A, Kim S, Salamone RJ, et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci U S A. 2009;106(1):268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118(5):635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Chen X, Calhoun-Davis T, Claypool K, Tang DG. PC3 human prostate carcinoma cell holoclones contain self-renewing tumor-initiating cells. Cancer Res. 2008;68(6):1820–1825. doi: 10.1158/0008-5472.CAN-07-5878. [DOI] [PubMed] [Google Scholar]
- 46.Qu W, Bortner CD, Sakurai T, et al. Acquisition of apoptotic resistance in arsenic-induced malignant transformation: role of the JNK signal transduction pathway. Carcinogenesis. 2002;23(1):151–159. doi: 10.1093/carcin/23.1.151. [DOI] [PubMed] [Google Scholar]
- 47.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 48.Domen J, Cheshier SH, Weissman IL. The role of apoptosis in the regulation of hematopoietic stem cells: overexpression of Bcl-2 increases both their number and repopulation potential. J Exp Med. 2000;191(2):253–264. doi: 10.1084/jem.191.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Liu Y, Goyer RA, et al. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenic. Toxicol Sci. 2000;55(2):460–467. doi: 10.1093/toxsci/55.2.460. [DOI] [PubMed] [Google Scholar]
- 50.Qu W, Fuquay R, Sakurai T, et al. Acquisition of apoptotic resistance in cadmium-induced malignant transformation: specific perturbation of JNK signal transduction pathway and associated metallothionein overexpression. Mol Carcinog. 2006;45(8):561–571. doi: 10.1002/mc.20185. [DOI] [PubMed] [Google Scholar]
- 51.Ghibelli L, Fanelli C, Rotilio G, et al. Rescue of cell from apoptosis by inhibition of active GSH extrusion. FASEB J. 1998;12(6):479–486. doi: 10.1096/fasebj.12.6.479. [DOI] [PubMed] [Google Scholar]
- 52.Borst P, Evers R, Kool M, et al. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92(16):1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 53.Kamat CD, Green DE, Curilla S, et al. Role of HIF signaling on tumorigenesis in response to chronic low-dose arsenic administration. Toxicol Sci. 2005;86(2):248–257. doi: 10.1093/toxsci/kfi190. [DOI] [PubMed] [Google Scholar]
- 54.Ramos-Gomez M, Kwak MK, Dolan PM, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers lost in Nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98(6):3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JM, Calkins MJ, Chan K, et al. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278(14):12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 56.Romach EH, Zhao CQ, Del Razo LM, et al. Studies on the mechanisms of arsenic-induced self tolerance developed in liver epithelial cells through continuous low-level arsenite exposure. Toxicol Sci. 2000;54(2):500–508. doi: 10.1093/toxsci/54.2.500. [DOI] [PubMed] [Google Scholar]
- 57.Pi J, He Y, Bortner C, et al. Low level, long-term inorganic arsenite exposure causes generalized resistance to apoptosis in cultured human keratinocytes: potential role in skin-carcinogenesis. Int J Cancer. 2005;116(1):20–26. doi: 10.1002/ijc.20990. [DOI] [PubMed] [Google Scholar]
- 58.Tang DG, Patrawala L, Calhoun T, et al. Prostate cancer stem/progenitor cells: identification, characterization, and implications. Mol Carcinog. 2007;46(1):1–14. doi: 10.1002/mc.20255. [DOI] [PubMed] [Google Scholar]
- 59.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cells paradigm. Science. 2009;324(5935):1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.