Abstract
The breeding female or “queen” naked mole-rat has a uniquely elongated body morphology attributed to the lengthening of the lumbar vertebral column that occurs during pregnancy. It is unknown whether this vertebral growth is a continuous process, or associated only with early reproductive experience. We compared pregnancy-related bone elongation in nascent primiparous queens and established queens to determine if this vertebral expansion was a lifelong process in these females. We also investigated the impact of lactation on vertebral elongation in these mole-rats because it is known to be a time of significant bone loss in other mammals. Our data show that after eight or more pregnancies, established queens no longer experienced a net gain in lumbar spine length over the reproductive cycle, whereas the nascent breeders demonstrated significant spine lengthening over this time. Despite the lack of net spine lengthening in established breeders, our results indicated that these queens still experienced some pregnancy-specific vertebral elongation. In naked mole-rats, pregnancy-induced bone elongation may serve the dual purposes of first lengthening the spine, and then once optimal spine size is achieved, serving as a homeostatic mechanism that prepares the spine for the mineral demands of lactation.
Keywords: longitudinal bone growth, lumbar vertebrae, pregnancy, lactation, rodents, eusocial
Introduction
Naked mole-rats are eusocial African rodents that inhabit a cooperative social environment that is characterized by a reproductive hierarchy and division of labor (Jarvis, 1981). Naked mole-rats live in large subterranean colonies where a single reproductively-active breeding female, or queen, will mate and produce offspring for the entire colony (Jarvis, 1981). The queen actively suppresses reproduction in the remaining subordinate “worker” mole-rats, and these animals engage in colony maintenance, defense, foraging and pup-rearing (Faulkes et al., 1990; Jarvis et al., 1991; Smith et al., 1997). Along with their unique status as sole breeding female of a colony, queen mole-rats are noticeably elongated, generally making them the largest animals in the colony (Buffenstein, 1996; Clarke and Faulkes, 1997; Henry et al., 2007; Jarvis et al., 1991; O'Riain et al., 2000).
The queen mole-rat's distinctive body morphology is largely attributed to the lengthening of the lumbar spine (Jarvis et al., 1991; O'Riain et al., 2000). This vertebral expansion is driven by two events: the onset of reproductive activity that occurs during the puberty-like period after removal from reproductive suppression (Dengler-Crish and Catania, 2007) and pregnancy (Buffenstein, 1996; Jarvis et al., 1991; O'Riain et al., 2000; Henry et al., 2007). Pregnancy-induced lumbar spine elongation increases as each pregnancy progresses, peaks just before parturition, and subsides after birth (Henry et al., 2007). As a result, the lumbar spine seems to lengthen with every pregnancy (Henry et al., 2007; O'Riain et al., 2000). Over the course of multiple pregnancies, queen mole-rats experience on average a 32% increase in the length of their lumbar vertebrae (Henry et al., 2007). One explanation for this growth is that it serves to extend the abdominal cavity along the body axis, thus increasing the capacity for gestating large numbers of fetuses without increasing girth (Jarvis et al., 1991). This may enable queens to produce large litters while preserving their ability to navigate through narrow underground tunnels (Buffenstein, 2008; Jarvis et al., 1991).
Queen mole-rats do not develop reproductive senescence as they age and can continue breeding late in their extremely long lifespan of almost 30 years (Buffenstein, 2008; Buffenstein and Jarvis, 2002). It is unknown whether there is an optimal spine length or number of reproductive cycles that must be achieved for a queen to acquire her distinctive morphology, or if reproduction-enhanced vertebral elongation continues over the queen's lifespan.
Although the pronounced elongation of the spine is a novel feature of pregnancy in naked mole-rats, pregnancy-induced changes in bone are not uncommon in other mammals. In rats, bone size and density can increase during pregnancy, and much of this growth is subsequently lost to a bone resorption process that occurs during lactation (Bowman and Miller, 1999; Vajda et al., 2001). Bone growth during pregnancy is thought to provide mineral for the development of the fetal skeleton, as well as prepare the maternal skeleton for the demands of lactation (Vajda et al., 2001). This bone growth process serves as a homeostatic mechanism for maintaining the integrity of the skeleton across many reproductive cycles (Kunkele and Kenagy, 1997; Bowman and Miller, 1999; Sengupta et al., 2005; Specker and Binkley, 2005). While it is known that pregnancy increases vertebral length in female mole-rats (Buffenstein, 1996; Jarvis et al., 1991; O'Riain et al., 2000; Henry et al., 2007), the role of lactation on this anabolic bone growth process has not been investigated. In this study, we measured longitudinal bone growth in queen mole-rats over a reproductive cycle consisting of pregnancy, parturition, and lactation in order to determine the respective roles each phase of the breeding cycle play in spine elongation. We compared skeletal growth in nascent primiparous queens and established multiparous queens in order to study changes in spine elongation that occurred with reproductive experience.
Materials and Methods
Subjects
Nine female naked mole-rats ranging from 1-6 years of age were used in this study. Each female mole-rat originated from a different colony. Additional male mole-rats were used as mates for these females but were not used for data collection. Naked mole-rats were maintained in colony rooms with ambient temperatures of ∼30 degrees Celsius and 40-60% relative humidity (for complete housing details, see Artwohl et al., 2002).
Female mole-rats were categorized into three separate groups based on reproductive experience. The first group was comprised of established queens, which were the active breeding females of their respective colonies. These females had produced 7-11 litters prior to the start of this study and were selected because they were the animals with the most reproductive experience in our colonies (Table 1). The second group was composed of nascent queens, which were reproductively-mature females that had been recently removed from reproductive suppression of their natal colony and were housed with a male. Prior to this study, these females had not produced any litters, but over the course of the study they became pregnant and produced their first litter. The third group consisted of non-breeder control females that lived within the confines of their natal colony and were reproductively suppressed by their queens. Mole-rats were identified with microchip implants (AVID; Norco, CA). All procedures involving mole-rats were approved by the Vanderbilt University Institutional Animal Care and Use Committee and complied with the National Institutes of Health guidelines.
Table 1.
Pups Born and Surviving Until Weaning During Observed Reproductive Cycle
| Established Queens | Nascent Queens | |||||
|---|---|---|---|---|---|---|
| Q3 | Q6 | QBG | CC6 | 02 | 04 | |
| Pregnancy # | 8 | 16 | 15 | 1 | 1 | 1 |
| # pups born | 15 | 3 | 11 | 5 | 11 | 9 |
| # pups weaned | 5 | 2 | 7 | 5 | 1 | 3 |
| Total litters to date* | 14 | 20 | 21 | 7 | 8 | 8 |
Total number of litters produced by each breeding female within 2 years after observation period
Measurements
Body mass and longitudinal bone growth were measured twice weekly for the duration of approximately 35 weeks. Bone measurements were derived from radiographs of the subjects. A Faxitron MX-20 specimen x-ray cabinet (Wheeling, IL) was used to obtain radiographs of each mole-rat. During the measurement period mole-rats were removed from their cages, weighed, and lightly anesthetized with isoflurane to provide immobilization for the x-ray procedure. Individual animals were placed in the x-ray cabinet and dorsal radiographs were taken at a magnification of 1.5× at 35 kV and 0.3 mA for 80 seconds. Once the x-ray was taken, animals were placed into a holding chamber until recovery from anesthesia, and then were returned to their housing facility. Radiography has not shown any deleterious effects on mole-rats or their reproductive activity (Dengler-Crish and Catania, 2007; Henry et al., 2007; O'Riain et al., 2000).
The goal of the experiment was to obtain spine measurements as each mole-rat progressed through one complete reproductive cycle that included a 10-week (∼70 day) pregnancy period (Jarvis, 1991), parturition, a 4-week lactation phase and any post-lactation time that preceded the next pregnancy. In order to accurately identify these periods, a second reproductive cycle that included pregnancy and parturition was also observed. Pregnancy onset was determined by counting 10 weeks prior to parturition. Lactation typically lasted 4 weeks. Pregnancy-free post-lactation periods varied in length from 1-4 weeks, and data for these periods were not available for all animals because several queens became pregnant again during the end of the lactation. Therefore, for statistical analysis, data was categorized as either “pregnancy” or “post-pregnancy” with the latter including any lactation and post-lactation measurements before the subsequent pregnancy. Non-breeder control animals were not reproductively active and therefore never experienced a pregnancy, so data from these animals was collected during time points yoked to the queens' reproductive cycles.
Radiograph Analysis
Digital calipers (accurate to 0.01 mm) were used to measure bones from the radiographic images and measurements were corrected for magnification before data analysis. Fetal skeletons were visible on radiographs around 5 weeks of gestation and this data was used to further confirm the onset of pregnancy. Although all lumbar vertebrae have been shown to elongate in queen mole-rats (Henry et al., 2007), the length of one lumbar vertebra, L4 was used as the main index of lumbar growth. Measurement of this vertebra is an accurate index of longitudinal growth because it is not confounded by the angle of the spine or changes in the size of intervertebral spaces (Henry et al., 2007; O'Riain et a., 2000). The entire length of the lumbar spine was also measured (L1-L8 vertebrae) along with the width of the zygomatic arch of the skull. Zygomatic arch measurement provides a reliable index of general skeletal growth that occurs with time (Henry et al., 2007; O'Riain et al., 2000). L4 values were divided by the corresponding weekly measurements of the zygomatic arch, making an index of L4 growth (L4/ZA) that was specific to reproduction and not affected by general skeletal growth.
Gains in bone length over the pregnancy and post-pregnancy periods were calculated for animals in each condition. Longitudinal bone growth rates were obtained by dividing the amount of bone length gained during each reproductive period by the duration of the observation period.
Analysis
Mean L4 length, gains, rates, and L4/ZA values were derived for each of the 10-week pregnancy periods and the post-pregnancy periods. Analyses of variance (ANOVAs) and t-tests were used to test for significant relationships. Pairwise comparisons using the Holm-Sidak method were used to follow-up significant ANOVA effects. SigmaStat software (Systat Software, San Jose, CA) was used to perform data analysis.
Results
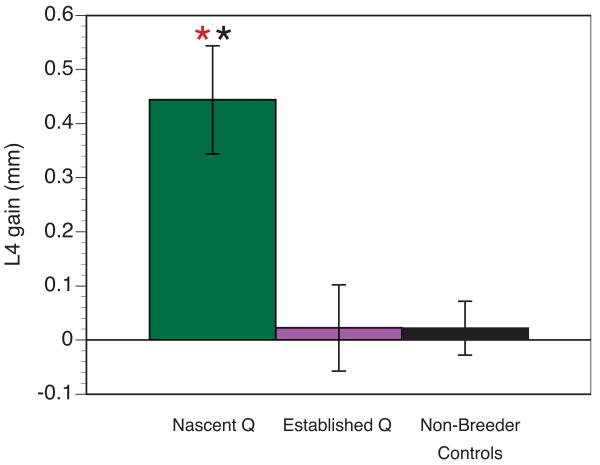
The net gain in L4 length experienced by females over the entire reproductive cycle (including both pregnancy and lactation) was analyzed with a one-way ANOVA, and the results indicated that nascent queens experienced significantly greater net gain in L4 over the reproductive cycle than established queens and non-breeder controls, F2,8 = 13.39, p < 0.01 (Fig. 1). The length of established queens' L4 did not differ from that of non-breeder controls over the reproductive cycle, t = 0.04, ns (Pairwise comparisons, Holm-Sidak method).
Figure 1.
Net gain in breeder L4 length over the course of one complete pregnancy-birth-lactation cycle or comparable time period in non-breeder controls. Nascent queens (n = 3) show a significantly greater gain in L4 length after their first reproductive cycle compared to established queens (n = 3) that have experienced eight or more pregnancies and non-breeder controls (n = 3). Non-breeder controls were not reproductively active and did not experience pregnancy, but data from these animals were collected during time points yoked to the queens' reproductive cycles. Established queens and non-breeder controls do not differ in net L4 gain over the breeding cycle, suggesting that reproduction-induced spine elongation was not achieved in established breeders. The red asterisk indicates a significant difference in L4 gain between nascent queens and established queens, and the black asterisk indicates a significant difference in L4 between nascent queens and controls (p < 0.05).
Nascent queens demonstrated a 4mm increase in the length of the total lumbar spine (L1-L8) over the course of the observed reproductive cycle of the study, whereas established queens and controls only gained about 1mm of length in their lumbar vertebrae. This demonstrated that while reproduction-related spine elongation did not occur in established queens, their skeletons were still experiencing longitudinal growth similar to that of the control animals. Body and bone measurements for female mole-rats in all three conditions of the study are shown in Table 2.
Table 2.
Body Mass, L4 Length, and Total Lumbar Spine Length at End of Breeding Cycle Observation Period
| Group | Body Mass (g) | L4L (mm) | Total Lumbar L (mm) |
|---|---|---|---|
| Established Queens | |||
| Q3 | 56.0 | 5.2 | 44.0 |
| Q5 | 43.7 | 4.8 | 35.7 |
| QBG | 49.7 | 5.9 | 43.7 |
| *Net gain in total lumbar length = 1.1mm | |||
| Nascent Queens | |||
| CC6 | 41.6 | 4.33 | 32.3 |
| 02 | -- | 4.29 | 32.7 |
| 04 | -- | 4.86 | 36.4 |
| *Net gain in total lumbar length = 3.8mm | |||
| Non-Breeder Controls | |||
| 051 | 34.1 | 3.4 | 26.7 |
| 581 | 24.9 | 3.0 | 30.4 |
| 095 | 26.8 | 3.4 | 31.4 |
| *Net gain in total lumbar length = 1.0mm | |||
Average net gain in lumbar spine length over the course of one reproductive cycle for each of the experimental groups
--Indicates missing data for these two animals
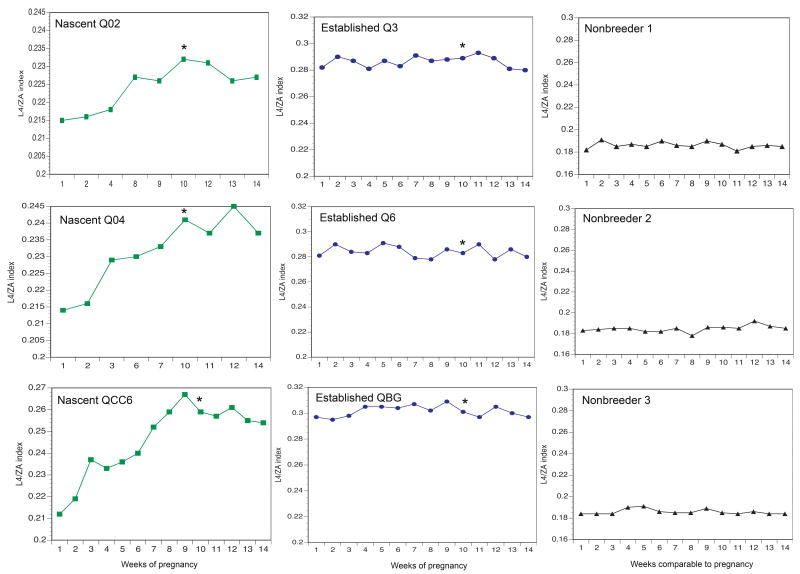
Weekly L4/ZA indexes during pregnancy are shown for each female mole-rat in Figure 2. Established queens began the study with higher L4/ZA indexes than females from the other two groups, and this was expected since established breeders had 7-15 pregnancies prior to this study. However, vertebral elongation in established breeders did not increase at the same rate as in nascent queens during the pregnancy period. Nascent queen L4/ZA indexes showed a demonstrable upward slope during pregnancy, whereas the established queens' indexes were relatively flat. The established queens' data resembled the data of the non-breeder controls in this sense, although the established queens' measurements did show more variability over the pregnancy period than those of the control animal.
Figure 2.
Attenuation of pregnancy-induced spine growth in established queen mole-rats. Weekly L4/ZA indexes for nascent queens (Q02, Q04, QCC6), established queens (Q3, Q6, QBG) and three non-breeder control females are plotted. Pregnancy begins at week 1 and parturition occurs around week 10 as noted by the asterisks. L4/ZA indexes from a non-pregnancy post-partum period (weeks 11-14) are plotted for reference. αNon-breeder controls were not reproductively active and did not experience pregnancy, but data from these animals was collected during a 10-week time yoked to the queens' pregnancy. The first pregnancy of each nascent queen is depicted, and an increase in L4/ZA index is noted throughout the 10-week pregnancy period. The 8th (Q3), 16th (Q6), and 15th (QBG) pregnancies of established queens are shown in this figure. Although the established queens exhibit an overall higher L4/ZA index, no obvious spine lengthening is seen over the 10-week pregnancy period. This pattern of growth is similar to the general skeletal growth experienced by the non-breeder controls. Note that values of x-axes differ between groups. These values also differ between some animals within groups due to high individual variability in growth.
L4 length gained specifically during pregnancy and the post-pregnancy period was compared between the females of the study. Overall, nascent queens gained significant length in L4 during pregnancy, but failed to demonstrate spine elongation in the period after pregnancy (paired t-test: t4 = 4.01, p = 0.02). Nascent queens exhibited higher bone elongation rates (mm/week) than established queens and controls during the pregnancy period (F 2, 8 = 9.43, p < 0.02). Nascent queens' L4 grew 3.5 times faster than established queens during this time, (t = 4.10, p < 0.02) and longitudinal growth rates shown by non-breeder controls during a comparable period were minimal (t = 3.30, p < 0.02). There was a trend in established queens that indicated a small amount of vertebral elongation occurred during pregnancy and this accounted for the variability seen in the L4/ZA indexes of established queens in Figure 2. However, this L4 lengthening was attenuated during the post-pregnancy period. Non-breeder control measurements were yoked to pregnancy and non-pregnancy periods of the breeders and did not significantly differ between these two observation periods (t4 = -0.15, ns).
Data from the lactation and post-lactation periods were distinguished and plotted for nascent and established queens. Both established queens and nascent queens experienced an attenuation of L4 lengthening during the 4-week lactation period, but some L4 elongation was seen in nascent queens after lactation was over. Established queens did not demonstrate any L4 elongation during the extended post-pregnancy period that included both lactation and post-lactation times. Post-lactation periods do not occur frequently in naked mole-rats because breeding females often become pregnant again during lactation (Jarvis, 1991), and the anabolic effects of pregnancy may lessen the impact of bone loss that commonly occurs in mammals during lactation. Data on the numbers of pups born, number of pups surviving to weaning, and number of past and present litters are shown for all the breeding females in Table 1.
Discussion
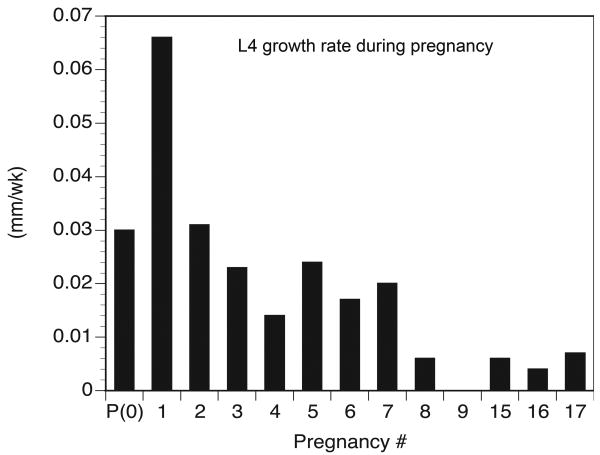
Pregnancy-induced spine elongation in queen mole-rats appears to be a finite growth process that is attenuated after multiple cycles of reproduction. In this study, we demonstrated that three established queens with a breeding history of eight or more pregnancies did not experience a net gain in spine length over the reproductive cycle and any spine elongation that did occur in these animals was no different than the non-breeder controls. However, the established breeders had already obtained the elongated morphology typical of queen mole-rats and had longer spines than all other females in the study (Table 2; Figure 3). The cessation of the reproduction-related spine-lengthening process in these breeding female mole-rats is an intriguing finding because an attenuation of this growth has not been previously defined, and these data suggest that the completion of spine elongation could be reached after as few as eight pregnancies. Queen mole-rats can produce eight litters within two years, and because they have an almost 30-year lifespan (Buffenstein and Jarvis, 2002), these females can complete the morphological transformation associated with their breeding status quite early in their reproductive experience. Figure 4 summarizes L4 growth rates from a sample of female mole-rats in our colonies with varying levels of reproductive experience.
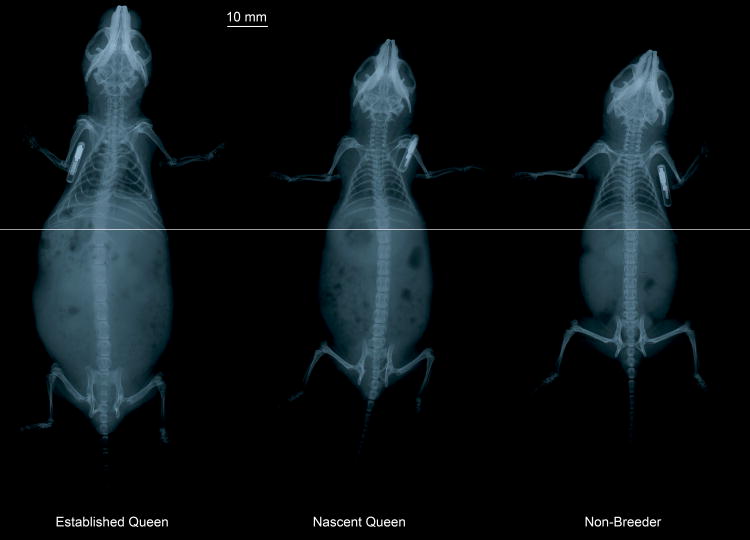
Figure 3.
Relative lumbar spine length for an established queen, nascent queen, and non-breeder female naked mole-rat. Radiographs were taken after pregnancy # 8 in the established queen, after pregnancy #1 in the nascent queen, and during a comparable time period in the non-breeder. The horizontal line in the center of the radiographs is aligned with the top of the vertebral body of L1 to allow comparison of lumbar spine length between the three mole-rats. The radiopaque cylindrical objects seen near the cervical spine are identification microchip implants.
Figure 4.
Most lumbar spine elongation in breeding female mole-rats occurs early in their reproductive experience. Representative L4 longitudinal growth rates (mm/week) over pregnancy (or comparable puberty period) in female mole-rats with varying levels of reproductive experience (n = 12) are shown. The y-axis displays longitudinal growth rate as a function of parity: rate during puberty is shown at 0(P); the data display growth rate by number of pregnancies (1-17, excluding 10-14 due to a lack of data from animals experiencing these numbers of pregnancies). L4 longitudinal growth rate is elevated during puberty, peaks with the first pregnancy peak, and remains elevated for seven pregnancies. This increased growth rate decreases by the 8th pregnancy. Representative growth rates are derived from an availability sample of breeders with varying degrees of reproductive experience. Some of the plotted values are derived from means of multiple breeders and other plots may represent data from only one available animal. See Appendix 1 for the longitudinal growth rate data and numbers of animals used to generate plots for this figure.
While they did not experience a significant net gain in vertebral length, our results suggest that established breeders may have developed a homeostatic mechanism for maintaining the integrity of their skeletons throughout the reproductive cycle. Our data suggests that established breeders' L4 still elongated to a small extent during the pregnancy period. In other mammals (such as rats and humans), pregnancy-induced bone growth occurs to prepare the skeleton for the demands of lactation--a time of rapid bone depletion (Bowman and Miller, 1999; Vajda et al., 2001; Specker and Binkley, 2005). While we did not measure bone loss in this study, it is possible that bone mineral resorption was occurring in naked mole-rats during the lactation period.
In naked mole-rats, pregnancy-induced bone elongation appears to serve a dual purpose. Early in a breeding female's reproductive experience, pregnancy induced elongation of the spine results in the morphologically distinct phenotype of the queen mole-rat. After this period of rapid spine growth, the pregnancy-facilitated bone elongation mechanism is apparently down-regulated to homeostatic levels, and may serve only to prepare the spine for bone loss that is typically associated with lactation in mammals. While our study suggests this focal spine growth period coincides with the first eight pregnancies, the exact time at which lumbar lengthening subsides remains to be defined, and more subtle growth of the spine correlated with general body size increases was not explored. It is interesting to note that bone acquisition rates during pregnancy are similar for tibia growth in rats (Bowman and Miller, 1999) and vertebral lengthening in naked mole-rats, but rats only experience this enhanced growth for a 3-week gestation period, whereas naked mole-rat spine elongation is sustained for their 10-week pregnancy duration.
The complexities of the queen mole-rat's reproductive cycle may also influence spine lengthening. Many queens become pregnant during the lactation period (Jarvis, 1991), so presumed bone loss during lactation could be counteracted by the anabolic growth of pregnancy. In the current study, we were able to analyze bone elongation specific to lactation and post-lactation periods. Our results indicated that while nascent breeders' L4 elongation was attenuated during the lactation period, they did experience some vertebral expansion in the period after weaning but before the next pregnancy. However, established queens did not experience any vertebral lengthening for the duration of the post-pregnancy period--even after lactation was complete. One possible explanation for this is that the more experienced, multiparous queens may nurse pups for a longer time. The research of Kunkele and Kenagy (1997) suggests that the first pregnancy cycle is not always the most efficient metabolically and energetically and it is possible that new mothers spend less time engaged in parental care than experienced mothers (Wang and Novak, 1994). Therefore, early cessation of nursing in nascent queens could lead to an attenuation of bone loss, and possibly even bone recovery (Miller and Bowman, 2004).
The association of lumbar spine elongation with pregnancy (and puberty: Dengler-Crish and Catania, 2007) suggests that reproductive hormones mediate skeletal growth in naked mole-rats (Buffenstein, 1996). Estrogens and progesterone are known to facilitate bone formation in mammals, and as pregnancy progresses, systemic levels of these hormones increase dramatically (Bowman and Miller, 1997; Eastell, 2005; Riggs et al., 2002). Changes in estrogen receptor expression and bone responsiveness to this hormone may account for the attenuation of lumbar spine elongation in experienced breeding females (Corvol et al., 1992; Riggs et al., 2002), since these queens likely experience high levels of these steroids with each subsequent pregnancy.
Acknowledgments
The authors thank Dr. Samuel Crish for assistance with experimental design and editorial support.
Grant Sponsorship: NIH grant DE 016061 and NSF Career Award IBN 028364 to K.C.C.
Literature Cited
- Artwohl J, Hill T, Comer C, Park T. Naked mole-rats: unique opportunities and husbandry challenges. Lab Anim. 2002;148:185–189. doi: 10.1038/5000156. [DOI] [PubMed] [Google Scholar]
- Buffenstein R. Ecophysiological responses to a subterranean habitat; a Bathyergid perspective. Mammalia. 1996;60:591–605. [Google Scholar]
- Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J Comp Physiol B. 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Jarvis JUM. The naked mole-rat--a new record for the oldest living rodent. Sci Aging Knowledge Environ. 2002;2002:7. doi: 10.1126/sageke.2002.21.pe7. [DOI] [PubMed] [Google Scholar]
- Bowman BM, Miller SC. Endochondral bone growth during early pregnancy compared with pseudopregnancy in rats. Endocrine. 1997;6:173–177. doi: 10.1007/BF02738961. [DOI] [PubMed] [Google Scholar]
- Bowman BM, Miller SC. Skeletal mass, chemistry, and growth during and after multiple reproductive cycles in the rat. Bone. 1999;25:553–559. doi: 10.1016/s8756-3282(99)00204-5. [DOI] [PubMed] [Google Scholar]
- Clark FM, Faulkes CG. Dominance and queen succession in captive colonies of the eusocial naked mole-rat, Heterocephalus glaber. Proc R Soc Lond B. 1997;264:993–1000. doi: 10.1098/rspb.1997.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvol M, Blanchard O, Tsagris L. Bone and cartilage responsiveness to sex steroid hormones. J Steroid Bioch Molec Biol. 1992;43:415–418. doi: 10.1016/0960-0760(92)90078-w. [DOI] [PubMed] [Google Scholar]
- Dengler-Crish CM, Catania KC. Phenotypic plasticity in female naked mole-rats after removal from reproductive suppression. J Exp Biol. 2007;210:4351–4358. doi: 10.1242/jeb.009399. [DOI] [PubMed] [Google Scholar]
- Eastell R. Role of oestrogen in the regulation of bone turnover at the menarche. J Endocrinol. 2005;185:223–234. doi: 10.1677/joe.1.06059. [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Abbott DH, Jarvis JUM. Social suppression of ovarian cyclicity in captive and wild colonies of naked mole-rats, Heterocephalus glaber. J Reprod Fert. 1990;88:559–568. doi: 10.1530/jrf.0.0880559. [DOI] [PubMed] [Google Scholar]
- Henry EC, Dengler-Crish CM, Catania KC. Growing out of a caste--reproduction and the making of the queen mole-rat. J Exp Biol. 2007;210:261–268. doi: 10.1242/jeb.02631. [DOI] [PubMed] [Google Scholar]
- Jarvis JUM. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science. 1981;212:571–573. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- Jarvis JUM. Reproduction of naked mole-rats. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat: Monographs in Behavior and Ecology. Oxford: Princeton University Press; 1991. pp. 384–425. [Google Scholar]
- Jarvis JUM, O'Riain MJ, McDaid E. Growth factors affecting body size in naked mole-rats. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat: Monographs in Behavior and Ecology. Oxford: Princeton University Press; 1991. pp. 358–383. [Google Scholar]
- Kunkele J, Kenagy GJ. Inefficiency of lactation in primiparous rats: the costs of first reproduction. Physiol Zool. 1997;70:571–577. doi: 10.1086/515862. [DOI] [PubMed] [Google Scholar]
- Miller SC, Bowman BM. Rapid improvements in cortical bone dynamics and structure after lactation in established breeder rats. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:143–149. doi: 10.1002/ar.a.10138. [DOI] [PubMed] [Google Scholar]
- O'Riain MJ, Jarvis JUM, Alexander R, Buffestein R, Peeters C. Morphological castes in a vertebrate. Proc Natl Acad Sci USA. 2000;97:13194–13197. doi: 10.1073/pnas.97.24.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- Sengupta W, Arshad M, Sharma S, Dubey M, Singh MM. Attainment of peak bone mass and bone turnover rate in relation to estrous cycle, pregnancy, and lactation in colony-bred Sprague-Dawley rats: suitability for studies on pathophysiology of bone and therapeutic measures for its management. J Steroid Biochem Mol Biol. 2005;94:421–429. doi: 10.1016/j.jsbmb.2004.12.039. [DOI] [PubMed] [Google Scholar]
- Smith TE, Faulkes CG, Abbott DH. Combined olfactory contact with the parent colony and direct contact with non-breeding animals does not maintain suppression of ovulation in female naked mole-rats. Horm Behav. 1997;31:277–288. doi: 10.1006/hbeh.1997.1384. [DOI] [PubMed] [Google Scholar]
- Specker B, Binkley T. High parity is associated with increased bone size and strength. Osteoporos Int. 2005;16:1969–1974. doi: 10.1007/s00198-005-1978-1. [DOI] [PubMed] [Google Scholar]
- Vajda EG, Bowman BM, Miller SC. Cancellous and cortical bone mechanical properties and tissue dynamics during pregnancy, lactation, and postlactation in the rat. Biol Reprod. 2001;65:689–695. doi: 10.1095/biolreprod65.3.689. [DOI] [PubMed] [Google Scholar]
- Wang Z, Novak MA. Parental care and litter development in primiparous and multiparous prairie voles (Microtus ochrogaster) J Mammal. 1994;75:18–23. [Google Scholar]