Abstract
Background
Incidental hyperglycemia in children generates concern about the presence of preclinical type 1 diabetes mellitus (T1DM).
Objective
To genetically evaluate two common forms of maturity-onset diabetes of youth (MODY), the short-term prognosis in children with mild hyperglycemia, and a positive family history of diabetes mellitus.
Subjects
Asymptomatic children and adolescents (n = 14), younger than 15 yr, with fasting hyperglycemia, a positive family history of mild non-progressive hyperglycemia, and negative pancreatic autoantibodies were studied.
Patients and methods
Glucokinase gene (GCK) and hepatocyte nuclear factor 1 alpha gene (HNF1A) causing two common forms of MODY were sequenced. The clinical outcome was evaluated after a follow-up period of 2.8 ± 1.3 yr.
Results
GCK mutations were present in seven children. The confirmation of this diagnosis allowed discontinuation of insulin in two families and oral medications in three families. Mutations of HNF1A were not detected in any of the families. During the follow-up period, all the GCK mutation carrier children remained asymptomatic without medication and the last hemoglobin A1c levels were 6.4 ± 0.7%. In the GCK-negative children (n = 7), one developed T1DM, corresponding to 7.2% of the total group. Mild fasting hyperglycemia persisted during follow-up in four GCK-negative children and normalized in the remaining two.
Conclusions
The presence of mild persistent hyperglycemia in any patient without autoantibodies should lead to genetic analysis of GCK, particularly if there is a positive family history. Furthermore, those without GCK mutations should be followed with repeat autoantibody testing, and other genetic types of diabetes should be considered if hyperglycemia worsens.
Keywords: DM, genetics, glucokinase, hyperglycemia, incidental hyperglycemia, MODY, prognosis
The presence of asymptomatic hyperglycemia in an apparently healthy child generates anxiety about the possibility of onset of type 1 diabetes mellitus (T1DM). Mild hyperglycemia in children is an infrequent finding (1) and is observed in only 0.013% of the children attending a pediatric office (2). Recently, the cutoff level of normal fasting blood glucose (BG) has been decreased to 100 mg/dL (3), which may be a reason for an increase in the referral of asymptomatic children with mildly elevated BG.
Several studies have evaluated the risk of progression to T1DM in children with mild hyperglycemia (4, 5), but these studies did not consider the study of monogenic forms of diabetes. In addition, they did not measure BG in the parents, who may have mild asymptomatic hyperglycemia too. Children with mild asymptomatic hyperglycemia with a positive family history of mild hyperglycemia or gestational diabetes may have maturity-onset diabetes of youth (MODY), caused by a heterozygous inactivating mutation in the glucokinase gene (GCK), which is not associated with progression to severe insulinopenia. Patients with this type of diabetes show mild fasting hyperglycemia with normal or near normal BG levels during the postprandial state (6). First-degree relatives carrying the familial GCK mutation show a similar abnormality, often with a history of lifelong hyperglycemia, without complications or requirement of insulin or oral medications (7).
We report the clinical and molecular outcome of 14 children with mild fasting hyperglycemia, without signs of autoimmunity, and their follow-up for 2.8 yr.
Methods
Subjects
Fourteen children with persistent mild hyperglycemia were studied. The families were referred from July 2003 to June 2007 to a group of pediatric endocrinologists working in hospitals or private clinics in Santiago, Chile, for evaluation of fasting, non-progressive hyperglycemia. All the probands had at least three fasting BG levels above 100 mg/dL, a parent and grandparent with a similarly elevated BG, and negative islet autoantibodies. All the relatives had mild hyperglycemia, which did not require insulin treatment and lacked a history of micro-vascular complications. All the families that fulfilled the inclusion criteria during this 4-yr period are included in this study except one family that did not sign the inform consent and a second case who had multiple positive islet autoantibodies. The molecular genetics of three of these families was previously reported (8). The study was approved by the Institutional Review Board at Columbia University. Parents signed an inform consent written in Spanish, and children gave verbal assent.
We performed a complete physical examination and pubertal development assessment according to Marshall and Tanner. Weight was measured using a conventional Seca scale with a precision of 100 g, and height was measured with a Harpenden Stadiometer. Standard deviation (SD) scores were calculated for height, weight, and body mass index (BMI) using current National Center for Health Statistics (NCHS) standard curves (9). In addition, birth weight SD was also calculated employing Chilean standards (10).
Laboratory evaluation
Plasma glucose concentrations were determined by glucose oxidase after a minimum of 8 h of fasting. Insulin concentration was determined by radioimmunoassay as previously described (11). Hemoglobin A1c (HbA1c) was determined by ionic exchange chromatography (DCA 2000; Bayer Diagnostics, Tarrytown, NY, USA). Oral glucose tolerance tests (OGTT) were performed with 1.75 g/kg of anhydrous glucose (maximum 75 g). Subjects were classified according to the American Diabetes Association Classification and diagnosed as having impaired fasting glucose, impaired glucose tolerance, or diabetes mellitus (DM) (3). Screening for serological anti-glutamic acid decarboxylase (GAD) 65, anti-islet antigen-2 (IA-2), and insulin autoantibodies was performed by immunoassay as previously described (8).
Molecular study of GCK was performed in all the patients because the phenotype of mild persistent familial hyperglycemia is highly suggestive of GCK mutations. Hepatocyte nuclear factor 1 alpha gene (HNF1A) was then sequenced in those without a GCK mutation because it is the second most common cause of MODY (12, 13). Hepatocyte nuclear factor 4 alpha gene (HNF4A) was not studied because of the low frequency of mutations (13). Analysis of hepatocyte nuclear factor 1 beta gene (HNF1B) was not considered in any of the families studied because HNF1B mutation carriers have renal abnormalities that were not present in any of the patients in the study.
Genomic DNA was isolated from leukocytes in whole blood by cell lysis followed by DNA extraction and precipitation according to manufacturer’s instructions (Promega, Madison, WI, USA). Each proband was amplified by polymerase chain reaction (PCR) and purified as previously described (8) Amplicons were bidirectionally sequenced for all the coding exons and splice sites of GCK and HNF1A with the BigDye Terminator kit using an ABI 377 sequencer (Applied Biosystems, Foster City, CA, USA). When a mutation was identified, all other family members were sequenced only for the exon containing the mutation. Sequence was analyzed using Sequencher software. In addition, each electropherogram was visually reviewed to identify any heterozygous DNA variants not detected by the automated sequencing software.
Statistical analysis
Comparisons of means between the group of patients with and without a GCK mutation were made using the Mann–Whitney U test. Differences in proportions between the two groups were evaluated using Fisher’s exact test. Results are expressed as mean age ± SD. All statistical calculations were run on spss for Windows, version 10.0. A p value less than 0.05 was considered statistically significant.
Results
Clinical characteristics of the patients and type of abnormality of the glucose metabolism
The clinical characteristics of the probands are shown in Table 1. Eleven patients had normal weight, 2 were overweight, and 1 was obese. Using a fasting BG cutoff level of 100 mg/dL, impaired fasting glucose and DM were diagnosed in 57.1 and 42.9% of the patients, respectively. However, in those patients with fasting hyperglycemia, only one patient had a fasting BG level higher than 140 mg/dL. She was a 1.6-yr-old girl who had a normal stimulated BG on the OGTT and an HbA1c of 6.6%.
Table 1.
Clinical and anthropometric characteristics of children with mild asymptomatic hyperglycemia*
| Mean ± SD | Range | |
|---|---|---|
| Number of children studied | 14 | |
| Male/female | 8/6 | |
| Age (yr) | 8.4 ± 3.9 | 1.6–15.6 |
| Body mass index (SD) | 0.4 ± 0.9 | −0.7 to 1.9 |
| Height (SD) | −0.5 ± 1.0 | −3.1 to 1.1 |
| Birth weight (SD) | −0.3 ± 1.0 | −2.3 to 1.6 |
| Hemoglobin A1c (%) | 6.2 ± 0.6 | 5.4 to 7.5 |
| Fasting glucose (mg/dL) | 121.4 ± 15.9 | 101–155 |
| Stimulated glucose level, 2-h OGGT (mg/dL) |
143.1 ± 29.7 | 105–201 |
| Fasting insulin (µUI/mL) | 5.2 ± 2.5 | 2–10.3 |
| Stimulated insulin level, 2-h OGTT (µUI/mL) |
26.2 ± 21.7 | 3.8–84.7 |
| Fasting glucose level (n) | ||
| Impaired fasting glucose | 8/14 | |
| Diabetes mellitus | 6/14 | |
| Stimulated glucose level on 2-h OGTT (n) |
||
| Normal | 7/14 | |
| Glucose intolerance | 6/14 | |
| Diabetes mellitus | 1/14 |
OGTT, oral glucose tolerance test.
Values listed are means ± SD.
Based on the stimulated glucose level on the OGTT, 50% of the patients were normal, 42.9% glucose intolerant, and 7.1% diabetic. The patient who was classified as diabetic based on the 2-h BG level on the OGGT was a 2.7-yr-old boy, who had a fasting BG level of 105 mg/dL and an HbA1c level of 5.7%. Mild hyperglycemia persisted for 6 months and normalized afterward.
Molecular study and treatment modifications after confirmation of GCK mutation
A GCK mutation was identified in 50% of the patients. Five different mutations were observed in these families (Gly258Asp, Arg303Trp, Thr209Met, Gly44Ser, and Arg191Gln). All these mutations have been previously reported, and Arg191Gln and Gly258Asp have previously been identified in children with MODY (14–16). Three unrelated families carried the same Arg191Gln mutation (Fig. 1). The remaining seven probands had normal GCK and HNF1A sequence, excluding the diagnosis of the most common forms of MODY in these kindreds.
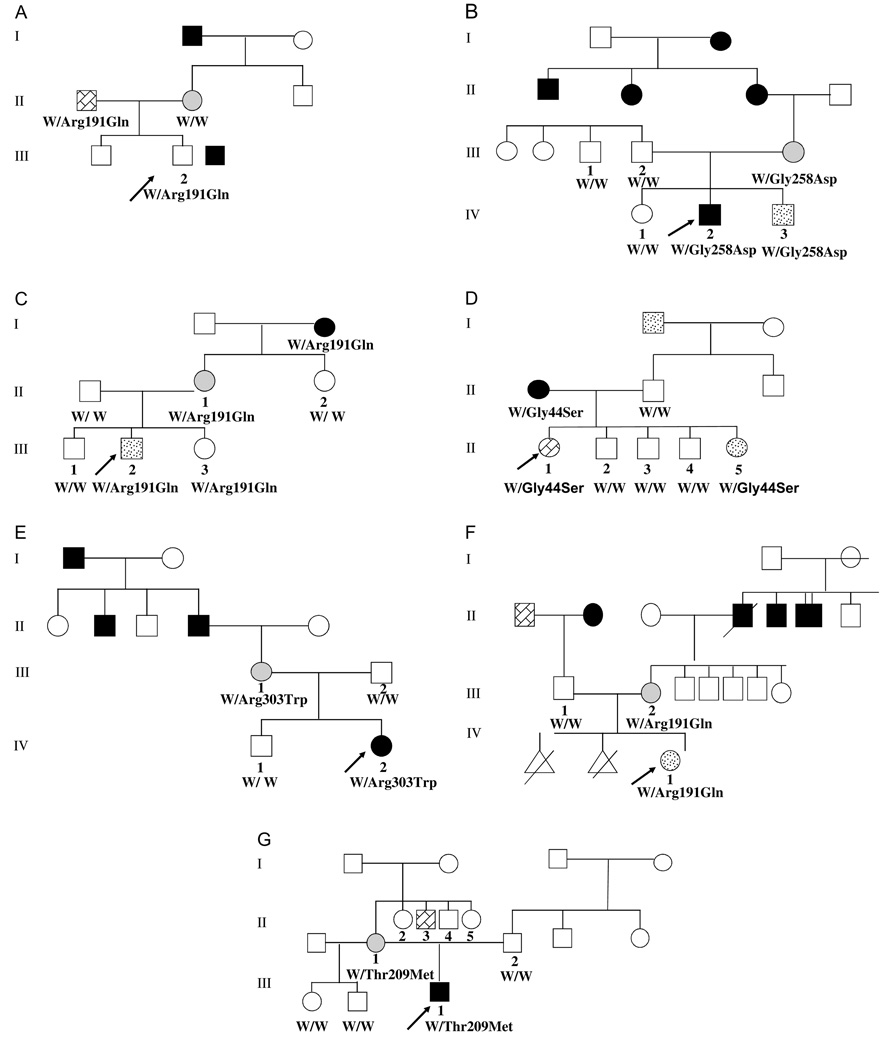
Fig. 1.
Pedigree and molecular analysis of the seven families that harbor a GCK mutation. ‘W’ denotes wild-type allele. The proband are indicated by an arrow. Black squares/circles: diabetes mellitus. Light gray circles: gestational diabetes. Stippled squares/circles: impaired glucose tolerance. (A) Affected subjects had an Arg191Gln mutation. (B) Affected subjects had a Gly258Asp mutation. (C) Affected subjects had an Arg191Gln mutation. (D) Affected subjects had a Gly44Ser mutation. (E) Affected subjects had a Arg303Trp mutation. (F) Affected subjects had a Arg191Gln mutation. (G) Affected subjects had a Thr209Met mutation.
The metabolic characteristics of the seven families with and without a GCK mutations are shown in Table 2. No statistical differences were observed between the groups regarding their anthropometry, BG, or insulin levels. However, many of the GCK probands were diabetic based on the fasting BG compared with the idiopathic group (71.4 vs. 14.3%, respectively, p = 0.05, chi-squared test).
Table 2.
Clinical and metabolic characteristics of probands with and without a GCK mutation*
| GCK | Idiopathic mild fasting hyperglycemia |
|
|---|---|---|
| Number of children studied |
7 | 7 |
| Age (yr) | 8.1 ± 4.5 | 8.7 ± 3.5 |
| Body mass index (SD) |
0.4 ± 0.9 | 0.4 ± 0.8 |
| Height (SD) | −0.7 ± 1.1 | −0.2 ± 0.9 |
| Birth weight (SD) | −0.4 ± 1.2 | −0.1 ± 0.7 |
| Hemoglobin A1c (%) | 6.4 ± 0.7 | 6.2 ± 0.7 |
| Fasting glucose (mg/dL) |
128.1 ± 17.2 | 114.7 ± 12.2 |
| Stimulated glucose level, 2-h |
141.9 ± 27.4 | 144.4 ± 34.1 |
| Fasting insulin (µUI/mL) |
4.8 ± 1.6 | 5.5 ± 3.3 |
| Stimulated insulin level, 2-h OGTT (µUI/mL) |
18.1 ± 14.7 | 34.3 ± 25.4 |
| Fasting glucose level (n) |
||
| Impaired fasting glucose |
2 | 6 |
| Diabetes mellitus | 5† | 1 |
| Stimulated glucose level on 2-h OGTT (n) |
||
| Normal | 3 | 3 |
| Glucose intolerance | 4 | 3 |
| Diabetes mellitus | 0 | 1 |
OGTT, Oral glucose tolerance test.
Values listed are means ± SD.
Proportion of patients with fasting glucose in the diabetic range p = 0.051.
In five families, corresponding to six subjects, medications were discontinued after identification of a GCK mutation. Insulin was discontinued in three children from two families after the molecular etiology was identified. HbA1c did not change after discontinuing the medications, remaining at the previous level of 6.5% before and after the intervention.
One of the families that could stop medications is shown in Fig. 1A. The proband (subject III.2), a 9-yr-old boy, had impaired fasting glucose and glucose intolerance and was treated with glargin insulin 1–2 units/d (<0.5 U/kg/d). Upon identification of an Arg191Gln GCK mutation, insulin treatment was discontinued and the HbA1c levels remained stable. The second family in which insulin treatment was discontinued has been reported previously (8) (Fig. 1B). Briefly, children IV.2 and IV.3 were initially treated with insulin until a Gly258Asp GCK mutation was identified. Insulin treatment was discontinued, and BG levels and HbA1c remained unchanged.
Other patients were able to discontinue taking oral medications. Illustrated in Fig. 1C, the mother (II.1) of the asymptomatic 8.9-yr-old proband (III.2) was initially treated with metformin and glybenclamide. Both drugs were successfully discontinued, and BG and HbA1c levels remained unchanged afterward.
In Fig. 1D, the proband (subject III.1) was a 15.6-yrold girl who was evaluated for hirsutism at which time impaired fasting glucose was diagnosed. She had a normal BMI of 19 kg/m2 (−0.4 SD). Her diabetic mother (subject II.1) had been treated with metformin and discontinued her medication after a Gly44Ser GCK mutation was identified. Her metabolic control remained unchanged after discontinuing metformin.
The mother (III.I) in Fig. 1Ewas diagnosed with DM at the age of 17 yr and was treated with sulfonylureas, which was associated with frequent hypoglycemic episodes, despite the use of 2.5–5 mg glyburide. Her medication was then changed to metformin, which was discontinued after an Arg303Trp mutation in GCK was identified. After discontinuation of oral medications, her BG remained unchanged, with fasting glucose of 100–140 mg/d.
Clinical outcome in children with a GCK mutation and those with idiopathic hyperglycemia
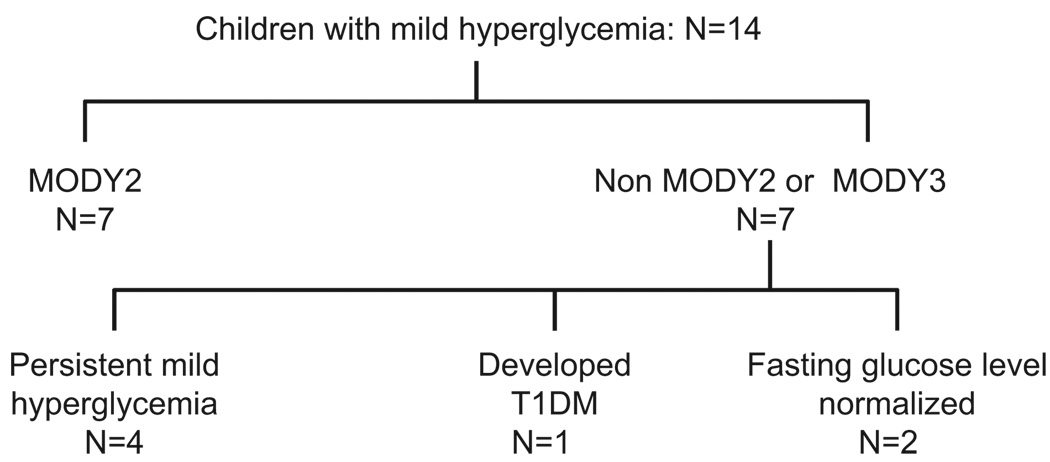
The clinical outcomes of the 14 children are shown in Fig. 2. The median time of observation was 2.8 ± 1.3 yr (range 1–4.8 yr). The seven children with GCK mutations have remained under observation with surveillance of BG and HbA1c levels. This group of patients has been followed for 3.2 ± 1.5 yr, and the last fasting BG and HbA1c levels were 114 ± 13 mg/dL and 6.4 ± 0.7%, respectively. None of them is currently receiving medication. Counseling on healthy lifestyle and diet was provided.
Fig. 2.
Outcome of the children with mild hyperglycemia 2.8 ± 1.3 yr after initial diagnosis. T1DM, type 1 diabetes mellitus.
One of the children with a negative sequence analysis for GCK, HNF1A, and HNF4A developed positive islet autoantibodies and progressive hyperglycemia 1 yr later leading to the diagnosis of T1DM. Initially, the boy was referred to a pediatric endocrinologist at the age of 11.1 yr for evaluation of short stature. The laboratory evaluation showed impaired fasting glucose (103–115 mg/dL), glucose intolerance on the OGTT, and negative islet autoantibodies. The father and paternal grandfather had a long-standing history of impaired fasting glucose. The child had stable BG for a year but then had progressive hyperglycemia subsequently. At the age of 12.9 yr, positive anti-GAD and anti-IA-2 autoantibodies were detected and fasting and postprandial BG increased to the 200 and 400 mg/dL, respectively. Treatment with glargine and multiple daily doses of aspart insulin was initiated. Currently, he is 13.4-yr-old and has been treated with multiple daily insulin injections for 6 months, with HbA1c of 7.2–8%.
Four children with idiopathic fasting hyperglycemia have had stable BG and two children normalized their BG. One of these two children has a fasting BG of 95 mg/dL with HbA1c level of 5.4% at the age of 16 yr. The second child who normalized his fasting BG had an HbA1c of 6.2% and fasting BG of 95 mg/dL at the age of 10 yr.
Discussion
We report on the genetic evaluation of GCK and HNF1A in a series of 14 young children and adolescents referred for asymptomatic hyperglycemia with a positive family history of mild hyperglycemia. We observed that half of these children carried a GCK mutation explaining their disturbed carbohydrate metabolism and accurately predicting a benign clinical course without pharmacological treatment. In the children without GCK or HNF1A mutations, 1 child of the 14 (7.2%) developed T1DM, despite negative islet autoantibodies at initial evaluation, suggesting the need for continued careful observation in these patients.
Development of T1DM has been reported in 2.1–32% of children with incidental hyperglycemia (4, 5, 17), which is similar to the findings in our report. Herskowitz-Dumont et al. prospectively followed children with incidental hyperglycemia for 18 months and observed a 32% incidence of T1DM. The risk of developing T1DM was associated with the presence of islet cell antibodies and a low stimulated insulin release during intravenous glucose tolerance test (17). Similar risk factors were also described by Schatz et al. in 1989 (5). More recently, Lorini et al. showed that children with incidental hyperglycemia exhibit a higher prevalence of the high-risk haplotypes and positive islet autoantibodies than the general population (4).
To characterize the etiology of mild hyperglycemia in children, the International Society of Pediatric and Adolescent Diabetes recommends molecular analysis of GCK in children who meet the following criteria: fasting BG of 100–150 mg/dL, hyperglycemia that is persistent and stable over a period of months or year, HbA1c just below or just above the upper normal limit, small increase of BG in the OGTT (Δ < 60 mg/dL), and a parent with a similar abnormality (18). The fact that half of the children in our series who all met these criteria had identifiable mutations in GCK confirms the clinical applicability of these criteria.
Four children had persistent hyperglycemia without identifiable GCK or HNF1A mutations and may be explained by several hypotheses. One possibility is that a mutation such as a large deletion or regulatory mutation in GCK may not have been identified by the assay based on PCR amplification of coding exons, as recently reported by Ellard et al. (19). It is possible that these patients have genetic variants in other genes for glycemic control besides GCK or HNF1A. It is also possible that some of these patients will develop T1DM, and the follow-up period is insufficient. Finally, it has been suggested by some authors that some people with a BG in the 100–110 mg/dL range do not have any disease and should be considered as part of the normal variation (20).
All the mutations we identified have been previously observed in many children (14, 15). Gly44Ser, Arg191Gln, Gly258Asp, and Thr209Met have previously observed in Italian subjects (14–16). Our results showed that three unrelated families share the same Arg191Gln GCK mutation. Several unrelated Italian families have also been identified with either the Arg191Gln or Arg191Trp GCK mutations, some of which are de novo (14). The increased frequency of mutations at this site is likely because of deamination of the cytosine leading to a C→T transversion, a common mutation mechanism. Mutations such as Arg191Gln cluster within the small domain of GCK, which is thought to be a mutational hot spot (21).
Most series evaluating the prevalence of GCK mutations have studied adult subjects. Recently, Sagen et al. studied adult subjects from a Norwegian registry of MODY subjects and determined that GCK mutations were less frequent than HNF1A mutations (12). However, these authors report adults with an average proband age of 32 yr. The high prevalence of GCK mutations in young children has also been observed in two Italian series (14, 21). These series suggest that the natural history of GCK and HNF1A is different, with decreased penetrance of the latter at young ages and suggest that GCK should be studied first in children with hyperglycemia.
There is significant clinical utility in making a genetic diagnosis of a GCK mutation in hyperglycemic children, providing reassurance of a good long-term prognosis and allowing successful discontinuation of medication highlighted by the two families able to discontinue insulin therapy in their children and three parents able to discontinue oral medications, without any detrimental effect on their metabolic control. Overtreatment of with oral hypoglycemic agents or insulin therapy has been previously reported (12) and may be especially risky for patients with GCK mutations because they have an altered counterregulatory response to hypoglycemia (22). The patients in our series did not benefit from drugs or insulin treatment, as has been previously suggested (12, 18, 23), and have remained stable off their medication.
The patients studied in our series with mild, non-progressive hyperglycemia, represent a group likely to have GCK mutations. The high prevalence of GCK mutations observed in our series emphasizes the concept that the presence of mild fasting non-progressive hyperglycemia should lead to the evaluation of fasting BG levels in other members of their family. In cases of mild familial hyperglycemia, without evidence of autoantibodies, GCK should be the first gene studied (24). If there is a family history of isolated, severe progressive hyperglycemia in the relatives, HNF1A should be also studied with consideration of reflexive testing of HNF4A if there is a strong family history consistent with autosomal dominant inheritance The study of other MODY genes was not recommended by a recent consensus for molecular genetic diagnosis for isolated hyperglycemia (24).
We conclude that the diagnosis of mild persistent hyperglycemia in children or adolescents should lead to the evaluation of fasting BG levels in the other members of their family. If the condition is familial, and no autoimmunity is present, genetic testing of GCK has a high yield for clarifying the diagnosis and avoiding unnecessary and potentially harmful treatment. Those patients without identified GCK mutations should be followed with repeat autoantibody testing, and other types of diabetes should be considered if hyperglycemia worsens.
Acknowledgements
We are grateful to all the families that were willing to participate in this study, to Patricia Lanzano for study coordination, to Dr Rossana Román, Hospital Clínico San Borja-Arriarán, Santiago, Chile, for referring patients, and to Dr Francisco Pérez-Bravo, INTA, University of Chile, for measuring autoantibodies. Sources of support: NIDDK 52431, DK63608 and DK26687.
References
- 1.Bhisitkul DM, Morrow AL, Vinik AI, Shults J, Layland JC, Rohn R. Prevalence of stress hyperglycemia among patients attending a pediatric emergency department. J Pediatr. 1994;124:547–551. doi: 10.1016/s0022-3476(05)83132-4. [DOI] [PubMed] [Google Scholar]
- 2.Steck AK, Eisenbarth GS. Genetic similarities between latent autoimmune diabetes and type 1 and type 2 diabetes. Diabetes. 2008;57:1160–1162. doi: 10.2337/db07-1786. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27 Suppl. 1:S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 4.Lorini R, Alibrandi A, Vitali L, et al. Risk of type 1 diabetes development in children with incidental hyperglycemia: a multicenter Italian study. Diabetes Care. 2001;24:1210–1216. doi: 10.2337/diacare.24.7.1210. [DOI] [PubMed] [Google Scholar]
- 5.Schatz DA, Kowa H, Winter WE, Riley WJ. Natural history of incidental hyperglycemia and glycosuria of childhood. J Pediatr. 1989;115:676–680. doi: 10.1016/s0022-3476(89)80641-9. [DOI] [PubMed] [Google Scholar]
- 6.Stride A, Vaxillaire M, Tuomi T, et al. The genetic abnormality in the beta cell determines the response to an oral glucose load. Diabetologia. 2002;45:427–435. doi: 10.1007/s00125-001-0770-9. [DOI] [PubMed] [Google Scholar]
- 7.Owen K, Hattersley AT. Maturity-onset diabetes of the young: from clinical description to molecular genetic characterization. Best Pract Res Clin Endocrinol Metab. 2001;15:309–323. doi: 10.1053/beem.2001.0148. [DOI] [PubMed] [Google Scholar]
- 8.Codner E, Deng L, Perez-Bravo F, et al. Glucokinase mutations in young children with hyperglycemia. Diabetes Metab Res Rev. 2006;22:348–355. doi: 10.1002/dmrr.622. [DOI] [PubMed] [Google Scholar]
- 9.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 10.Juez G. Intrauterine growth curve for the appropriate diagnosis of intrauterine growth retardation. Rev Med Chil. 1989;117:1311. [PubMed] [Google Scholar]
- 11.Bazaes RA, Salazar TE, Pittaluga E, et al. Glucose and lipid metabolism in small for gestational age infants at 48 hours of age. Pediatrics. 2003;111:804–809. doi: 10.1542/peds.111.4.804. [DOI] [PubMed] [Google Scholar]
- 12.Sagen JV, Bjorkhaug L, Molnes J, et al. Diagnostic screening of MODY2/GCK mutations in the Norwegian MODY Registry. Pediatr Diabetes. 2008;9:442–449. doi: 10.1111/j.1399-5448.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- 13.Frayling TM, Evans JC, Bulman MP, et al. β-cell genes and diabetes: molecular and clinical characterization of mutations in transcription factors. Diabetes. 2001;50 Suppl. 1:S94–S100. doi: 10.2337/diabetes.50.2007.s94. [DOI] [PubMed] [Google Scholar]
- 14.Massa O, Meschi F, Cuesta-Munoz A, et al. High prevalence of glucokinase mutations in Italian children with MODY. Influence on glucose tolerance, first-phase insulin response, insulin sensitivity and BMI. Diabetes Study Group of the Italian Society of Paediatric Endocrinology and Diabetes (SIEDP) Diabetologia. 2001;44:898–905. doi: 10.1007/s001250100530. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani V, Salardi S, Cerreta V, et al. Identification of eight novel glucokinase mutations in Italian children with maturity-onset diabetes of the young. Hum Mutat. 2003;22:338. doi: 10.1002/humu.9179. [DOI] [PubMed] [Google Scholar]
- 16.Gragnoli C, Cockburn BN, Chiaramonte F, et al. Early-onset type II diabetes mellitus in Italian families due to mutations in the genes encoding hepatic nuclear factor 1 alpha and glucokinase. Diabetologia. 2001;44:1326–1329. doi: 10.1007/s001250100644. [DOI] [PubMed] [Google Scholar]
- 17.Herskowitz-Dumont R, Wolfsdorf JI, Jackson RA, Eisenbarth GS. Distinction between transient hyperglycemia and early insulin-dependent diabetes mellitus in childhood: a prospective study of incidence and prognostic factors. J Pediatr. 1993;123:347–354. doi: 10.1016/s0022-3476(05)81731-7. [DOI] [PubMed] [Google Scholar]
- 18.Hattersley A, Bruining J, Shield J, Njolstad P, Donaghue K. ISPAD Clinical Practice Consensus Guidelines 2006–2007. The diagnosis and management of monogenic diabetes in children. Pediatr Diabetes. 2006;7:352–360. doi: 10.1111/j.1399-5448.2006.00217.x. [DOI] [PubMed] [Google Scholar]
- 19.Ellard S, Thomas K, Edghill EL, et al. Partial and whole gene deletion mutations of the GCK and HNF1A genes in maturity-onset diabetes of the young. Diabetologia. 2007;50:2313–2317. doi: 10.1007/s00125-007-0798-6. [DOI] [PubMed] [Google Scholar]
- 20.Schriger DL, Lorber B. Lowering the cut point for impaired fasting glucose: where is the evidence? Where is the logic? Diabetes Care. 2004;27:592–595. doi: 10.2337/diacare.27.2.592. [DOI] [PubMed] [Google Scholar]
- 21.Tinto N, Zagari A, Capuano M, et al. Glucokinase gene mutations: structural and genotype-phenotype analyses in MODY children from South Italy. PLoS ONE. 2008;3:e1870. doi: 10.1371/journal.pone.0001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guenat E, Seematter G, Philippe J, Temler E, Jequier E, Tappy L. Counterregulatory responses to hypoglycemia in patients with glucokinase gene Mutations. Diabetes Metab. 2000;26:377–384. [PubMed] [Google Scholar]
- 23.Schnyder S, Mullis PE, Ellard S, Hattersley AT, Fluck CE. Genetic testing for glucokinase mutations in clinically selected patients with MODY: a worth-while investment. Swiss Med Wkly. 2005;135:352–356. doi: 10.4414/smw.2005.11030. [DOI] [PubMed] [Google Scholar]
- 24.Ellard S, Bellanne-Chantelot C, Hattersley AT. Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia. 2008;51:546–553. doi: 10.1007/s00125-008-0942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]