Abstract
The importance of mitochondria in spinal cord injury has mainly been attributed to their participation in apoptosis at the site of injury. But another aspect of mitochondrial function is the generation of more than 90% of cellular energy in the form of ATP, mediated by the oxidative phosphorylation (OxPhos) process. Cytochrome c oxidase (CcO) is a central OxPhos component and changes in its activity reflect changes in energy demand. A recent study suggests that respiratory muscle function in chronic obstructive pulmonary disease (COPD) patients is compromised via alterations in mitochondrial function. In an animal model of cervical spinal cord hemisection (C2HS) respiratory dysfunction, we have shown that theophylline improves respiratory function. In the present study, we tested the hypothesis that theophylline improves respiratory function at the cellular level via improved mitochondrial function in the C2HS model. We demonstrate that CcO activity was significantly (33%) increased in the spinal cord adjacent to the site of injury (C3–C5), and that administration of theophylline (20 mg/kg 3× daily orally) after C2HS leads to an even more pronounced increase in CcO activity of 62% compared to sham-operated animals. These results are paralleled by a significant increase in cellular ATP levels (51% in the hemidiaphragm ipsilateral to the hemisection). We conclude that C2HS increases energy demand and activates mitochondrial respiration, and that theophylline treatment improves energy levels through activation of the mitochondrial OxPhos process to provide energy for tissue repair and functional recovery after paralysis in the C2HS model.
Keywords: spinal cord injury, respiratory function, cytochrome c oxidase, mitochondria, oxidative phosphorylation, energy
Introduction
Axons arising from motoneurons at the C3–C6 spinal cord level form the phrenic nerve that controls the diaphragm’s motility (Goshgarian et al., 1991; Moreno et al., 1992). Transection of the spinal cord rostral to that level (C3–C6) interrupts inputs deriving from brainstem/pontine sources thus impairing the function of phrenic motoneurons and consequently disturbing the normal activity of the phrenic nerve as a whole. This leads to impaired diaphragm motility and ultimately to dysfunctional respiration (Nantwi et al., 1996; Goshgarian, 2003). At the cellular level spinal cord injury triggers numerous responses including cell death and tissue remodeling. Mitochondria play an important role in these processes because cell death can be executed through the mitochondrial pathway and because tissue repair requires energy, which is mainly provided by the mitochondrial oxidative phosphorylation (OxPhos) process (Exline and Crouser, 2008; Hüttemann et al., 2008a).
Earlier studies demonstrated that mitochondria play an important role in spinal cord injury (SCI) through type II apoptosis. Cell death as a consequence of damage was shown to involve Bax translocation to the mitochondria and cytochrome c release from the mitochondria, followed by caspase 3 and subsequently caspase 9 activation (Martin and Liu, 2002, Springer, et al., 1999, Wu, et al., 2007). Cell death in the damaged area seems to be paralleled by dysfunctional OxPhos as was shown in studies assessing the OxPhos enzyme cytochrome c oxidase (CcO). CcO is the terminal and proposed rate-limiting enzyme of the mitochondrial electron transport chain in intact cells (Villani, et al., 1998; Dalmonte, et al., 2009), and transfers electrons from cytochrome c to molecular oxygen. In a dog model of SCI induced by a weight drop, CcO activity was strongly reduced 15 min after injury in the affected area (Ito, et al., 1978), and a rabbit SCI study showed significantly decreased CcO activities 24 h post injury (Brambilla, et al., 1996). In contrast, indirect injury to spinal motoneurons caused by peripheral nerve avulsion was associated with elevated CcO activity in the degeneration of motoneurons observed in this condition (Martin, et al., 1999). Consequently, decreased CcO activities can negatively impact cellular function. For example, we have recently shown in a rat weight drop model of traumatic brain injury that CcO activity was reduced via nitric oxide, a competitive inhibitor of CcO, produced by the inducible form of nitric oxide synthase (iNOS) after trauma, and that this inhibition was accompanied by significantly reduced ATP levels (Hüttemann, et al., 2008a). In SCI, increased CcO activity was observed in sites adjacent to the injury (Liu, et al., 2001), which may be beneficial for spontaneous regeneration after spinal cord transection, due to increased energy production. Others have shown that in injured spinal cords, which received grafts, CcO upregulation is associated with enhanced vascularization benefiting the retention and preservation of the graft (Horner, et al., 1996). Interestingly, toxic injury to the spinal cord induced by application of dimethyl sulfoxide (DMSO) did not result in changes in CcO activity in the injured animals, but the drug induced upregulation of CcO activity in the control, non-injured spinal cords (Goodnough, et al., 1980).
The above studies suggest that changes in mitochondrial function may be important for SCI and that CcO is a useful marker enzyme for mitochondrial function. Some caution is necessary, however, since the experimental approaches were different, e.g., complete spinal cord transection versus hemisection or contusion, and the use of different species like rats, dogs, and rabbits further complicates the interpretation and comparison of the results.
In the present study, we employed a well-established model cervical (C2) spinal cord hemisection (C2HS) of spinal cord injury to assess CcO activity. The C2HS model is used as an injury model for respiratory-related impairment, and it is clinically relevant to identifying targets in respiratory insufficiency via activation of a latent respiratory motor pathway (Goshgarian, 2003, Moreno, et al., 1992; Nantwi et al., 1996). The model has been employed in many studies to demonstrate that activation of the latent pathway restores respiratory function after paralysis (Nantwi et al., 1996; Golder et al. 2001; Fuller et al., 2005; 2008). However, the effects of injury at the cellular level in terms of energy demands have not been elucidated in the model. In the present study, we therefore assessed changes in CcO activity in the C2HS model and analyzed cellular energy levels. In addition, a pharmacological intervention with theophylline revealed significantly increased CcO activity and increased energy levels. We propose pharmacological improvement of mitochondrial energy metabolism as a target for spinal cord injury therapy in the future.
Materials and methods
Experimental animals
All surgical procedures employed in the present study were conducted strictly in accordance with the guidelines set out by the National Institutes of Health and followed by the Division of Laboratory Animal Research (DLAR) at Wayne State University. Adult female Sprague Dawley rats (250–320 g) were used in this study. Animals were divided into three experimental groups (n=4/group): 1) controls sham-operated, 2) animals subjected to a left C2 hemisection only (C2HS), and 3) C2HS rats that were administered theophylline (C2HST). Two separate groups of control, C2HS and C2HST were employed for histoanalysis of CcO activity and ATP measurements, respectively. Prior to surgery, rats were anesthetized (IP) (ketamine, 70 mg/kg; xylazine 7 mg/kg) and then subjected to a left upper cervical (C2) hemisection as described previously (Goshgarian, 2003, Liou and Goshgarian, 1994). Theophylline was administered by oral gavage (20 mg/kg, 3 × daily) for 3 days. The drug dose and route of administration were based on a previous study showing the drug to be effective in restoring function after C2 hemisection (Nantwi, et al., 2003).
Electrophysiology
Twenty-four hours after hemisection, experimental animals were re-anesthetized as above and prepared for electrophysiologic assessment of respiratory activity in the left and right hemidiaphragm. Briefly, a horizontal incision was made across the abdomen at the base of the rib cage to expose the abdominal surface of the diaphragm of spontaneously-breathing C2 hemisected adult female rats. A pair of sterile platinum electrodes was then inserted into the crural area of the left and right hemidiaphragm to record electromyographic (EMG) activity according to previously described methods (Nantwi et al., 1996). After recording stable levels of activity the recording was stopped, the abdominal incisions closed with fine sutures, and all skin incisions closed with stainless steel wound clips. Respiratory activity in C2HST rats was assessed as previously described (Nantwi et al., 2003).
Diaphragmatic recordings
Diaphragmatic recordings were filtered (bandwidth 0.1–3 kHz), amplified (Tektronix, gain 5–20 K), and displayed on line using a Cambridge Electronic Design (CED) 1401 data acquisition system. The recordings were then fed into a Spike 2 software (CED).
Tissue harvesting
Rats were anesthetized with chloral hydrate (400 mg/kg, IP) and decapitated with a guillotine. Tissue harvesting was done at 3 days post C2HS. The spinal cords at levels C3–C6 were dissected at the region containing the phrenic motoneurons and prepared for cryostat sectioning (Goshgarian, 2003). Three series of 18 µm thick sections through C3–C5 spinal cord levels were collected on poly-L-lysine-slides and stored at −80 °C as previously described (Petrov, et al., 2000). Special care was taken with tissue specimens for ATP analysis. For this experiment, other groups (control, C2HS and C2HST, N=4) of rats were used. Specifically, since ATP turnover is high, the time from sacrificing the animal until storing the tissue samples at −80 °C was kept within 90 sec. Spinal cords were dissected as above and saggitally sectioned into the left (ipsilateral) and right (contralateral) part and immediately frozen in tubes pre-cooled to −80 °C.
CcO histochemistry and quantitative assessment of the histochemical reaction
This assay allows the measurement of CcO activity on tissue sections (not to be confused with immuno-histochemical staining, which measures protein amount), and the signal obtained is directly proportional to CcO activity. Reagents were obtained from Sigma (St. Louis, MO, USA). Tissues were processed according to an established protocol (Hüttemann, et al., 2008a, Wong-Riley, 1979). Briefly, slides containing spinal cord sections (C3–C5) were incubated at room temperature with a reaction buffer (100 mM KH2PO4, pH 7.4, 4% sucrose, 0.50 mg/mL diaminobenzidine (DAB), 200 mg/mL catalase, 0.15 mg/mL cow heart cytochrome c). Incubation was carried out in a moist chamber for 20 min at 37 °C in the dark. The reaction was stopped by washing the slides 3× for 1 min in 100 mM KH2PO4 (pH 7.4). Slides were rinsed once with dH2O, dried and mounted. Images were captured using a Leica Axiophot light microscope as described (Hüttemann, et al., 2008). Images from the ventral horn where the phrenic motoneurons are located (15–20 slides through C3–C5 segments of the spinal cord of each rat) were analyzed with the MetaMorph image analysis system (Fryer, Huntley, IL). The relative intensity of the signal was determined by optical densitometry. The background intensity (in the white matter) was measured for each individual section and was subtracted from the intensity registered in the areas of interest (the ventral horn) to obtain an absolute value as described previously (Hüttemann et al., 2008; Petrov et al., 2000; Steiner et al., 2004). Statistical analysis (one-way ANOVA) was used to determine differences between groups after individual average intensities were pooled for all animals in each experimental group. Significant difference (p < 0.05) between groups was established by the use of the Student-Neuman-Keuls test.
Determination of ATP levels using the bioluminescent method
ATP was released from spinal cords (C3–C5) separately for the left (ipsilateral) and right (contralateral) using the boiling method. Three hundred mL boiling buffer (100 mM Tris-Cl (pH 7.75), 4 mM EDTA) was added and tubes were immediately transferred to a boiling water bath for 2 min. Samples were put on ice and sonicated. ATP concentration was determined with the ATP biolumescence assay kit HS II (Roche) according to the manufacturer's manual. Experiments were performed in triplicates for each specimen (n = 4 animals) and data were standardized to the protein concentration using the DC protein assay kit (Bio-Rad). One-way ANOVA was used to assess the significance of differences between treatment groups. Pair-wise significance between groups is defined as p < 0.05 and was determined with Student’s t-test.
Results
C2 hemisection abolishes respiratory-related activity in the ipsilateral hemidiaphragm
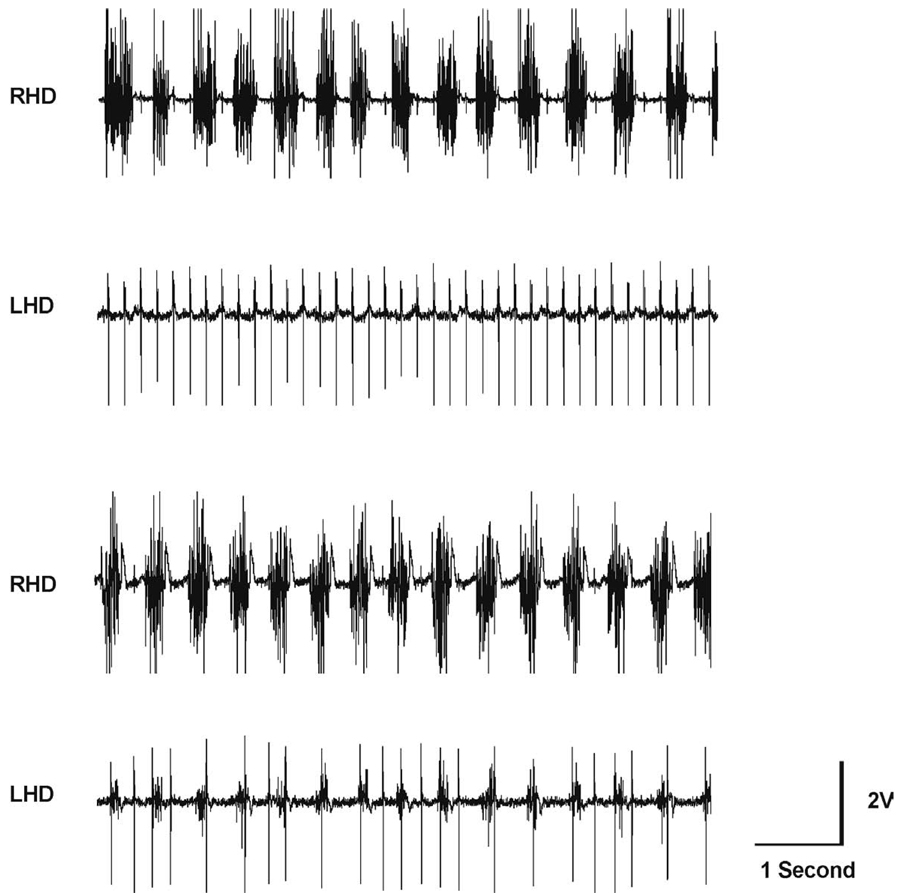
The C2HS model resulted in an absence of EMG activity in the ipsilateral diaphragm as previously described (Nantwi et al., 1996; Fig. 1A, compare the injured left hemidiaphragm (LHD) with the right hemidiaphragm (RHD). In contrast, administration of theophylline for three days post injury partially restores function to the paralyzed hemidiaphragm (compare LHD in Fig. 1B and A). It must be emphasized that electrophysiologic recordings were conducted in rats under spontaneously-breathing conditions. We therefore describe a qualitative aspect of drug-induced functional improvement, which supports our previous quantitative findings of theophylline-induced recovery (Nantwi, et al., 2003a, Nantwi, et al., 2003b, Nantwi, et al., 1996, Nantwi and Goshgarian, 1998a, Nantwi and Goshgarian, 1998b).
Fig. 1.
A set of representative electromyographic (EMG) tracings recorded from an anesthetized and spontaneously-breathing C2HS rat (A). The EMG recordings were taken 24 h after hemisection. The total absence of respiratory-related activity in the left hemidiaphragm (LHD) is indicative of a functionally complete hemisection. In contrast, the right hemidiaphragm (RHD) shows respiratory-related activity. EKG activity is also evident. EMG tracings recorded from the same rat after theophylline treatment (C2HST) (B). Respiratory-related activity in the LHD is now evident, and synchronous with activity in the RHD. Theophylline administration induced recovery of respiratory-related activity in the LHD (compare with A, bottom trace). The amplification setting is at 20,000× in both sets of tracings.
Cytochrome c oxidase activity is increased after C2 hemisection (C2HS), and is further pronounced after theophylline treatment (C2HST)
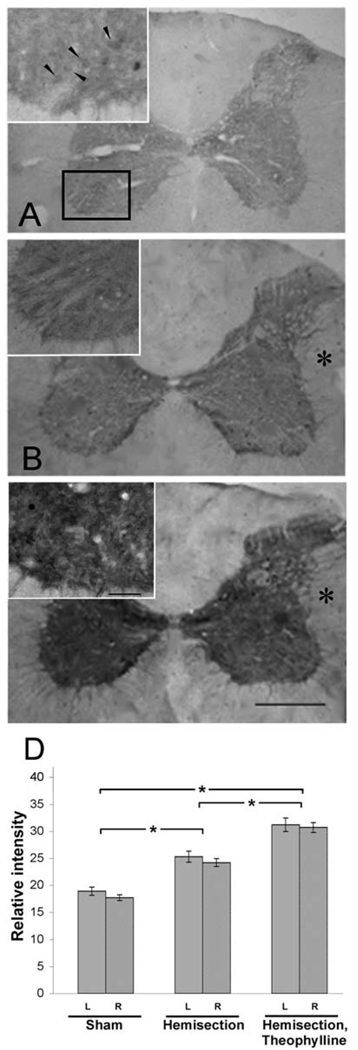
CcO is the terminal enzyme of the electron transport chain (ETC) and its complicated regulation through reversible phosphorylations, allosteric control, and the expression of tissue-specific isoforms (reviewed in Hüttemann, et al., 2008b) support the notion that it is the rate-limiting enzyme of the ETC in intact cells (Villani, et al., 1998; Dalmonte, et al., 2009) and therefore a marker enzyme of OxPhos function. CcO activity was determined on spinal cord section (C3–C5) using the histochemical method. The reaction couples the oxidation of DAB via cytochrome c with CcO activity. The oxidized DAB forms a precipitate, which is then quantitatively analyzed. There was no statistically significant difference in CcO activity between the ipsi- and contralateral sides in control and all experimental paradigms. However, CcO activity was enhanced 3 days after C2HS and even more so in C2HST rats (Fig. 2). More specifically, histochemistry revealed that C2HS leads to 33% increased CcO activity (25.3±3) in comparison to controls (19.1±2.5) on sections through the cervical spinal cord (Fig. 2 A, B, and D). Importantly, CcO activity was increased by 62% (31.0±4.1) in the C2HST experimental group compared to controls (Fig. 2 A, C, and D).
Fig. 2.
Cytochrome c oxidase activity is increased after spinal cord injury. Photomicrographs depicting the CcO histochemical activity in controlnoninjured, (A), C2HS (B), or C2HST rats (C,). Note that the intensity of the COX reactivity gradually increases from A to C. (*) = contralateral to the injury. In higher power photomicrographs (insets) neuronal-like profiles are occasionally observed (arrowheads in A). The rectangle in A encompasses the approximate borders of the area including the phrenic nucleus as shown in the insets.
Quantitation of the intensity of COX histochemical reaction (D). C2HS leads to increased CcO activity (25.3±3) in comparison to controls (19.1±2.5) on sections through the cervical spinal cord (A, B). CcO activity was further increased (31.0±4.1) in the C2HST experimental group (C). Differences (*, on the ordinate) between experiments (on the abscissa) are significant (p < 0.05). Also note the lack of significant difference between the ipsi- and contralateral sides to the injury (gray and striped columns respectively). Bars= 250 and 50 µm for low and high power photomicrographs respectively.
Cellular energy levels after spinal cord injury significantly improve after theophylline treatment
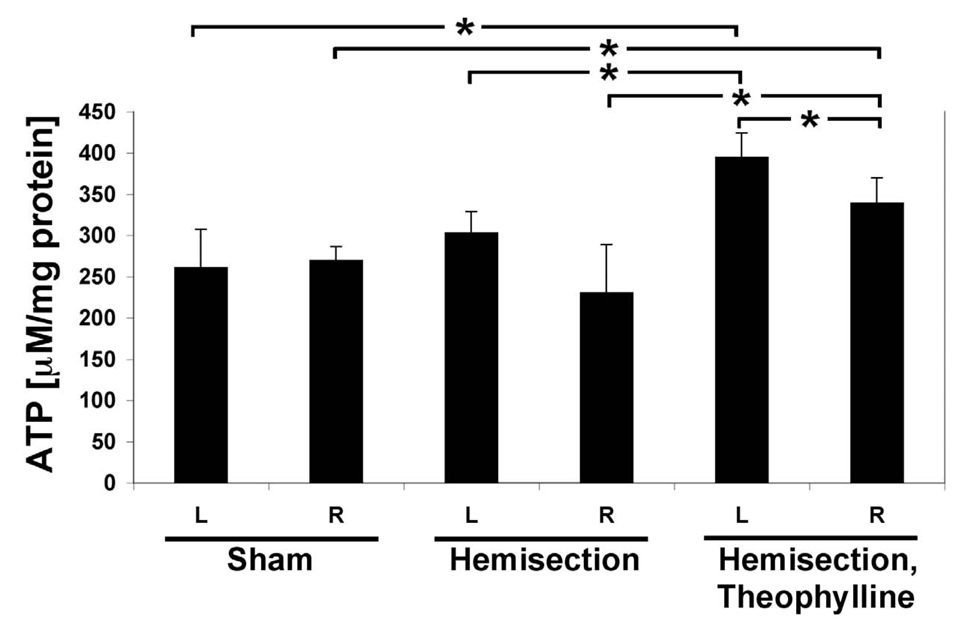
Tissue remodeling after injury is an energy-expensive process. The mitochondrial OxPhos process provides more than 90% of cellular energy in the form of ATP. We analyzed energy levels separately for the left (injured but not including the damaged area) and right cervical spinal cord segments. In the controls there was no difference between the left and the right cervical spinal cord segments (Fig. 3). Interestingly, in the C2HS rats we observed a trend in which ATP levels were on average increased 31% in the left cervical spinal cord segments, i.e., the side that was injured. This trend becomes a significant difference in the C2HST rats with a 17% increase in ATP levels in the left compared to the right hemisphere (Fig. 3). Importantly, theophylline treatment after injury (i.e., C2HST rats) produced a significant 51% increase of ATP levels compared to controls and a significant 31% increase compared to untreated C2HS rats. The increased levels of ATP (particularly in the ipsilateral cervical spinal cord segments) in C2HST rats are important inasmuch as energy demand is increased after injury/trauma. Thus at the cellular level, theophylline appears to act by improving ATP levels.
Fig. 3.
ATP levels increase in C2SH and C2HST rats. ATP concentrations in spinal cords at levels C3–C6 were determined with the bioluminescence method for the left (L) and right (R) cervical spinal cord segments. There is no difference in ATP levels in the left and right side in sham operated animals. C2HS results in ATP levels which are 17% elevated in the left cervical spinal cord segments (not significant, p = 0.24) compared to the control. C2HST results in a significant 51% increase of ATP levels compared to sham-operated animals and a significant 31% increase compared to untreated C2 hemisected rats. The side ipsilateral to injury (L) shows increased ATP levels in C2HS rats, which is significant in the theophylline-treated animals (n = 4 animals, measured in triplicates each; *, p < 0.05).
Discussion
Spinal cord injury affects approximately 12,000 individuals annually excluding deaths, and it is often associated with long term disability. According to the National Spinal Cord Injury Center the leading cause of mortality following SCI is respiratory impairment (National Spinal Cord Injury Center, 2009). Therefore, studies elucidating respiratory deficits after injury are important. The C2HS model of SCI is a good system for such studies. The results from the present study will be discussed in terms of changes in energy demand after injury and how pharmacologic manipulations may improve mechanisms to meet increased energy demand after injury. Understanding the molecular mechanisms that participate in tissue remodeling, repair, and functional recovery after injury may allow an improved targeted treatment in the future.
Mitochondria are increasingly recognized as organelles that play an important role in numerous human diseases including amyotrophic lateral sclerosis and COPD (Boilee et al., 2006). Their pathologic contribution may result from decreased energy production or increased production of reactive oxygen species (ROS). For example, we have previously shown that the inflammatory pathway triggered by TNFα targets CcO for phosphorylation on Tyr304 of catalytic subunit I, which leads to strong enzyme inhibition and dramatically reduced ATP levels (Samavati, et al., 2008). This mechanism might be important in sepsis, which is characterized by poor tissue oxygen extraction and utilization in such patients. In contrast, in a cell culture model for spinal muscular atrophy we observed increased CcO activity as a likely compensatory response to decreased ATP levels, as well as increased ROS, both of which may negatively impact cellular function (Acsadi, et al., 2009). In the present study we used theophylline, which is a non-specific adenosine receptor antagonist, a non-specific phosphodiesterase (PDE) inhibitor, and a respiratory stimulant. We have previously shown that theophylline leads to an inhibition of CcO in liver tissue (Lee, et al., 2005). This contrasts with the findings of this study showing increased CcO activity after theophylline administration (Fig. 2), indicating a tissue-specific difference in cell signaling to the mitochondria. Very little is known about mitochondrial signaling components, and how they affect OxPhos (reviewed in Hüttemann, et al., 2007). However, neuronal tissue has a clearly distinct kinase and phosphatase composition as was demonstrated for PTP-1B, SHP-2, and Src, which were found in mitochondria from rat brain but not in mitochondria from several other tissues including liver (Arachiche, et al., 2008). Interestingly, Src kinase can phosphorylate subunit II, leading to enzyme activation (Miyazaki, et al., 2003), which is one possible scenario explaining increased CcO activity.
In the present study, we did not observe significant differences in CcO activity between the ipsilateral spinal cord segments (C3–C5) and contralateral segments (C3–C5). However, a trend towards greater enhanced activity in ipsilateral segments compared to the contralateral segments was evident. The significance of this observation is unclear at the present time and more work is necessary to identify the molecular mechanism of CcO activation following theophylline application in SCI.
Energy demand is expected to be increased after injury (at the injury site and sites distal to injury), and interventions that up-regulate energy production and enhance the availability of substrates may provide a therapeutic strategy to improve tissue repair and function in SCI. The finding that theophylline enhances ATP levels in the C2HS model are important for a better understanding of cellular mechanisms of respiration from the standpoint that increased ATP levels were more pronounced on the injured side compared to the intact side (contralateral segments). This suggests another therapeutic potential of theophylline and raises the possibility that compounds similar to theophylline may be therapeutically beneficial to alleviate respiratory-related deficits via activation of mitochondria respiration.
Acknowledgements
This work was supported by the Center for Molecular Medicine and Genetics (MH) and NIH grant 35766 (KDN). We thank Dr. Jeffrey Doan (Center for Molecular Medicine and Genetics) for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acsadi G, Lee I, Li X, Khaidakov M, Pecinova A, Parker GC, Hüttemann M. Mitochondrial dysfunction in a neural cell model of spinal muscular atrophy. J Neurosci Res. 2009;87:2748–2756. doi: 10.1002/jnr.22106. [DOI] [PubMed] [Google Scholar]
- 2.Arachiche A, Augereau O, Decossas M, Pertuiset C, Gontier E, Letellier T, Dachary-Prigent J. Localization of PTP-1B, SHP-2, and Src exclusively in rat brain mitochondria and functional consequences. J Biol Chem. 2008;283:24406–24411. doi: 10.1074/jbc.M709217200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boilee S, Velde VC, Cleveland DW. ALS: Disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Brambilla G, Sangiovanni G, Pugliese R, Campagna G, Imberti R, Bernocchi G, Facchetti G, Curti D. Alteration of complex IV and acetylcholine-related enzymes in experimental spinal cord injury. J Neurosurg Sci. 1996;40:213–219. [PubMed] [Google Scholar]
- 5.Dalmonte ME, Forte E, Genova ML, Giuffre A, Sarti P, Lenaz G. Control of respiration by cytochrome c oxidase in intact cells: role of the membrane potential. J Biol Chem. 2009;284:32331–32335. doi: 10.1074/jbc.M109.050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Exline MC, Crouser ED. Mitochondrial mechanisms of sepisis-induced organ failure. Front Biosci. 2008;13:5030–5041. doi: 10.2741/3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal cord hemisection in rats. J Appl. Physiol. 2005;100:800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- 8.Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp. Neurol. 2008:97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golder FJ, Reier PJ, Davenport PW, Bolser DC. Cervical spinal cord injury alters the pattern of breathing in anesthetized rats. J. Appl. Physiol. 2001;91:2451–2458. doi: 10.1152/jappl.2001.91.6.2451. [DOI] [PubMed] [Google Scholar]
- 10.Goodnough J, Allen N, Nesham ME, Clendenon NR. The effect of dimethyl sulfoxide on gray matter injury in experimental spinal cord trauma. Surg Neurol. 1980;13:273–276. [PubMed] [Google Scholar]
- 11.Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- 12.Goshgarian HG, Ellenberger HH, Feldman JL. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei in adult rats: a possible substrate for the crossed phrenic phenomenon. Exp. Neurol. 1991;111:135–139. doi: 10.1016/0014-4886(91)90061-g. [DOI] [PubMed] [Google Scholar]
- 13.Horner PJ, Reier PJ, Stokes BT. Quantitative analysis of vascularization and cytochrome oxidase following fetal transplantation in the contused rat spinal cord. J Comp Neurol. 1996;364:690–703. doi: 10.1002/(SICI)1096-9861(19960122)364:4<690::AID-CNE7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Hüttemann M, Lee I, Kreipke CW, Petrov T. Suppression of the inducible form of nitric oxide synthase prior to traumatic brain injury improves cytochrome c oxidase activity and normalizes cellular energy levels. Neuroscience. 2008;151:148–154. doi: 10.1016/j.neuroscience.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 15.Hüttemann M, Lee I, Pecinova A, Pecina P, Przyklenk K, Doan JW. Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J Bioenerg Biomembr. 2008;40:445–456. doi: 10.1007/s10863-008-9169-3. [DOI] [PubMed] [Google Scholar]
- 16.Hüttemann M, Lee I, Samavati L, Yu H, Doan JW. Regulation of mitochondrial oxidative phosphorylation through cell signaling. Biochim Biophys Acta. 2007;1773:1701–1720. doi: 10.1016/j.bbamcr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Ito T, Allen N, Yashon D. A mitochondrial lesion in experimental spinal cord trauma. J Neurosurg. 1978;48:434–442. doi: 10.3171/jns.1978.48.3.0434. [DOI] [PubMed] [Google Scholar]
- 18.Lee I, Salomon AR, Ficarro S, Mathes I, Lottspeich F, Grossman LI, Hüttemann M. cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J Biol Chem. 2005;280:6094–6100. doi: 10.1074/jbc.M411335200. [DOI] [PubMed] [Google Scholar]
- 19.Liou WW, Goshgarian HG. Quantitative assessment of the effect of chronic phrenicotomy on the induction of the crossed phrenic phenomenon. Exp Neurol. 1994;127:145–153. doi: 10.1006/exnr.1994.1088. [DOI] [PubMed] [Google Scholar]
- 20.Liu YY, Wong-Riley MT, Liu HL, Jia Y, Jiao XY, Wang CT, You SW, Ju G. Increase in cytochrome oxidase activity in regenerating nerve fibers of hemitransected spinal cord in the rat. Neuroreport. 2001;12:3239–3242. doi: 10.1097/00001756-200110290-00019. [DOI] [PubMed] [Google Scholar]
- 21.Martin LJ, Kaiser A, Price AC. Motor neuron degeneration after sciatic nerve avulsion in adult rat evolves with oxidative stress and is apoptosis. J Neurobiol. 1999;40:185–201. [PubMed] [Google Scholar]
- 22.Martin LJ, Liu Z. Injury-induced spinal motor neuron apoptosis is preceded by DNA single-strand breaks and is p53- and Bax-dependent. J Neurobiol. 2002;50:181–197. doi: 10.1002/neu.10026. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki T, Neff L, Tanaka S, Horne WC, Baron R. Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J Cell Biol. 2003;160:709–718. doi: 10.1083/jcb.200209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreno DE, Yu XJ, Goshgarian HG. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol. 1992;116:219–228. doi: 10.1016/0014-4886(92)90001-7. [DOI] [PubMed] [Google Scholar]
- 25.Nantwi KD, Basura GJ, Goshgarian HG. Effects of long-term theophylline exposure on recovery of respiratory function and expression of adenosine A1 mRNA in cervical spinal cord hemisected adult rats. Exp Neurol. 2003a;182:232–239. doi: 10.1016/s0014-4886(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 26.Nantwi KD, El-Bohy A, Goshgarian HG. Actions of systemic theophylline on hemidiaphragmatic recovery in rats following cervical spinal cord hemisection. Exp Neurol. 1996;140:53–59. doi: 10.1006/exnr.1996.0114. [DOI] [PubMed] [Google Scholar]
- 27.Nantwi KD, Goshgarian HG. Effects of chronic systemic theophylline injections on recovery of hemidiaphragmatic function after cervical spinal cord injury in adult rats. Brain Res. 1998a;789:126–129. doi: 10.1016/s0006-8993(98)00024-9. [DOI] [PubMed] [Google Scholar]
- 28.Nantwi KD, Goshgarian HG. Theophylline-induced recovery in a hemidiaphragm paralyzed by hemisection in rats: contribution of adenosine receptors. Neuropharmacology. 1998b;37:113–121. doi: 10.1016/s0028-3908(97)00190-1. [DOI] [PubMed] [Google Scholar]
- 29.Alabama B. National Spinal Cord Injury Statistical Center. Published by The University of Alabama at Birmingham; 2009. [Google Scholar]
- 30.Petrov T, Page AB, Owen CR, Rafols JA. Expression of the inducible nitric oxide synthase in distinct cellular types after traumatic brain injury: an in situ hybridization and immunocytochemical study. Acta Neuropathol (Berl) 2000;100:196–204. doi: 10.1007/s004019900167. [DOI] [PubMed] [Google Scholar]
- 31.Petrov T, Steiner J, Braun B, Rafols JA. Sources of endothelin-1 in hippocampus and cortex following traumatic brain injury. Neuroscience. 2002;115:275–283. doi: 10.1016/s0306-4522(02)00345-7. [DOI] [PubMed] [Google Scholar]
- 32.Samavati L, Lee I, Mathes I, Lottspeich F, Hüttemann M. Tumor necrosis factor α inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J Biol Chem. 2008;283:21134–21144. doi: 10.1074/jbc.M801954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat Med. 1999;5:943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- 34.Steiner J, Rafols D, Park HK, Katar MS, Rafols JA, Petrov T. Attenuation of iNOS mRNA exacerbates hypoperfusion and upregulates endothelin-1 expression in hippocampus and cortex after brain trauma. Nitric Oxide. 2004;10:162–169. doi: 10.1016/j.niox.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Villani G, Greco M, Papa S, Attardi G. Low reserve of cytochrome c oxidase capacity in vivo in the respiratory chain of a variety of human cell types. J Biol Chem. 1998;273:31829–31836. doi: 10.1074/jbc.273.48.31829. [DOI] [PubMed] [Google Scholar]
- 36.Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- 37.Wu KL, Hsu C, Chan JY. Impairment of the mitochondrial respiratory enzyme activity triggers sequential activation of apoptosis-inducing factor-dependent and caspase-dependent signaling pathways to induce apoptosis after spinal cord injury. J Neurochem. 2007;101:1552–1566. doi: 10.1111/j.1471-4159.2006.04445.x. [DOI] [PubMed] [Google Scholar]