Abstract
Nerve grafts are often required to replace tissue damaged by disease, surgery, or extensive trauma. Limitations such as graft availability, donor site morbidity, and immune rejection have led investigators to develop strategies to engineer nerve tissue. The goal of this study was to fabricate a scaffoldless three-dimensional (3D) nerve construct using a co-culture of fetal nerve cells with a fibroblast monolayer and allow the co-culture to remodel into a 3D construct with an external fibroblast layer and an internal core of interconnected neuronal cells. Primary fibroblasts were seeded on laminin-coated plates and allowed to form a confluent monolayer. Neural cells isolated from E-15 spinal cords were seeded on top of the fibroblast monolayer and allowed to form a networked monolayer across the monolayer of fibroblasts. Media shifts initiated contraction of the fibroblast monolayer and a remodeling of the co-culture into a 3D construct held statically in place by the two constraint pins. Immunohisto-chemistry using S100 (Schwann cell), β3-tubulin, DAPI, and collagen I indicated an inner core of nerve cells surrounded by an external layer of fibroblasts. Conduction velocities of the 3D nerve and control (fibroblast-only) constructs were measured in vitro and compared to in vivo measures of neonatal sciatic nerve. The conduction velocities of the nerve constructs were comparable to 24-d-old neonatal nerve. The presence of Schwann cells and the ability to conduct neuronal signals in vitro suggest the scaffoldless 3D nerve constructs will be a viable option for nerve repair.
Keywords: Nerve, Tissue engineering, Fibroblast
Introduction
Nerve transection is the most severe neural injury (Schmidt and Leach 2003). Following transection, the proximal segments of nerves in the peripheral nervous system are capable of regenerating to restore nerve function as long as the defect is less than 3 cm (Rutkowski et al. 2004; Aszmann et al. 2008). Myelin debris is removed, and neurotrophic factors are released by Schwann cells and macrophages that guide the regenerating axons and allow for restored function (Huang et al. 2006). The current “gold standard” for repairing critical nerve defects, greater than a 2-cm defect, in the peripheral nervous system is an autologous nerve graft (Lundborg and Richard 2003; Schmidt and Leach 2003; Meek 2008). However, there are several concerns that arise with nerve grafting. Harvesting of a donor nerve graft can lead to donor site neuroma, loss of function, and scarring (Taras et al. 2005). Concerns also arise over the limited supply of nerve graft donors (Millesi 1990). The functional recovery from this type of procedure is also variable, only approaching 80% on average (Hudson et al. 2000; Tseng et al. 2003).
The drawbacks of autologous nerve grafting have led to an increased focus in neural tissue engineering research. Current research focuses on finding an ideal nerve guidance channel for peripheral nerve repair. Nerve guidance channels help direct regenerating axons with nerve-compatible biomaterials and neurotrophic factors (Hudson et al. 2000). They avoid the need for a second surgery as well as the potential risks involved with nerve grafting, including donor site morbidity (Taras et al. 2005). They also provide additional benefits in that they prevent axon escape and collateral sprouting (Meek 2008). Several requirements must be considered when manufacturing a nerve guidance channel including shape, biocompatibility, wall porosity, degradation rate, mechanical strength, and material electrical conductivity (Huang et al. 2006). The material must be appropriate to allow for the penetration of regenerating axons while not damaging the axons (Balgude et al. 2001). The need for a longitudinal distribution of axons also makes it difficult to design scaffolds for nerve repair (Wang et al. 2009). These requirements place limits on the types of materials that may be used for nerve repair.
Nerve guidance channels are fabricated using synthetic or natural materials, both of which have specific benefits and drawbacks. Synthetic materials are beneficial because they allow for alteration of various properties, including porosity, mechanical strength, and degradation rate (Hudson et al. 2000; Schmidt and Leach 2003). Drawbacks to synthetic materials include biocompatibility, immune rejection, poor cell adhesion, and mediocre tissue repair (Schmidt and Leach 2003). Native tissues are beneficial because they are more biocompatible and less toxic (Hudson et al. 2000; Schmidt and Leach 2003; Taras et al. 2005). However, procurement of native tissues is always a potential problem (Schmidt and Leach 2003). Unfortunately, there are still several complications that may arise with nerve guidance channels regardless of the material used. Most importantly, guidance channels can only currently be used for distances less than 3 cm, and nerve grafts must still be used for large gaps (Meek 2008). Current research in tissue engineering has focused on improving nerve guidance channels to enhance nerve regeneration (Hudson et al. 2000; Wang et al. 2009). Current projects have focused on five main conduit adaptations: (1) porous channel walls (Huang et al. 2006), (2) neurotrophic factor release (Piotrowicz and Shoichet 2006), (3) incorporation of Schwann cells (Galla et al. 2004), (4) aligned intraluminal matrix (Lu et al. 2009), and (5) electrical properties (Bryan et al. 2004). All of these methods are still faced with the drawbacks of using scaffolds for tissue engineering as mentioned above.
Current experiments aimed at creating 3-D nerve constructs mainly focus on the use of agarose gels and other hydrogel scaffolds (Bellamkonda et al. 1995). Hydrogels are attractive for scaffolds due to their biocompatibility (Luo and Shoichet 2004). The addition of extracellular matrix and neurotrophic factors such as laminin and nerve growth factors to hydrogel scaffolds have led to enhanced neurite extension (Yu et al. 1999). The factor-infused hydrogel can be used to fill nerve guidance channels to enhance neurite growth and allow for the development of 3-D nerve repair (Yu et al. 1999). Matrigel ™ has also been shown to promote neural growth but is unappealing due to its tumorogenic origins (Bellamkonda et al. 1995). Collagen and collagen-glycosaminoglycan matrices have also proven successful in peripheral nerve repair (Spilker et al. 2001). Again, all of these experiments still involve scaffolds and all of the problems that are associated with the use of scaffolds.
Our laboratory has the technical ability to fabricate 3-D fibroblast constructs and muscle constructs from a tissue monolayer (Calve et al. 2004; Larkin et al. 2006a, b). Unlike fibroblast and muscle cell monolayers, a nerve monolayer does not contract and remodel into a 3-D construct. Previous work from our lab indicated that neural cells would proliferate and form a network of cells across an established monolayer of muscle (Larkin et al. 2006b). The objective of this research was to use similar technologies to grow a nerve monolayer on an existing fibroblast monolayer so that when the fibroblast monolayer contracts due to passive tension between the fibroblast cells, a 3-D construct with an external fibroblast sheath and an internal core of interconnected nerve cells forms. To accomplish this, nerve cells were seeded on a confluent monolayer of fibroblasts. Once the neural cells had proliferated and migrated across the fibroblast monolayer, the medium was changed to stimulate the fibroblast contraction-induced formation of a 3-D construct resulting in a 3-D fibroblast-nerve construct. The 3-D fibroblast-nerve constructs may eventually be used for surgical repair of nerve transection.
Materials and Methods
Animal model and animal care
Fischer 344 rats (Charles River Laboratories, Wilmington, MA) were used to obtain connective tissue, and E15 prenatal rats from pregnant Fischer 344 rats were used to obtain spinal cords. All animals were acclimated to the light cycle and temperature conditions of our colony for 1 wk before surgery. At day E15, fetuses were Caesarean delivered, and fetal spinal cords were obtained. The animals were killed with an overdose of pentobarbital. All animal care and animal surgery were in accordance with The Guide for Care and Use of Laboratory Animals (Public Health Service 1996, NIH Publication No. 85-23); the experimental protocol was approved by the University of Michigan’s Committee for the Use and Care of Animals.
Preparation of media
All media were stored at 4°C until use and warmed to 37°C in a heated water bath for 20 min before use. Growth medium with ABAM (GMA) consisted of 400 mL HAM F-12 nutrient mixture (Gibco BRL), 100 mL fetal bovine serum (Gibco BRL), and 5 mL antibiotic anti-mycotic (ABAM). Differentiation medium with ABAM (DMA) consisted of 465 mL Dulbecco’s modified Eagle medium (DMEM; Gibco BRL), 35 mL 100% horse serum albumin (Gibco BRL), and 5 mL ABAM. Neural basal medium consisted of 500 mL neural basal medium with 5 mL N2 supplement and 5 ml ABAM.
Preparation of culture dishes
Each 35-mm plate was coated with 1.5 mL of Sylgard (Dow Chemical Corporation, Midland, MI; type 184 silicon elastomer) and cured for 3 wk before use. The plates were then sprayed with 70% ethanol and washed with 3 mL Dulbecco’s phosphate-buffered saline (DPBS; Gibco, BRL). The DPBS was aspirated off, and then the plates were coated with a solution of 3 mL DPBS with 20 μg of laminin. The plates were left to dry overnight in a biosafety containment cabinet then rinsed again with 2 mL DPBS for 5 min. Each plate was then covered with 1 mL GMA and decontaminated with UV light for 1 h. The plates were stored in a 37°C, 5% carbon dioxide incubator for 1 d before plating the tendon cells.
Preparation of-self organized fibroblast monolayer
Achilles tendons were surgically removed from Fischer 344 retired breeder rats (Charles River Laboratories, Durham, NC). Tendon fibroblasts were isolated from the Achilles tendon by dissociating the tendons in 1 mg/mL type II collagenase (Worthington Biochemical, Lakewood, NJ) in DMEM plus 2% ABAM. The cells were placed in the 37°C incubator overnight to break down the extra cellular matrix. The cell solution was then centrifuged at 100 g for 5 min, and the supernatant was aspirated off just above the cell pellet. The cells were resuspended in 8 mL of GMA and plated on 100-mm-diameter tissue culture dishes. The media on the plate were changed every 48 h and at 90% confluence, the cells were passaged on to two new 100-mm plates.
After two to three passages, the cells were treated with 1 mL of 0.25% trypsin and placed on the cell shaker for 20 min. The cells were then plated at a density of 2.0×105 cells per plate with GMA and 3 mg of L-ascorbic-acid-2 phosphate (Sigma-Aldrich, St Louis, MO) on to the 35-mm laminin-coated plates. Fresh media consisting of 1.5 mL GMA with 0.2 mg ascorbic acid and 3 mg TGFβ were added to each plate every 48 h. When the plates were at 90% confluence, the medium was removed, and 1.5 ml of nerve cells suspended in neural basal medium (1.0×105 cells) were added to the plates.
Isolation of nerve cells
Spinal cords from E-15 fetal rats were surgically removed and placed in a 100-mm dish with HAM F-12 nutrient mixture (Gibco BRL). The spinal cords were collectively rinsed with fresh Ham F-12 and placed in a new 100-mm dish with 8 mL Ham F-12 nutrient mixture (Gibco BRL). The spinal cords were then cut into approximately 1–2 mm long pieces, placed in a 50-mL conical with 1 mL 0.25% trypsin and 10 mL Ham F-12 nutrient mixture (Gibco BRL), and placed in the 37°C hot water bath for 20 min. A 100-mm cell strainer was used to strain the spinal cord mixture, and the strained spinal cord pieces were discarded. An additional 0.5 mL bovine serum albumin 4% weight volume was added to the nerve cell suspension, and the solution was then centrifuged at 1,500 RPM for 10 min. The supernatant was aspirated off just above the cell pellet, and 5 mL NBM was added to the conical. The cell suspension was mixed thoroughly, cell density determined, and the volume of NBM was adjusted to achieve a final plating density of 1.0×105 cells per 1.5 ml. Neural cells were then seeded on to the 35-mm plates containing the fibroblast monolayer, replacing the GMA medium currently on the fibroblast plates. The plates were stored in the 37°C incubator, and NBM medium was changed every 48 h.
Formation of 3-D nerve-fibroblast construct
Once the nerve cells had proliferated and migrated to create a network that covered approximately 70–80% of the plate, the medium was switched from NBM to 1.5 mL DMA with 0.2 mg ascorbic acid and 3.0 mg TGFβ. Each plate was pinned with two sets of constraint pins at 12 and 14 mm apart. The medium was replaced every 48 h. Construct formation was usually complete after approximately 4–5 d on DMA.
Histology
Immunofluorescent staining was performed to detect the presence of Collagen I, S100, β3-tubulin, and double-stranded DNA. The fibroblast-nerve monolayer was rinsed three times for 10 min each with DPBS then incubated 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) for 1 h. Each plate was rinsed twice with DPBS for 10 min then incubated with 0.5% Triton X100 (Sigma, St. Louis, MO) for 10 min. The plates were then rinsed two times for 10 min each with DPBS and then blocked with DPBS containing 5% donkey serum (DS; Jackson ImmunoResearch Labs, Inc, West Grove, PA) for 30 min. The plates were then incubated for 2 h in collagen I (from goat host; 1:200) and S100 or β3-tubulin (from rabbit host; 1:200) in DPBS containing 1% DS. After three additional rinses with DPBS, the plates were incubated with both Cy-3-conjugated AffiniPure donkey anti-goat IgG and fluorescein isothiocyanate-conjugated AffiniPure donkey anti-rabbit IgG (1:500 dilution; Jackson ImmunoResearch Labs, Inc, West Grove, PA) for 1 h. Each plate was then incubated with DAPI (1:500 dilution; Sigma, St. Louis, MO) for 10 min then rinsed a final time with DPBS. A Leica DMIRB inverted microscope fitted with an Olympus DP-30 high sensitivity grayscale CCD camera was used to image the constructs. Images were viewed at either ×50 or ×100.
Nerve conduction velocity
Sciatic nerve conduction velocities of neonatal rats ranging from 1 to 4 wk of age were measured following the Karagoz et al. (2009) method. Briefly, the sciatic nerve was isolated and exposed. The stimulator of a Viasys TECA Synergy N2 EMG machine was placed proximal to the sciatic nerve. The recording and reference wires were placed in the gastrocnemius muscle. The nerve was stimulated with EMG settings of 0.3 mA and conduction velocities were measured in triplicate at 23°C.
Conduction velocities were measured for both the nerveonly construct and the control (fibroblast-only) construct within 1 wk of construct formation. Medium was replaced with DPBS (Gibco BRL) to limit background signal. The stimulator was placed under one end of the construct and the recording and reference wires under the other. A ground wire was placed in the dish. Approximately 20 mm away from the construct, nerve conduction velocities were measured in triplicate at 23°C.
Results
Our laboratory has successfully engineered 3-D nervefibroblast constructs from rat fibroblasts and fetal nerve cells. After approximately 3 d, the fibroblast monolayer became 80–90% confluent, and nerve cells suspended in NBM were added to the plates. After approximately 10 d of co-culture in NBM, the nerve cells reached 70–80% confluence across the fibroblast monolayer. At this point, the medium was switched to DMA containing ascorbic acid and TGFb. After approximately 4 d on DMA, the monolayer contracted around the constraint pins and formed a 3-D configuration. Immunohistochemistry showed an inner core of nerve cells surrounded by an external layer of fibroblasts.
The timeline and methodology chosen resulted from several experiments that studied the influence of various media and co-culturing techniques on the proliferation of nerve and fibroblast cells in co-culture. Experiments were conducted to test the viability of fibroblasts and nerve cells in NBM, GMA, and 50/50 mixtures of NBM and GMA. Experiments were also conducted to investigate the effect of the timing of the seeding of the nerve cells with the fibroblasts. Thus, the two cell types were seeded simultaneously versus allowing the fibroblasts to become confluent before the addition of nerve cells. It was found that when fibroblasts and nerve cells were plated simultaneously, the fibroblasts would dominate the plate, and the nerve cells were out competed for space on the plate and did not have a chance to proliferate. This brought us to the conclusion that the fibroblasts would need to form a monolayer before the nerve cells were added to the plates if we wanted a fibroblast exterior and neural interior. We also found that fibroblasts would appear to become dormant on NBM, neither proliferating nor dying, and grow slower on a mixture of 50% GMA/50% NBM compared to GMA (Fig. 1). Once these cells were switched back to GMA, the fibroblasts immediately began proliferating once again (Fig. 1D). We hypothesized that this occurs due to the lack of serum in the NBM. Addition of serum to NBM allowed the fibroblasts to proliferate and form a confluent monolayer (image looked just like cells in 1D). This provided us with the insight that a fibroblast monolayer may be able to survive and be held in a static state (not contract into a 3-D construct) on NBM while the nerve cells had a chance to grow. Medium studies also showed that the nerve cells did not grow as well on GMA or a 50/50 mixture of GMA and NBM as opposed to NBM only. We therefore concluded that the best protocol would include developing a monolayer of fibroblasts on GMA prior to the addition of NBM. The nerve cells were seeded onto the fibroblast monolayer suspended in NBM and allowed to form a network on top of the fibroblast monolayer before switching to DMA to promote the growth of fibroblasts once again (Fig. 2). With the growth of fibroblasts, the monolayer contracted around the constraint pins effectively forming a core of nerve cells surrounded by an exterior of fibroblasts (Fig. 3). These images show the collagen sheath, stained with Collagen I, surrounding neural cell bodies, stained with S100 or β3-tubulin, running along the interior.
Figure 1.

The effect of media on the formation of a confluent monolayer of fibroblasts. Fibroblasts were seeded a density of 1×105 cells per plate and viewed 7 d after proliferation of laminin-coated sylgaard plated and fed with (A) GMA, (B) 50% GMA/50% NBM, (C) NBM, and (D) NBM for 5 d then GMA. Images were viewed at ×50.
Figure 2.

The effect of co-culturing E-15 neural cells on an established monolayer of fibroblasts. (A) Neural cells on fibroblast monolayer after 3 d on neural basal medium, (B) neural cells on fibroblast monolayer stained with S100 and DAPI, and (C) neural cells on fibroblast monolayer after 7 d on neural basal medium formed a confluent network. Notice the decreased presence of fibroblasts. Images A and B are viewed at ×100. Image C was viewed at ×50.
Figure 3.
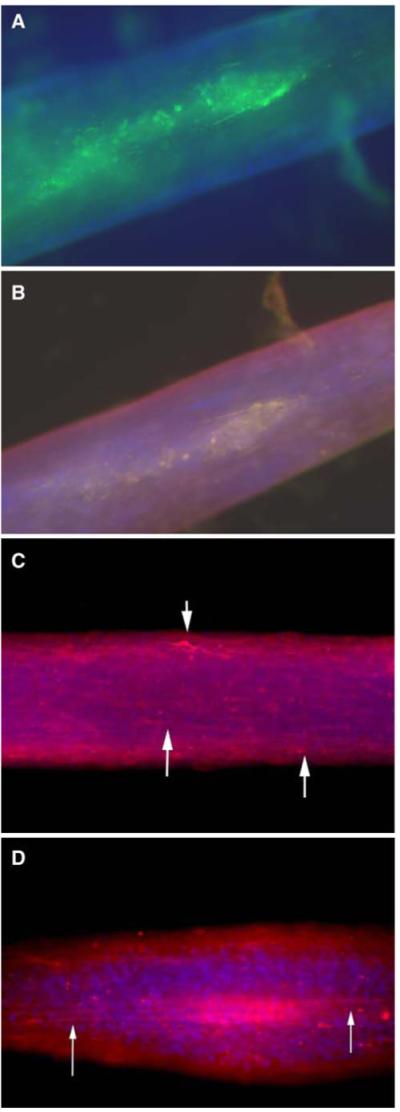
Immunohistological characterization of 3-D nerve-fibroblast construct stained with (A) S100 and DAPI, (B) S100, DAPI, and collagen 1, and (C, D) β3-tubulin and DAPI. Images viewed at ×50.
The nerve conduction velocities increased with age in the neonatal sciatic nerve, ranging from 2.21±0.12 m/s at 1 wk to 15.30±0.27 m/s at 4 wk (Fig. 4). The engineered scaffoldless 3-D nerve construct was equivalent to that observed in the sciatic nerve of the 4-wk-old neonatal rat and approximately 50% of that observed in the 12-wk-old adult sciatic nerve, 12.50±0.13 and 21.24±0.77 m/s, respectively. In contrast, the fibroblast-only construct reached a velocity of 2.38±0.16 m/s, comparable to a 1 wk neonatal sciatic nerve.
Figure 4.

Nerve conduction velocities of native sciatic nerve ranging from 1 to 4 wk old compared to measures taken of adult (12 wk old) native sciatic nerve and in vivo measures of nerve-only constructs and fibroblast-only constructs. Data represent mean ± standard deviations.
Discussion
The purpose of this study was to co-culture fetal nerve cells with an existing fibroblast monolayer and allow the coculture to form a 3-D construct with an external fibroblast layer and an internal core of interconnected nerve cells. Immunohistochemistry indicates that a 3-D nerve construct with a fibroblast exterior was successfully engineered. Such constructs avoid the complications found with scaffolds such as immune rejection and degradation rate issues, as well as those associated with grafting such as availability and donor site morbidity. They are also unique in that they have a fibroblast sheath consisting of Collagen I similar to that found in native peripheral nerve tissue (Bunge et al. 1989). This scaffoldless 3-D nerve-fibroblast construct may show potential for replacement of damaged nerve tissue.
Current technologies related to neural tissue engineering focus on using nerve guidance channels for the repair of peripheral nerves. These guidance channels may be incorporated with Schwann cells and/or neurotrophic factors for the enhanced growth of nerve cells. These experiments have found success with peripheral nerve growth but are faced with several drawbacks associated with the use of scaffolds, including biocompatibility, immune rejection, poor cell adhesion, and mediocre tissue repair. The experiments conducted here present the technology for the development of a scaffoldless 3-D nervefibroblast construct that will avoid the drawbacks associated with scaffolds.
The ability of the engineered scaffoldless 3-D nerve construct to conduct an electrical signal may make it advantageous for nerve repair. The conductive property of the construct may enhance the restoration of the electrical pathways to the target tissue. One might assume that the conduction velocity of the construct will improve following in vivo implantation in a nerve repair model.
In conclusion, future studies will include using electrophysiological techniques to test the conduction of electrical impulses along the 3-D construct and the usefulness of these constructs for surgical repair. Comparisons of the nerve conduction velocity along the engineered constructs will be compared to that along the sciatic nerve in vivo and in vitro. Studies will also be conducted to examine co-cultures of engineered nerve constructs with muscle monolayers to develop 3-D nerve-fibroblast-muscle constructs.
Acknowledgments
This research was supported by the NIH through a NIAMS and NIBIB funded grant (R01 AR054778-02) and by a gift from the Barbara and Richard Raynor Medical Foundation Award.
Contributor Information
Jennifer Baltich, Biomedical Engineering, University of Michigan, Room #2025 Biomedical Science Research Building (BSRB), 109 Zina Pitcher Place, Ann Arbor, MI 48109-2200, USA.
Leah Hatch-Vallier, Biomedical Engineering, University of Michigan, Room #2025 Biomedical Science Research Building (BSRB), 109 Zina Pitcher Place, Ann Arbor, MI 48109-2200, USA.
Aaron M. Adams, Molecular and Integrated Physiology, University of Michigan, Room #2025 Biomedical Science Research Building (BSRB), 109 Zina Pitcher Place, Ann Arbor, MI 48109-2200, USA
Ellen M. Arruda, Mechanical Engineering, University of Michigan, 3126 GGB, Ann Arbor, MI 48109-2125, USA; Macromolecular Science and Engineering, University of Michigan, 3126 GGB, Ann Arbor, MI 48109-2125, USA
Lisa M. Larkin, Biomedical Engineering, University of Michigan, Room #2025 Biomedical Science Research Building (BSRB), 109 Zina Pitcher Place, Ann Arbor, MI 48109-2200, USA; Molecular and Integrated Physiology, University of Michigan, Room #2025 Biomedical Science Research Building (BSRB), 109 Zina Pitcher Place, Ann Arbor, MI 48109-2200, USA
References
- Aszmann OC, et al. Bridging critical nerve defects through an acellular homograft seeded with autologous schwann cells obtained from a regeneration neuroma of the proximal stump. J Reconstr Microsurg. 2008;24(3):151–158. doi: 10.1055/s-2008-1076091. [DOI] [PubMed] [Google Scholar]
- Balgude AP, et al. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22(10):1077–1084. doi: 10.1016/s0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- Bellamkonda R, et al. Hydrogel-based three-dimensional matrix for neural cells. J Biomed Mater Res. 1995;29(5):663–671. doi: 10.1002/jbm.820290514. [DOI] [PubMed] [Google Scholar]
- Bryan DJ, et al. Enhanced peripheral nerve regeneration through a poled bioresorbable poly(lactic-co-glycolic acid) guidance channel. J Neural Eng. 2004;1(2):91–98. doi: 10.1088/1741-2560/1/2/004. [DOI] [PubMed] [Google Scholar]
- Bunge MB, et al. Role of peripheral nerve extracellular matrix in Schwann cell function and in neurite regeneration. Dev. Neurosci. 1989;11(4–5):348–360. doi: 10.1159/000111911. [DOI] [PubMed] [Google Scholar]
- Calve S, et al. Engineering of functional tendon. Tissue Eng. 2004;10(5–6):755–761. doi: 10.1089/1076327041348464. [DOI] [PubMed] [Google Scholar]
- Galla TJ, et al. Fibrin/Schwann cell matrix in poly-epsilon-caprolactone conduits enhances guided nerve regeneration. Int. J. Artif. Organs. 2004;27(2):127–136. doi: 10.1177/039139880402700208. [DOI] [PubMed] [Google Scholar]
- Huang M, et al. Preparation of chitosan derivative with polyethylene glycol side chains for porous structure without specific processing technique. Int. J. Biol. Macromol. 2006;38(3–5):191–196. doi: 10.1016/j.ijbiomac.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Hudson TW, Evans GR, Schmidt CE. Engineering strategies for peripheral nerve repair. Orthop. Clin. North Am. 2000;31(3):485–498. doi: 10.1016/s0030-5898(05)70166-8. [DOI] [PubMed] [Google Scholar]
- Karagoz H, et al. Comparison of regeneration results of prefabricated nerve graft, autogenous nerve graft, and vein graft in repair of nerve defects. Microsurgery. 2009;29(2):138–143. doi: 10.1002/micr.20586. [DOI] [PubMed] [Google Scholar]
- Larkin LM, et al. Structure and functional evaluation of tendon-skeletal muscle constructs engineered in vitro. Tissue Eng. 2006a;12(11):3149–3158. doi: 10.1089/ten.2006.12.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin LM, et al. Functional evaluation of nerve-skeletal muscle constructs engineered in vitro. In Vitro Cell. Dev. Biol. Anim. 2006b;42(3–4):75–82. doi: 10.1290/0509064.1. [DOI] [PubMed] [Google Scholar]
- Lu MC, et al. Evaluation of a multi-layer microbraided polylactic acid fiber-reinforced conduit for peripheral nerve regeneration. J Mater Sci Mater Med. 2009;20(5):1175–1180. doi: 10.1007/s10856-008-3646-4. [DOI] [PubMed] [Google Scholar]
- Lundborg G, Richard P. Bunge memorial lecture. Nerve injury and repair—a challenge to the plastic brain. J Peripher Nerv Syst. 2003;8(4):209–226. doi: 10.1111/j.1085-9489.2003.03027.x. [DOI] [PubMed] [Google Scholar]
- Luo Y, Shoichet MS. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat Maters. 2004;3(4):249–253. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- Meek M. Photochemical tissue bonding: a promising technique for peripheral nerve repair. J Surg Res. 2008;149(2):169–170. doi: 10.1016/j.jss.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Millesi H. Peripheral nerve surgery today: turning point or continuous development? J Hand Surg Br. 1990;15(3):281–287. doi: 10.1016/0266-7681_90_90004-n. [DOI] [PubMed] [Google Scholar]
- Piotrowicz A, Shoichet MS. Nerve guidance channels as drug delivery vehicles. Biomaterials. 2006;27(9):2018–2027. doi: 10.1016/j.biomaterials.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Rutkowski GE, et al. Synergistic effects of micropatterned biodegradable conduits and Schwann cells on sciatic nerve regeneration. J Neural Eng. 2004;1(3):151–157. doi: 10.1088/1741-2560/1/3/004. [DOI] [PubMed] [Google Scholar]
- Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- Spilker MH, et al. Contraction of collagen-glycosaminoglycan matrices by peripheral nerve cells in vitro. Biomaterials. 2001;22(10):1085–1093. doi: 10.1016/s0142-9612(00)00345-8. [DOI] [PubMed] [Google Scholar]
- Taras JS, Nanavati V, Steelman P. Nerve conduits. J Hand Ther. 2005;18(2):191–197. doi: 10.1197/j.jht.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Tseng CY, et al. Histologic analysis of Schwann cell migration and peripheral nerve regeneration in the autogenous venous nerve conduit (AVNC) J Reconstr Microsurg. 2003;19(5):331–340. doi: 10.1055/s-2003-42502. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. Interaction of olfactory ensheathing cells with nerve repairing scaffolds. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2009;34(5):382–387. [PubMed] [Google Scholar]
- Yu X, Dillon GP, Bellamkonda RB. A laminin and nerve growth factor-laden three-dimensional scaffold for enhanced neurite extension. Tissue Eng. 1999;5(4):291–304. doi: 10.1089/ten.1999.5.291. [DOI] [PubMed] [Google Scholar]


