Summary
This study compared two schedules of low-dose gemtuzumab ozogamicin (GO) as induction monotherapy for untreated acute myeloid leukemia in older patients unfit for intensive chemotherapy, to identify the more promising regimen for further study. Patients were randomized to receive either best supportive care or a course of GO according to one of two schedules: 3 mg/m2 on days 1, 3 and 5 (arm A), or GO 6 mg/m2 on day 1 and 3 mg/m2 on day 8 (arm B). Primary endpoint was the rate of disease non-progression (DnP), defined as the proportion of patients either achieving a response or maintaining a stable disease following GO induction in each arm. Fifty-six patients were randomized in the two GO arms (A, n=29; B, n=27). The rate of DnP was 38% (90% confidence interval [CI], 23%–55%) in arm A, and 63% (90% CI, 45%–78%) in arm B. Peripheral cytopenias were the most common adverse events for both regimens. The all-cause early mortality rate was 14% in arm A and 11% in arm B. The day 1+8 schedule, which was associated with the highest rate of DnP, met the statistical criteria to be selected as the preferred regimen for phase III comparison with best supportive care.
Keywords: acute myeloid leukemia, gemtuzumab ozogamicin, targeted therapy, elderly, supportive care
The treatment of acute myeloid leukemia (AML) in the elderly is a difficult challenge (Estey, 2009). While progress has been made over the last three decades in younger adults, the same has not occurred in the older population. Most elderly patients, particularly those over the age of 75 years and those with comorbidities, are deemed unsuitable for standard chemotherapy, but palliative treatment offers a limited survival of about 3 months (Appelbaum et al, 2006; Menzin et al, 2002). There is therefore an urgent need to find innovative treatments for this patient subgroup who are traditionally not catered for in most clinical trials.
Gemtuzumab ozogamicin (GO) is a humanized IgG4 anti-CD33 monoclonal antibody conjugated to calicheamicin, a potent antitumour antibiotic (Stasi et al, 2008). The immunoconjugate binds to the CD33 antigen typically expressed on the surface of AML cells. The toxin is then internalized causing DNA strand breaks leading to cell death. When used as single agent, GO has shown significant antileukemic activity in older patients with relapsed AML (Larson et al, 2005; Sievers et al, 2001). On the other hand, results in unselected older patients with newly diagnosed AML have been rather disappointing. In particular, we have previously reported a complete response rate of only 17% when the licensed dose/schedule of GO (9 mg/m2 on days 1 and 15) was used as frontline monotherapy for older unfit patients (Amadori et al, 2005). Excessive hematological and liver toxicity, particularly in patients over 75 years of age, suggested that dosing and scheduling changes were needed to improve feasibility. In this regard, a recent French study suggested that the fractionated dosing of a reduced total dose of GO (9 mg/m2 in three fractions for a single course) had similar efficacy but a better safety profile in patients with relapsed AML compared to the results reported in the pivotal phase II trials, and may represent a valuable alternative for frailer patients (Taksin et al, 2007). Based on these experiences, the European Organisation for Research and Treatment of Cancer- Gruppo Italiano Malattie Ematologiche dell’Adulto (EORTC-GIMEMA) intergroup designed a sequential randomized phase II/III trial (AML-19) for newly diagnosed AML in older patients not considered suitable for an intensive treatment approach. Two different schedules of low-dose GO induction monotherapy were investigated in the initial phase II part of the study. The schedule with the more favorable efficacy profile will be selected for further phase III comparison with best supportive care (BSC). This report describes the final results of the randomized phase II part of the trial, which have guided the choice of the preferred regimen for full-scale phase III evaluation.
Patients and methods
Study design
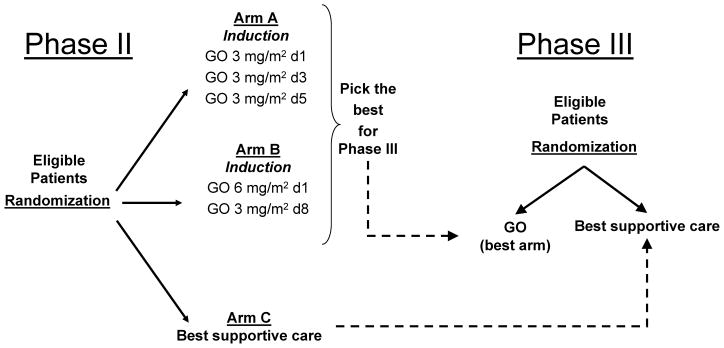
AML-19 is an open label, randomized, multicenter trial with a sequential phase II–III design (Fig 1). The main objective of the initial phase II stage was to determine which of the two schedules of low-dose GO induction monotherapy was more promising to continue phase III comparison with BSC in the study population. A third arm offering BSC only was also included in the initial randomization, but the patients entered onto this arm will only be used for comparative evaluation against the selected GO regimen in the subsequent phase III portion of the study, and will not be further analyzed in this report. The primary endpoint of the phase II study was the rate of disease non-progression (DnP), defined as the proportion of patients either achieving a clinical response or maintaining a stable disease (SD) following GO induction in each experimental arm. Secondary endpoints included the estimation of the complete response rates as well as toxicity for the two GO schedules under evaluation. As the phase II study was not powered to detect differences in overall and progression-free survival between the randomized arms, such information will only be provided in the context of the subsequent phase III part of the study. The primary objective of the ongoing phase III stage is to assess the effect on overall survival of the selected best schedule of GO monotherapy compared to BSC, and for this purpose patients from the phase II GO selected arm will also be included in the comparative analysis.
Fig 1.
AML-19 trial design. The study integrates an initial phase II with a sequential phase III stage. Aim of the phase II was to identify the more promising schedule of GO induction monotherapy to be compared with best supportive care in the following phase III stage. See text for details.
The final protocol was approved by the EORTC Protocol Review Committee and by the Ethical Committee of each participating centre. Written, informed consent according to national/local regulations was obtained from each patient before study entry. This trial was registered with clinicaltrials.gov under identifier NCT00091234.
Eligibility
Patients were eligible for the study if they had previously untreated de novo or secondary AML as defined by the World Health Organization (WHO) classification (Vardiman et al, 2002), and were not considered candidates for intensive chemotherapy. This included all patients over the age of 75 years, as well as those of 61–75 years with a WHO performance score of greater than 2 or unwilling to receive intensive chemotherapy. Patients had to have adequate renal and hepatic function, and the white blood cell (WBC) count had to be less than 30×109/l at the time of registration (cytoreduction with hydroxycarbamide for up to 14 days was permitted). Exclusion criteria included acute promyelocytic leukemia, central nervous system leukemia, blast crisis of chronic myeloid leukemia or AML developing after other myeloproliferative disorders, concomitant malignant disease, severe cardiac or pulmonary dysfunction, active uncontrolled infection, and human immunodeficiency virus (HIV) positivity.
Treatment plan
On entry, patients were allocated randomly to receive a single course of GO in 1 of 2 investigational schedules (arm A and B), or BSC (arm C). While the planned total dose of GO (Mylotarg, Wyeth Pharmaceuticals, Collegeville, PA, USA) was the same for all patients (9 mg/m2), patients in arm A received GO at a dose of 3 mg/m2 on days 1, 3 and 5 (hyperfractionated schedule); those in arm B received GO at a dose of 6 mg/m2 on day 1 and 3 mg/m2 on day 8 (condensed schedule). Each dose of GO was administered intravenously over 2 h following premedication with steroids to prevent allergic reactions. Patients with SD or better response following GO induction, then received continuation treatment with monthly outpatient infusions of GO at 2 mg/m2 for a maximum of 8 months in the absence of disease progression/relapse or unacceptable toxicity. Policies with regard to blood product support, prophylaxis and treatment of febrile neutropenia were determined by institutional guidelines, but the use of growth factors was not permitted. Patients assigned to arm C received supportive care only (blood components, antimicrobials, palliative chemotherapy with hydroxycarbamide or other cytostatics to control the leucocyte count) according to the local policy, and as outpatients whenever possible.
Assessment of toxicity and response
Patients were monitored with physical examination, complete blood count and chemistry profile at least twice weekly during induction, and then at least every 2 weeks as long as they received GO continuation therapy. Response to treatment was assessed by bi-weekly peripheral blood examinations, and by bone marrow aspiration on study day +36. Standard criteria were used to define clinical response as previously reported (Amadori et al, 2005). Complete remission (CR) required a normal bone marrow aspirate with <5% blasts and no Auer rods, absence of circulating blasts, no evidence of extramedullary leukemia, and regeneration of the peripheral neutrophil count to 1 × 109/l and the platelet count to 100 × 109/l. Complete remission with incomplete platelet recovery (CRp) was defined as for CR, but with transfusion-independent platelet counts remaining below 100 × 109/l. Partial remission (PR) was similar to CR except for the persistence of 5–10% marrow blasts (or <5% if Auer rod positive). Progressive disease (PD) was defined as any of the following: new appearance of >5% circulating blasts; >25% increase in the absolute number of circulating blasts from baseline; development of extramedullary leukemia. SD (stable disease) was defined as any response not meeting CR, CRp, PR or PD criteria. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria version 3.0 (http://ctep.cancer.gov/reporting/ctc.html).
Laboratory investigations
Diagnostic bone marrow smears were reviewed centrally according to the standardized procedures of the EORTC-GIMEMA intergroup. Cytogenetic studies on pretreatment bone marrow were also reviewed centrally. Cytogenetic abnormalities were grouped according to published EORTC criteria as favorable, intermediate, unfavorable, other, or unknown (Amadori et al, 2005). CD33 expression by the leukemic blasts was evaluated at diagnosis in all patients with adequate samples of bone marrow aspirate by standard flow cytometry.
Statistical methods
Patient registration and data collection were managed by the EORTC Headquarters. Randomization was computer generated using minimization to ensure balance overall and within the following stratification parameters: age (61–75 years, 76–80 years, and ≥81 years), CD33-positivity of bone marrow blasts (<20%, 20–80%, >80%, unknown), WBC count at diagnosis (<30×109/l, ≥30×109/l), WHO performance status (0–1, 2, 3–4), and participating centre. The sample size of the phase II study was calculated based on the primary endpoint of DnP rate using a Fleming 1-stage design. This design called for a total of 50 patients to be randomized with equal allocation to each of the 2 GO arms, with 25 additional patients assigned to the control arm (BSC). The primary goal was to test within each of the 2 experimental arms the null hypothesis that the DnP rate would be ≤35% versus the alternative hypothesis that the DnP rate would be ≥60%. If ≤11 patients had evidence of DnP, then the null hypothesis would be accepted. If ≥12 patients had evidence of DnP, then the alternative hypothesis would be accepted and that arm would be selected for phase III comparison with BSC. This 1-stage design had an actual power of 92.2% and a type I error rate of 12.5%. Efficacy and toxicity analyses were performed on the intent-to-treat population, with all patients analyzed in their allocated arms, irrespective of whether or not they actually received their assigned treatment.
Results
Patient characteristics
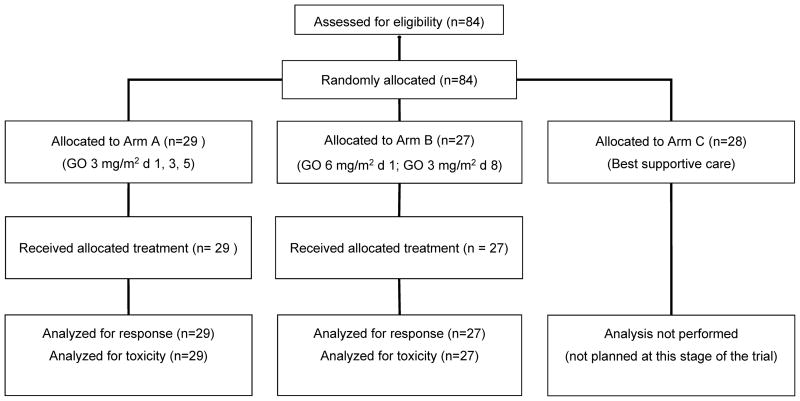
Between June 2004 and December 2006, a total of 84 patients were randomized from 30 participating centres: 56 patients in the two experimental arms (arm A, n = 29; arm B, n = 27), and 28 in the BSC arm (arm C). Patient disposition as per the CONSORT criteria is shown in Fig 2. The baseline characteristics of the 56 patients randomized to receive GO induction monotherapy were balanced well between the two experimental arms (Table I). The median age was 77 years (range 65–84 years) in arm A, and 78 years (range 62–86 years) in arm B. The median WBC count at diagnosis was 8.2 × 109/l (range 0.9–132 × 109/l) in arm A, and 9.1 × 109/l (range 0.7–183 × 109/l) in arm B. Pre-treatment with hydroxycarbamide was given to 10 patients (34%) in arm A, and 11 patients (41%) in arm B.
Fig 2.
Patient disposition as per the CONSORT (Consolidated Standards of Reporting Trials) criteria.
Table I.
Patient characteristics by treatment arm.
| No. of patients (%) | ||
|---|---|---|
| Variable | Arm A | Arm B |
| Total | 29 (100) | 27 (100) |
| Age (years) | ||
| 61–75 | 10 (34.5) | 7 (25.9) |
| >75 | 19 (65.5) | 20 (74.1) |
| Sex | ||
| Male | 16 (55.2) | 13 (48.1) |
| Female | 13 (44.8) | 14 (51.9) |
| Performance Status | ||
| 0 | 10 (34.5) | 10 (37) |
| 1 | 10 (34.5) | 10 (37) |
| 2 | 7 (24.1) | 6 (22.3) |
| 3 | 2 (6.9) | 1 (3.7) |
| Type of AML | ||
| De novo | 16 (55.2) | 17 (63) |
| Secondary | 13 (44.8) | 10 (37) |
| Pretreatment with hydroxycarbamide | ||
| No | 19 (65.5) | 16 (59.3) |
| Yes | 10 (34.5) | 11 (40.7) |
| WBC (×109/l) at diagnosis | ||
| Median | 8.2 | 9.1 |
| Range | 0.9–132 | 0.7–183 |
| Cytogenetic profile* | ||
| Favorable | 0 | 0 |
| Intermediate | 11 (38) | 11 (41) |
| Unfavorable | 4 (14) | 3 (11) |
| Other | 4 (14) | 6 (22) |
| Unknown | 10 (34) | 7 (26) |
| CD33 expression | ||
| <20% | 4 (14) | 4 (15) |
| ≥20% | 25 (86) | 23 (85) |
AML, acute myeloid leukemia; WBC, white blood cell count.
Defined according to the EORTC criteria reported by Amadori et al (2005).
Feasibility and treatment outcome
All 56 eligible patients in the GO arms completed the planned induction course of treatment and were included in the intent-to-treat analyses for efficacy and toxicity, although 2 of the 29 patients in arm A were considered not assessable for response due to inadequate documentation: one patient had symptomatic deterioration and died of “cachexia” 41 days after the beginning of therapy, but with no response evaluation having been performed; the other patient was taken off study early during induction (day +16) for unknown reasons. To avoid any possible risk of bias due to an informative censorship, both patients were included in the denominator of arm A and considered as having failed GO therapy. Induction response data are presented in Table II. Across both randomized groups, 11 patients (20%) achieved CR, 1 (2%) had CRp, 1 (2%) had PR and 15 (26%) maintained a SD, for an overall DnP rate of 50% (28 of 56 patients). The DnP rate was higher with the condensed schedule (arm B: 63%; 90% CI, 45%–78%) than with the hyperfractionated schedule (arm A: 38%; 90% CI, 23%–55%), with the former regimen meeting the prespecified targeted value of 60% DnP rate. While the proportion of patients achieving a complete (CR+CRp) or a PR was similar in the two arms (arm A: 7/29, 24%; arm B: 6/27, 22%), more patients maintained a SD in arm B (11/27, 41%) than in arm A (4/29, 14%). Twelve patients (41%) in arm A and 7 (26%) in arm B had evidence of progressive disease. The all-cause early mortality rate (within 6 weeks of treatment start) was 12% (7 of 56 patients) for the whole cohort, including 2 patients with infection, 4 patients with disease progression and 1 with both. Early deaths occurred in 4 patients (14%) in arm A and in 3 patients (11%) in arm B.
Table II.
Summary of clinical responses by treatment arm.
| Treatment arm |
|||
|---|---|---|---|
| All patients (N=56) | A (N=29) | B (N=27) | |
| Response | N (%) | N (%) | N (%) |
| CR | 11 (20) | 6 (21) | 5 (18) |
| CRp | 1 (2) | 0 | 1 (4) |
| PR | 1 (2) | 1 (3) | 0 |
| SD | 15 (26) | 4 (14) | 11 (41) |
| PD | 19 (34) | 12 (41) | 7 (26) |
| Death (≤6 weeks) | 7 (12) | 4 (14) | 3 (11) |
| Un-assessable | 2 (4) | 2 (7) | 0 |
CR, complete remission; CRp, complete remission without platelet recovery; PR, partial remission; SD, stable disease; PD, progressive disease.
Toxicity
Toxicity data were evaluated for all 56 patients who received the assigned GO therapy. Most toxicities were less than grade 3 in both arms and included nausea/vomiting, diarrhoea, stomatitis and transient elevations of serum transaminases and bilirubin. Treatment-emergent grade 3–4 neutropenia and thrombocytopenia occurred in all patients. Hematological recovery for patients who achieved CR/CRp was the same in both patient groups irrespective of GO schedule: median time to neutrophil (≥0.5 × 109/l) and platelet (≥50 × 109/l) recovery from the first GO infusion was 26 (range 17–32) and 25 (range 1–28) days, respectively in arm A; 25 (range 1–41) and 26 (range 1–50+) days, respectively in arm B. Additional grade 3–5 toxicities that occurred during therapy are shown in Table III. Overall severity and type of toxic incidences were comparable between the two treatment groups except for infectious and haemorrhagic complications, which tended to be more common in arm B than arm A. Invasive fungal infections were responsible for the death of 3 of the 7 patients who died within 6 weeks of treatment start: in arm A, one patient died of candida sepsis, and a second one died of cerebral aspergillosis in the setting of progressive AML; one patient in arm B died of aspergillus pneumonia. One additional patient in arm B developed myocardial infarction and died with SD on day +66. Notably, signs and/or symptoms suggestive of hepatic veno-occlusive disease (VOD) were not observed in any patient receiving GO.
Table III.
Most common (>5%) grade 3–5 toxicities by treatment arm.
| Treatment arm |
||
|---|---|---|
| A (N=29) | B (N=27) | |
| Category | N (%) | N (%) |
| Documented infection | ||
| grade 3 | 7 (24) | 11 (41) |
| grade 4 | 0 | 2 (7) |
| grade 5 | 2 (7) | 1 (4) |
| Febrile neutropenia | ||
| grade 3 | 8 (28) | 3 (11) |
| grade 4 | 1 (3) | 0 |
| Haemorrhage | ||
| grade 3 | 0 | 2 (7) |
| grade 4 | 0 | 1 (4) |
| Cardiac | ||
| grade 3 | 2 (7) | 1 (4) |
| grade 5 | 0 | 1 (4) |
| Other | ||
| grade 3 | 12 (41) | 9 (33) |
| grade 4 | 2 (7) | 1 (4) |
Discussion
A substantial proportion of individuals diagnosed with AML are elderly and either decline or are not considered medically fit for intensive chemotherapy. There is no established treatment for these patients, and the majority of them are generally offered a palliative approach consisting of BSC with or without low-intensity chemotherapy. In this patient population the use of low-dose cytarabine was recently shown to be superior to BSC and hydroxycarbamide because it had greater success in achieving CR (Burnett et al, 2007). Nevertheless, even in responders to low-dose cytarabine the outlook remains unsatisfactory and additional clinical investigations are warranted. Recent advances in unraveling the pathophysiology of AML have resulted in the development of a variety of novel agents directed at specific molecular targets that may provide new, potentially more effective and less toxic opportunities for these patients. As the first targeted agent approved for relapsed AML in elderly patients, GO has generated great interest for potential use, alone or in combination with other agents, in patients with newly diagnosed AML, including those ineligible for intensive chemotherapy.
The current trial showed that a single course of low-dose GO is a tolerable and clinically effective induction therapy for older AML patients deemed unsuitable for intensive chemotherapy. Overall, 28 patients (50%) had evidence of DnP, including complete remission (CR+CRp) in 12 (22%). The rate of DnP was observed to be higher for the condensed schedule (63%) than for the hyperfractionated schedule (38%), and thus, the day 1+8 schedule met the statistical criteria to be selected as the preferred regimen for further phase III evaluation. In light of the above-mentioned results of the UK National Cancer Research Institute AML-14 trial (Burnett et al, 2007) showing a CR rate of 18% in a comparable patient population treated frontline with low-dose cytarabine, it would have been interesting to consider that regimen rather than BSC as the control arm for phase III comparison with the selected GO schedule. However, by the time the AML-14 data were published, our phase II trial had been completed and patients were already being recruited into the phase III stage of the study.
The clinical hypothesis underlying the choice of DnP as a primary endpoint is that survival in very old patients can be prolonged without necessarily reaching a CR. This concept is suggested by the often smoldering course of AML in the elderly (Estey, 2007; Latagliata et al, 2006). DnP is a composite clinical endpoint that broadens the traditional response criteria used in AML, and also makes it possible to compare innovative regimens in much smaller trials (Freemantle & Calvert, 2007). Although views on this subject are sometimes contradictory (Freemantle et al, 2003), both large trials, using traditional endpoints (i.e., CR rate and survival duration), and smaller trials, utilizing a composite outcome endpoint like the one we have chosen, can be used in a complementary fashion. A new regimen could first be tested using the composite outcome endpoint; if it showed particular promise, it could then become a candidate for testing in a larger trial. Conversely, if it did not show any superiority, futile research in hundreds of patients might be prevented.
The GO regimens investigated in this study differed considerably from the licensed schedule (9 mg/m2 on days 1 and 15) used in the previous AML-15B trial of the EORTC/GIMEMA intergroup in the same category of patients (Amadori et al, 2005). The results of that trial indicated excessive hematological and liver toxicity. Dose modifications to improve feasibility and treatment results have involved both dose reduction and fractionation. This approach was based on analyses of GO internalization kinetics and membrane CD33 renewal performed ex vivo on fresh blast cells from patients (van Der Velden et al, 2001). A continuous renewed expression of CD33 antigenic sites on the cell surface after exposure to GO was observed. This finding led to the hypothesis that repeated infusions of low doses of GO may be able to enhance the internalization process and thereby the intracellular accumulation of the drug. Both the hyperfractionated schedule of arm A (3 mg/m2 on days 1, 3 and 5) and the condensed schedule of arm B (6 mg/m2 on day 1 and 3 mg/m2 on day 8) appeared to have an improved side effect profile compared to the previous trial, with an overall induction death rate of 12%, equally distributed between the two arms of treatment. Our data are particularly remarkable concerning liver toxicity. In the previous AML-15B trial, manifestations of grade 3–4 liver toxicity, elevation of total bilirubin and/or transaminases, were reported in 10% of patients. In the current study, as well as in another recent French study using a fractionated regimen (Taksin et al, 2007), no patient developed grade 3 or 4 liver toxicity and hepatic VOD.
With all the caveats of a study based on a limited number of patients, there appears to be a difference in the antileukemic activity of the two regimens, with fewer patients showing evidence of early disease progression on arm B than on arm A. No pharmacokinetic study has directly related GO plasma concentrations with response to treatment, but a near complete saturation of CD33 sites on circulating blasts was reported after intravenous administration of a radio-iodinated anti-CD33 antibody at doses ≥ 5 mg/m2 (Scheinberg et al, 1991). Furthermore, in a phase I dose-escalation study the proportion of patients who experienced reductions in peripheral blast cell counts was higher among patients treated with either 6 or 9 mg/m2 of GO compared with those treated at lower dose levels (Sievers et al, 1999). Hence, the hypothesis that the administration of a higher upfront dose of GO might result in a higher degree of saturation of CD33 sites, which in turn could facilitate intracellular drug loading and enhance leukemia cell killing, seems plausible.
In conclusion, a single course of low-dose GO monotherapy provides encouraging clinical activity in older, previously untreated AML patients considered unfit for intensive chemotherapy, with an acceptable safety profile. Of the two schedules under evaluation, the condensed regimen (day 1+8) resulted in a superior early disease control and was selected as the preferred regimen for further study. Whether this regimen has any advantage in terms of survival over BSC is the focus of the ongoing phase III part of the trial.
Acknowledgments
This work was supported in part by grants 5U10 CA11488-33 through 5U10 CA11488-39 from National Cancer Institute (Bethesda, Maryland, USA), and by a donation from the EORTC Charitable Trust. Its contents are solely the responsibility of the authors and do not represent the official views of the National Cancer Institute. Supported also by a grant from the Italian Association against Leukemias, Lymphoma and Myeloma (AIL). Wyeth Pharmaceuticals provided an educational grant as well as free gemtuzumab ozogamicin.
The Authors would like to thank Filip Beeldens, EORTC Headquarters, and Francesca Cotugno, GIMEMA Data center, for expert data management.
Appendix
In addition to the authors, the following investigators (institutions) recruited patients into the study: D. Bron (Institut Jules Bordet, Brussels, Belgium); W. Feremans (ULB-Hospital Erasme, Brussels, Belgium); J.F. Pruijt (Jeroen Bosch Hospital, ‘s-Hertogenbosch, The Netherlands); O. Leeksma (OLV Hospital, Amsterdam, The Netherlands); R. Willemze (University Medical Center, Leiden, The Netherlands); G.L. Castoldi (University Hospital, Ferrara, Italy); G. Avvisati (Campus Biomedico University Hospital, Rome, Italy); M. Musso (La Maddalena Hospital, Palermo, Italy); A. Zaccaria (S. Maria delle Croci Hospital, Ravenna, Italy); F. Falzetti (University Hospital, Perugia, Italy); G. Visani (S. Salvatore Hospital, Pesaro, Italy); V. Liso (University Hospital, Bari, Italy); E. Angelucci (A. Businco Hospital, Cagliari, Italy); M. Sborgia (Civic Hospital, Pescara, Italy); E. Mitra (P. Giaccone Hospital, Palermo, Italy); M. Pizzuti (S. Carlo Hospital, Potenza, Italy); M. Longinotti (UNiversity Hospital, Sassari, Italy); G. La Nasa (R. Binaghi Hospital, Cagliari, Italy).
Footnotes
Authorship and discosures
SA, SS, PM, DS and TdW conceived the study. SA, SS and RS analyzed the data and wrote the paper. LB and MV coordinated the data collection. SS performed the statistical analyses. SA, DS, GA, VR, EB, GG, DM, GT, PM, AV, EC, and FL recruited patients. All authors reviewed the manuscript and contributed to the final version. The Authors reported no potential conflicts of interest.
References
- Amadori S, Suciu S, Stasi R, Willemze R, Mandelli F, Selleslag D, Denzlinger C, Muus P, Stauder R, Berneman Z, Pruijt J, Nobile F, Cassibba V, Marie JP, Beeldens F, Baila L, Vignetti M, de Witte T. Gemtuzumab ozogamicin (Mylotarg) as single-agent treatment for frail patients 61 years of age and older with acute myeloid leukemia: final results of AML-15B, a phase 2 study of the European Organisation for Research and Treatment of Cancer and Gruppo Italiano Malattie Ematologiche dell’Adulto Leukemia Groups. Leukemia. 2005;19:1768–1773. doi: 10.1038/sj.leu.2403901. [DOI] [PubMed] [Google Scholar]
- Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE, Petersdorf SH. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK, Wheatley K. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–1124. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- Estey EH. Advances in the management of AML in the elderly. Clinical Advances in Hematology and Oncology. 2007;5:185–187. [PubMed] [Google Scholar]
- Estey EH. Treatment of acute myeloid leukemia. Haematologica. 2009;94:10–16. doi: 10.3324/haematol.2008.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemantle N, Calvert M. Composite and surrogate outcomes in randomised controlled trials. British Medical Journal. 2007;334:756–757. doi: 10.1136/bmj.39176.461227.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials: greater precision but with greater uncertainty? Journal of the American Medical Association. 2003;289:2554–2559. doi: 10.1001/jama.289.19.2554. [DOI] [PubMed] [Google Scholar]
- Larson RA, Sievers EL, Stadtmauer EA, Lowenberg B, Estey EH, Dombret H, Theobald M, Voliotis D, Bennett JM, Richie M, Leopold LH, Berger MS, Sherman ML, Loken MR, van Dongen JJ, Bernstein ID, Appelbaum FR. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer. 2005;104:1442–1452. doi: 10.1002/cncr.21326. [DOI] [PubMed] [Google Scholar]
- Latagliata R, Bongarzoni V, Carmosino I, Mengarelli A, Breccia M, Borza PA, D’Andrea M, D’Elia GM, Mecarocci S, Morano SG, Petti MC, Mandelli F, Alimena G. Acute myelogenous leukemia in elderly patients not eligible for intensive chemotherapy: the dark side of the moon. Annals of Oncology. 2006;17:281–285. doi: 10.1093/annonc/mdj112. [DOI] [PubMed] [Google Scholar]
- Menzin J, Lang K, Earle CC, Kerney D, Mallick R. The outcomes and costs of acute myeloid leukemia among the elderly. Archives of Internal Medicine. 2002;162:1597–1603. doi: 10.1001/archinte.162.14.1597. [DOI] [PubMed] [Google Scholar]
- Scheinberg DA, Lovett D, Divgi CR, Graham MC, Berman E, Pentlow K, Feirt N, Finn RD, Clarkson BD, Gee TS, et al. A phase I trial of monoclonal antibody M195 in acute myelogenous leukemia: specific bone marrow targeting and internalization of radionuclide. Journal of Clinical Oncology. 1991;9:478–490. doi: 10.1200/JCO.1991.9.3.478. [DOI] [PubMed] [Google Scholar]
- Sievers EL, Appelbaum FR, Spielberger RT, Forman SJ, Flowers D, Smith FO, Shannon-Dorcy K, Berger MS, Bernstein ID. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood. 1999;93:3678–3684. [PubMed] [Google Scholar]
- Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H, Karanes C, Theobald M, Bennett JM, Sherman ML, Berger MS, Eten CB, Loken MR, van Dongen JJ, Bernstein ID, Appelbaum FR. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. Journal of Clinical Oncology. 2001;19:3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- Stasi R, Evangelista ML, Buccisano F, Venditti A, Amadori S. Gemtuzumab ozogamicin in the treatment of acute myeloid leukemia. Cancer Treatment Reviews. 2008;34:49–60. doi: 10.1016/j.ctrv.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Taksin AL, Legrand O, Raffoux E, de Revel T, Thomas X, Contentin N, Bouabdallah R, Pautas C, Turlure P, Reman O, Gardin C, Varet B, de Botton S, Pousset F, Farhat H, Chevret S, Dombret H, Castaigne S. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21:66–71. doi: 10.1038/sj.leu.2404434. [DOI] [PubMed] [Google Scholar]
- van Der Velden VH, te Marvelde JG, Hoogeveen PG, Bernstein ID, Houtsmuller AB, Berger MS, van Dongen JJ. Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood. 2001;97:3197–3204. doi: 10.1182/blood.v97.10.3197. [DOI] [PubMed] [Google Scholar]
- Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]