Abstract
The anti-epileptic drug Vigabatrin induces an irreversible constriction of the visual field, but is still widely used to treat infantile spasms and some forms of epilepsy. We recently reported that vigabatrin-induced cone damage is due to a taurine deficiency. However, optic atrophy and thus retinal ganglion cell degeneration was also reported in children treated for infantile spasms. We here show in neonatal rats treated from postnatal day 4 to 29 that the vigabatrin treatment triggers not only cone photoreceptor damage, disorganisation of the photoreceptor layer and gliosis but also retinal ganglion cell loss. Furthermore, we demonstrate in these neonatal rats that taurine supplementation partially prevents these retinal lesions and in particular the retinal ganglion cell loss. These results provide the first evidence of retinal ganglion cell neuroprotection by taurine. They further confirm that taurine supplementation should be administered with the vigabatrin treatment for infantile spasms or epilepsy.
Introduction
Infantile spasms are severe epileptic encephalopathies with heterogeneous aetiologies that develop in infancy and early childhood. Vigabatrin (VGB) has been found to be effective for treating infantile spasms and complex partial seizures in adults (Ben-Menachem et al., 2008; Curatolo et al., 2006). VGB or gamma-vinyl GABA is an irreversible and highly selective inhibitor of γ-aminobutyric acid (GABA) transaminase, resulting in an increase in tissue GABA concentrations. Unfortunately, long-term VGB treatment generates irreversible adverse secondary events leading to a bilateral concentric constriction of the visual field in 10 to 40% of adult patients depending on which study was considered (Eke et al., 1997; Krauss et al., 1998; Ruether et al., 1998). This loss in visual field is associated with amplitude decreases of both the photopic electroretinogram (ERG) b-wave and the 30 Hz flicker response (Krauss et al., 1998; Miller et al., 1999; van der Torren et al., 2002). In infants, visual losses were indicated by a decrease of both the photopic ERG b-wave amplitude and the flicker responses (Westall et al., 2002). Furthermore, atrophy of the optic nerve was detected in some VGB-treated infants using in vivo imaging technique (Buncic et al., 2004; Frisen and Malmgren, 2003). Such optic nerve atrophy were subsequently also reported in VGB-treated adult patients with visual field loss (Wild et al., 2006). Optic nerve atrophy was confirmed by histological analysis of post-mortem retinal tissues (Ravindran et al., 2001). In this case, not only had the photoreceptors been damaged, but there was also evidence for the loss of retinal ganglion cell (RGC) bodies and the loss of their axons in the retinal fibre layer. Despite these damaging secondary effects, VGB remains the first line treatment for infantile spasms in Europe and was recently approved in the US for this application (Ben-Menachem et al., 2008; Curatolo et al., 2006).
Retinal toxicity of VGB was first described in albino rats (Butler et al., 1987) and has subsequently been investigated in rabbits (Ponjavic et al., 2004) and mice (Wang et al., 2008). The most obvious retinal damage reported was the disorganisation of the peripheral retinal photoreceptor layer in VGB-treated animals (Butler et al., 1987; Duboc et al., 2004; Wang et al., 2008). Cone photoreceptors were later found to degenerate (Duboc et al., 2004; Wang et al., 2008); glial cells significantly increased their expression of the glial fibrillary acidic protein (GFAP), a classic marker for retinal lesions (Duboc et al., 2004; Ponjavic et al., 2004) and a major synaptic plasticity was observed at rod terminals with the formation of ectopic synapses (Wang et al., 2008). Indeed, rod bipolar and horizontal cells were found to grow long dendrites into the photoreceptor nuclear layer contacting displaced rod terminals. Finally, ERG measurements showed changes such as amplitude decreases of both the photopic ERG and the flicker response (Duboc et al., 2004; Ponjavic et al., 2004). Considering the mechanisms of retinal toxicity, it was first suggested that VGB mediates phototoxicity, as albino animals alone are susceptible to the toxic effects of VGB (Butler et al., 1987). This suggestion was further supported by photoreceptor degeneration observed in retinal explants exposed to light of strong intensity in the presence of VGB; however, this degeneration was not observed in the presence of GABA (Izumi et al., 2004). Recently, we confirmed that VGB mediates phototoxicity by showing that albino animals maintained in darkness experienced no features of retinal toxicity (Jammoul et al., 2009). Furthermore, we found that VGB-treated animals had lower taurine plasma levels and that taurine supplementation prevented the development of retinal lesions (Jammoul et al., 2009). The clinical relevance of these findings was observed in five infants with undetectable taurine plasma levels or at least levels below normal ranges (Jammoul et al., 2009). These effects of taurine deficiency are consistent with mechanisms of phototoxicity because retinal lesions are known to be exacerbated by light in taurine-deficient animals (Rapp et al., 1988). However, no RGC degeneration was demonstrated in VGB-treated animals or in taurine-deficient animals.
As all previous preclinical studies involved adult animals, we investigated the nature of retinal lesions in VGB-treated neonatal rats, providing an animal model that more closely resembled infants treated for infantile spasms. An important aim was to examine whether RGCs degenerate in VGB-treated neonatal rats, as found in infants. Furthermore, we wanted to define whether this eventual cell loss and other retinal damage were also caused by taurine deficiency in these neonatal animals. This work was previously presented as an abstract (Jammoul., ARVO abstract [3605], 2009).
Results
We had demonstrated previously that VGB leads to taurine deficiency in adult animals (Jammoul et al., 2009). In the current study, VGB (0.6 mg/day, representing 50mg/kg for the smallest animal sizes) was injected into 4-day-old rats for 25 days (age of retinal maturation) to model retinal lesions observed in VGB-treated infants and a group of VGB-treated neonatal rats was supplemented with taurine by intraperitoneal injections (5 mg/day representing 420mg/kg for the smallest animal size) (10 animals per group). To examine whether VGB treatment produced similar taurine deficiency during the developmental period, rat blood was collected at the end of the injection period. Table 1 provides the amino acid measurements in three groups: control rats (Group I), VGB-treated animals (Group II) and VGB-treated animals receiving taurine supplementation (group III). For all tested amino acids, a statistically significant decrease was observed only for taurine in the plasma of VGB-treated animals (group II) (Fig.1). Taurine levels were 26.8% lower in VGB-treated neonatal rats (group II: 178.7±23.6μM, n=10, s.e.m., p<0.05) than in the control group (group I: 244.9±54.3μM, s.e.m., n= 10). Taurine supplementation restored taurine plasma concentrations to normal (group III: 243.1±43.1μM, n=10, s.e.m.) (Fig.1). These measurements indicated that VGB also triggers a taurine decrease in neonatal rats.
Table 1.
Amino acid levels in the plasma of neonatal rats untreated (control), treated with vigabatrin (VGB) or treated with both vigabatrin and taurine (VGB + taurine). The differences were statistically significant for taurine level between VGB-treated animals and either the control group or VGB-treated animals administered with taurine.
| Amino acids | Control | VGB | VGB+taurine |
|---|---|---|---|
| Taurine | 244.9±54.3 | 178.7±23.6 | 244.3±42.5* |
| Threonine | 153.2±32.7 | 158.3±30.3 | 169.3±35.1 |
| Serine | 198.7±40.6 | 206.4±40.2 | 224.3±32 |
| Glutamic acid | 110.5±34.2 | 104.8±37.7 | 113.8±25.5 |
| Valine | 189.1±27.4 | 184.5±33 | 176.8±28.1 |
| Citrulline | 135.5±18.3 | 126.7±16 | 142.8±18 |
| Alanine | 521.2±99.6 | 516.3±115.1 | 531±77.3 |
| Glycine | 225.4±46.3 | 222.6±36.6 | 254.2±52 |
| Proline | 318.4±62.2 | 310±50.3 | 313.6±62.5 |
| Glutamine | 694.2±71 | 621±88.5 | 614.1±76.4 |
| Methionine | 54.9±10.5 | 57.3±8 | 58.2±9.8 |
| Isoleucine | 93.6±12.1 | 92.7±15.3 | 88.8±13.6 |
| Leucine | 169.1±20.5 | 193.3±36.7 | 191.2±46.2 |
| Tyrosine | 64.3±12.2 | 68.5±16.4 | 67.6±18.1 |
| Phenylalanine | 62.4±6.4 | 62.5±8.3 | 64.7±7.3 |
| Arginine | 135.7±19.5 | 125.1±30.9 | 128.4±22.5 |
| Histidine | 46.3±5.5 | 51.8±10.5 | 48.7±4.4 |
| Lysine | 265±47.7 | 223.8±89.1 | 241.6±24.5 |
| Ornithine | 85.8±13.9 | 84±16.7 | 90.1±14.6 |
Measurements are provided in μM (n=10, s.e.m.)
p<0.05.
Figure 1.
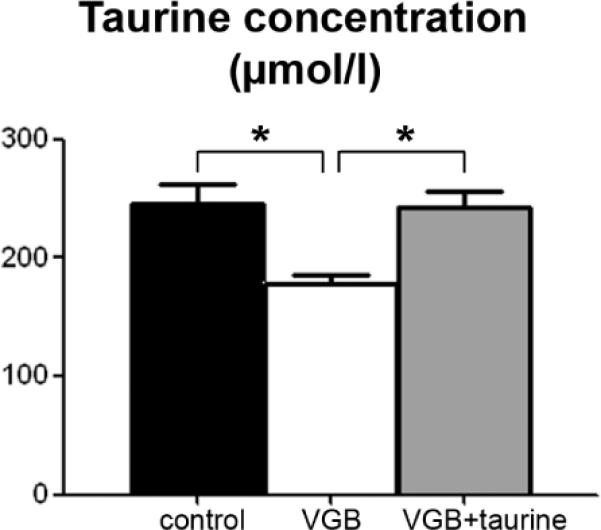
Taurine deficiency in VGB-treated neonatal rats. Plasma taurine levels were measured in control animals (n=10) and in rats treated with VGB from postnatal day 4 to postnatal day 29 with (VGB + taurine, n=10) or without (VGB, n=10) taurine administration. (s.e.m., *p<0.05).
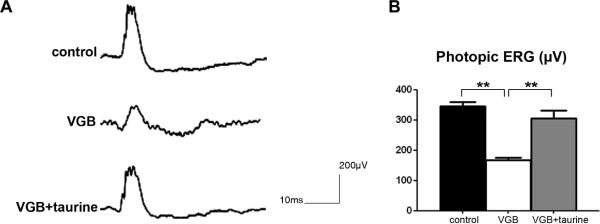
To determine if VGB treatment and subsequent taurine plasma level decreases induced retinal dysfunction, photopic ERGs were measured in the three animal groups. Figure 2A illustrates representative photopic ERGs in these groups. VGB-treated rats (group II) exhibited significantly lower ERG amplitudes than the control group (Fig. 2B, p<0.001). Taurine supplementation (group III) restored 77.2% of the decrease (51,7%) in photopic ERG amplitude resulting from the VGB treatment (Fig.2); the difference with the group of VGB-treated animals (group II) was statistically significant (p<0.001) but that with the control group (group I) was no longer statistically significant. Thus, VGB induces retinal dysfunction in neonatal rats that could be restored by taurine supplementation.
Figure 2.
Retinal cell function in VGB-treated neonatal rats with or without taurine administration. A) Photopic electroretinograms (ERGs) recorded in a control animal, and in VGB-treated rats with (VGB + taurine) or without (VGB) taurine administration. B) Quantification of photopic ERG amplitudes in control, and VGB-treated animals with or without taurine administration showing that taurine administration prevented the VGB-induced decrease in photopic ERG amplitudes. (s.e.m., **p<0.001)
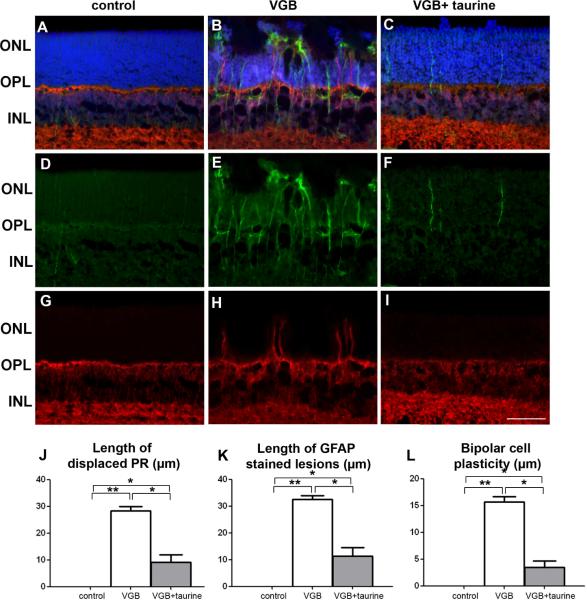
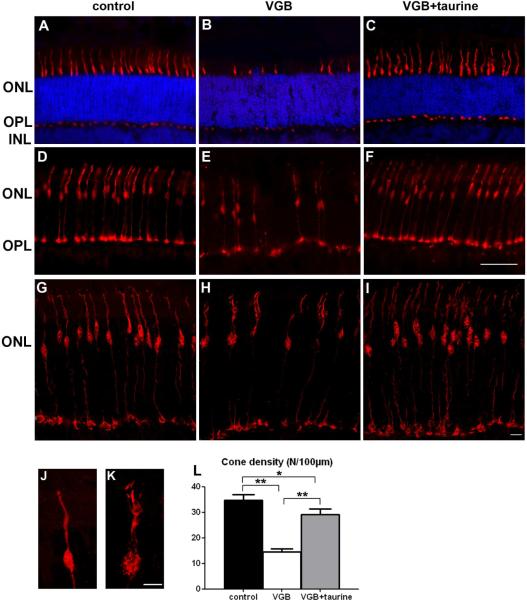
To examine whether retinal dysfunction was related to cellular damage, a histological examination of retinal vertical sections was performed (Fig. 3). As originally described in adult animals (Butler et al., 1987; Duboc et al., 2004; Jammoul et al., 2009), VGB induced a disorganisation of the photoreceptor nuclear layer with nuclei displaced toward the retinal pigment epithelium in neonatal rats (Fig.3A–B). If VGB-treated animals were administered taurine, the disorganised retinal layer persisted (Fig. 3C), but its length was reduced by 67.5% (Fig. 3J, p<0.05). To further analyse the extent of retinal lesions, retinal sections were immuno-labelled against the glial fibrillary acidic protein (GFAP), a classic marker for retinal lesions (Picaud et al., 1993). In control animals, few glial proteins were immuno-labelled (Fig. 3D). By contrast, areas with disorganised retinal layers and their surrounding regions were intensely labelled GFAP-immunopositive (Fig.3E). In VGB-treated animals supplemented with taurine, GFAP-immunopositive glial cells were still present (Fig. 3F). The quantification of retinal areas with intense GFAP immuno-labelling showed that taurine supplementation reduced the extent of such stained retinal lesions (Fig.3K). In adult VGB-treated animals, the disorganisation of the photoreceptor layer is associated with plasticity of rod bipolar cell dendrites (Wang et al., 2008). In neonatal rats, VGB treatment triggered similar plasticity (Fig.3H). Again, taurine supplementation reduced neuronal plasticity by 78%, but did not completely prevent it (Fig. 3H–I,L). The loss of vision in adult animals is mainly attributed to a loss of cone photoreceptors (Duboc et al., 2004; Wang et al., 2008); we therefore examined cone photoreceptor in VGB-treated neonatal rats, detailing cone damage and subsequent cone loss (Fig. 4). Cone photoreceptors were labelled with the peanut agglutinin lectin (PNA) (Fig. 4A–C) or the cone arrestin antibody (Fig. 4D–I). In both cases, the number of cone photoreceptors appeared to be lower in VGB-treated animals (group II) than in controls, whereas the numbers of cone photoreceptors appeared to be preserved by taurine supplementation (group III). An enlarged view of a single cone photoreceptor in a control animal (Fig. 4J) and a VGB-treated rat (Fig. 4K) illustrates the morphological changes induced by VGB treatment. The quantification of PNA immunopositive outer/inner segments confirmed that VGB generated a 58% cone inner/outer segment loss (p<0.001). The number of cones after taurine administration was only 16% lower than that observed in controls, indicating that taurine supplementation prevented 72% of the VGB-induced cone cell loss (Fig.3J). These results were consistent with those for adult animals, for which a similar reduction of retinal lesions and cone damage was observed after taurine supplementation.
Figure 3.
Cell plasticity and retinal gliosis in VGB-treated neonatal rats with or without taurine administration. (A–I) Retinal sections showing that VGB-elicited retinal lesions are less extensive in rats with taurine administration (C, F, I, VGB + taurine) than in rats without (B, E, H, VGB), but were still greater than in control animals (A, D, G). These sections were stained with DAPI (blue in A–C) and were immuno-labelled with antibodies directed against Goα (red in A–C, G–I), GFAP (green in A-F). Photoreceptor nuclei displaced above the outer nuclear layer (ONL) are observed in both groups of VGB-treated rats treated with or without taurine supplementation (B, C), but not in control animals (A). GFAP-positive processes extending vertically throughout the retina were observed in VGB-treated rats (B, E), but staining was less dense in VGB-treated rats receiving taurine administration (C, F), with no staining in control animals (A, D). Neuronal plasticity was indicated by the extension of Goα-immunopositive bipolar cell dendrites into the ONL of VGB-treated animals without taurine administration (B, H), whereas control animals (A, G) and animals receiving taurine (C, I) exhibited no such cell changes. (J–L). Length quantification of retinal areas with displaced photoreceptor (PR) nuclei (J), of areas with increased GFAP expression (K) and areas with bipolar cell neurite extension (L). The scale bar represents 50 μm (ONL=outer nuclear layer, INL=inner nuclear layer, OPL=outer plexiform layer, s.e.m., n=10, *p<0.05; **p<0.001).
Figure 4.
Taurine preservation of cone photoreceptors in VGB-treated neonatal rats. (A–K) Retinal sections from control animals (A, G, J), VGB-treated rats (B, H, K) and VGB-treated rats administered with taurine (C, I) stained with peanut lectin (PNA, in A–C) or immuno-labelled with an antibody directed against the cone arrestin (D–K): sections were examined by light (A–F) or confocal microscopy (G–K). Note the decrease in PNA-positive inner/outer segments of photoreceptors (B) or cone-arrestin immunpositive cells (F, H) in VGB-treated rats in comparison with control animals (A, D, G) and neonatal VGB-treated rats that had taurine injections (C, F, I). Enlarged views of cone-arrestin immuno-labelled photoreceptors showing the morphological changes in a VGB-treated rat (K) in comparison with a normal cone photoreceptor (J). (L) Quantification of cone inner/outer segment densities in control rats (n=10), in VGB-treated animals with (VGB + taurine, n=10) or without (VGB, n=10) taurine administration. The scale bar represents 50 μm (A–F), 10μm (G–I) and 1μm (J–K). (ONL=outer nuclear layer, INL=inner nuclear layer, OPL=outer plexiform layer, s.e.m., *p<0.05; **p<0.001).
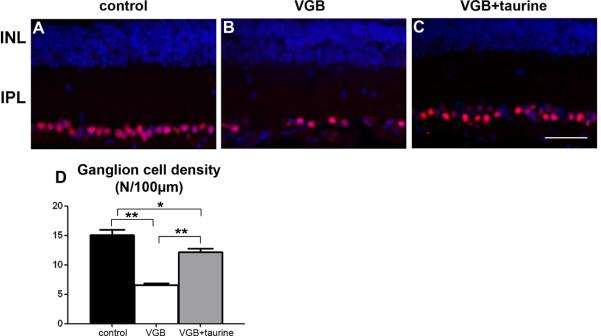
In VGB-treated infants, optic nerve atrophy suggests that RGCs degenerate during the treatment. To examine if RGCs degenerate in VGB-treated neonatal rats, vertical sections along the dorso-ventral axis were immunolabeled with the antibody against Brn3a (also named Pou4f1), which reacts with 92.2% of dye backfilled RGC cell bodies (Nadal-Nicolas et al., 2009). Half of Brn3A-positive RGCs (56%) were lost, confirming that the VGB treatment is highly toxic to RGCs (Fig.5A–B, D) (p<0.001, n=10). To determine whether VGB toxicity was related to taurine deficiency, Brn3a-positive RGCs were quantified in VGB-treated animals receiving taurine supplementation. Although this supplementation preserved 76% of RGCs, we still observed a statistically significant decrease (19%) in RGC density (Fig. 4, p<0,001, n=10). These results indicated that taurine deficiency plays an important role in VGB toxicity and its consecutive RGC loss.
Figure 5.
Taurine protection of retinal ganglion cells in VGB-treated neonatal rats. (A-C). Retinal sections from a control animal (A), a VGB-treated rat (B) or a VGB-treated rat with taurine administration (C) that were stained with DAPI to visualise nuclei (blue in A–C) and immuno-labelled with a Brn3A antibody (red in A–C). Note the decrease in Brn3A-positive ganglion cells in the neonatal VGB-treated rats (B), whereas the number of retinal ganglion cells appeared to be relatively maintained in the VGB-treated animal receiving taurine (C) in comparison with the control rat (A). Quantification of retinal ganglion cells (RGC, D) in control rats (n=10), and in VGB-treated animals with (VGB + taurine, n=10) or without (VGB, n=10) taurine injections. The scale bar represents 50 μm. (ONL=outer nuclear layer, INL=inner nuclear layer, OPL=outer plexiform layer, IPL=inner plexiform layer, s.e.m., *p<0.05; **p<0.001).
Experimental Methods
Animal Treatments
Wistar rats Rj Wi IOPS Han were purchased from Janvier (Le Genest-St-Isle, France) at postnatal day 4. VGB dissolved in 0.9% NaCl was administered at 0.6 mg/day (6mg/ml, 0.1ml) to neonatal rats by daily intraperitoneal injection for 25 days. This daily dose (50mg/kg) for the smallest animal sizes at the start of treatment was consistent with those described for the treatment of infantile spasms (100mg/kg (Aicardi et al., 1996)). Taurine was injected at a concentration of 5 mg/day (0.1 ml at 50 mg/ml, representing 420mg/kg for the smallest animal size). Light intensities in the animal cages were between 125 and 130 lux during the day period of the 12h/12h light/dark cycle.
Electroretinogram
Photopic ERGs were recorded after the last VGB injection, as described previously (20). Rats were anesthetised by intraperitoneal injection (0.8 −1.2ml/kg) with a solution containing ketamine (40mg/ml) and xylazine (4mg/ml Rompum). Animals were light-adapted for 10 minutes with a background light of 25cdm−2. Animals were then subjected to light flashes and background light; the light intensity of the flash was 25 cdm−2. Ten recordings were averaged with an interstimulus interval of 30 seconds.
Histology
Eye cups were fixed overnight at 4°C in 4% (wt/vol) paraformaldehyde in phosphate-buffered saline (PBS; 0.01M, pH7.4). The tissue was cryoprotected in successive solutions of PBS containing 10, 20, and 30% sucrose at 4°C, oriented along the dorsoventral axis and embedded in OCT (Labonord,Villeneuve d'Ascq, France). Retinal sections (8–10μm in thickness) were permeabilised for 5 minutes in PBS containing 0.1% Triton X-100 (Sigma, St. Louis, MO), rinsed, and were incubated in PBS containing 1% bovine serum albumin (Eurobio, Les-Ulis, France), 0.1% Tween 20 (Sigma), and 0.1% sodium azide (Merck, Fontenay-Sous-Bois, France) for 2 hours at room temperature. The primary antibody added to the solution was incubated for 2 hours at room temperature. Polyclonal antibodies were directed against rabbit GFAP (1/400; Dako, Carpinteria, CA), and mouse cone arrestin (Luminaire junior, LUMIj; 1:20,000)(Zhu et al., 2002). Monoclonal antibodies were directed against Goα (1:2,000; Chemicon) and Brn3A (1:125; Chemicon). Sections were rinsed and then incubated with the secondary antibody, goat anti–rabbit IgG or rabbit anti–mouse IgG conjugated to either Alexa TM594 or Alexa TM488 (1:500; Molecular Probes, Eugene, OR) for 2 hours. Inner/outer segments of cone photoreceptors were stained with a peanut agglutinin lectin (PNA; 1/80, Sigma) during 12 hours of incubation at 4°C. The dye, diamidiphenyl-indole (DAPI), was added during the final incubation period. Sections were rinsed, mounted with Fluorsave reagent (Calbiochem, San Diego, CA), and viewed with a fluorescence microscope (LEICA DM 5000B; Leica, Deerfield, IL) equipped with a Ropper scientific camera (Photometrics cool SNAP TM FX; Photometrics, Tucson, AZ).
For quantification, vertical sections along the dorsoventral axis were selected at the optic nerve. After nuclear DAPI staining, the lengths of disorganised retinal areas were measured. The cone photoreceptor and the ganglion cell densities were calculated after PNA and Brn3A labelling to visualise the inner/outer segments of cone photoreceptors and ganglion cells, respectively; areas with disorganised retinal layering were excluded.
Statistical Analysis
Statistical analysis of the results was performed by one-way analysis of variance using the Student-Newman–Keul's test (Sigmastat) for all measurements.
Discussion
In adult albino rats, the toxic effects of VGB on retina to be first described were the disorganisation of the photoreceptor nuclear layer with nuclei bordering upon the retinal pigment epithelium (Butler et al., 1987). We subsequently confirmed this observation in mice and rats and showed that damage to cone photoreceptors occurs before nuclear layer rearrangement (Duboc et al., 2004; Wang et al., 2008). This cell damage is generally associated with retinal gliosis, indicated by a marked increase in GFAP expression (Duboc et al., 2004; Ponjavic et al., 2004). There is also an unexpected neuronal plasticity of rods and their postsynaptic neurons (Wang et al., 2008). Here, we extended these observations to VGB-treated neonatal rats. Furthermore, we found that VGB induced a decrease in RGC density. In animal models of and patients with retinitis pigmentosa, a secondary degeneration of RGCs was reported following photoreceptor loss (Humayun et al., 1999; Villegas-Perez et al., 1998). In VGB-treated animals, RGCs appeared instead to undergo degeneration in parallel with cone photoreceptors. Indeed, VGB-treated neonatal rats (group II) exhibited 58% and 56% decreases in cone photoreceptor and RGC density, respectively. In group III, these decreases were reduced to 16% and 19%, respectively. These observations suggest that the RGC degeneration is not a consequence of photoreceptor cell loss, but a primary consequence of VGB toxicity.
We recently provided evidence showing that VGB induces taurine deficiency, which can be responsible for cone damage, neuronal plasticity and retinal gliosis in adult animals (Duboc et al., 2004). In this study, we extended these results to neonatal animals and showed that taurine deficiency also triggers RGC degeneration. In fact, taurine supplementation was unable to completely prevent either cone or RGC losses. A similar conclusion was reached for all features of retinal lesions in adult animals (Jammoul et al., 2009). This partial prevention of the degenerative process suggests that taurine supplementation does not fully restore retinal taurine concentrations, despite recovery of normal plasma levels. Taurine uptake in the retina occurs on both sides of the retinal blood barrier in retinal capillary endothelial cells (Tomi et al., 2008), in retinal pigment epithelial cells and in retinal glial Müller cells (El-Sherbeny et al., 2004). Therefore, VGB-induced inhibition of taurine uptake in the retina could alter local taurine concentrations despite normal blood levels. Taurine uptake could be inhibited directly by VGB or indirectly by the high increase in retinal GABA. Indeed, VGB concentrations are known to be 5-fold greater in the retina than in the brain (Sills et al., 2001). This increase in the concentration of VGB is associated with a significant increase in retinal GABA concentrations (Neal et al., 1989). Furthermore, GABA is a known substrate for the taurine transporter and could thus limit taurine uptake into the retina by competitive inhibition (Tomi et al., 2008). Future studies will have to measure retinal taurine levels in VGB-treated animals with or without taurine supplementation.
Although the dependence of photoreceptors for taurine is well established (Hayes et al., 1975), similar observations relating to RGCs is a new finding. Photoreceptor degeneration has been reported in other examples: in cats receiving a taurine free-diet (Hayes et al., 1975; Leon et al., 1995), in baby monkeys also on a taurine-free diet (Imaki et al., 1987), in taurine transporter-deficient mice (Rascher et al., 2004) and in rats receiving a taurine transporter blocker (Lake and Malik, 1987). In none of these animals, was RGC degeneration reported. The parallel decrease in cone photoreceptors and RGCs suggests that RGC degeneration does not occur secondarily to photoreceptor loss, as in patients with and animal models of retinitis pigmentosa (see discussion above) (Humayun et al., 1999; Villegas-Perez et al., 1998). Expression of the taurine transporter and taurine transport have been previously demonstrated in RGC1, a RGC line (El-Sherbeny et al., 2004). The observed RGC degeneration in VGB-treated animals and taurine expression in RGC1 cell lines are consistent with the suggestion that taurine deficiency could directly affect RGC survival. Future studies will have to therefore define whether RGCs are also degenerating in animals with taurine deficiency or taurine uptake impairments.
In VGB-treated patients, the irreversible loss in visual field was associated with a decrease in the photopic ERG and flicker response amplitudes (Miller et al., 1999; van der Torren et al., 2002; Westall et al., 2002). These observations were consistent with a selective impairment of the cone pathway. The reversible change in the electrooculogram response was also in agreement with a dysfunction of the functional relationship between photoreceptors and the retinal pigment epithelium (Arndt et al., 1999; Comaish et al., 2002; van der Torren et al., 2002). As in VGB-treated animals (Butler et al., 1987; Duboc et al., 2004; Wang et al., 2008), an histological examination of the retina from a VGB-treated patient confirmed major photoreceptor degeneration (Ravindran et al., 2001). However, in this more recent study, the primary site of injury was located within RGCs (Ravindran et al., 2001). This location was subsequently confirmed in more patients using in vivo imaging techniques of the retina, in both VGB-treated infants (Buncic et al., 2004; Frisen and Malmgren, 2003) and VGB-treated adult patients (Viestenz et al., 2003; Wild et al., 2006). Although this may be related to the plasticity of the photoreceptor synapse (Wang et al., 2008), the decrease in the scotopic ERG b-wave amplitude could also be attributed to RGC loss (Comaish et al., 2002; Coupland et al., 2001; Hardus et al., 2003; van der Torren et al., 2002; Viestenz et al., 2003). Indeed, the selective degeneration of RGCs is often associated with a decrease in the scotopic b-wave amplitude, with no major modification of the corresponding a-wave in animals (Fortune et al., 2004). Our RGC quantification in neonatal VGB-treated animals is consistent with the notion that RGCs are directly affected by VGB. After having demonstrated that taurine deficiency is responsible for the photoreceptor degeneration in VGB-treated rats (Jammoul et al., 2009), we showed that taurine deficiency also causes RGC degeneration. The clinical relevance of these results was provided in our previous study, in which plasma taurine levels were undetectable or below the normal range in VGB-treated infants (Jammoul et al., 2009). Therefore, taurine supplementation may prevent or slow down visual field loss and optic nerve atrophy in VGB-treated patients. Inversely, VGB-induced visual field constriction in adults and infants may therefore provide the first description of human retinal damages caused by taurine deficiency. Indeed, although the taurine dependence for photoreceptor survival was first described in the 70s (Hayes et al., 1975), no clinical photoreceptor degeneration had since been attributed to taurine deficiency. Our characterisation of the toxic effects of VGB on the retina clearly indicates that VGB-induced retinal lesions likely result from taurine deficiency in patients. Therefore, VGB-elicited visual field constriction may serve as a reference to identify future cases of clinical taurine deficiency.
Conclusion
We confirmed that taurine deficiency may explain the different retinal damages observed in VGB-treated patients. Indeed, taurine supplementation prevented both cone and RGC damage, which were both described in VGB-treated patients and may explain the visual field constriction. If the taurine deficiency had already been incriminated in cone damage occurring in adult VGB-treated animals and other animal models, this study is the first demonstration that it could also trigger RGC degeneration. Future studies will have to investigate whether a co-treatment of taurine/VGB can preserve the anti-epileptic efficacy of VGB without inducing any visual defects. However, a taurine-rich diet and reduced sunlight exposure may already significantly reduce the extent of VGB-induced retinal lesions.
Acknowledgements
We would like to thank Stephen Collins (NeuroTherapeutics Pharma, USA), Stephen Sagar, Mark Walzer, Chris Siber and Charles Krikorian (Lundbeck US, USA) for their help and comments, and Dr Villey for project management support. This work was supported by INSERM, Université Pierre et Marie Curie (Paris VI), the Fondation Ophtalmologique A. de Rothschild (Paris), Agence Nationale pour la Recherche (ANR: GABARET), the Fédération des Aveugles de France, the city of Paris, the Regional Council of Ile de France and Lundbeck US (USA). FJ received a fellowship from the University of Tichcrine (Syria). Dr. Cheryl M. Craft received the Mary D. Allen Chair in Vision Research, Doheny Eye Institute (DEI). She acknowledges Mary D. Allen for her generous endowment, NIH (EY015851, EY03040 [DEI Core]) and RPB for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aicardi J, Mumford JP, Dumas C, Wood S. Vigabatrin as initial therapy for infantile spasms: a European retrospective survey. Sabril IS Investigator and Peer Review Groups. Epilepsia. 1996;37:638–642. doi: 10.1111/j.1528-1157.1996.tb00627.x. [DOI] [PubMed] [Google Scholar]
- Arndt CF, Derambure P, Defoort-Dhellemmes S, Hache JC. Outer retinal dysfunction in patients treated with vigabatrin. Neurology. 1999;52:1201–1205. doi: 10.1212/wnl.52.6.1201. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E, Dulac O, Chiron C. Vigabatrin. In: Jerome Engel, Jr, Timothy A Pedley., editors. Epilepsy: a comprehensive textbook. Second edition Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 1683–1693. [Google Scholar]
- Buncic JR, Westall CA, Panton CM, Munn JR, MacKeen LD, Logan WJ. Characteristic retinal atrophy with secondary “inverse” optic atrophy identifies vigabatrin toxicity in children. Ophthalmology. 2004;111:1935–1942. doi: 10.1016/j.ophtha.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler WH, Ford GP, Newberne JW. A study of the effects of vigabatrin on the central nervous system and retina of Sprague Dawley and Lister-Hooded rats. Toxicologic pathology. 1987;15:143–148. doi: 10.1177/019262338701500203. [DOI] [PubMed] [Google Scholar]
- Comaish IF, Gorman C, Brimlow GM, Barber C, Orr GM, Galloway NR. The effects of vigabatrin on electrophysiology and visual fields in epileptics: a controlled study with a discussion of possible mechanisms. Doc Ophthalmol. 2002;104:195–212. doi: 10.1023/a:1014603229383. [DOI] [PubMed] [Google Scholar]
- Coupland SG, Zackon DH, Leonard BC, Ross TM. Vigabatrin effect on inner retinal function. Ophthalmology. 2001;108:1493–1496. doi: 10.1016/s0161-6420(01)00638-8. discussion 1497–1498. [DOI] [PubMed] [Google Scholar]
- Curatolo P, Bombardieri R, Cerminara C. Current management for epilepsy in tuberous sclerosis complex. Curr Opin Neurol. 2006;19:119–123. doi: 10.1097/01.wco.0000218225.50807.12. [DOI] [PubMed] [Google Scholar]
- Duboc A, Hanoteau N, Simonutti M, Rudolf G, Nehlig A, Sahel JA, Picaud S. Vigabatrin, the GABA-transaminase inhibitor, damages cone photoreceptors in rats. Annals of neurology. 2004;55:695–705. doi: 10.1002/ana.20081. [DOI] [PubMed] [Google Scholar]
- Eke T, Talbot JF, Lawden MC. Severe persistent visual field constriction associated with vigabatrin. BMJ (Clinical research ed. 1997;314:180–181. doi: 10.1136/bmj.314.7075.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sherbeny A, Naggar H, Miyauchi S, Ola MS, Maddox DM, Martin PM, Ganapathy V, Smith SB. Osmoregulation of taurine transporter function and expression in retinal pigment epithelial, ganglion, and muller cells. Investigative ophthalmology & visual science. 2004;45:694–701. doi: 10.1167/iovs.03-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune B, Bui BV, Morrison JC, Johnson EC, Dong J, Cepurna WO, Jia L, Barber S, Cioffi GA. Selective ganglion cell functional loss in rats with experimental glaucoma. Investigative ophthalmology & visual science. 2004;45:1854–1862. doi: 10.1167/iovs.03-1411. [DOI] [PubMed] [Google Scholar]
- Frisen L, Malmgren K. Characterization of vigabatrin-associated optic atrophy. Acta ophthalmologica Scandinavica. 2003;81:466–473. doi: 10.1034/j.1600-0420.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- Hardus P, Verduin W, Berendschot T, Postma G, Stilma J, van Veelen C. Vigabatrin: longterm follow-up of electrophysiology and visual field examinations. Acta ophthalmologica Scandinavica. 2003;81:459–465. doi: 10.1034/j.1600-0420.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- Hayes KC, Carey RE, Schmidt SY. Retinal degeneration associated with taurine deficiency in the cat. Science (New York, N.Y. 1975;188:949–951. doi: 10.1126/science.1138364. [DOI] [PubMed] [Google Scholar]
- Humayun MS, Prince M, de Juan E, Jr., Barron Y, Moskowitz M, Klock IB, Milam AH. Morphometric analysis of the extramacular retina from postmortem eyes with retinitis pigmentosa. Investigative ophthalmology & visual science. 1999;40:143–148. [PubMed] [Google Scholar]
- Imaki H, Moretz R, Wisniewski H, Neuringer M, Sturman J. Retinal degeneration in 3-month-old rhesus monkey infants fed a taurine-free human infant formula. Journal of neuroscience research. 1987;18:602–614. doi: 10.1002/jnr.490180414. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Ishikawa M, Benz AM, Izumi M, Zorumski CF, Thio LL. Acute vigabatrin retinotoxicity in albino rats depends on light but not GABA. Epilepsia. 2004;45:1043–1048. doi: 10.1111/j.0013-9580.2004.01004.x. [DOI] [PubMed] [Google Scholar]
- Jammoul F, Wang Q, Nabbout R, Coriat C, Duboc A, Simonutti M, Dubus E, Craft CM, Ye W, Collins SD, Dulac O, Chiron C, Sahel JA, Picaud S. Taurine deficiency is a cause of vigabatrin-induced retinal phototoxicity. Annals of neurology. 2009;65:98–107. doi: 10.1002/ana.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss GL, Johnson MA, Miller NR. Vigabatrin-associated retinal cone system dysfunction: electroretinogram and ophthalmologic findings. Neurology. 1998;50:614–618. doi: 10.1212/wnl.50.3.614. [DOI] [PubMed] [Google Scholar]
- Lake N, Malik N. Retinal morphology in rats treated with a taurine transport antagonist. Exp Eye Res. 1987;44:331–346. doi: 10.1016/s0014-4835(87)80169-0. [DOI] [PubMed] [Google Scholar]
- Leon A, Levick WR, Sarossy MG. Lesion topography and new histological features in feline taurine deficiency retinopathy. Exp Eye Res. 1995;61:731–741. doi: 10.1016/s0014-4835(05)80024-7. [DOI] [PubMed] [Google Scholar]
- Miller NR, Johnson MA, Paul SR, Girkin CA, Perry JD, Endres M, Krauss GL. Visual dysfunction in patients receiving vigabatrin: clinical and electrophysiologic findings. Neurology. 1999;53:2082–2087. doi: 10.1212/wnl.53.9.2082. [DOI] [PubMed] [Google Scholar]
- Nadal-Nicolas FM, Jimenez-Lopez M, Sobrado-Calvo P, Nieto-Lopez L, Canovas-Martinez I, Salinas-Navarro M, Vidal-Sanz M, Agudo M. Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Investigative ophthalmology & visual science. 2009;50:3860–3868. doi: 10.1167/iovs.08-3267. [DOI] [PubMed] [Google Scholar]
- Neal MJ, Cunningham JR, Shah MA, Yazulla S. Immunocytochemical evidence that vigabatrin in rats causes GABA accumulation in glial cells of the retina. Neuroscience letters. 1989;98:29–32. doi: 10.1016/0304-3940(89)90368-6. [DOI] [PubMed] [Google Scholar]
- Picaud S, Peichl L, Franceschini N. Dye-induced photolesion in the mammalian retina: glial and neuronal reactions. Journal of neuroscience research. 1993;35:629–642. doi: 10.1002/jnr.490350606. [DOI] [PubMed] [Google Scholar]
- Ponjavic V, Granse L, Kjellstrom S, Andreasson S, Bruun A. Alterations in electroretinograms and retinal morphology in rabbits treated with vigabatrin. Doc Ophthalmol. 2004;108:125–133. doi: 10.1023/b:doop.0000036780.96560.74. [DOI] [PubMed] [Google Scholar]
- Rapp LM, Thum LA, Anderson RE. Synergism between environmental lighting and taurine depletion in causing photoreceptor cell degeneration. Exp Eye Res. 1988;46:229–238. doi: 10.1016/s0014-4835(88)80080-0. [DOI] [PubMed] [Google Scholar]
- Rascher K, Servos G, Berthold G, Hartwig HG, Warskulat U, Heller-Stilb B, Haussinger D. Light deprivation slows but does not prevent the loss of photoreceptors in taurine transporter knockout mice. Vision research. 2004;44:2091–2100. doi: 10.1016/j.visres.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Ravindran J, Blumbergs P, Crompton J, Pietris G, Waddy H. Visual field loss associated with vigabatrin: pathological correlations. Journal of neurology, neurosurgery, and psychiatry. 2001;70:787–789. doi: 10.1136/jnnp.70.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruether K, Pung T, Kellner U, Schmitz B, Hartmann C, Seeliger M. Electrophysiologic evaluation of a patient with peripheral visual field contraction associated with vigabatrin. Archives of ophthalmology. 1998;116:817–819. [PubMed] [Google Scholar]
- Sills GJ, Patsalos PN, Butler E, Forrest G, Ratnaraj N, Brodie MJ. Visual field constriction: accumulation of vigabatrin but not tiagabine in the retina. Neurology. 2001;57:196–200. doi: 10.1212/wnl.57.2.196. [DOI] [PubMed] [Google Scholar]
- Tomi M, Tajima A, Tachikawa M, Hosoya KI. Function of taurine transporter (Slc6a6/TauT) as a GABA transporting protein and its relevance to GABA transport in rat retinal capillary endothelial cells. Biochimica et biophysica acta. 2008;1778:2138–2142. doi: 10.1016/j.bbamem.2008.04.012. [DOI] [PubMed] [Google Scholar]
- van der Torren K, Graniewski-Wijnands HS, Polak BC. Visual field and electrophysiological abnormalities due to vigabatrin. Doc Ophthalmol. 2002;104:181–188. doi: 10.1023/a:1014615517996. [DOI] [PubMed] [Google Scholar]
- Viestenz A, Viestenz A, Mardin CY. Vigabatrin-associated bilateral simple optic nerve atrophy with visual field constriction. A case report and a survey of the literature. Ophthalmologe. 2003;100:402–405. doi: 10.1007/s00347-002-0745-3. [DOI] [PubMed] [Google Scholar]
- Villegas-Perez MP, Lawrence JM, Vidal-Sanz M, Lavail MM, Lund RD. Ganglion cell loss in RCS rat retina: a result of compression of axons by contracting intraretinal vessels linked to the pigment epithelium. The Journal of comparative neurology. 1998;392:58–77. [PubMed] [Google Scholar]
- Wang QP, Jammoul F, Duboc A, Gong J, Simonutti M, Dubus E, Craft CM, Ye W, Sahel JA, Picaud S. Treatment of epilepsy: the GABA-transaminase inhibitor, vigabatrin, induces neuronal plasticity in the mouse retina. Eur J Neurosci. 2008;27:2177–2187. doi: 10.1111/j.1460-9568.2008.06175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westall CA, Logan WJ, Smith K, Buncic JR, Panton CM, Abdolell M. The Hospital for Sick Children, Toronto, Longitudinal ERG study of children on vigabatrin. Doc Ophthalmol. 2002;104:133–149. doi: 10.1023/a:1014656626174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM, Robson CR, Jones AL, Cunliffe IA, Smith PE. Detecting vigabatrin toxicity by imaging of the retinal nerve fiber layer. Investigative ophthalmology & visual science. 2006;47:917–924. doi: 10.1167/iovs.05-0854. [DOI] [PubMed] [Google Scholar]
- Zhu X, Li A, Brown B, Weiss ER, Osawa S, Craft CM. Mouse cone arrestin expression pattern: light induced translocation in cone photoreceptors. Molecular vision. 2002;8:462–471. [PubMed] [Google Scholar]