Abstract
Objective
To determine if bimatoprost (Lumigan) causes increased lash length when used in gel suspension applied to the base of the eyelashes.
Design
Randomized controlled trial.
Participants
Nineteen subjects were enrolled.
Methods
Subjects recruited from the Bascom Palmer Eye Institute were screened and those who met inclusion criteria were enrolled. Each participant received two vials of gel suspension, which contained bimatoprost and normal saline, respectively, each mixed 1:1 with Gonak™ gel and labeled “right eye” and “left eye” according to randomization. The suspension was applied to the upper eyelid eyelashes every evening on the designated eye for 6 weeks.
Main Outcome Measures
Lash length was measured with a caliper at enrollment, at weekly intervals during the application of the gel, and at 1 and 3 months after discontinuation of its use. Visual acuity, ocular symptoms, intraocular pressure and photographs were documented at these same intervals.
Results
The mean eyelash growth from baseline in the bimatoprost group was 2.0 mm vs. a mean of 1.1 mm in the placebo group, which was a statistically significant difference (p=0.009). The average intraocular pressure decreased equally in both groups (2 mmHg). No change in visual acuity or iris discoloration was noted in any of the subjects.
Conclusion
Our data showed an increase in eyelash length with the use of bimatoprost in gel suspension, suggesting the product’s eyelash lengthening properties.
Eyelash growth has been reported to be a side effect of the prostaglandin analogs since their introduction in the late 1990s. In 1997, Johnstone reported findings of a retrospective unmasked trial in which 43 patients receiving topical monocular latanoprost treatment developed hypertrichosis and pigmentation of the eyelashes.1 Sugimoto et al performed a quantitative analysis of eyelash lengthening following monocular topical latanoprost therapy and found a significant increase in eyelash length in the treated eye.2 A study by Chiba et al evaluating topical latanoprost-induced iridial pigmentation and eyelash change in Japanese patients with glaucoma found 46.3% had a change in the length and thickness of the eyelashes, and there was no significant correlation between iridial pigmentation and eyelash change.3 Elgin et al measured eyelash length in children and adults using topical latanoprost for glaucoma therapy and found that the mean difference in eyelash lengths at baseline and sixth months was 0.5–0.7mm in adults and 0.4–1.2 in children.4 Uno et al. used a macaque model of androgenetic alopecia and showed that treatment with 50 mcg/ml of latanoprost daily over 5 months caused minimal hair growth, whereas 500 mcg/ml daily over 3 months induced moderate-to-marked hair regrowth. 5 Each of the above mentioned studies, with the exception of Uno et al., used prostaglandin analogs topically on the surface of the eye. We are not aware of any previously published randomized control trial that has evaluated the effect on eyelash length of bimatoprost applied monocularly to the base of the eyelashes compared to control solution applied in the same fashion to the other eye.
Prostaglandin receptors are present in the dermal papilla and the outer root sheath of the hair follicle and thus may be involved in the development and growth of the hair follicle. It has been postulated that the prostaglandin analogs may prolong the eyelash anagen phase, the active growth phase. Several investigators have also hypothesized that minoxidil, a well known dermatologic treatment for alopecia, may stimulate hair growth via activation of prostaglandins.6 Michelet et al assayed oxygen consumption and prostaglandin production and demonstrated that minoxidil is an activator of purified PGHS-1, which was also seen by increased prostaglandin (PGE2) production in human dermal papilla fibroblasts in culture.7 These findings suggest that the mechanism behind the hair-growth-stimulating effect of minoxidil is stimulation of PGE2 synthesis. A more recent study by Torii et al8 showed that prostaglandin receptor mRNAs were expressed in the dermal papilla cells and the outer-root-sheath cells located in the hair bulb region, in 3-week-old mouse dorsal skin (in the anagen phase). In the telogen phase, however, these signals had disappeared. On days 8 and 12 after depilation, the mRNA were reexpressed, and induction of cyclooxygenase (COX)-2 mRNA was also observed, suggesting that prostaglandin receptors are involved in the development and regrowth of the hair follicles.
Previous studies have also shown that bimatoprost appears to have a more substantial effect on eyelash growth than latanoprost. Bimatoprost 0.03% is a synthetic prostamide analogue that has a chemical structure similar to the PGF2a analogs with a free acid identical to latanoprost except for a single bond instead of a double bond at one of the carbon positions. In a six-month randomized clinical trial by Noecker et al comparing bimatoprost and latanoprost, eyelash growth was noted in 14 of 133 patients in the bimatoprost group versus no patients in the latanoprost group.9 Other studies by Eisenberg et al and Tosti et al have found similar results, also reporting that hypertrichosis usually appears earlier with bimatoprost than with latanoprost.10, 11 Given these findings, we were interested in evaluating the effect of bimatoprost applied to the eyelashes on eyelash length.
There has been growing interest among cosmetic companies as more and more products are being released on the market claiming to promote eyelash growth. Many of these products contain prostaglandin analogs. Recently, Allergan released Latisse, the first FDA-approved treatment for hypotrichosis of the eyelashes. There has been no previously published randomized controlled trial comparing the effect of monocular application of a prostaglandin or prostamide analogue to application of a control solution on eyelash length when applied to the base of the eyelashes.
METHODS
This prospective, randomized controlled double blind study was approved by the Institutional Review Board at the University of Miami and followed the tenets of the Declaration of Helsinki. Funding was provided by the Bascom Palmer Eye Institute, University of Miami. The clinical trial was registered at www.clinicaltrials.gov (trial number NCT00773136, accessed October 15, 2008). Through study advertisement with IRB approved flyers at the Bascom Palmer Eye Institute, 23 subjects were recruited for initial evaluation. Study recruitment and enrollment began in February 2008 and the study was completed in July of 2008, including visits up to 3 months after cessation of the gel suspension. Exclusion criteria included history of glaucoma, uveitis, pregnancy, and allergic reaction to prostaglandins or the gel suspension. Of the 23 subjects screened, 2 were not eligible due to underlying conditions (1 was diagnosed with glaucoma during the initial screening and 1 had a previous history of an idiopathic choroidal neovascular membrane). Two additional subjects did not complete enrollment and thus were not included in the study. Four subjects withdrew from the study during the trial period and one subject had inconsistent use. All 19 subjects who were randomized in the trial were included in the analysis up to the point of withdrawal in an intent-to-treat analysis. We aimed to recruit about 20 subjects given that a sample size of 18 is adequate to find a large effect size with a type 1 error of 0.05 and a power of 0.90.12
Enrolled subjects received two vials of gel suspension, one of which contained bimatoprost 0.03% (therapeutic intervention) and one of which contained normal saline (control group), each mixed 1:1 with Gonak™. Gonak is Hydroxypropyl Methylcellulose in 2.5% solution and is used to lubricate the cornea for application of ophthalmic lenses. In a pre-study comparison, the investigators found that the bimatoprost solution was not well miscible in petroleum based gels and appeared to mix well with Hydroxypropyl Methylcellulose, which is why this solution was chosen. The tubes were identical in appearance and were labeled “right eye” and “left eye” and were prepared and recorded in a double-blind fashion. Random allocation was performed by study personnel prior to subject enrollment by individual assignment, and containers were labeled with study numbers and “right eye” or “left eye” and recorded in a separate database which identified which tube contained the bimatoprost solution for each study number. These data were concealed until study completion. Subjects were each given study numbers by the trial administrators which corresponded to the previous randomization and preparation of the solutions. The database with the allocation sequence was kept in a separate locked locker until study completion and no participants had access to this data. The subjects were also given matching labeled brushes. Subjects were instructed to shake the tube and then apply the gel suspension to base of the respective upper eyelashes every evening for six weeks using the matching labeled brushes. Each subject had weekly follow-up during the six weeks of gel application and at one month and three months after discontinuing its use. Lash length was measured using surgical calipers, and photos were taken during the study. The longest measured eyelashes were plucked from the upper eyelid of 14 of the 15 total enrolled patients at the end of the six weeks for comparison (although this data was not used in the comparative analysis as there was no baseline measurement of plucked eyelashes). Throughout the study, we used the same measuring technique with only two investigators performing the measurements and always measuring the length of the longest lash from the base of the lash to the eyelash tip (without any manual straightening of the lash) using a surgical caliper. The caliper was marked to the nearest millimeter, and the reader interpolated to the nearest tenth of a millimeter. At the conclusion of the study, we also performed a reproducibility study to assess inter and intra-reader reproducibility by having two examiners measure seven individuals’ eyelashes (a total of 14 lashes) seven days apart masked to their own readings and those of the other reader. Visual acuity, ocular symptoms, intraocular pressure and slit lamp exam were recorded at these same intervals.
The eyelash length difference was compared between the treated and the untreated eyes at baseline and at the different follow-up times using paired t-tests. The changes from baseline to follow-up were also compared using paired t-tests. The measurements of eyelash length and IOP were done by personnel masked to which eye received bimatoprost or the control suspension.
RESULTS
A total of 19 subjects with 38 eyes were recruited in the study. All participants were female. The average age of the subjects was 46 years old (Range: 23–69). A total of 10 right eyes (52.6%) and 9 left eyes (47.4%) were treated with gel suspension mixed with bimatoprost.
The mean length of the eyelashes at the beginning of the study was 6.6mm in the bimatoprost group and 6.6mm in the control group, a difference which was not significantly significant (p=0.77)(Table 1). The average eyelash growth at 6 weeks was 2.0 mm in the bimatoprost group which was highly significant with p<0.001. Interestingly, the eyelashes in the eyes with the control gel suspension also grew, with an average growth of 1.1 mm, which was also statistically significant with p=0.001. Although the eyelashes grew in both groups, the amount of eyelash growth was higher in the bimatoprost group (p=0.009) (Table 2). A graphical depiction of the data shows a clear trend (Figure 1).
Table 1.
Baseline of two randomized groups
| Bimatoprost Group | Control Group | Mean Difference ± SD | P-value* | |||
|---|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | |||
| Eyelash Length | 19 | 6.6±1.1 | 19 | 6.6±1.1 | 0 ± 0.5 | 0.77 |
| IOP | 19 | 17±3 | 19 | 17±2 | 0 ± 1 | 0.76 |
Paired t-test.
SD = Standard Deviation
IOP = Intraocular Pressure
Table 2.
Change in Eyelash Length
| Bimatoprost Group | Control Group | Mean Difference± SD | P-value* | |||||
|---|---|---|---|---|---|---|---|---|
| N | Changes (mean±SD) | P-value | N | Changes (mean±SD) | P-value | |||
| Changes from Baseline to Cessation of Gel Application (6 weeks) | 15 | 2.0±1.5 | <0.001 | 15 | 1.1±1.1 | 0.001 | 0.9 ± 1.1 | 0.009 |
| Changes from Baseline to 1 month after Cessation | 13 | 1.9±1.3 | <0.001 | 13 | 1.0±1.1 | 0.009 | 0.9 ± 1.2 | 0.013 |
| Changes from Baseline to 3 Month after Cessation | 15 | 1.9±1.2 | <0.001 | 15 | 0.8±1.2 | 0.017 | 1.1 ± 0.7 | <0.001 |
| Changes from Cessation of Gel Application to 3 Months after Cessation | 15 | −0.1±0.6 | 0.58 | 15 | −0.3±0.7 | 0.09 | 0.2 ± 1.0 | 0.39 |
Paired t-test.
SD = Standard Deviation
Figure 1.
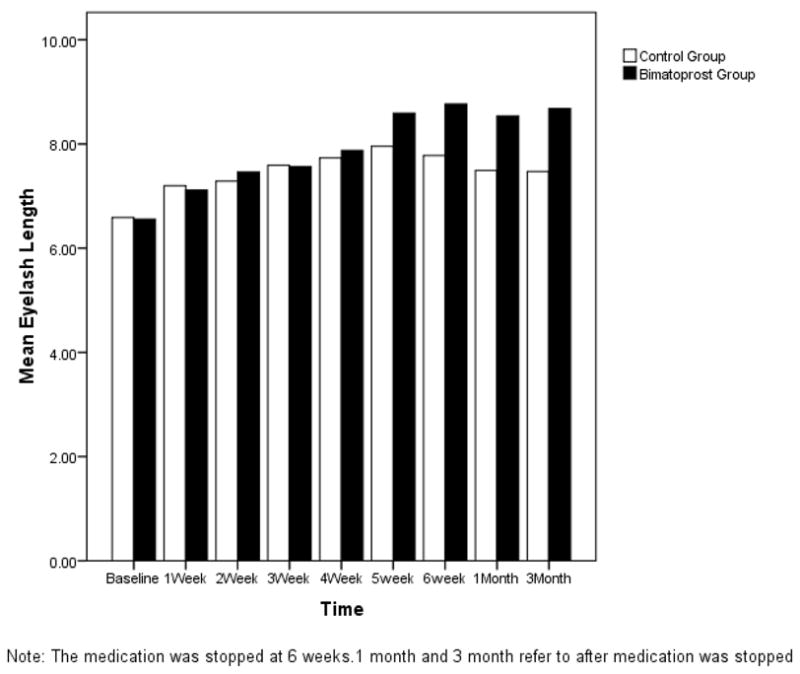
Change in Eyelash Length over Time
In order to ensure the lash measurement changes were not subject only to chance, we also performed a post-study reproducibility study to examine inter and intra-examiner variability in lash measurements. Two investigators measured seven individuals’ eyelashes (a total of 14 lashes), performing two separate measurements seven days apart masked to their own readings and those of the other reader. The intra-reader reproducibility was excellent.13 For reader 1, the intraclass correlation (ICC) coefficient was .98 for the right eye and .99 for the left; for reader 2 the ICC was .98 for the right eye and .96 for the left. The inter-reader reproducibility was also excellent. For day 1, the ICC was .97 for the right eye and .96 for the left; for day 2, the ICC was .96 for the right and for the left eye. The coefficient of variation was also calculated for measurements for each person, it averaged 2.1% for right eyes and 2.4% for left eyes.
At three months after treatment completion, the mean length of eyelash decreased 0.3 mm in the control group vs. 0.1mm in the bimatoprost group which was not statistically significant with p=0.09 and p=0.58 respectively. The amount of eyelash length decrease in the control group was a little higher than in bimatoprost group but there was no statistically significant difference (p=0.39) (Table 2). A representative case is highlighted in Figure 2, showing the initial eyelash length as well as the changes at two weeks, three weeks, five weeks and at three months after cessation of the gel application (Figure 2).
Figure 2.
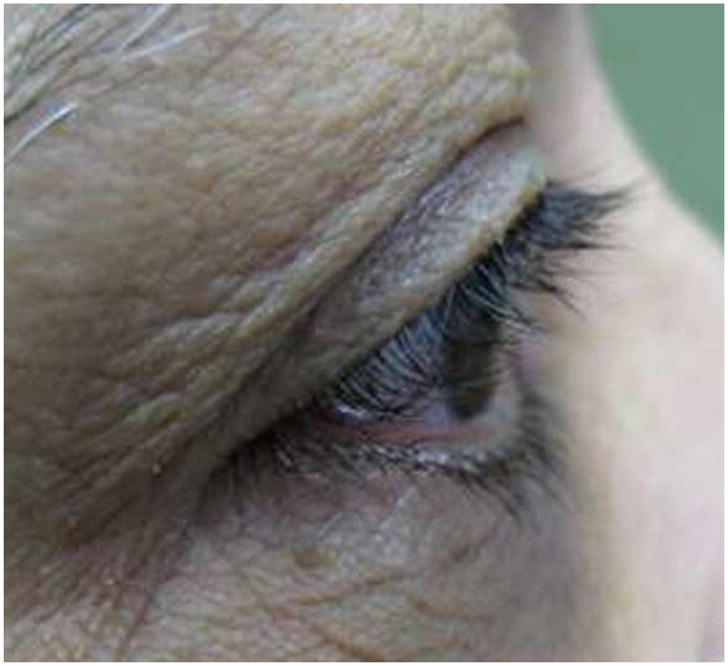
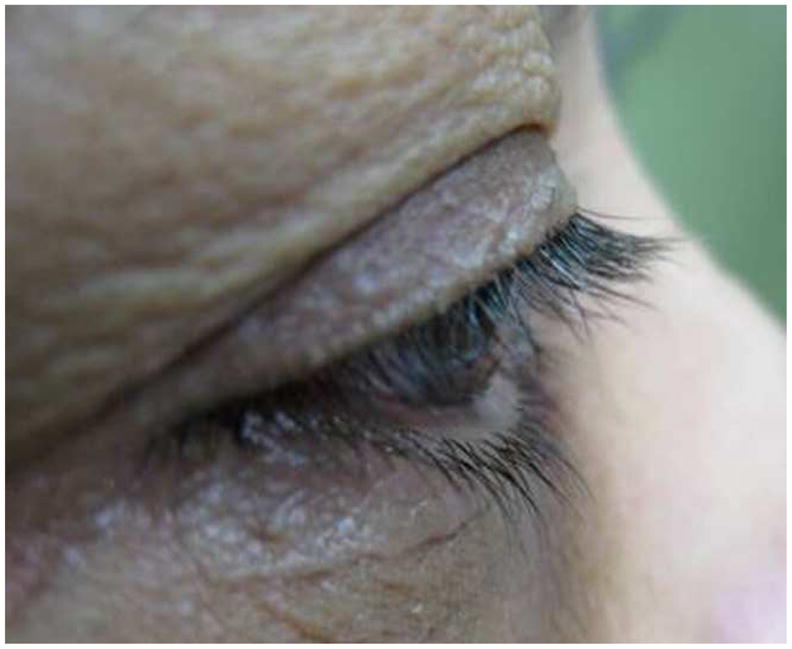
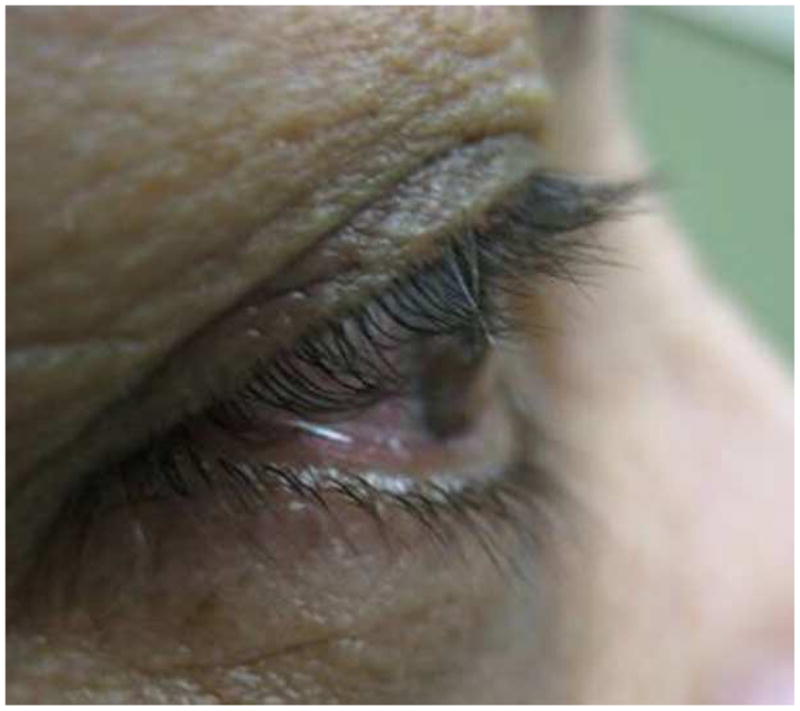
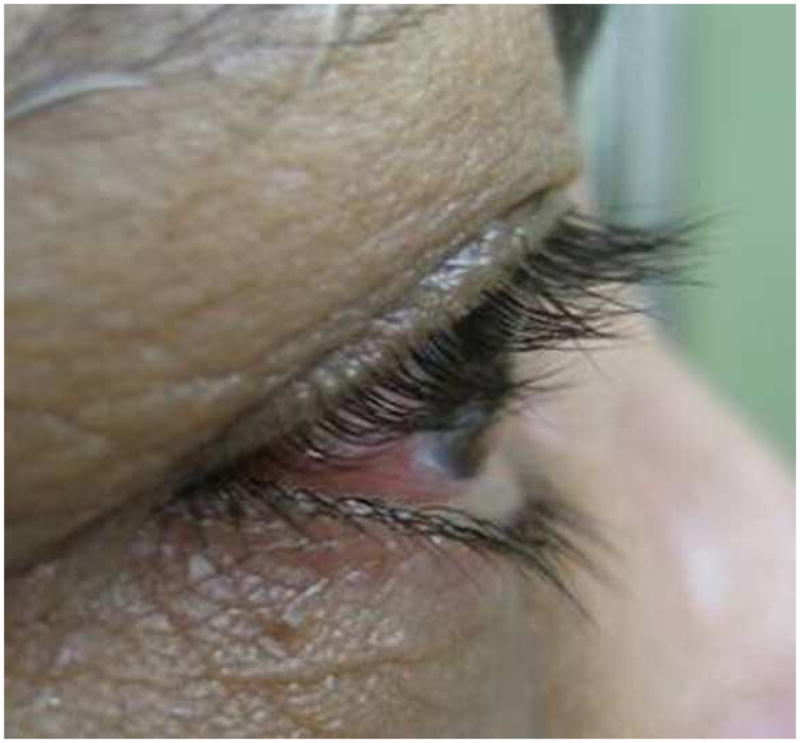
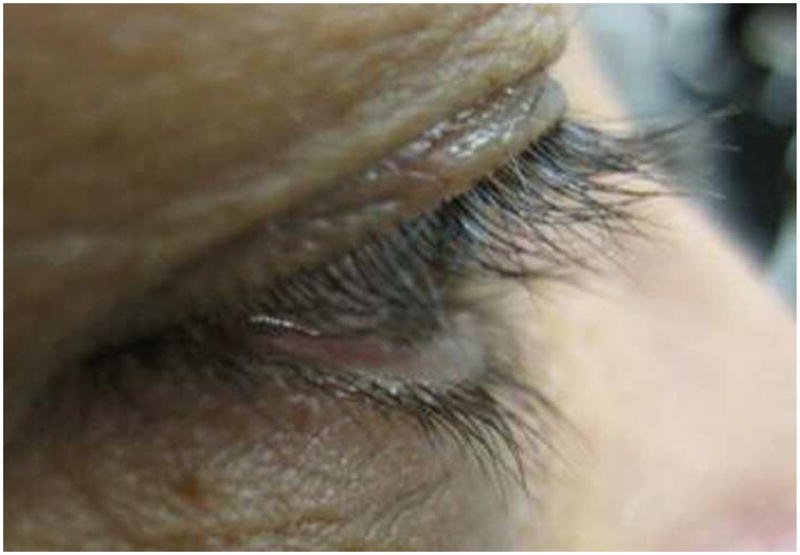
Pictures demonstrating lash growth in subject 6 at several study intervals
a) Pre-study photo
b) 2 Weeks after treatment
c) 3 Weeks after treatment
d) 5 Weeks after treatment
e) 3 Months after cessation of treatment
There was one patient in whom the eyelashes grew more in the eye with the control than the one with bimatoprost. Both eyelashes had significant growth (2.4mm in the bimatoprost eye, and 3.4mm in the control) and the patient later admitted to switching the tubes. The intraocular pressure decreased from 20 to 14 in the control eye (which was the eye that had more eyelash growth) versus 20 to 17 in the eye randomized to bimatoprost. This asymmetric IOP difference supports the patient’s admission that the suspensions were switched, although as mentioned below the IOP was noted to have decreased in both groups, so the power of this finding is limited.
There was another patient whose eyelashes both were measured to be shorter at the end of the study. There was also one patient with shorter eyelashes in the control eye at the end of the study. This patient had 0.7mm of eyelash growth in the eye with the bimatoprost and 0.4mm loss of eyelash length in the control.
The mean intraocular pressure at the beginning of the study in the bimatoprost group was 17mmHg versus 17mmHg in the control group. There was no statistical difference between the two groups (p=0.76) (Table 1). At the cessation of application of the suspension, there was a 2 mmHg average decrease in intraocular pressure in the control group which was highly significant with p=0.008. The average intraocular pressure decrease in the bimatoprost group was the same at 2mmHg, which was also significant with p=0.022. Since both groups had the same average decrease, obviously there was no difference between them in terms of change in intraocular pressure (p=1.00). (Table 3)
Table 3.
Change in Intraocular Pressure
| Bimatoprost Group | Control Group | Mean Difference ± SD | P-value* | |||||
|---|---|---|---|---|---|---|---|---|
| N | Changes(mean±SD) | P- value | N | Changes (mean±SD) | P-value | |||
| Changes from Baseline to Cessation of Gel Application (6 weeks) | 14 | −2±3 | 0.022 | 14 | −2±3 | 0.008 | 0 ± 2 | 1.00 |
| Changes from Baseline to 1 month after Cessation | 11 | −1±4 | 0.224 | 11 | −1±3 | 0.24 | 0 ± 2 | 0.553 |
| Changes from Baseline to 3 Month after Cessation | 14 | −2±2 | 0.005 | 14 | −3±3 | 0.004 | 1 ± 2 | 0.405 |
| Changes from Cessation of Gel Application to 3 Months after Cessation | 13 | 0±3 | 0.616 | 13 | −1±3 | 0.49 | 1 ± 1 | 0.016 |
Paired t-test
SD = Standard Deviation
At three months after cessation of gel application, there was still an average decrease of 2mmHg from baseline in intraocular pressure in the bimatoprost group (p=0.005). In the control group, the average decrease from baseline was 3mmHg (p=0.004) at three months after cessation of gel application. There was no significant difference in the amount of intraocular pressure decrease between groups (as shown in Table 3 and Figure 3) (p=0.41).
Figure 3.
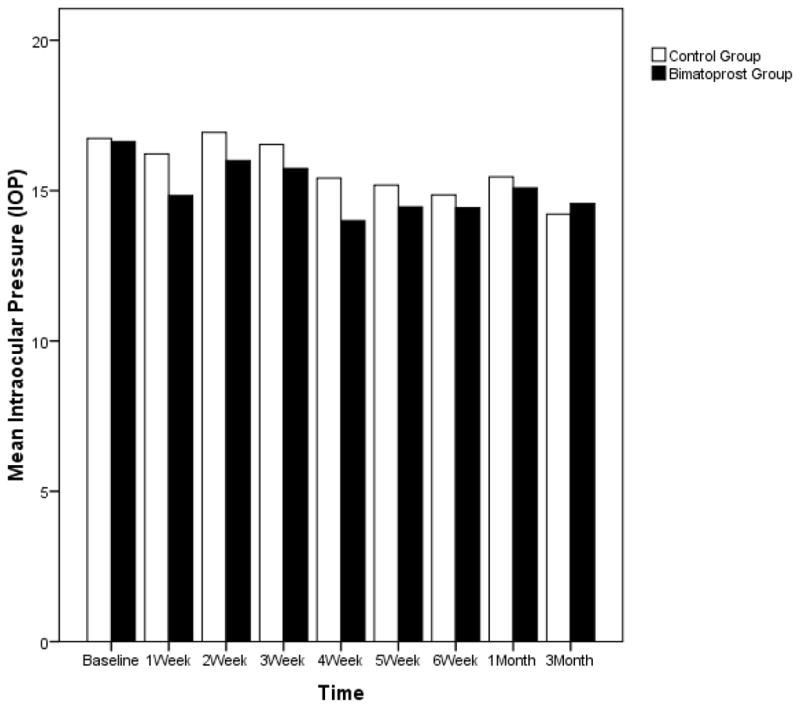
Change in Intraocular Pressure (IOP) over Time
The median visual acuity was 20/20 in both groups at the onset of the study. No patients had any change in visual acuity, subjective or objective, throughout the study. No patients had objective or subjective documentation of iris pigmentary changes throughout the study. One patient stated that she had temporary pigmentation of the lower eyelids, to which she applied fade cream, and which disappeared prior to her next follow-up visit. She wished to continue the study despite this pigmentation and had no further recurrence. One patient stated that she developed an additional row of hair above her eyelashes which she shaved and did not recur. She removed the hairs between visits and thus there was no photo documentation of this. Of note, vellus hairs were noted above the eyelashes on the opposite eyelid. On examination of these vellus hairs, she stated that these were the same hairs she noticed on the eyelid and that they may have been present previously. She also chose to continue with the study and had no recurrence of these “abnormal” lashes.
Four subjects withdrew from the study during the study period. Of these four subjects, two stated they withdrew because of complaints of irritation, one of whom noted significantly worse symptoms in one eye. Neither of these had conjunctival injection or eyelid margin inflammation noted on exam. In addition, after completion of the study and unmasking, we discovered that the subject with asymmetric complaints had more irritation in the eye that received the control suspension (evidence for successful masking among participants). The other subject later stated that she withdrew because of fear of pigmentary changes and denied actual symptoms of irritation. The other subject who withdrew had significant meibomian gland disease and blepharitis at baseline as well as a history of extremely sensitive eyelid skin and allergies. She noted worsening symptoms with use of the gel suspension on both eyes. The final subject who withdrew did so because of concerns of possible future side effects, but had no complaints at the time of withdrawal.
DISCUSSION
Bimatoprost and several other prostaglandin analogs have been shown to cause increased lash length and pigmentation as a side effect of ocular surface application of the medications.1–7 Based on these data, several cosmetics companies have released products, some of which contain prostaglandin analogs, as non-drug cosmetics, not regulated by the Food and Drug Administration (FDA), including Age Intervention Eyelash by Jan Marini Skin Research, RevitaLash by Athena Cosmetics Corp. and MD Lash Factor by PhotoMedex Inc.
Some of these products, such as Age Intervention Eyelash, were seized by the FDA in November of 2007 as an “unapproved and misbranded drug.”14, 15 Recently, Allergan announced the release of Latisse, the first FDA-approved treatment for hypotrichosis of the eyelashes which was clinically tested in a Phase III study to assess safety and efficacy.16 Of note, as detailed in the Latisse prescribing insert, Latisse contains the active ingredient bimatoprost 0.3 mg/mL, which is the same active ingredient used in our solution. Inactive ingredients in Latisse include the preservative benzalkonium chloride 0.05 mg/mL, sodium chloride; sodium phosphate, dibasic, citric acid, purified water and sodium hydroxide and/or hydrochloric acid may be added to adjust the pH. Our preparation included the same active ingredient bimatoprost 0.3mg/ml as well as the same inactive ingredients (all of which are part of the Lumigan solution), but we used Hydroxypropyl Methylcellulose 2.5% (Gonak) as an additional inactive ingredient. The Latisse study showed a statistically significant greater improvement in subjects treated with Latisse than those in the placebo group in the measurements of eyelash prominence, length, thickness and darkness (Subjects were randomized to bilateral treatment of either vehicle or control). At the time of our study, this study had not been completed and there had been no previously published randomized controlled double blind prospective study of the effect of prostaglandin analogs applied in a gel suspension to the base of the eyelashes on eyelash growth. Our study was also unique in comparing monocular treatment versus control solution for each patient whereas the Allergan trial randomized both eyelashes of each subject to placebo versus control.
We found a statistically significant growth of 2.0 mm in eyelash length in the eyes with bimatoprost gel suspension applied to the eyelash. Our reproducibility study confirmed that the variation was not likely to be attributed to inter-rater or intra-rater variation in measurement.
Interestingly, we also noted a statistically significant growth of eyelashes in eyes with the control gel suspension applied to the eyelash, although the growth was less at only 1.1 mm. The difference in eyelash growth between the control and therapeutic intervention of 0.9mm was noticeable to patients and is demonstrated in Figure 3. In addition, in the recent study by Allergan, the average eyelash growth (mean change from baseline) was 1.4mm in the Latisse group (a 25% increase), which was found to be significant. This was compared to 0.1mm growth in the vehicle group. These similar findings support the clinical significance of our findings.
Our finding of eyelash growth in the control eyes was more significant than that in the Allergan study and is worthy of mention. It is possible that the increased lash growth noted in the control eyes may have been due to hydration of the lashes with the suspension. Gonak is Hydroxypropyl Methylcellulose in 2.5% solution and is used to lubricate the cornea for application of ophthalmic lenses. Given its lubricating properties, it may possibly have caused hydration and lubrication of the eyelashes and contributed to growth. Systemic absorption may also have contributed to the bilateral eyelash growth, supported by the fact that intraocular pressure decreased in both eyes. The limited amount of medication and focal delivery on the base of the eyelash, however, make that a less likely explanation. Wrong eye application as well as chance may have contributed to the increased growth of lashes as well as the decrease in intraocular pressure in control eyes. One subject admitted to applying what she thought was the “medicated suspension” to the other eye so that the lash growth would be more symmetric, which was also supported by the lower intraocular pressure in the eye that was supposed to be the control eye. It is possible that other subjects did this as well, although they denied it. In addition, it is possible that subjects mixed up brushes and mistakenly applied the bimatoprost suspension to the incorrect eye. Other possible factors that may have contributed to eyelash lengthening in the control eyes include weekly fluctuation in eyelash length, researcher variability, and caliper inaccuracy. As mentioned, we epilated lashes in both the control and treatment groups at the end of the six week application period. Interestingly, we found that the difference between eyelash length in the eyes treated with lumigan versus the control length when using the epilated lashes was 1.4 mm. This is interesting as the “difference” between the control and lumigan growth using non-epilated lashes was 0.9mm. This suggests an even greater difference noted between the two groups when using epilated lash measurements. Future studies may benefit from epilation prior to initiation of treatment as well as there may be a slight reduction in variation due to measurement when the lashes are epilated.
We found no significant ocular adverse events in subjects that completed the study. Several studies have documented the ocular side effects of topical ocular prostaglandin application. 3,17, 18 A study by Sherwood et al. demonstrated the most common side effects of topical ocular application of prostaglandin analogs.19 These included mild hyperemia of the eye in approximately 31% of patients, ocular pruritis in about 14% of patients, and ocular dryness in 7% of patients. Less common side effects (1% of patients) included hyperpigmentation, swelling of periorbital skin and heterochromia, a permanent darkening of the iris more commonly seen in blue/brown or green/brown irises. As mentioned, there were no periocular or iris pigmentation changes documented on our study visits, however two patients did note subjective periocular changes which they self-treated in the interim period between visits and which did not recur. Neither subject felt the changes were significant enough that they wanted to withdraw from the study. In fact, both subjects wanted to continue use of the product on both eyes after study completion. In addition, there was no subjective or objective change in visual acuity documented in any of the patients.
It is important to note as well, that two patients did withdraw because of complaints of irritation. On later discussion with these patients, one patient stated she withdrew because of fear of pigmentary changes and denied actual symptoms of irritation. The other patient had complained of worse symptoms in the eye that had been receiving the control suspension. In addition, on exam, no conjunctival injection or eyelid margin inflammation was noted. Another subject who withdrew had significant meibomian gland disease and extremely sensitive eyelid skin at baseline to such an extent that she was unable to wear any eye makeup. She noted worsening symptoms with use of the gel suspension on both eyes.
In addition, several subjects did note sporadic lengthening of certain lashes in an asymmetric fashion, two of which admitted to self-epilation to even out the eyelash appearance. This effect may be due to uneven mixture of the gel solution, inherent properties in the prostaglandin analogs, or uneven application of the solution. The possibility of inconsistent lash growth may be secondary to unequal distribution of the solution either from imperfect miscibility or uneven application. It also may be secondary to the natural variation in the phases of hair growth of the eyelashes and thus should be noted as a possible side effect.
A decrease in intraocular pressure was noted in both the control and bimatoprost eyes. This is possibly due to systemic absorption of the solution with bimatoprost, although it is unlikely due to the focal nature of application. Diurnal fluctuations were also possible contributing factors, although most subjects had follow up visits at the same time of the morning as the initial visit. Wrong eye application, as discussed previously, may have also contributed to the symmetric decrease in intraocular pressure that was noted. This is difficult to quantify because only one patient admitted to wrong eye application.
At three months after treatment completion, the mean eyelash length in the bimatoprost group decreased an average of only 0.1 mm from the study completion length. This suggests the possibility of a longer term effect on eyelash length.
Our study supports the hypothesis that gel suspension mixed with bimatoprost has eyelash lengthening properties. While we had no adverse ocular events in the subjects that completed the study, a larger study would help assess the incidence of ocular irritation and side effects related to the use of these and similar products for eyelash lengthening properties. In addition, this randomized controlled study evaluated patients after only 6 weeks of application of the drug. Additional studies are needed to assess longer term effects of treatment. As mentioned previously, Allergan recently announced Federal Drug Administration (FDA) approval of Latisse bimatoprost ophthalmic solution, which was launched earlier this year. In their study, full results were seen at 16 weeks, suggesting longer term application may show even more growth. In our study, increased lash length was sustained 3 months after cessation of the medication. A study with long term follow up beyond 3 months may reveal if the lash length recedes after a given time.
Our finding of increased lash growth in control eyes may be due to a variety of previously highlighted confounding factors. It would be interesting, however, to evaluate the effect of Hydroxypropyl Methylcellulose applied to the eyelashes on lash length. It is possible that the Hydroxypropyl Methylcellulose solution has a hydrating effect which indirectly contributes to eyelash lengthening. Given these findings, follow up studies comparing bilateral intervention versus control groups in addition to the monocular intervention versus control would be interesting to assess this effect.
This short study showed a statistically significant increase in eyelash length from application of bimatoprost to the base of the lashes, supporting its use as a cosmetic pharmaceutical. Application to the base of the eyelashes not only showed the benefit of lash lengthening, but also demonstrated the advantage of avoiding other unwanted side effects often seen with topical use of the drug to the ocular surface, such as skin hyperpigmentation and hyperemia. Although we had no adverse events in our small study, given the properties of prostaglandin analogs and the potential for side effects, we recommend evaluation, prescription and monitoring by an ophthalmologist if use of such a product is going to be considered, especially in the growing cosmetic industry where many non-physicians will be eager to recommend such treatment.
Acknowledgments
Supported by grant EY014801 from the National Eye Institute, National Institutes of Health, Bethesda, Maryland, and by Research to Prevent Blindness, Inc., New York, New York.
Footnotes
All authors have no financial or proprietary interest in any products or devices mentioned in this article.
Meeting Presentation: Skin Disease Education Foundation, February 2009
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997;124:544–7. doi: 10.1016/s0002-9394(14)70870-0. [DOI] [PubMed] [Google Scholar]
- 2.Sugimoto M, Sugimoto M, Uji Y. Quantitative analysis of eyelash lengthening following topical latanoprost therapy. Can J Ophthalmol. 2002;37:342–5. doi: 10.1016/s0008-4182(02)80004-7. [DOI] [PubMed] [Google Scholar]
- 3.Chiba T, Kashiwagi K, Ishijima K, et al. A prospective study of iridial pigmentation and eyelash changes due to ophthalmic treatment with latanoprost. Jpn J Ophthalmol. 2004;48:141–7. doi: 10.1007/s10384-003-0039-6. [DOI] [PubMed] [Google Scholar]
- 4.Elgin U, Batman A, Berker N, Ilhan B. The comparison of eyelash lengthening effect of latanoprost therapy in adults and children. Eur J Ophthalmol. 2006;16:247–50. doi: 10.1177/112067210601600209. [DOI] [PubMed] [Google Scholar]
- 5.Uno H, Zimbric ML, Albert DM, Stjernschantz J. Effect of latanoprost on hair growth in the bald scalp of the stump-tailed macaque: a pilot study. Acta Derm Venereol. 2002;82:7–12. doi: 10.1080/000155502753600803. [DOI] [PubMed] [Google Scholar]
- 6.Mansberger SL, Cioffi GA. Eyelash formation secondary to latanoprost treatment in a patient with alopecia. Arch Ophthalmol. 2000;118:718–9. doi: 10.1001/archopht.118.5.718. [DOI] [PubMed] [Google Scholar]
- 7.Michelet JF, Commo S, Billoni N, et al. Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair growth-stimulating effect. J Invest Dermatol. 1997;108:205–9. doi: 10.1111/1523-1747.ep12334249. [DOI] [PubMed] [Google Scholar]
- 8.Torii E, Segi E, Sugimoto Y, et al. Expression of prostaglandin E(2) receptor subtypes in mouse hair follicles. Biochem Biophys Res Commun. 2002;290:696–700. doi: 10.1006/bbrc.2001.6256. [DOI] [PubMed] [Google Scholar]
- 9.Noecker RS, Dirks MS, Choplin NT, et al. Bimatoprost/Latanoprost Study Group. A six-month randomized clinical trial comparing the intraocular pressure-lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J Ophthalmol. 2003;135:55–63. doi: 10.1016/s0002-9394(02)01827-5. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg DL, Toris CB, Camras CB. Bimatoprost and travoprost: a review of recent studies of two new glaucoma drugs. Surv Ophthalmol. 2002;47(suppl):S105–15. doi: 10.1016/s0039-6257(02)00327-2. [DOI] [PubMed] [Google Scholar]
- 11.Tosti A, Pazzaglia M Voudouris S, Tosti G. Hypertrichosis of the eyelashes caused by bimatoprost. J Am Acad Dermatol. 2004;51(suppl):S149–50. doi: 10.1016/j.jaad.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: L. Erlbaum Assoc; 1988. pp. 27–40. [Google Scholar]
- 13.Fleiss JL. Statistical Methods for Rates and Proportions. 2. New York: Wiley; 1981. pp. 223–4. [Google Scholar]
- 14.Rundle RL. Drug that lengthens eyelashes sets off flutter. [Accessed May 26, 2009];Wall Street Journal. 2007 November 19;B1 Available at: http://online.wsj.com/article/SB119543055372597359.html.
- 15.MSNBC: FDA Seizes $2 Million Of Potentially Harmful SJ Eye Product KNTV-TV 2:44 p.m. ET, Sat. [Accessed June 22, 2009];2007 Nov 17; Available at: http://www.lipotreatmentfacts.org/downloads/WiredScienceBlog_11-2007.pdf.
- 16.Irvine, CA: Allergan; Dec 26, 2008. [Accessed May 26, 2009]. Allergan announces U.S. Food And Drug Administration (FDA) approval of Latisse--first and only treatment approved by the FDA for hypotrichosis of eyelashes [press release] Available at: http://agn.client.shareholder.com/releasedetail.cfm?ReleaseID=356159. [Google Scholar]
- 17.Grierson I, Jonsson M, Cracknell K. Latanoprost and pigmentation. Jpn J Ophthalmol. 2004;48:602–12. doi: 10.1007/s10384-004-0110-y. [DOI] [PubMed] [Google Scholar]
- 18.Herndon LW, Williams RD, Wand M, Asrani S. Increased periocular pigmentation with ocular hypotensive lipid use in African Americans. Am J Ophthalmol. 2003;135:713–4. doi: 10.1016/s0002-9394(02)02146-3. [DOI] [PubMed] [Google Scholar]
- 19.Sherwood M, Brandt J. Bimatoprost Study Groups 1 and 2. Six-month comparison of bimatoprost once-daily and twice-daily with timolol twice-daily in patients with elevated intraocular pressure. Surv Ophthalmol. 2001;45(suppl):S361–8. doi: 10.1016/s0039-6257(01)00219-3. [DOI] [PubMed] [Google Scholar]


