Abstract
Environmental exposures suspected of contributing to the pathophysiology of Parkinson’s disease (PD) include potentially neurotoxic pesticides, which have been linked to an increased risk of PD. Conversely, possible protective factors such as the adenosine antagonist caffeine have been linked to a reduced risk of the disease. Here we assessed whether caffeine alters dopaminergic neuron loss induced by exposure to environmentally relevant pesticides (paraquat and maneb) over 8 weeks. The number of nigral neurons positive for tyrosine hydroxylase immunoreactivity (TH+) was assessed using stereological methods and found to be significantly reduced (to 60% of control) by combined pesticide treatment. Caffeine at 20 mg/kg significantly reduced TH+ neuron loss (to 85% of the respective control). The results demonstrate the neuroprotective potential of caffeine in a chronic pesticide exposure model of model of PD.
Keywords: substantia nigra, striatum, mouse, stereology, pesticide, Parkinson’s disease, caffeine
Parkinson’s disease (PD) is a chronic neurodegenerative condition with cardinal clinical features of rest tremor, rigidity and bradykinesia attributed to an underlying neurodegeneration of dopaminergic neurons of the substantia nigra. Evidence suggests both genetic and environmental risk factors for PD (Warner and Shapira, 2003; Mizuno et al., 1999). Non-genetic factors appear to be prominent in the majority of sporadic PD patients, in whom typical symptoms develop after age 50. Twin studies (Tanner et al., 1987; Wirdefeldt et al., 2004) have generally found indistinguishable concordance rates for PD diagnosed after age 50 between monozygotic and dizygotic twins, supporting a prominent role for environmental risk factors.
Amongst environmental factors, exposure to pesticides has been linked to an increase in the risk of PD (Chade et al., 2006; Tanner et al., 2009; Costello et al., 2009). In addition to case-control studies, several prospective epidemiological investigations have supported an elevated risk of developing PD amongst those exposed to pesticides. In Hawaiian men of Japanese ancestry the relative risk of developing PD tended to be increased (though did not reach statistical significance) for those individuals exposed to pesticides (Petrovitch et al., 2002). In an investigation of elderly subjects residing in France a significant increase in the relative risk of PD among men, but not women, was associated with occupational pesticide exposure (Baldi et al., 2003).
Recently, a large prospective epidemiological study (Ascherio et al., 2006) of both men and women in the US found that current or regular past exposure to pesticides was associated with an increased risk for developing PD. Pesticide use was associated with a 70% increased risk of developing PD amongst men and women whose pesticide exposure history was reported prior to diagnosis. Though this study addressed the concern of recall bias that limited interpretation of prior studies, it did not identify specific pesticides as potential environmental risk factors for PD. While most studies have shown a positive association between pesticide exposure and PD, no specific single agent has been implicated consistently. The herbicide paraquat (PQ) has emerged as a putative human risk factor based on epidemiologic and occupational exposure data (Hertzman et al., 1990; Liou et al., 1997; Tanner et al., 2009) as well as experimental cell (McCarthy et al., 2004) and rodent data (Brooks et al., 1999; McCormack et al., 2002; Prasad et al., 2007).
Exposures to pesticides such as PQ are likely to occur in combination with other pesticides, as single agents are often applied in overlapping geographical areas (Thiruchelvam et al., 2000a). For example, diethyldithiocarbamates of which maneb (MB) is a member, are heavily used alongside PQ in certain parts of the US (United States Geographic Service 1998). Occupational exposure to MB has been anecdotally linked to cases of parkinsonism in humans (Ferraz et al., 1988; Meco et al., 1994).
Thiruchelvam et al., 2000a,b established a dual pesticide model of environmental parkinsonism in mice based on repeated systemic exposure to a combination of PQ and MB. Co-administration of these toxins was shown to produce selective loss of nigrostriatal dopaminergic neurons and loss of dopamine in the striatum. In addition to its potential environmental significance, this ‘dual pesticide’ mouse model entails exposure to PQ and MB over months and thus parallels the long term exposure in humans to putative environmental neurotoxicants, further enhancing its utility in the characterization of promising neuroprotective candidates for PD.
In contrast to pesticide exposure, the consumption of coffee and other caffeinated beverages has been repeatedly linked to a reduced risk of developing PD (Xu et al., 2005). Caffeine itself appears to be the component that accounts for the association given that caffeinated, but not decaffeinated coffee was found to be associated with a lower PD risk in a large prospective study (Ascherio et al., 2001).
Laboratory studies from our and other groups have complemented these epidemiologic findings to suggest that the inverse association between caffeine and PD risk may be due to a direct neuroprotective action of caffeine. We found caffeine at a dose in mice corresponding to as little as a single cup of coffee in humans could significantly attenuate the loss of striatal dopamine induced by acute exposure to the dopaminergic neuron toxin MPTP (Chen et al., 2001). Similarly, caffeine can reduce nigrostriatal neuron injury triggered by a single dose of locally administered 6-OHDA in rats (Joghataie et al., 2004; Aguiar et al., 2006). The biological plausibility of a neuroprotective action of caffeine in PD was strengthened by the demonstration that antagonists of the adenosine A2A (but not A1) receptor mimic the effects of caffeine in these acute MPTP and 6-OHDA toxin models of PD (Chen et al., 2001; Ikeda et al., 2002; Pierri et al., 2005; Bove et al., 2005). Genetic disruption of the A2A receptor similarly attenuated acute neurotoxicity in the MPTP mouse model of PD (Chen et al., 2001).
These convergent epidemiologic and laboratory data have prompted the inclusion of caffeine on a short list of candidate neuroprotective agents warranting consideration for disease-modifying therapy in clinical neuroprotection trials for PD (Ravina et al., 2003). However, the recent failure of several clinical neuroprotection trials of agents that had emerged as promising candidates from the preclinical pipeline for PD has prompted a reexamination of the animal models supporting the pipeline as well as the clinical trial methodology used to test the candidates (Hung et al., 2007; Suchowersky et al., 2006). These concerns have encouraged the development and use of additional animal models of PD (beyond the historical acute toxin models), with particular emphasis on models that are more intuitively relevant to PD, for example, because they better mimic the pathology or time course of the disease (Fornai et al., 2005; Anderson et al., 2006). Accordingly, the current study seeks to better assess the neuroprotective potential of caffeine by testing its ability to alter the dopaminergic nigral neuron degeneration induced by chronic exposure to pesticides (Thiruchelvam et al., 2000a,b.).
Two-month-old male C57BL/6NCrl mice were obtained from Charles River Laboratories; Wilmington, MA and housed under a 12:12 hr light:dark cycle. Food and water was provided ad libitum. All experiments were performed in accordance with Massachusetts General Hospital and NIH guidelines on the ethical use of animals, with adequate measures taken to minimize pain and discomfort. Mice were injected i.p. with saline (vehicle), caffeine 5mg/kg or caffeine 20 mg/kg (Sigma), followed 10 minutes later by a pair of i.p. injections, either saline for both or 10 mg/kg PQ (1,1′-dimethyl-4,4′-bipyridinium) dichloride hydrate (Sigma) first and 30 mg/kg MB (manganese bisethylenedithiocarbamate) (Chem Service) second. PQ and MB were dissolved separately in saline on the day of administration. Mice were treated chronically (twice weekly for 8 weeks) in the following initial randomly assigned groups: Saline control (n=8); PQ and MB (n=12); caffeine (5mg/kg) control, (n=8); caffeine (5mg/kg) + (PQ and MB) (n=12); caffeine (20mg/kg) control (n=8); and caffeine (20mg/kg) + (PQ and MB) (n=12). A mortality rate of 42% in the toxin-treated groups consistent with the upper range of prior experience in this PQ and MB paradigm (M. Thiruchelvam, personal communication) occurred equally (5 of 12 mice) across these three pesticide groups, and thus is unlikely to affect comparisons among them. Toxin treatment-related deaths occurred between the 2nd and 13th dose with the majority of mice expiring between the 4th and 5th dose. Mortality was low (0–25%) in the three control groups not treated with toxins, leaving final n’s of 8,7 and 6 for all analyses in control groups pretreated with 0, 5 and 20 mg/kg caffeine, respectively. Body weights were obtained twice a week during the course of the experiment. No differences in body weight were produced by any of the treatments, consistent with previous observations (Thiruchelvam et al., 2000a). Animals were sacrificed one week after the last injection.
During the experimental paradigm, horizontal locomotor activity subdivided into ambulation and fine movement, was assessed by an automated recording system (San Diego Instruments) in standard polypropylene cages (15 × 25 cm) placed into frames equipped with 5 infrared photocell beams (5 cm apart). Ambulation was measured as the number of sequential breaks in 2 adjacent beams and fine motor activity (which can reflect grooming and other stereotyped activities) was measured as the number of sequential breaks in a single beam. Photobeam breaks were recorded in consecutive 10 min periods for 3 hours. Mice were not tested on treatment days but 24–48 h later. Motor assessments were performed at baseline (day 0), and on the 4th, 6th and 8th week time-points over the 8-week treatment period for a total of 4 sessions. No differences in motor activity (measured over the full 3 hour testing session, or just the first 45 min or last 60 min periods) were observed during the course of the experiment due to any of the treatments (data not shown).
One week after the last injection (week 9), mouse brains were removed and immediately fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) and stored at 4 °C overnight. The paraformaldehyde-fixed brains were then transferred to 30% sucrose for cryoprotection. Brains were rinsed in PBS, placed in aluminum foil and stored at −80°C. The fixed brains were cut on a Leica microtome into 40 μm-thick sections for immunolabelling studies. Free floating sections were washed three times in PBS and treated with a 20:1 mixture of 3% hydrogen peroxide and Triton X-100. The sections were washed again three times in PBS before pre-incubation in a blocking solution of 3% milk in PBS for one hour. After a further three washes, sections were incubated overnight at 4° C with a primary antibody to tyrosine hydroxylase (monoclonal anti-TH; 1:800, Sigma). The next day the sections were washed three times before incubation with a secondary biotinylated goat anti-mouse antibody (Jackson Immunoresearch Laboratories) at 1:200 for one hour followed by three washes and incubation for one hour in ABC solution (Vectastatin, Vector Labs), before the sections were further washed in PBS and developed in 0.3 mg/ml 3-3′-diaminobenzidine tetrachloride (DAB). The stained free floating sections were mounted on glass slides and cover-slipped. These slides were counterstained with cresyl violet acetate (9-amino-5-imino-5H-benzo[a]phenoxazine acetate salt, Sigma). Complete antibody penetration was verified to cover the full thickness of sections using confocal microscopy (Melvin and Sutherland).
Stereological assessment of neuronal loss in midbrain sections was limited to the substantia nigra pars compacta (SNpc) because Thiruchelvam et al., 2000b had previously demonstrated that neuronal cell counts of the ventral tegmental area were unaffected in mice exposed to paraquat and maneb. After delineation of the SNpc at low magnification (10× objectives) the entire region was sampled at higher magnification (40× objectives). The total number of TH+ and TH− neurons in the SNpc were counted following specified criteria (West et al., 1993; Chan et al., 1997) using the optical dissector technique in 40 μm coronal sections throughout the entire substantia nigra bilaterally. The entire volume of the SN was estimated according to the principle of Cavalieri using the Bioquant Image Analysis System (R&M Biometrics, Nashville, TN). TH immunoreactive neurons were counted using 75 μm by 75 μm optical disectors at 40× power, excluding neurons in the superficial plane of section. All counts were performed by a single investigator blinded as to the treatments. The average coefficient of error (CE) from the sampling technique was 0.089 and 0.083 for the TH+ and TH− counts, respectively.
All values are expressed as mean ± SEM. Multiple group comparisons were performed using one-way ANOVA followed by Bonferroni post hoc analysis.
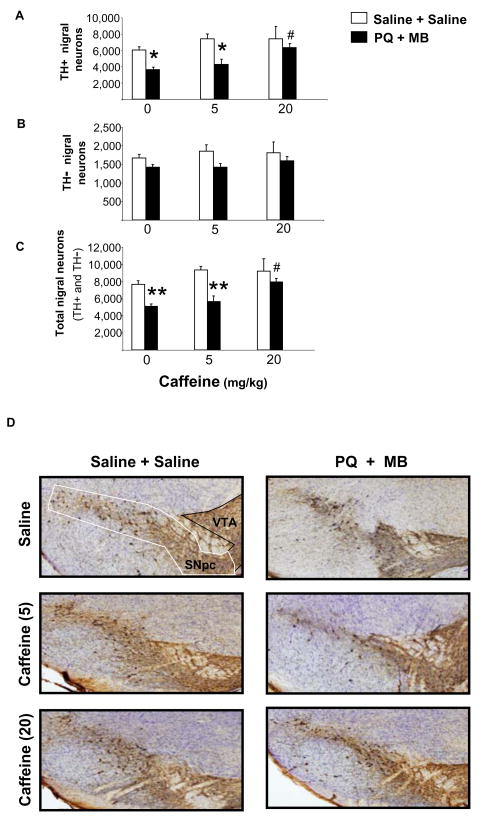
Combined PQ and MB treatments significantly decreased the number of TH+ neurons in the SNpc by approximately 40% compared to the corresponding saline-treated group (Figure, panels A and D; p<0.005). Prior caffeine treatments at 20 mg/kg but not 5 mg/kg provided a significant (p<0.05), attenuation in the loss of TH+ neurons, suggesting almost complete neuroprotection (compared to respective caffeine control group) against pesticide-induced neuronal cell loss. Total Nissl-positive neurons (i.e., total number of neurons) showed a similar profile to TH+ neuronal cell counts suggesting that these pesticides targeted the dopaminergic neurons of the SNpc (Figure, Panel C). Direct assessment of TH− neurons confirmed that there was no effect of the pesticides on this subpopulation of nigral neurons (Figure, Panel B; p>0.05). Sub-analysis of the data confirmed that changes in total nigral neuron density were comparable to changes in TH+ neuronal cell counts. Assessment of the total volume of the SNpc between groups showed no significant difference.
FIGURE 1.
Effect of drug treatments on neuronal cell counts of the substantia nigra pars compacta. Animals were sacrificed 1 week after the last treatment. (A) Total number of TH+ neurons per substantia nigra (bilateral). (B) Total number of TH− neurons per substantia nigra (bilateral). (C) Total neuron counts (TH+ and TH−) per substantia nigra (bilateral). Data are represented as group mean ± SEM. *p<0.005, **p<0.001 vs. respective control, #p<0.05 vs. saline + paraquat and maneb treatment; one-way ANOVA followed by Bonferroni post-hoc analysis). (D) Low power photomicrographs of representative sections showing PQ and MB-induced loss of TH+ neurons. Low power (4×) photomicrograph of the substantia nigra pars compacta (SNpc) stained for TH-IR and counterstained with cresyl violet acetate (Nissl) in representative tissue sections from mice pretreated with Saline, Caffeine (5 mg/kg) or Caffeine (20 mg/kg) and treated with Saline + Saline or PQ + MB. Typical delineations for stereological counting are shown for the SNpc and ventral tegmental area (VTA).
This study sought to determine whether long-term caffeine pretreatment would afford protection against dopaminergic neuron toxicity in mice chronically exposed to a combined PQ and MB. In this setting, caffeine exposure did in fact reduce the loss of TH+ neurons triggered by pesticide treatment suggesting a neuroprotective effect. However, an alternative explanation is that caffeine up-regulated TH as indicated by the tendency toward an elevation of TH+ neurons in caffeine control groups compared to the saline control group. Chronic caffeine treatment at 20mg/kg has previously been shown to increase expression of TH in nigral neurons (Datta et al., 1996), and recent studies have highlighted the importance of addressing the potential confound of up-regulated TH expression in studies of neuroprotection in models of PD (Aumann et al., 2008). If caffeine were indeed up-regulating TH expression in our experiment, then the observed increase in TH+ cell counts, albeit not significant, should correlate with a complementary decrease in TH− neurons. Such a conversion of nigral neuron phenotype appeared not to be the case, because TH-nigral cells were not reduced in number, indicating that caffeine was neuroprotective in this study. Note that the premature death of two (25%) of the mice in 20 mg/kg caffeine control group represents a potential confound of caffeine-induced systemic toxicity, though it is unlikely to have altered our interpretation.
It is also important to consider the basis of the relatively high mortality (42%) observed after pesticide treatment because interpretation of the results amongst the surviving mice may not necessarily generalize to those with greater susceptibility to systemic toxicity. Moreover, sub-lethal systemic toxicity in the surviving mice might have contributed to nigrostriatal toxicity in this model, which would lessen its significance for PD in which there is little evidence of primary systemic pathology. In rats lethal pulmonary injury is readily observed in response to paraquat exposure (Satomi et al., 2004). In a rat toxicological study that reported substantial mortality after repeated treatment with both paraquat and maneb at the same concentrations used in the present study, half the rats showed progressive weight loss before death or respiratory decline with pulmonary pathology on post-mortem analysis (Saint-Pierre et al., 2006). By contrast, the remaining half all regained their normal weight after initial loss and when later sacrificed were found to have normal organ histology, suggesting that lethal organ toxicity in a subset of animals does not necessarily imply substantial toxicity in those that survive. Moreover, in the present study the fact that mortality after chronic pesticide treatment was identical with and without caffeine pretreatment argues against an interaction between caffeine and pesticide-induced systemic mortality as the basis for the apparent neuroprotection by PD.
Locomotor activity was monitored throughout this experiment to provide a behavioral surrogate of pesticide toxicity. However, PQ and MB treatment failed to reduce basal motor activity after 8 weeks of toxin treatment. Thiruchelvam et al. 2000b had administered PQ and MB twice a week for 6 weeks to 6-week-old mice and demonstrated a motor deficit 24 hours after the last injection (6 week) time-point. This discrepancy between studies may be due to methodological differences. For example, the different substrains of C57Bl/6 mice employed in their study (C57Bl/6J) and ours (C57BL/6NCrl) may contribute to different functional manifestations of neurotoxin-induced injury to nigrostriatal dopaminergic neurons in C57Bl/6 substrains (Heikkila, 1985; Giovanni et al., 1991).
The antiparkinsonian potential of A2A antagonism has been bolstered by convergent epidemiological studies and laboratory data showing that A2A antagonists protect against acute toxin exposure in the MPTP and 6-OHDA models of PD (Xu et al., 2005). Here we demonstrate that the neuroprotective potential of caffeine extends to a chronic and potentially more environmentally relevant ‘dual pesticide’ model of PD. These data also extend our preliminary findings in the MPTP model (Oztas et al., 2002) that caffeine can prevent the degeneration per se of dopaminergic neurons, as well as their dysfunction.
Although adenosine A2A receptor antagonism likely accounts the protective effects of caffeine in MPTP models of PD (Xu et al., 2005, 2006) the mechanism by which blocking A2A receptors protects dopaminergic neurons remains unsettled. Because A2A receptors are known to facilitate potentially excitotoxic glutamate release in the CNS, it has been suggested that the neuroprotective effects of caffeine and more specific A2A antagonists may be mediated by attenuation of neuronal glutamate release (Xu et al., 2005; Popoli et al., 1995). A key role for neuronal A2A receptors in the chronic neurodegeneration of PD is supported by the recent finding that conditional knockout mice lacking neuronal A2A receptors are resistant to dopaminergic neuron lesions induced by relatively chronic (Carta et al, 2009)) though not acute (Yu et al., 2008) MPTP exposure.
Establishing the ability of caffeine to protect dopaminergic neurons in a chronic pesticide model further supports (but does not prove) the hypothesis that a neuroprotective effect of caffeine is the basis for its inverse epidemiological association with the risk of PD. The study also strengthens the rationale for consideration of caffeine and more specific A2A antagonists as therapeutic tools for slowing the underlying degenerative process.
Acknowledgments
This work was supported by National Institutes of Health Grant ES010804, the American Federation on Aging Research Paul Beeson Scholars Program, and Department of Defense grant W81XWH-04-1-0881. The authors thank Dr Eric K. Richfield, Kavita Prasad and the Molecular Histology Center at the Environmental and Occupational Health Sciences Institute (EOHSI) (http://eohsi.rutgers.edu/mhc/) for their expert advice and further training in the stereological methods employed here. We thank Mona Thiruchelvam for technical advice and Deborah Brown-Jermyn for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguiar LMV, Nobre HV, Jr, Macedo DS, Oliveira AA, Freitas RM, Vasconcelos SM, Cunha GMA, Sousa FCF, Viana GSB. Neuroprotective effects of caffeine in the model of 6-hydroxydopamine lesions in rats. Pharmacol Biochem and Behav. 2006;84(3):415–9. doi: 10.1016/j.pbb.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Anderson DW, Bradbury KA, Schneider JS. Neuroprotection in Parkinson models varies with toxin administration protocol. Eur J Neurosci. 2006;24:3174–3182. doi: 10.1111/j.1460-9568.2006.05192.x. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Zhang SM, Hernan MA, Kawachi I, Colditz GA, Speizer FE, Willett WC. Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol. 2001;50:56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Chen H, Weisskopf MG, O’Reilly E, McCullough ML, Calle EE, Schwarzschild MA, Thun MJ. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- Aumann TD, Gantois I, Egan K, Vais A, Tomas D, Drago J, Horne MK. SK channel function regulates the dopamine phenotype of neurons in the substantia nigra pars compacta. Exp Neurol. 2008;213:419–430. doi: 10.1016/j.expneurol.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Baldi I, Lebailly P, Mohammed-Brahim B, Letenneur L, Dartigues JF, Brochard P. Neurodegenerative diseases and exposure to pesticides in the elderly. Am J Epidemiol. 2003;157:409–14. doi: 10.1093/aje/kwf216. [DOI] [PubMed] [Google Scholar]
- Bove J, Serrats J, Mengod G, Corte R, Tolosa E, Marin C. Neuroprotection induced by the adenosine A2A antagonist CSC in the 6-OHDA rat model of parkinsonism: effect on the activity of striatal output pathways. Exp Brain Res. 2005;165(3):362–74. doi: 10.1007/s00221-005-2302-1. [DOI] [PubMed] [Google Scholar]
- Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron Loss. Brain Res. 1999;823:1–10. doi: 10.1016/s0006-8993(98)01192-5. [DOI] [PubMed] [Google Scholar]
- Carta AR, Kachroo A, Schintu N, Xu K, Schwarzschild MA, Wardas J, Morelli M. Inactivation of neuronal forebrain A2A receptors protects dopaminergic neurons in a mouse model of Parkinson’s disease. Journal of Neurochemistry. 2009;111:1478–1489. doi: 10.1111/j.1471-4159.2009.06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri B. Geometria degli indivisibili. Torino: Unione Tipografico. Editrice; 1966. [Google Scholar]
- Chade AR, Kasten M, Tanner CM. Non genetic causes of Parkinson’s disease. J Neural Transm Suppl. 2006;70:147–51. doi: 10.1007/978-3-211-45295-0_23. [DOI] [PubMed] [Google Scholar]
- Chan P, Dimonte DA, Langston JW, Janson AM. (+) MK-801 does not prevent MPTP-induced loss of nigral neurons in mice. J Pharmacol Exp Ther. 1997;280:439–446. [PubMed] [Google Scholar]
- Chen J-F, Xu K, Petzer JP, Staal R, Xu Y-H, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N, Jr, Schwarzschild MA. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson’s disease. J Neurosci. 2001;21:RC143, 1–6. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epi. 2009;169:919–926. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta U, Noailess AHP, Rodriguez M, Kraft M, Zhang Y. Accumulation of tyrosine hydroxylase messenger RNA molecules in the rat mesencephalon by chronic caffeine treatment. Neurosci Lett. 1996;230:77–80. doi: 10.1016/s0304-3940(96)13213-4. [DOI] [PubMed] [Google Scholar]
- Ferraz HB, Bertolucci PHF, Pereira JS, Lima JGC, Andrade LAF. Chronic exposure to the fungicide maneb may produce symptoms and signs of CNS manganese intoxication. Neurol. 1988;38:550–553. doi: 10.1212/wnl.38.4.550. [DOI] [PubMed] [Google Scholar]
- Fornai F, Schluter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, Lazzeri G, Busceti GL, Pontarelli F, Battaglia G, Pelligrini A, Nicoletti F, Ruggieri S, Paparelli A, Sudhof TC. Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin–proteasome system and alpha-synuclein. Proc Natl Acad Sci U S A. 2005;102:3413–3418. doi: 10.1073/pnas.0409713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanni A, Sieber BA, Heikkila RE, Sonsalla PK. Correlation between the neostriatal content of the 1-Methyl-4-Phenylpyridinium species and dopaminergic neurotoxicity following 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine administration to several strains of mice. J Pharmacol Exp Ther. 1991;257(2):691–697. [PubMed] [Google Scholar]
- Heikkila RE. Differential neurotoxicity of 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) in Swiss-Webster mice from different sources. Eur J Pharmacol. 1985;117:131–133. doi: 10.1016/0014-2999(85)90482-0. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Wiens M, Bowering D, Snow B, Caine D. Parkinson’s disease: a case-control study of occupational and environmental risk factors. Am J Ind Med. 1990;17:349–355. doi: 10.1002/ajim.4700170307. [DOI] [PubMed] [Google Scholar]
- Hung AY, Schwarzschild MA. Clinical trials for neuroprotection in Parkinson’s disease: overcoming angst and futility? Curr Opin Neurol. 2007;20:477–83. doi: 10.1097/WCO.0b013e32826388d6. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Kurokawa M, Aoyama S, Kuwana Y. Neuroprotection by adenosine A2A receptor blockade in experimental models of Parkinson’s disease. J Neurochem. 2002;80:262–270. doi: 10.1046/j.0022-3042.2001.00694.x. [DOI] [PubMed] [Google Scholar]
- Joghataie MT, Roghani M, Negahdar F, Hashemi L. Protective effect of caffeine against neurodegeneration in a model of Parkinson’s Disease in rat: behavioral and histochemical evidence. Parkinsonism Relat Discord. 2004;10(8):465–8. doi: 10.1016/j.parkreldis.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC. Environmental risk factors and Parkinson’s disease: a case control study in Taiwan. Neurol. 1997;48:1583–1588. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- McCarthy S, Somayajulu M, Sikorska M, Borowy-Borowski H, Pandey S. Paraquat induces oxidative stress and neuronal cell death:neuroprotection by water-soluble Coenzyme Q10. Toxicol Appl Pharmacol. 2004;201(1):21–31. doi: 10.1016/j.taap.2004.04.019. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, DiMonte DA. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10:119–27. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- Meco G, Bonifati V, Vanacore N, Fabrizio E. Parkinsonism after chronic exposure to the fungicide maneb (manganese ethylene-bisdithiocarbamate. Scand J Work Enviro Health. 1994;20:301–305. doi: 10.5271/sjweh.1394. [DOI] [PubMed] [Google Scholar]
- Melvin NR, Sutherland RJ. Quantitative Caveats of Standard Immunohistochemical Procedures: Implications for Optical Disector-based Designs. J Histochem Cytochem. 2009;10:1–28. doi: 10.1369/jhc.2009.954164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Shimoda-Matsubayashi S, Matsumine H, Morikawa N, Hattori N, Kondo T. Genetic and environmental factors in the pathogenesis of Parkinson’s disease. Adv Neurol. 1999;80:171–9. [PubMed] [Google Scholar]
- Oztas E, Kalda A, Xu K, Irizarry MC, Schwarzschild MA, Chen JF. Caffeine attenuates MPTP-induced loss of dopaminergic neurons in substantia nigra in mice. Soc. for Neurosci. Annual Meeting; 2002. Abstract # A487.6. [Google Scholar]
- Petrovitch H, Ross GW, Abbott R, Sanderson WT, Sharp DS, Tanner CM, Masaki KH, Blanchette PL, Popper JS, Foley D, Launer L, White LR. Plantation work and risk of Parkinson disease in a population-based longitudinal study. Arch Neurol. 2002;59:1787–92. doi: 10.1001/archneur.59.11.1787. [DOI] [PubMed] [Google Scholar]
- Pierri M, Vaudano E, Sager T, Englund U. KW-6002 protects from MPTP induced dopaminergic toxicity in the mouse. Neuropharmacol. 2005;48:517–524. doi: 10.1016/j.neuropharm.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Popoli P, Betto P, Reggio R, Ricciarello G. Adenosine A2A receptor stimulation enhances striatal extracellular glutamate levels in the rat striatum. Eur J Pharmacol. 1995;287:215–217. doi: 10.1016/0014-2999(95)00679-6. [DOI] [PubMed] [Google Scholar]
- Prasad K, Winnik B, Thiruchelvam MJ, Buckley B, Mirochnitchenko O, Richfield EK. Prolonged toxicokinetics and toxicodynamics of paraquat in mouse brain. Environmental Health Perspectives. 2007;115(10):1448–1453. doi: 10.1289/ehp.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravina BM, Fagan SC, Hart RG, Hovinga CA, Murphy DD, Dawson TM, Marler JR. Neuroprotective agents for clinical trials in Parkinson’s disease: a systematic assessment. Neurol. 2003;60:1234–1240. doi: 10.1212/01.wnl.0000058760.13152.1a. [DOI] [PubMed] [Google Scholar]
- Saint-Pierre M, Tremblay ME, Sik A, Gross RE, Cicchetti F. Temporal effects of Paraquat/Maneb on microglial activation and dopamine neuronal loss in older rats. J Neurochem. 2006;98:760–772. doi: 10.1111/j.1471-4159.2006.03923.x. [DOI] [PubMed] [Google Scholar]
- Satomi Y, Tsuchiya W, Mihara K, Ota M, Kasahara Y, Akahori F. Gene expression of the lung following paraquat administration in rats using DNA microarray. J Toxicol Sci. 2004;29:91–100. doi: 10.2131/jts.29.91. [DOI] [PubMed] [Google Scholar]
- Seale TW, Johnson P, Carney JM, Rennert OM. Interstrain variation in acute toxic response to caffeine among inbred mice. Pharmacol Biochem Behav. 1984;20:567–573. doi: 10.1016/0091-3057(84)90306-x. [DOI] [PubMed] [Google Scholar]
- Suchowersky O, Gronseth G, Perlmutter J, Reich S, Zesiewicz T, Weiner WJ. Quality Standards Subcommittee of the American Academy of Neurology. Practice Parameter: neuroprotective strategies and alternative therapies for Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurol. 2006;66:976–982. doi: 10.1212/01.wnl.0000206363.57955.1b. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Ross GW, Jewell SA, Hauser RA, Jankovic J, Factor SA, Bressman SA, Deligtisch A, Marras C, Lyons KE, Bhudhikanok GS, Roucoux DF, Meng C, Abbott RD, Langston JW. Occupation and risk of parkinsonism: a multicenter case control study. Arch Neurol. 2009;66(9):1106–1113. doi: 10.1001/archneurol.2009.195. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Chen WZ, Wang ML, Peng ZL, Liu X, Liang LC, Kao DW, Gilley DW, Schoenberg BS. Environmental factors in the etiology of Parkinson’s disease. Can J Neurol Sci. 1987;14:419–423. doi: 10.1017/s0317167100037835. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Brockel BJ, Richfield EK, Baggs RB, Cory-Slechta DA. Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: environmental risk factors for Parkinson’s disease? Brain Res. 2000a;873(2):225–234. doi: 10.1016/s0006-8993(00)02496-3. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, Baggs RB, Tank WA, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: Implications for Parkinson’s disease. J Neurosci. 2000b;20(24):9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchelvam M, McCormack A, Richfield EK, Baggs RB, Tank AW, Di Monte DA, Cory-Slechta DA. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson’s disease phenotype. Eur J of Neurosci. 2003;18:589–600. doi: 10.1046/j.1460-9568.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- United States Geographic Service. Pesticide National Synthesis Project. Washington, DC: USGS; 1998. [Google Scholar]
- Warner TT, Schapira AH. Genetic and environmental factors in the cause of Parkinson’s disease. Ann Neurol. 2003;53(Suppl):3S16–23. doi: 10.1002/ana.10487. discussion S23–5. [DOI] [PubMed] [Google Scholar]
- West M, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivision of the rat hippocampus using the optical fractionators. Anat Record. 1993;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wirdefeldt K, Gatz M, Schalling M, Pedersen NL. No evidence for heritability of Parkinson’s disease in Swedish twins. Neurol. 2004;63:305–311. doi: 10.1212/01.wnl.0000129841.30587.9d. [DOI] [PubMed] [Google Scholar]
- Xu K, Bastia E, Schwarzschild MA. Therapeutic potential of adenosine A(2A) receptor antagonists in Parkinson’s disease. Pharmacol Ther. 2005;105:267–310. doi: 10.1016/j.pharmthera.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Xu K, Xu Y, Chen J-F, Schwarzschild MA. Neuroprotection by caffeine in the MPTP model of Parkinson’s disease: the role of adenosine A2A receptor. Soc. for Neurosci. Annual Meeting; 2006. Abstract # 470.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Shen HY, Coelho JE, Araujo IM, Huang QY, Day YJ, Rebola N, Canas PM, Rapp EK, Ferrara J, Taylor D, Muller CE, Linden J, Cunha RA, Chen JF. Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann Neurol. 2008;63:338–346. doi: 10.1002/ana.21313. [DOI] [PubMed] [Google Scholar]