Abstract
Purpose
To describe associations of MC1R variants and melanoma in a US population and to investigate whether genetic risk is modified by pigmentation characteristics and sun exposure measures.
Methods
Melanoma patients (n=960) and controls (n=396) self-reported phenotypic characteristics and sun exposures via structured questionnaire and underwent a skin examination. Logistic regression was used to estimate associations of high [R] and low [r] risk MC1R variants and melanoma, overall and within phenotypic and sun exposure strata. A meta-analysis of results from published studies was undertaken.
Results
Carriage of two [r] or any [R] variant was associated with increased risk of melanoma (odds ratio (OR) = 1.7; 95% CI, 1.0-2.8; OR=2.2; 95% CI 1.5-3.0, respectively). However, risk was stronger in or limited to individuals with protective phenotypes and limited sun exposure such as those who tanned well after repeated sun exposure (OR=2.4; 95% CI 1.6-3.6), had dark hair (OR=2.4; 95% CI 1.5-3.6), or had dark eyes (OR=3.2, 95% CI 1.8-5.9). We noted this same pattern of increased melanoma risk among persons who did not freckle, tanned after exposure to first strong summer sun, reported little or average recreational or occupational sun exposure, or reported no sun burning events. Meta-analysis of published literature supported these findings.
Conclusions
These data indicate that MC1R genotypes provide information about melanoma risk in those individuals who would not be identified as high risk based on their phenotypes or exposures alone.
Keywords: melanoma, melanocortin-1 receptor, pigmentation phenotype, genetic variation
Introduction
The melanocortin-1 receptor gene (MC1R [MIM *155555]) encodes the melanocyte stimulating hormone receptor, a membrane-bound protein central to pathways that signal the production of melanins. Inherited variation in MC1R is a robust genetic marker for increased risk of melanoma. However, the translational impact of MC1R genotype depends upon whether this genetic “exposure” can provide information about melanoma risk beyond that already known for phenotypic risk markers such as red hair, fair complexion, high nevus counts, and presence of dysplastic nevi.1, 2 The frequency of MC1R variants in the general population suggests that a considerable proportion of melanoma risk may be attributable to these genetic variants.3
Several studies have noted that the association of MC1R genotype with risk was stronger in or limited to persons with “protective” cutaneous phenotypes, i.e. persons with darker hair and darker skin color.4-6 Although the impact of MC1R variants on melanoma risk within strata of phenotypic measures was not directly addressed in a recent meta-analysis of eleven published studies, heterogeneity of effect of the p.D294H variant was observed when comparing studies set in northern European countries (odds ratio (OR)=1.3; 95% confidence interval (CI) 0.76-2.1) to those in southern European countries (OR=2.8; 95% CI 0.16-4.7), presumably related to deeper pigmentation of southern European populations.7
To assess the association of MC1R genotype and melanoma risk, we present results from a case-control study of melanoma set in the Mid-Atlantic region of the United States that strongly suggest that the effect of certain MC1R variants is confined to persons without traditional risk factors for melanoma. We also undertook a meta-analysis of data available from the published literature to validate our finding that the risk associated with inherited MC1R variants is greater among persons with “low risk” phenotypes such as dark hair and dark skin. Our data indicate that MC1R genotyping should be considered in prediction models assessing melanoma risk.
Methods
Study participants were recruited into a case-control of melanoma susceptibility from the University of Pennsylvania Health System Pigmented Lesion Clinic (PLC) between September 1997 and December 2006. Prior work from the PLC has shown its patient population to be reasonably representative of the general population with early stage melanoma.8 Information about the study methodology previously has been published.9, 10 Briefly, melanoma subjects had a first invasive cutaneous melanoma diagnosed within the past year. We asked each enrolled case for the name of a contact without melanoma and who was not a blood relative to serve as a potential control. Because only a modest proportion (36%) of melanoma cases were willing to disclose information about potential controls, additional controls were obtained from patients with clinically dysplastic nevi who did not have melanoma and who were referred to the PLC. The majority (85.1%) of controls were spouses or partners of PLC patients, the remaining were friends (11.9%), or persons related by law (3.0%). The University of Pennsylvania Institutional Review Board approved this study; and informed consent was obtained from all participants. Information on cutaneous phenotypes and sun exposure history was obtained from a self-administered questionnaire. Each participant underwent a skin examination by a trained research nurse who recorded nevus counts, eye color, and degree of freckling. DNA was collected using a sterile buccal swab. MC1R genotypes were determined as previously described.10, 11 We used previously suggested nomenclature and definitions to group MC1R variants as higher-risk [R] variants (D84E, R151C, R160W, and D294H) or lower-risk [r] variants (all other variants excluding synonymous changes).12
Meta-analysis
We undertook a literature search for publications that presented results of MC1R associations with melanoma stratified by at least one phenotype (e.g., hair color, skin type) or sun exposure (e.g., sun burns). We searched MEDLINE through January Week 3, 2009 for publications that were referenced under the MeSH subject heading of “Melanoma” and either the MeSH subject heading of “Receptor, Melanocortin, Type 1” or the keyword “MC1R.” After limiting the search findings to human studies, 105 articles were returned including four non-English publications that were review articles. Overall, 18 reported results of associations between MC1R variants and risk of first sporadic melanoma by comparison of a melanoma group to a referent group. After cross-referencing these publications with those cited in a recent meta-analysis,7 one publication was added that was referenced under ‘Skin Neoplasms” rather than “Melanoma”.
We excluded one study in which the majority of the case group (73%) was targeted for study enrollment based on increased likelihood of underlying genetic susceptibility. Of the remaining 18 publications, we excluded studies 1) for which data on MC1R∼melanoma associations were available in a second article; 2) that did not include information on phenotypic or sun exposure measures; 3) that enrolled fewer than 50 melanoma cases and 50 controls; and 4) for which information on stratum-specific associations could not be abstracted. We also excluded results from one genome-wide association study because of fundamental differences in this study methodology compared to more traditional case-control approaches. After applying these inclusion/exclusion criteria, data were available from seven publications.4-6, 13-16
We abstracted stratum-specific associations in the form of adjusted odds ratio (aOR) and 95% confidence interval (CI) in three publications.4, 6, 13 Data in the form of number of cases and controls with and without MC1R variants were abstracted from the remaining four publications.5, 14-16 We noted whether stratum-specific melanoma associations were based on MC1R genotype categories corresponding to carriage of i) only [r] variants, ii) [R] variants (regardless of carriage of [r] variants), or iii) either [r] or [R] variants when the determination of (i) or (ii) was not possible. In the two studies that reported stratum-specific melanoma associations with MC1R [r] variants,4, 13 it was not possible to distinguish between carriage of only one [r] variant or two [r] variants.
Statistical analysis
The PLC Study
For all models, independent variables including phenotypes, exposures and MC1R genotypes were entered as class indicator variables and aOR and CI were estimated as an indirect measure of risk for each level compared to the referent level. Independent variables with more than two levels were considered as ordinal variables, and trend across categories was assessed by the chi-square test for trend. To evaluate whether phenotypic characteristics or sun exposure measures modified associations of MC1R variants and melanoma status, we determined aORs and 95% CIs within strata. Age of melanoma diagnosis within strata was compared using the non-parametric Kruskal-Wallis test. To maximize sample sizes within strata of eye color and freckling, we substituted self-reported values for their clinically assessed counterparts for those participants who did not complete a skin examination (n=170; 12.5%).
Meta-analysis
Meta-analyses were run using the Comprehensive Meta Analysis software v2.2.046 (Biostat, Inc., Englewood, NJ). We report pooled OR (pOR) estimates derived from random effects models and assessed heterogeneity of study results by the Q statistic. Within strata, a pOR was determined separately for three MC1R genotype categories, each compared to carriage of no MC1R variants: i) only [r] variants, ii) [R] variants regardless of carriage of [r] variants, and iii) either [R] or [r] variant. The primary pOR of interest represents likely carriage of at least one [R] variant and was derived by combining pORs determined for the [R] variant (ii) and either [r] or [R] variant (iii) groups, where appropriate. Absence of publication bias in studies of MC1R variants and melanoma previously has been reported.7
Results
The PLC Study
The PLC study sample consisted of 960 melanoma cases and 396 controls, all of whom reported being white of non-Hispanic origin. On average, cases were slightly older (49.8±14.5 years) than controls (47.7±13.4 years; p=0.014) and more likely to be male (49% and 45%, respectively; p=0.16).
Table 1 presents adjusted odds ratios for melanoma and cutaneous phenotype and sun exposure measures collected by questionnaire or clinical examination. We found statistically significant associations with all known risk factors. We did not find an association of occupational sun exposure with melanoma. A family history of melanoma among first degree relatives was reported by 13% of melanoma cases and 9% of controls, and was associated with a 50% increased risk of melanoma (aOR=1.5, 95% CI 1.0-2.2).
Table 1. Associations of self-reported and clinically assessed cutaneous phenotypic and sun exposure measures and melanoma status: PLC Study.
| Control (n=396) na (%) | Case (n=960) na (%) | OR (95% CI) | aORb (95% CI) | Ptrend | |
|---|---|---|---|---|---|
| Hair color | |||||
| Dark | 309 (78.0) | 623 (64.9) | 1.0 | 1.0 | |
| Blond | 66 (16.7) | 216 (22.5) | 1.6 (1.2-2.2) | 1.7 (1.2-2.3) | |
| Red | 21 (5.3) | 121 (12.6) | 2.9 (1.8-4.6) | 3.0 (1.9-4.9) | p<0.001 |
| Eye color | |||||
| Brown | 148 (37.4) | 255 (26.6) | 1.0 | 1.0 | |
| Green or hazel | 106 (26.8) | 307 (32.0) | 1.7 (1.2-2.3) | 1.7 (1.3-2.3) | |
| Blue or grey | 142 (35.9) | 397 (41.4) | 1.6 (1.2-2.1) | 1.6 (1.2-2.1) | p=0.0012 |
| Skin reaction to first strong summer sun | |||||
| No burn | 33 (8.4) | 44 (4.6) | 1.0 | 1.0 | |
| Mild burn then tan | 208 (52.7) | 393 (41.3) | 1.4 (0.88-2.3) | 1.5 (0.90-2.4) | |
| Burn without blister | 119 (30.1) | 381 (40.1) | 2.4 (1.5-3.9) | 2.6 (1.5-4.2) | |
| Burn and blister | 35 (8.9) | 133 (14.0) | 2.9 (1.6-5.1) | 3.0 (1.7-5.5) | p<0.001 |
| Skin reaction to long and repeated exposure to sun | |||||
| Medium or dark tan | 296 (75.5) | 583 (61.7) | 1.0 | 1.0 | |
| Light tan | 84 (21.4) | 297 (31.4) | 1.8 (1.4-2.4) | 1.9 (1.4-2.5) | |
| No tan | 12 (3.1) | 65 (6.9) | 2.8 (1.5-5.2) | 2.9 (1.5-5.4) | p<0.001 |
| Freckling | |||||
| None | 163 (41.3) | 198 (20.7) | 1.0 | 1.0 | |
| Some | 141 (35.7) | 357 (37.4) | 2.1 (1.6-2.8) | 2.1 (1.6-2.8) | |
| A lot | 91 (23.0) | 401 (42.0) | 3.6 (2.7-4.9) | 3.8 (2.8-5.2) | p<0.001 |
| Recreational sun exposure | |||||
| A little | 10 (2.5) | 32 (3.3) | 1.5 (0.74, 3.2) | 1.5 (0.71, 3.1) | |
| Average | 230 (58.1) | 480 (50.2) | 1.0 | 1.0 | |
| A lot | 156 (39.4) | 445 (46.5) | 1.4 (1.1, 1.7) | 1.4 (1.1, 1.8) | p=0.028c |
| Occupational sun exposure | |||||
| A little | 248 (62.6) | 606 (63.3) | 1.0 | 1.0 | |
| Average | 104 (26.3) | 237 (24.8) | 0.93 (0.71, 1.2) | 0.88 (0.67, 1.2) | |
| A lot | 44 (11.1) | 114 (11.9) | 1.1 (0.73, 1.5) | 1.0 (0.70, 1.5) | p=0.83 |
| Number of sunburns (before age 18) | |||||
| 0 | 80 (22.3) | 138 (16.1) | 1.0 | 1.0 | |
| 1-3 | 145 (40.4) | 317 (37.0) | 1.3 (0.90-1.8) | 1.3 (0.95-1.9) | |
| 4-10 | 100 (27.9) | 263 (30.7) | 1.5 (1.1-2.2) | 1.6 (1.1-2.4) | |
| 11 or more | 34 (9.5) | 139 (16.2) | 2.4 (1.5-3.8) | 2.5 (1.6-4.0) | p<0.001 |
| Number of sunburns (after age 18) | |||||
| 0 | 96 (25.2) | 199 (22.3) | 1.0 | 1.0 | |
| 1-3 | 193 (50.7) | 434 (48.6) | 1.1 (0.81-1.5) | 1.1 (0.83-1.5) | |
| 4-10 | 76 (20.0) | 198 (22.2) | 1.3 (0.88-1.8) | 1.3 (0.91-1.9) | |
| 11 or more | 16 (4.2) | 63 (7.1) | 1.9 (1.0-3.5) | 1.9 (1.0-3.5) | p=0.023 |
| Clinically assessed cutaneous phenotypes | |||||
| Eye color | |||||
| Brown | 117 (39.4) | 240 (27.2) | 1.0 | 1.0 | |
| Green or hazel | 70 (23.6) | 235 (26.6) | 1.6 (1.2-2.3) | 1.6 (1.2-2.3) | |
| Blue or grey | 110 (37.0) | 407 (46.2) | 1.8 (1.3-2.4) | 1.8 (1.3-2.4) | p<0.001 |
| Freckling | |||||
| None | 45 (15.1) | 45 (5.1) | 1.0 | 1.0 | |
| Mild | 84 (28.2) | 164 (18.5) | 2.0 (1.2-3.2) | 2.0 (1.2-3.2) | |
| Moderate | 57 (19.1) | 229 (25.9) | 4.0 (2.4-6.7) | 4.1 (2.4-6.8) | |
| Heavy | 112 (37.6) | 448 (50.6) | 4.0 (2.5-6.4) | 4.1 (2.5-6.6) | p<0.001 |
| Total nevus count | |||||
| 0-8 | 134 (44.6) | 196 (22.1) | 1.0 | 1.0 | |
| 9-20 | 86 (28.7) | 184 (20.8) | 1.5 (1.0-2.1) | 1.7 (1.2-2.4) | |
| 21-53 | 60 (20.0) | 231 (26.1) | 2.6 (1.8-3.8) | 3.2 (2.2-4.7) | |
| 54 or more | 20 (6.7) | 275 (31.0) | 9.4 (5.7-15) | 13 (7.7-22) | p<0.001 |
| Number of dysplastic nevi | |||||
| 0 | 249 (84.4) | 472 (53.5) | 1.0 | 1.0 | |
| 1 | 20 (6.8) | 134 (15.2) | 3.5 (2.2-5.8) | 3.7 (2.3-6.1) | |
| 2 | 14 (4.8) | 108 (12.2) | 4.1 (2.3-7.3) | 4.4 (2.5-7.9) | |
| 3 or more | 12 (4.1) | 169 (19.1) | 7.4 (4.1-14) | 8.6 (4.7-16) | p<0.01 |
| Number of large (≥8mm) nevi | |||||
| 0 | 251 (83.7) | 570 (64.3) | 1.0 | 1.0 | |
| 1 | 32 (10.7) | 160 (18.0) | 2.2 (1.5-3.3) | 2.3 (1.5-3.5) | |
| 2 | 8 (2.7) | 58 (6.6) | 3.2 (1.5-6.8) | 3.8 (1.7-8.2) | |
| 3 or more | 9 (3.0) | 98 (11.1) | 4.8 (2.4-9.6) | 7.2 (3.3-16) | p<0.001 |
Totals may vary due to missing data.
OR adjusted for age and sex; OR for clinically assessed cutaneous phenotypes further adjusted for examiner.
P-value for χ2 analysis testing heterogeneity among categories is reported.
Genomic DNA was obtained from 952 (99.2%) cases and 330 (98.5%) controls. MC1R genotypes were obtained from 779 (81.2%) cases and 325 (82.1%) controls. We detected 44 unique MC1R variants (Table 2). We found a statistically significant trend (p<0.001) of increasing melanoma risk comparing carriage of multiple and higher-risk variants to carriage of the MC1R consensus sequence alone (Table 3). After adjustment for age, sex, and hair color, carriage of two MC1R [r] variants was associated with a 70% increased risk of melanoma (aOR=1.7; 95% CI 1.0-2.8), while carriage of at least one high risk MC1R [R] variant was associated with a near 2-fold risk of melanoma (aOR=1.9; 95% CI 1.3-2.8). Carriage of only one low risk MC1R [r] variant was not associated with melanoma. We found similar results when separately adjusting for other phenotypic characteristics, including eye color, freckling, and skin reaction to first strong summer sun or repeated sun exposure (data not tabulated).
Table 2. MC1R variants, allele and genotype frequencies in individuals with (cases) and without (controls) melanoma: PLC Study.
| Nucleotide change | Amino acid change | Control (n=325) | Case (n=779) | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Allele Frequencya | |||||
| Nonsynonymous | |||||
| g.178T>G | p.V60L | 95 | 14.6 | 226 | 14.5 |
| g.252C>A | p.D84E | 6 | 0.9 | 14 | 0.9 |
| g.274G>A | p.V92M | 62 | 9.5 | 144 | 9.2 |
| g.425G>A | p.R142H | 9 | 1.4 | 11 | 0.7 |
| g.451C>T | p.R151C | 35 | 5.4 | 156 | 10.0 |
| g.464T>C | p.I155T | 8 | 1.2 | 16 | 1.0 |
| g.478C>T | p.R160W | 44 | 6.8 | 151 | 9.7 |
| g.488G>A | p.R163Q | 23 | 3.5 | 60 | 3.9 |
| g.880G>C | p.D294H | 10 | 1.5 | 58 | 3.7 |
| Rareb,f | 8 | 1.4 | 29 | 1.9 | |
| Insertion/deletionc,f | 0 | 0 | 14 | 0.9 | |
| Synonymous | |||||
| g.942A>G | p.T314T | 71 | 10.9 | 175 | 11.2 |
| Rared,f | 6 | 0.9 | 17 | 1.1 | |
| Genotype Frequencye | |||||
| Any variant (excluding synonymous) | |||||
| 0 | 96 | 29.5 | 174 | 22.3 | |
| 1 | 157 | 48.3 | 336 | 43.1 | |
| 2 | 72 | 22.2 | 264 | 33.9 | |
| 3 | 0 | 0.0 | 5 | 0.64 | |
| Any [R] variant | |||||
| 0 | 239 | 73.5 | 446 | 57.3 | |
| 1 | 77 | 23.7 | 287 | 36.8 | |
| 2 | 9 | 2.8 | 46 | 5.9 | |
| Any [r] variant | |||||
| 0 | 148 | 45.5 | 373 | 47.9 | |
| 1 | 148 | 45.5 | 315 | 40.4 | |
| 2 | 29 | 8.9 | 88 | 11.3 | |
| 3 | 0 | 0.0 | 3 | 0.39 | |
Allele frequency is determined from the total number of chromosomes genotyped.
A group indicating carriage of any of 24 nsSNP.
A group indicating carriage of g.86_87insA, g.411delC, or g.537_538insC.
A group indicating carriage of any of nine sSNP.
Genotype frequency is determined from the total number of individuals genotyped.
A detailed listing of variants is available upon request.
Table 3. Associations of MC1R genotype categories and melanoma status: PLC Study.
| MC1R genotype | Control | Case | |||
|---|---|---|---|---|---|
| n (%) | n (%) | OR (95% CI) | aORa (95% CI) | aORb (95% CI) | |
| consensusa / consensus | 96 (29.5) | 174 (22.3) | 1.0 | 1.0 | 1.0 |
| [r] / consensus | 114 (35.1) | 183 (23.5) | 0.89 (0.63, 1.2) | 0.88 (0.62, 1.2) | 0.89 (0.63, 1.3) |
| [r] / [r] | 29 (8.9) | 89 (11.4) | 1.7 (1.0, 2.8) | 1.7 (1.0, 2.7) | 1.7 (1.0, 2.8) |
| [R] / con | 43 (13.2) | 153 (19.6) | 2.0 (1.3, 3.0) | 2.0 (1.3, 3.0) | 1.8 (1.2, 2.8) |
| [R] / [r] | 34 (10.5) | 134 (17.2) | 2.2 (1.4, 3.4) | 2.2 (1.4, 3.5) | 1.9 (1.2, 3.1) |
| [R] / [R] | 9 (2.8) | 46 (5.9) | 2.8 (1.3, 6.0) | 2.9 (1.3, 6.0) | 1.9 (0.84, 4.4) |
| Ptrend<0.001 | Ptrend<0.001 | ||||
| Any [R] | 86 (26.5) | 333 (42.8) | 2.1 (1.5, 3.0) | 2.2 (1.5, 3.0) | 1.9 (1.3, 2.8) |
Adjusted for age and sex.
Adjusted for age, sex, and hair color.
Consensus indicates no observed MC1R variants.
Results from analyses of MC1R stratified by phenotypic and sun exposure measures are shown in Table 4. Compared to persons who inherited no MC1R variants, carriage of any [R] variants increased melanoma nearly 2½-fold (OR=2.4; 95% CI 1.6-3.6) among those who tanned moderately or deeply after long and repeated sun exposure, while among those who tanned only lightly or not at all, no association with MC1R [R] variants was noted (OR=1.0; 95% CI 0.44-2.4; and OR=0.60; 95% CI 0.06-5.9, respectively). Similarly, carriage of any MC1R [R] variant was also associated with increased risk among participants with dark hair (OR=2.4; 95% CI 1.5-3.6), while no increased risk was evident among those with blond (OR=1.1; 95% CI 0.44-2.5) or red (OR=0.81; 95% CI 0.16-4.1) hair. Without exception for all other phenotypes and sun exposure measures, the strongest effect of MC1R [R] variants on melanoma risk was seen in those “protected” individuals. We also noted a similar pattern of increased risk associated with carriage of two MC1R [r] variants among those with the more protective phenotypic and sun exposure measures. To explore whether skin type accounted for our observed associations, we adjusted for skin reaction to long and repeated sun exposure and skin reaction in response to the first strong summer sun in analyses of hair color, eye color, and freckling as well as those of sun exposure measures. Although we noted some change in stratum-specific associations (Table 4), none impacted the interpretation of the results.
Table 4. Associations of MC1R genotype and melanoma stratified by cutaneous phenotype, nevus phenotype, and sun exposure measures.
| Phenotype or Exposure Category | MC1R genotype | Controlsa | Casesa | ORb (95% CI) | ORc (95% CI) |
|---|---|---|---|---|---|
| Cutaneous Phenotype | |||||
| Hair Color | |||||
| Red | [r] / consensus | 3 ∣ 5 | 5 ∣ 18 | 0.25 (0.03 - 2.1) | 0.28 (0.02, 3.7) |
| [r] / [r] | 0 ∣ 2 | 7 ∣ 20 | n.e.d | n.e.d | |
| Any [R] | 14 ∣ 16 | 71 ∣ 84 | 0.81 (0.16 - 4.1) | 0.86 (0.14, 5.4) | |
| Blond | [r] / consensus | 16 ∣ 25 | 30 ∣ 64 | 0.50 (0.19 - 1.3) | 0.47 (0.18, 1.2) |
| [r] / [r] | 3 ∣ 12 | 11 ∣ 44 | 1.0 (0.23 - 4.4) | 0.98 (0.22, 4.4) | |
| Any [R] | 25 ∣ 34 | 96 ∣ 130 | 1.1 (0.44 - 2.5) | 0.99 (0.41, 2.4) | |
| Dark | [r] / consensus | 95 ∣ 180 | 148 ∣ 275 | 1.0 (0.70 - 1.5) | 0.98 (0.66, 1.4) |
| [r] / [r] | 26 ∣ 111 | 71 ∣ 198 | 1.8 (1.0 - 3.0) | 1.6 (0.95, 2.9) | |
| Any [R] | 47 ∣ 132 | 166 ∣ 293 | 2.4 (1.5 - 3.6) | 2.2 (1.4, 3.3) | |
| Eye Color | |||||
| Blue/grey | [r] / consensus | 39 ∣ 67 | 75 ∣ 139 | 0.86 (0.48 - 1.6) | 0.86 (0.47, 1.6) |
| [r] / [r] | 9 ∣ 37 | 44 ∣ 108 | 2.2 (0.94 - 5.1) | 2.6 (1.1, 6.3) | |
| Any [R] | 39 ∣ 67 | 158 ∣ 222 | 1.8 (1.0 - 3.1) | 1.8 (1.0, 3.2) | |
| Green/hazel | [r] / consensus | 21 ∣ 42 | 56 ∣ 109 | 1.0 (0.51 - 2.1) | 1.0 (0.48, 2.1) |
| [r] / [r] | 7 ∣ 28 | 23 ∣ 76 | 1.3 (0.48 - 3.4) | 1.2 (0.42, 3.3) | |
| Any [R] | 23 ∣ 44 | 85 ∣ 138 | 1.5 (0.73 - 2.9) | 1.3 (0.64, 2.7) | |
| Darker | [r] / consensus | 53 ∣ 99 | 51 ∣ 106 | 0.77 (0.44 - 1.3) | 0.72 (0.41, 1.3) |
| [r] / [r] | 13 ∣ 59 | 22 ∣ 77 | 1.4 (0.63 - 3.1) | 1.2 (0.51, 2.7) | |
| Any [R] | 23 ∣ 69 | 88 ∣ 143 | 3.2 (1.8 - 5.9) | 2.6 (1.4, 4.9) | |
| Skin reaction to first strong summer sun | |||||
| Burn and blister | [r] / consensus | 13 ∣ 17 | 19 ∣ 35 | 0.35 (0.09 - 1.3) | |
| [r] / [r] | 3 ∣ 7 | 13 ∣ 29 | 1.1 (0.20 - 5.8) | ||
| Any [R] | 10 ∣ 14 | 56 ∣ 72 | 1.4 (0.37 - 5.0) | ||
| Burn without blister | [r] / consensus | 29 ∣ 49 | 70 ∣ 138 | 0.72 (0.37 - 1.4) | |
| [r] / [r] | 9 ∣ 29 | 29 ∣ 97 | 0.94 (0.38 - 2.3) | ||
| Any [R] | 37 ∣ 57 | 141 ∣ 209 | 1.1 (0.60 - 2.1) | ||
| Mild burn then tan | [r] / consensus | 60 ∣ 119 | 86 ∣ 165 | 1.1 (0.66 - 1.7) | |
| [r] / [r] | 17 ∣ 59 | 38 ∣ 117 | 1.6 (0.83 - 3.2) | ||
| Any [R] | 37 ∣ 96 | 118 ∣ 197 | 2.3 (1.4 - 3.8) | ||
| Tan or no change | [r] / consensus | 12 ∣ 25 | 5 ∣ 16 | 0.37 (0.09 - 1.5) | |
| [r] / [r] | 0 ∣ 13 | 6 ∣ 17 | n.e.d | ||
| Any [R] | 2 ∣ 15 | 16 ∣ 27 | 9.1 (1.6 - 50) | ||
| Skin reaction to long and repeated sun exposure | |||||
| No tan | [r] / consensus | 3 ∣ 4 | 9 ∣ 19 | 0.31 (0.03 - 3.7) | |
| [r] / [r] | 1 ∣ 1 | 3 ∣ 13 | 0.20 (0.01 - 5.3) | ||
| Any [R] | 5 ∣ 6 | 31 ∣ 41 | 0.60 (0.06 - 5.9) | ||
| Light tan | [r] / consensus | 22 ∣ 31 | 49 ∣ 92 | 0.49 (0.20 - 1.2) | |
| [r] / [r] | 8 ∣ 17 | 30 ∣ 73 | 0.79 (0.27 - 2.3) | ||
| Any [R] | 24 ∣ 33 | 116 ∣ 159 | 1.0 (0.44 - 2.4) | ||
| Medium or dark tan | [r] / consensus | 88 ∣ 174 | 124 ∣ 243 | 0.99 (0.67 - 1.5) | |
| [r] / [r] | 19 ∣ 105 | 53 ∣ 172 | 2.0 (1.1 - 3.6) | ||
| Any [R] | 55 ∣ 141 | 180 ∣ 299 | 2.4 (1.6 - 3.6) | ||
| Freckling | |||||
| Heavy | [r] / consensus | 28 ∣ 44 | 73 ∣ 143 | 0.60 (0.30 - 1.2) | 0.63 (0.31, 1.3) |
| [r] / [r] | 10 ∣ 26 | 50 ∣ 120 | 1.1 (0.47 - 2.7) | 1.4 (0.55, 3.3) | |
| Any [R] | 46 ∣ 62 | 174 ∣ 244 | 0.85 (0.45 - 1.6) | 0.81 (0.42, 1.6) | |
| Moderate | [r] / consensus | 18 ∣ 32 | 55 ∣ 98 | 1.0 (0.46 - 2.4) | 0.92 (0.40, 2.1) |
| [r] / [r] | 6 ∣ 20 | 19 ∣ 62 | 1.0 (0.34 - 3.1) | 0.71 (0.22, 2.3) | |
| Any [R] | 20 ∣ 34 | 89 ∣ 132 | 1.5 (0.68 - 3.3) | 1.3 (0.57, 2.9) | |
| Mild | [r] / consensus | 33 ∣ 67 | 37 ∣ 82 | 0.83 (0.44 - 1.6) | 0.82 (0.42, 1.6) |
| [r] / [r] | 9 ∣ 43 | 14 ∣ 59 | 1.2 (0.45 - 3.0) | 1.0 (0.38, 2.7) | |
| Any [R] | 17 ∣ 51 | 56 ∣ 101 | 2.5 (1.2 - 5.0) | 2.2 (1.1, 4.6) | |
| No | [r] / consensus | 35 ∣ 67 | 18 ∣ 34 | 1.0 (0.45 - 2.4) | 1.0 (0.44, 2.5) |
| [r] / [r] | 4 ∣ 36 | 6 ∣ 22 | 2.9 (0.72 - 12) | 2.7 (0.61, 12) | |
| Any [R] | 3 ∣ 35 | 12 ∣ 28 | 8.2 (2.0 - 33) | 8.3 (1.9, 37) | |
| Nevus Phenotype | |||||
| Total nevus count | |||||
| 54+ | [r] / consensus | 6 ∣ 12 | 54 ∣ 94 | 1.3 (0.39 - 4.4) | |
| [r] / [r] | 0 ∣ 6 | 24 ∣ 64 | n.e.d | ||
| Any [R] | 2 ∣ 8 | 112 ∣ 152 | 9.0 (1.7 - 47) | ||
| 21-53 | [r] / consensus | 19 ∣ 30 | 46 ∣ 86 | 0.68 (0.29 - 1.6) | |
| [r] / [r] | 5 ∣ 16 | 28 ∣ 68 | 1.6 (0.50 - 5.1) | ||
| Any [R] | 16 ∣ 27 | 82 ∣ 122 | 1.5 (0.62 - 3.5) | ||
| 9-20 | [r] / consensus | 20 ∣ 44 | 27 ∣ 77 | 0.64 (0.30 - 1.4) | |
| [r] / [r] | 8 ∣ 32 | 17 ∣ 67 | 1.1 (0.40 - 3.0) | ||
| Any [R] | 18 ∣ 42 | 50 ∣ 100 | 1.4 (0.69 - 3.0) | ||
| 0-8 | [r] / consensus | 43 ∣ 71 | 38 ∣ 74 | 0.68 (0.34 - 1.3) | |
| [r] / [r] | 9 ∣ 37 | 18 ∣ 54 | 1.5 (0.59 - 4.1) | ||
| Any [R] | 32 ∣ 60 | 62 ∣ 98 | 1.3 (0.68 - 2.6) | ||
| Number of dysplastic nevi | |||||
| 4+ | [r] / consensus | 3 ∣ 3 | 29 ∣ 53 | 1.0 (0.18 - 5.9) | |
| [r] / [r] | 1 ∣ 4 | 21 ∣ 45 | 2.6 (0.24 - 28) | ||
| Any [R] | 1 ∣ 4 | 68 ∣ 92 | 9.6 (0.89 - 103) | ||
| 2-3 | [r] / consensus | 2 ∣ 4 | 26 ∣ 41 | 1.7 (0.21 - 14) | |
| [r] / [r] | 2 ∣ 4 | 10 ∣ 25 | 0.67 (0.08 - 5.7) | ||
| Any [R] | 6 ∣ 8 | 47 ∣ 62 | 1.2 (0.21 - 6.7) | ||
| 1 | [r] / consensus | 7 ∣ 11 | 26 ∣ 50 | 0.55 (0.14 - 2.2) | |
| [r] / [r] | 1 ∣ 5 | 17 ∣ 41 | 2.5 (0.25 - 25) | ||
| Any [R] | 5 ∣ 9 | 48 ∣ 72 | 1.6 (0.38 - 6.4) | ||
| 0 | [r] / consensus | 75 ∣ 135 | 83 ∣ 186 | 0.63 (0.40 - 0.98) | |
| [r] / [r] | 18 ∣ 78 | 39 ∣ 142 | 1.3 (0.66 - 2.4) | ||
| Any [R] | 55 ∣ 115 | 143 ∣ 246 | 1.5 (0.98 - 2.4) | ||
| Number of large nevi | |||||
| 3+ | [r] / consensus | 2 ∣ 4 | 17 ∣ 33 | 0.76 (0.08 - 6.9) | |
| [r] / [r] | 2 ∣ 4 | 13 ∣ 29 | 0.68 (0.07 - 6.2) | ||
| Any [R] | 2 ∣ 4 | 38 ∣ 54 | 2.6 (0.32 - 21) | ||
| 2 | [r] / consensus | 0 ∣ 2 | 11 ∣ 18 | n.e.d | |
| [r] / [r] | 2 ∣ 4 | 4 ∣ 11 | 0.36 (0.03 - 4.7) | ||
| Any [R] | 4 ∣ 6 | 22 ∣ 29 | 0.99 (0.12 - 8.1) | ||
| 1 | [r] / consensus | 7 ∣ 16 | 28 ∣ 60 | 1.2 (0.37 - 3.7) | |
| [r] / [r] | 0 ∣ 9 | 20 ∣ 52 | n.e.d | ||
| Any [R] | 6 ∣ 15 | 53 ∣ 85 | 2.7 (0.82 - 8.7) | ||
| 0 | [r] / consensus | 79 ∣ 135 | 109 ∣ 220 | 0.68 (0.44 - 1.0) | |
| [r] / [r] | 18 ∣ 74 | 50 ∣ 161 | 1.4 (0.73 - 2.6) | ||
| Any [R] | 56 ∣ 112 | 193 ∣ 304 | 1.7 (1.1 - 2.7) | ||
| Sun Exposure | |||||
| Recreational sun | |||||
| Lots | [r] / consensus | 49 ∣ 87 | 81 ∣ 168 | 0.70 (0.41 - 1.2) | 0.67 (0.39, 1.2) |
| [r] / [r] | 15 ∣ 53 | 40 ∣ 127 | 1.2 (0.57 - 2.3) | 1.0 (0.51, 2.2) | |
| Any [R] | 36 ∣ 74 | 158 ∣ 245 | 2.0 (1.2 - 3.3) | 1.6 (0.94, 2.8) | |
| Little or average | [r] / consensus | 65 ∣ 123 | 101 ∣ 188 | 1.0 (0.66 - 1.6) | 1.0 (0.64, 1.6) |
| [r] / [r] | 14 ∣ 72 | 49 ∣ 136 | 2.3 (1.2 - 4.6) | 2.2 (1.1, 4.5) | |
| Any [R] | 50 ∣ 108 | 174 ∣ 261 | 2.3 (1.5 - 3.7) | 2.1 (1.3, 3.4) | |
| Occupational sun | |||||
| Lots | [r] / consensus | 10 ∣ 19 | 17 ∣ 37 | 0.68 (0.22 - 2.1) | 0.61 (0.18, 2.1) |
| [r] / [r] | 6 ∣ 15 | 10 ∣ 30 | 0.67 (0.18 - 2.5) | 0.58 (0.14, 2.4) | |
| Any [R] | 12 ∣ 21 | 43 ∣ 63 | 1.7 (0.58 - 4.7) | 1.2 (0.37, 3.8) | |
| Average | [r] / consensus | 28 ∣ 56 | 47 ∣ 92 | 1.0 (0.53 - 2.0) | 1.0 (0.51, 2.0) |
| [r] / [r] | 10 ∣ 38 | 14 ∣ 59 | 0.84 (0.33 - 2.2) | 0.81 (0.29, 2.3) | |
| Any [R] | 21 ∣ 49 | 87 ∣ 132 | 2.7 (1.4 - 5.2) | 2.2 (1.1, 4.5) | |
| Little | [r] / consensus | 76 ∣ 135 | 118 ∣ 227 | 0.83 (0.54 - 1.3) | 0.78 (0.50, 1.2) |
| [r] / [r] | 13 ∣ 72 | 65 ∣ 174 | 2.6 (1.3 - 5.1) | 2.3 (1.2, 4.6) | |
| Any [R] | 53 ∣ 112 | 202 ∣ 311 | 2.1 (1.3 - 3.2) | 1.8 (1.2, 2.9) | |
| Number of sunburns (after age 18) | |||||
| 11+ | [r] / consensus | 5 ∣ 7 | 6 ∣ 20 | 0.17 (0.03 - 1.2) | 0.11 (0.01, 0.87) |
| [r] / [r] | 3 ∣ 5 | 3 ∣ 17 | 0.15 (0.02 - 1.3) | 0.11 (0.01, 1.2) | |
| Any [R] | 5 ∣ 7 | 28 ∣ 42 | 0.81 (0.14 - 4.9) | 0.70 (0.11, 4.4) | |
| 4-10 | [r] / consensus | 16 ∣ 34 | 33 ∣ 68 | 1.0 (0.45 - 2.4) | 0.92 (0.39, 2.2) |
| [r] / [r] | 6 ∣ 24 | 21 ∣ 56 | 1.7 (0.57 - 4.9) | 1.6 (0.50, 5.4) | |
| Any [R] | 23 ∣ 41 | 74 ∣ 109 | 1.6 (0.78 - 3.4) | 1.3 (0.62, 2.9) | |
| 1-3 | [r] / consensus | 52 ∣ 100 | 97 ∣ 176 | 1.1 (0.69 - 1.9) | 1.1 (0.64, 1.8) |
| [r] / [r] | 13 ∣ 61 | 34 ∣ 113 | 1.6 (0.76 - 3.3) | 1.4 (0.66, 3.0) | |
| Any [R] | 42 ∣ 90 | 142 ∣ 221 | 2.1 (1.3 - 3.4) | 1.8 (1.1, 3.0) | |
| 0 | [r] / consensus | 31 ∣ 59 | 35 ∣ 65 | 1.0 (0.51 - 2.1) | 1.1 (0.54, 2.4) |
| [r] / [r] | 6 ∣ 34 | 24 ∣ 54 | 3.6 (1.3 - 10) | 3.6 (1.3, 10) | |
| Any [R] | 12 ∣ 40 | 69 ∣ 99 | 5.3 (2.4 - 12) | 4.8 (2.1, 11) | |
| Number of sunburns (before age 18) | |||||
| 11+ | [r] / consensus | 10 ∣ 17 | 20 ∣ 43 | 0.64 (0.20 - 2.0) | 0.58 (0.17, 2.0) |
| [r] / [r] | 3 ∣ 10 | 13 ∣ 36 | 1.4 (0.31 - 6.5) | 2.1 (0.35, 12) | |
| Any [R] | 10 ∣ 17 | 58 ∣ 81 | 1.9 (0.64 - 5.8) | 1.9 (0.59, 6.0) | |
| 4-10 | [r] / consensus | 23 ∣ 44 | 54 ∣ 95 | 1.2 (0.59 - 2.5) | 1.2 (0.56, 2.5) |
| [r] / [r] | 11 ∣ 32 | 14 ∣ 55 | 0.65 (0.25 - 1.7) | 0.52 (0.19, 1.4) | |
| Any [R] | 29 ∣ 50 | 107 ∣ 148 | 1.8 (0.94 - 3.6) | 1.6 (0.79, 3.2) | |
| 1-3 | [r] / consensus | 41 ∣ 77 | 61 ∣ 114 | 0.97 (0.54 - 1.7) | 1.0 (0.56, 1.8) |
| [r] / [r] | 8 ∣ 44 | 37 ∣ 90 | 3.0 (1.2 - 7.2) | 2.9 (1.2, 7.1) | |
| Any [R] | 27 ∣ 63 | 104 ∣ 157 | 2.5 (1.4 - 4.6) | 2.4 (1.3, 4.5) | |
| 0 | [r] / consensus | 25 ∣ 51 | 27 ∣ 57 | 0.92 (0.43 - 2.0) | 0.93 (0.42, 2.1) |
| [r] / [r] | 3 ∣ 29 | 16 ∣ 46 | 4.5 (1.2 - 17) | 4.8 (1.2, 19) | |
| Any [R] | 9 ∣ 35 | 38 ∣ 68 | 3.6 (1.5 - 9.0) | 4.1 (1.5, 11) |
Numbers of individuals with MC1R variants and total number of individuals (# with ∣ total #).
OR are adjusted for age and sex; the referent group is individuals who do not carry any MC1R variant (consensus / consensus) within that stratum.
OR additionally adjusted for skin reaction to long and repeated sun exposure and skin reaction in response to the first strong summer sun; the referent group is individuals who do not carry any MC1R variant (consensus / consensus) within that stratum.
OR not estimable due to zero cell count.
For counts of total, dysplastic, and large nevi, we did not find the same pattern of association between MC1R variants and melanoma risk (Table 4). In contrast, risk of melanoma associated with MC1R [R] variants among persons with few total moles (OR=1.3, 95% CI 0.68-2.6 for ≤8) or no dysplastic nevi (OR=1.5, 95% CI 0.98-2.4) were similar to or less than those among persons with increased numbers of total nevi (OR=9.0, 95% CI 1.7-47 for ≥54; OR=1.5, 95% CI 0.62-3.5 for 21-53) or dysplastic nevi (OR=9.6, 95% CI 0.89-103 for ≥4; OR=1.2, 95% CI 0.21-6.7 for 2-3).
We also explored whether age at melanoma diagnosis was associated with MC1R genotype. For most comparisons, median age of diagnosis was not statistically significantly different across genotype categories within strata of phenotypic variables (data not tabulated). In those strata where differences were noted, there was no consistent pattern of diagnosis age across genotypic categories. However, we found a difference in the median age of diagnosis by MC1R status among persons without a family history of melanoma in first degree relatives (p=0.01), with melanoma cases who carried at least one [R] variant tending to have earlier median age at diagnosis (46 years, interquartile range 36-55) than those in other MC1R genotype categories. No difference in age at diagnosis by genotype status was noted among those with a family history of melanoma (p=0.48).
Meta-analysis of MC1R variants and melanoma by level of cutaneous phenotype
Summary information on the seven studies4, 5, 13-16 included in the meta-analysis is available from the corresponding author upon request. We calculated pORs for measures for which data were available from at least three publications, including our present results. Forest plots for associations of MC1R genotypes and melanoma by for phenotype and sun exposure are given in Figure 1a-l. Results from these analyses indicated that pORs for associations of MC1R [R] variants were stronger among individuals with dark hair (pOR=2.5, 95% CI 2.0-3.1) than those with light hair (pOR=1.4, 95% CI, 0.97-2.1), with dark eyes (pOR=2.8, 95% CI 1.7-4.5) than those with light eyes (pOR=1.8, 95% CI 1.3-2.6), with dark skin (pOR=2.1, 95% CI 1.2-3.9) than those with light skin (pOR=1.4, 95% CI 0.81-2.5). Further, associations were as strong among individuals with skin type III/IV (pOR=2.3, 95% CI 1.5-3.5) than those with type I/II skin (pOR=2.2, 95% CI 0.90-5.1) and among those reporting low recreational sun exposure (pOR=2.2, 95% CI 1.5-3.2) than those with high recreational sun exposure (pOR=2.0, 95% CI 1.2-3.3).
Figure 1. Meta-analysis of associations of MC1R genotype and melanoma stratified by hair color (a, b), eye color (c,d), skin color (e, f), skin type (g, h), sun exposure (i, j), and nevus count (k, l).
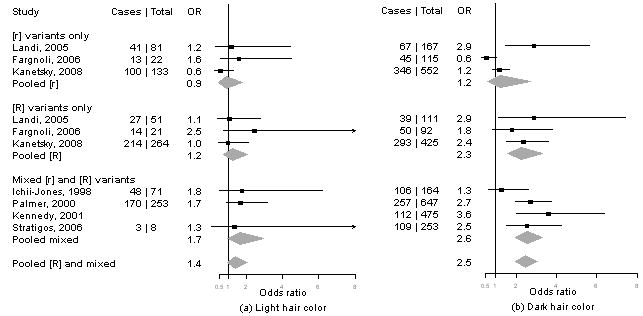
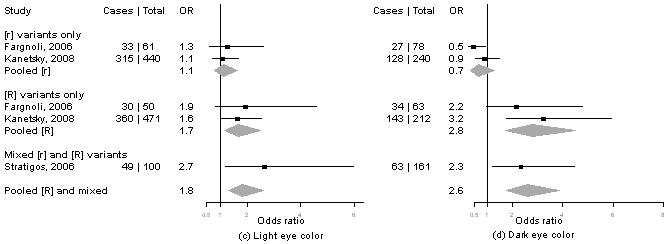
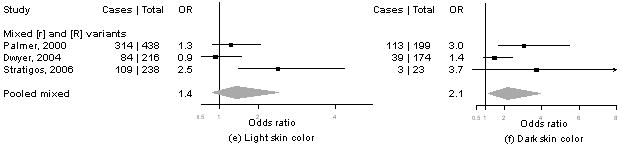
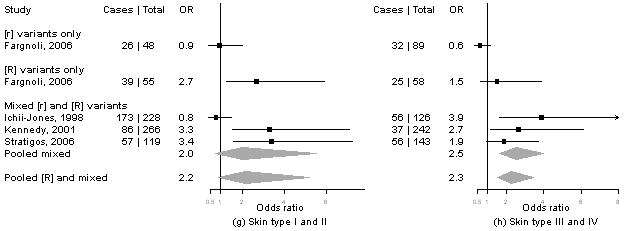
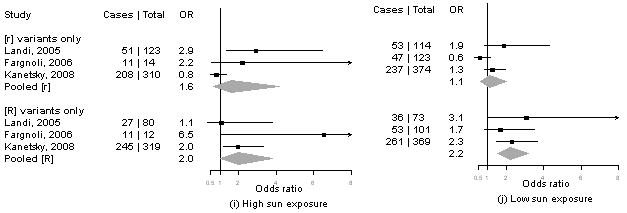
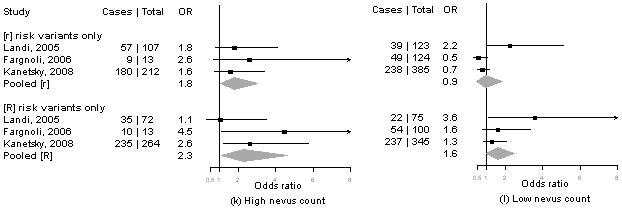
Study-specific odds ratios (OR, squares) and 95% confidence intervals (CI, horizontal lines) and pooled odds ratios and 95% CI (diamonds) for carriage of MC1R variants are shown; for all comparisons, the referent group is individuals who do not carry any MC1R variant. Number of cases and total number of individuals within each stratum are indicated.
In contrast, the pOR for associations of MC1R [R] variants and melanoma were smaller among individuals with low nevus counts (pOR=1.6, 1.0-2.5) and no dysplastic nevi (pOR=1.4, 95% CI 0.90-2.1) than those with high nevus counts (pOR=2.3, 95% CI 1.1-4.6) or any dysplastic nevi (pOR=3.1, 95% CI 0.62-16).
Discussion
Since first shown to be associated with human pigmentation characteristics, numerous investigators have demonstrated that natural variation in MC1R is associated with increase risk of melanoma. Palmer et al. first reported that the melanoma risk conferred by MC1R genotypes was strongest among persons with darker skin tones even after adjustment for hair color and suggested that risk associated with MC1R may be modified by pigmentation characteristics.5 This effect measure modification was later noted in a second study set in Australian and one set in Italy.4, 6 Here, we confirmed that MC1R variants are associated with increased melanoma risk in a U.S. population and extended previous findings to show that genetic risk is greater not only in those with darker hair or skin, but is largely limited to those characterized by phenotypes and sun exposure levels considered protective against melanoma development. The results of our meta-analyses further demonstrate increased risk of melanoma among person with dark hair, dark eyes, dark skin color, skin type III or IV, and low levels of recreational sun exposure. Thus, results from the PLC study together with results from these meta-analyses strongly suggest that MC1R genotype provides information about melanoma risk beyond that of oculocutaneous phenotype and sun exposure. We conclude that the combination of MC1R genotype and phenotype or sun exposure data may be vital to melanoma risk prediction in persons with otherwise “protective” phenotypes. Without knowledge of MC1R genotypes, these individuals would otherwise be considered at low melanoma risk.
We considered several potential sources of bias in the PLC study. First, we compared melanoma cases who referred a control for study recruitment (n=339, 35%) to those who did not provide a referred control (n=621, 65%) and found no difference for most associations of pigmentation or sun exposure phenotypes; further, MC1R genotype categories did not differ between these cases. Second, we compared characteristics of the 339 controls referred by melanoma cases to the 57 controls referred by patients with a clinically dysplastic nevus, all of whom were seen in the same ascertainment clinic. We did not observe a difference in MC1R genotypes between these groups. As expected, controls referred by clinically dysplastic nevus patients were younger (mean age=42.3) than controls referred by melanoma cases (mean age=48.7; p=0.0007); they were also more likely to have a dysplastic nevus (χ2=5.80, df=1, p=0.016) and more extensive freckling (χ2=8.42, df=1, p=0.038). This suggests that patients diagnosed with dysplastic nevi were more likely to refer a control based on perceived increased risk of melanoma and the need to undergo a free full-body skin examination as part of this research. This selection pressure would tend to create a control group that overall was more similar to melanoma cases and a potential bias toward the null hypothesis. Despite these potential biases, all traditional risk factors were statistically significantly associated with melanoma status in our study; and strengths of associations were consistent with previously published work.1, 2
We defined high risk MC1R [R] variants as p.D84E, p.R151C, p.R160W, p.D294H based on prior work,12 but other classification schemes are possible. Secondary analysis considering the p.R142H, p.I155T, g.86_87insA, g.411delC, and g.537_538insC as [R] variants did not meaningfully alter interpretation of results. Our finding that carriage of two MC1R [r] variants increases risk of melanoma by 70% (95% CI 1.0-2.7) is consistent with recent results demonstrating a per allele risk of 1.2 (95% CI 1.1-1.3) associated with carriage of the p.V60L, p.V92M, p.I155I, or p.R163Q variant17 and with functional analysis demonstrating that the activity of the p.V60L and p.R163Q variant receptor is compromised compared to native MC1R function.18
We acknowledge that for several of the meta-analyses, the total number of studies contributing information was small and power to detect heterogeneity of effect was modest. Interestingly, while many meta-analyses did demonstrate significant heterogeneity, it is notable that we did not find heterogeneity in the pooled estimate for any meta-analysis of MC1R [R] variants within the “protective” phenotypic or sun exposure strata. This suggests that the MC1R-phenotype relationship with melanoma risk is robust across various studies and further supports the credibility of this finding.
There is potential for a substantial public health impact of using MC1R genetic information in conjunction with phenotype and/or exposure data. Raimondi et al. reported a combined etiologic fraction (EF) for the p.D84E, p.R151C, p.R160W, and p.D294H variants of 15.0%.7 Under the assumption of a causal relationship between MC1R and melanoma, this EF would mean that nearly 15% of melanomas are attributable to the genetic effects of these four MC1R variants. This figure, however, likely underestimates the EF among those persons with protective phenotype and sun exposure measures because associations with [R] variants are stronger in these groups.
Using data from the present study and focusing on only the four MC1R [R] variants for simplicity, the estimated EFs {[(OR-1) / OR] × proportion of cases carrying MC1R [R] variants} ranged from 33% among dark haired individuals to 42% among dark eyed individuals. We applied these EFs to population estimates of the proportion of melanoma occurring in individuals within each protective phenotype as reported by the Genes, Environment, and Melanoma study. This study enrolled over 2400 cases with first primary melanoma from across nine international ascertainment centers.19 These results suggest that between 8 to 33% of all melanomas could be detected early in their natural history and potentially cured by screening for MC1R [R] variants among persons with protective phenotypes. Although two risk estimation models for melanoma have been published,20, 21 neither had MC1R genotypes available for analysis. Echoing prior commentary by Whiteman and Green,22 we believe that this study establishes the carriage of MC1R [R] variants as a risk factor to be considered when developing and testing new multivariable risk models. Its addition may improve a model's clinically utility by increasing calibration, improving risk categorization and enhancing classification accuracy.23 Knowing MC1R status can empower clinicians to emphasize skin self-examination and sun-protection behavior for those patients who otherwise believe that they are at lower risk for melanoma based on their phenotypic characteristics alone.
Acknowledgments
We thank the patients of the PLC for their willingness to participate in this research. We also acknowledge the contributions of Lindsey Ackerman, Zoe Harris, Robin Holmes, Derek Najarian, Sharon Sandel, Jennifer Swoyer, Sharon Voros, Amy Walker, and former PLC clinicians.
Research support: This work was supported by the National Cancer Institute (CA75434 to DG, CA80700 to PAK, CA092428 to TRR and PAK), and CA016520 to the Abramson Cancer Center of the University of Pennsylvania in support of the Melanoma Core.
Footnotes
Financial disclosures: None
References
- 1.Bliss JM, Ford D, Swerdlow AJ, et al. Risk of cutaneous melanoma associated with pigmentation characteristics and freckling: systematic overview of 10 case-control studies. Int J Cancer. 1995;62:367–376. doi: 10.1002/ijc.2910620402. [DOI] [PubMed] [Google Scholar]
- 2.Chang YM, Newton-Bishop JA, Bishop DT, et al. A pooled analysis of melanocytic nevus phenotype and the risk of cutaneous melanoma at different latitudes. Int J Cancer. 2009 Jan 15;124(2):420–428. doi: 10.1002/ijc.23869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerstenblith MR, Goldstein AM, Fargnoli MC, Peris K, Landi MT. Comprehensive evaluation of allele frequency differences of MC1R variants across populations. Hum Mutat. 2007 May;28(5):495–505. doi: 10.1002/humu.20476. [DOI] [PubMed] [Google Scholar]
- 4.Landi MT, Kanetsky PA, Tsang S, et al. MC1R, ASIP, and DNA repair in sporadic and familial melanoma in a Mediterranean population. Journal of the National Cancer Institute. 2005 Jul 6;97(13):998–1007. doi: 10.1093/jnci/dji176. [see comment][erratum appears in. [DOI] [PubMed] [Google Scholar]; J Natl Cancer Inst. 2005 Sep 21;97(18):1385. doi: 10.1093/jnci/dji285. [DOI] [PubMed] [Google Scholar]
- 5.Palmer JS, Duffy DL, Box NF, et al. Melanocortin-1 receptor polymorphisms and risk of melanoma: is the association explained solely by pigmentation phenotype? Am J Hum Genet. 2000;66(1):176–186. doi: 10.1086/302711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dwyer T, Stankovich JM, Blizzard L, et al. Does the addition of information on genotype improve prediction of the risk of melanoma and nonmelanoma skin cancer beyond that obtained from skin phenotype? Am J Epidemiol. 2004 May 1;159(9):826–833. doi: 10.1093/aje/kwh120. [DOI] [PubMed] [Google Scholar]
- 7.Raimondi S, Sera F, Gandini S, et al. MC1R variants, melanoma and red hair color phenotype: a meta-analysis. Int J Cancer. 2008 Jun 15;122(12):2753–2760. doi: 10.1002/ijc.23396. [DOI] [PubMed] [Google Scholar]
- 8.Gimotty PA, Elder DE, Fraker DL, et al. Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J Clin Oncol. 2007 Mar 20;25(9):1129–1134. doi: 10.1200/JCO.2006.08.1463. [DOI] [PubMed] [Google Scholar]
- 9.Kanetsky PA, Holmes R, Walker A, et al. Interaction of glutathione S-transferase M1 and T1 genotypes and malignant melanoma. Cancer Epidemiol Biomarkers Prev. 2001;10(5):509–513. [PubMed] [Google Scholar]
- 10.Kanetsky PA, Ge F, Najarian D, et al. Assessment of polymorphic variants in the melanocortin-1 receptor gene with cutaneous pigmentation using an evolutionary approach. Cancer Epidemiol Biomarkers Prev. 2004;13(5):808–819. [PubMed] [Google Scholar]
- 11.Kanetsky PA, Rebbeck TR, Hummer AJ, et al. Population-based study of natural variation in the melanocortin-1 receptor gene and melanoma. Cancer Res. 2006 Sep 15;66(18):9330–9337. doi: 10.1158/0008-5472.CAN-06-1634. [DOI] [PubMed] [Google Scholar]
- 12.Sturm RA, Duffy DL, Box NF, et al. The role of melanocortin-1 receptor polymorphism in skin cancer risk phenotypes. Pigment Cell Res. 2003;16(3):266–272. doi: 10.1034/j.1600-0749.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 13.Fargnoli MC, Altobelli E, Keller G, Chimenti S, Hofler H, Peris K. Contribution of melanocortin-1 receptor gene variants to sporadic cutaneous melanoma risk in a population in central Italy: a case-control study. Melanoma Research. 2006 Apr;16(2):175–182. doi: 10.1097/01.cmr.0000198454.11580.b5. [DOI] [PubMed] [Google Scholar]
- 14.Stratigos AJ, Dimisianos G, Nikolaou V, et al. Melanocortin receptor-1 gene polymorphisms and the risk of cutaneous melanoma in a low-risk southern European population. Journal of Investigative Dermatology. 2006 Aug;126(8):1842–1849. doi: 10.1038/sj.jid.5700292. see comment. [DOI] [PubMed] [Google Scholar]
- 15.Ichii-Jones F, Lear JT, Heagerty AH, et al. Susceptibility to melanoma: influence of skin type and polymorphism in the melanocyte stimulating hormone receptor gene. Journal of Investigative Dermatology. 1998 Aug;111(2):218–221. doi: 10.1046/j.1523-1747.1998.00287.x. see comment. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy C, ter Huurne J, Berkhout M, et al. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J Invest Dermatol. 2001;117(2):294–300. doi: 10.1046/j.0022-202x.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- 17.Gudbjartsson DF, Sulem P, Stacey SN, et al. ASIP and TYR pigmentation variants associate with cutaneous melanoma and basal cell carcinoma. Nat Genet. 2008 May 18; doi: 10.1038/ng.161. [DOI] [PubMed] [Google Scholar]
- 18.Beaumont KA, Shekar SN, Cook AL, Duffy DL, Sturm RA. Red hair is the null phenotype of MC1R. Hum Mutat. 2008 May 16; doi: 10.1002/humu.20788. [DOI] [PubMed] [Google Scholar]
- 19.Berwick M, Orlow I, Hummer AJ, et al. The prevalence of CDKN2A germ-line mutations and relative risk for cutaneous malignant melanoma: an international population-based study. Cancer Epidemiol Biomarkers Prev. 2006 Aug;15(8):1520–1525. doi: 10.1158/1055-9965.EPI-06-0270. [DOI] [PubMed] [Google Scholar]
- 20.Fears TR, Guerry Dt, Pfeiffer RM, et al. Identifying Individuals at High Risk of Melanoma: A Practical Predictor of Absolute Risk. J Clin Oncol. 2006 May 25; doi: 10.1200/JCO.2005.04.1277. [DOI] [PubMed] [Google Scholar]
- 21.Cho E, Rosner BA, Feskanich D, Colditz GA. Risk factors and individual probabilities of melanoma for whites. J Clin Oncol. 2005 Apr 20;23(12):2669–2675. doi: 10.1200/JCO.2005.11.108. [DOI] [PubMed] [Google Scholar]
- 22.Whiteman DC, Green AC. A risk prediction tool for melanoma? Cancer Epidemiol Biomarkers Prev. 2005 Apr;14(4):761–763. doi: 10.1158/1055-9965.EPI-14-4-ED. [DOI] [PubMed] [Google Scholar]
- 23.Janes H, Pepe MS, Gu W. Assessing the value of risk predictions by using risk stratification tables. Ann Intern Med. 2008 Nov 18;149(10):751–760. doi: 10.7326/0003-4819-149-10-200811180-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]


